Ethosuximide, sodium valproate or lamotrigine for absence seizures in children and adolescents
Abstract
Background
This is an updated version of the Cochrane Review previously published in 2019.
Absence seizures (AS) are brief epileptic seizures which present in childhood and adolescence. Depending on clinical features and electroencephalogram (EEG) findings they are divided into typical, atypical absences, and absences with special features. Typical absences are characterised by sudden loss of awareness and an EEG typically shows generalised spike wave discharges at three cycles per second. Ethosuximide, valproate and lamotrigine are currently used to treat absence seizures. This review aims to determine the best choice of antiepileptic drug for children and adolescents with AS.
Objectives
To review the evidence for the effects of ethosuximide, valproate and lamotrigine as treatments for children and adolescents with absence seizures (AS), when compared with placebo or each other.
Search methods
For the latest update we searched the Cochrane Register of Studies (CRS Web, 22 September 2020) and MEDLINE (Ovid, 1946 to September 21, 2020). CRS Web includes randomised or quasi‐randomised, controlled trials from PubMed, Embase, ClinicalTrials.gov, the World Health Organization International Clinical Trials Registry Platform (ICTRP), the Cochrane Central Register of Controlled Trials (CENTRAL), and the Specialized Registers of Cochrane Review Groups including Epilepsy. No language restrictions were imposed. In addition, we contacted Sanofi Winthrop, Glaxo Wellcome (now GlaxoSmithKline) and Parke Davis (now Pfizer), manufacturers of sodium valproate, lamotrigine and ethosuximide respectively.
Selection criteria
Randomised parallel group monotherapy or add‐on trials which include a comparison of any of the following in children or adolescents with AS: ethosuximide, sodium valproate, lamotrigine, or placebo.
Data collection and analysis
Outcome measures were: 1. proportion of individuals seizure free at one, three, six, 12 and 18 months post randomisation; 2. individuals with a 50% or greater reduction in seizure frequency; 3. normalisation of EEG and/or negative hyperventilation test; and 4. adverse effects. Data were independently extracted by two review authors. Results are presented as risk ratios (RR) with 95% confidence intervals (95% CIs). We used GRADE quality assessment criteria to evaluate the certainty of evidence for the outcomes derived from all included studies.
Main results
On the basis of our selection criteria, we included no new studies in the present review. Eight small trials (total number of participants: 691) were included from the earlier review. Six of them were of poor methodological quality (unclear or high risk of bias) and seven recruited less than 50 participants. There are no placebo‐controlled trials for ethosuximide or valproate, and hence, no evidence from randomised controlled trials (RCTs) to support a specific effect on AS for either of these two drugs. Due to the differing methodologies used in the trials comparing ethosuximide, lamotrigine and valproate, we thought it inappropriate to undertake a meta‐analysis. One large randomised, parallel double‐blind controlled trial comparing ethosuximide, lamotrigine and sodium valproate in 453 children with newly diagnosed childhood absence epilepsy found that at 12 months, seizure freedom was higher in patients taking ethosuximide (70/154, 45%) than in patients taking lamotrigine (31/146, 21%; P < 0.001), with no difference between valproate (64/146, 44%) and ethosuximide (70/154, 45%; P > 0.05).
In this study, the frequency of treatment failures due to intolerable adverse events was significantly different among the treatment groups, with the largest proportion of adverse events in the valproic acid group (48/146, 33%) compared to the ethosuximide (38/154, 25%) and the lamotrigine (29/146, 20%) groups (P < 0.037). Overall, this large study demonstrates the superior effectiveness of ethosuximide and valproic acid compared to lamotrigine as initial monotherapy aimed to control seizures without intolerable adverse effects in children with childhood absence epilepsy. This study provided high certainty of the evidence for outcomes for which data were available. However, the certainty of the evidence provided by the other included studies was low, primarily due to risk of bias and imprecise results because of the small sample sizes. Hence, conclusions regarding the efficacy of ethosuximide, valproic acid and lamotrigine derive mostly from this single study.
Authors' conclusions
Since the last version of this review was published, we have found no new studies. Hence, the conclusions remain the same as the previous update. With regards to both efficacy and tolerability, ethosuximide represents the optimal initial empirical monotherapy for children and adolescents with AS. However, if absence and generalised tonic‐clonic seizures coexist, valproate should be preferred, as ethosuximide is probably inefficacious on tonic‐clonic seizures.
PICO
Plain language summary
Ethosuximide, sodium valproate or lamotrigine for absence seizures in children and adolescents
Background
Epilepsy is a disorder where seizures are caused by abnormal electrical discharges from the brain. Absence epilepsy involves seizures that cause a sudden loss of awareness. It often starts in childhood or adolescence. Three antiepileptic drugs are often used for absence epilepsy: valproate, ethosuximide and lamotrigine.
This review aims to determine which of these three antiepileptic drugs is the best choice for the treatment of absence seizures in children and adolescents.
Results
The review found some evidence (based on eight small trials) that individuals taking lamotrigine are more likely to be seizure free than those using placebos. The review found robust evidence that patients taking ethosuximide or valproate are more likely to be seizure free than those using lamotrigine. However, because of the lower risk of adverse effects, the use of ethosuximide is preferred over valproate in patients with absence childhood epilepsy.
With regards to both efficacy and tolerability, ethosuximide represents the optimal initial empirical monotherapy for children and adolescents with absence seizures.
The evidence is current to September 2020.
Authors' conclusions
Summary of findings
| Ethosuximide compared to valproate for absence seizures in children and adolescents | ||||||
| Patient or population: absence seizures in children and adolescents | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with valproate | Risk with ethosuximide | |||||
| Seizure freedom at 12 months | Study population | ‐ | 365 | ⊕⊕⊕⊝ | None of the included trials found a difference for this outcome. Length of follow‐up in included studies: from 6 weeks to 4 years. | |
| see comment | see comment | |||||
| 80% or greater reduction in seizure frequency | Study population | RR 0.70 | 29 | ⊕⊝⊝⊝ | No difference was found, but the confidence interval is wide and equivalence cannot be inferred. Length of follow‐up: 6 weeks. | |
| 286 per 1,000 | 200 per 1,000 | |||||
| 50% or greater reduction in seizure frequency | Study population | ‐ | 49 | ⊕⊕⊝⊝ | No difference was found, but the confidence interval is wide and equivalence cannot be inferred. Length of follow‐up in included studies: from 1 to 4 years. | |
| see comment | see comment | |||||
| Normalisation of the EEG ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Adverse effects (Table 1; Table 2) Ethosuximide treatment was mostly associated with nausea, vomiting, and behavioural/psychiatric changes. The most common adverse effects of treatment with valproate were fatigue, nausea, vomiting, increased appetite with weight gain, behavioural/psychiatric changes (decreased concentration, personality change, hyperactivity, attention problems, hostility), and thrombocytopenia | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Most information is from studies at low or unclear risk of bias; plausible bias is likely to seriously alter the results. 2 Information is from a study with unclear and high risk of bias; plausible bias is likely to seriously alter the results. 3 Small number of patients included in this study (29) (see also footnote below). 4 Information is from two small studies with unclear or high risk of bias; plausible bias is likely to seriously alter the results. 5 Information is from two studies with small number of patients included. aIn this study, one patient in the ethosuximide group was subsequently treated with valproate, but failed to respond to either single drug and did not improve when both drugs were used in combination. The outcomes of this patient on combined treatment were therefore counted twice ("no remission"), since the patient received both drugs. | ||||||
| Event | ||||||
|---|---|---|---|---|---|---|
| Acute pancreatitis | 1 | |||||
| Obesity/Weight gain | 1 | 1 | 14 | |||
| Drowsiness | 4 | |||||
| Nausea | 5 | 3 | 12* | |||
| Vomiting | 1 | 2 | 12* | |||
| Decreased platelet numbers | 2 | 4 | ||||
| Increased appetite | 15 | |||||
| Poor appetite | 1 | 8 | ||||
| Diarrhoea | 1 | 7 | ||||
| Dizziness | 1 | 2 | ||||
| Hyperactivity | 23 | |||||
| Attention problems | 24 | |||||
| Hostility | 22 | |||||
| Concentration decreased | 18 | |||||
| Personality change | 17 | |||||
| Sleep problem | 17 | |||||
| Depression | 11 | |||||
| Slow process speed | 11 | |||||
| Memory problem | 10 | |||||
| Apathy | 9 | |||||
| Fatigue | 27 | |||||
| Headache | 1 | 18 | ||||
| Leukopenia | 2 | |||||
| Elevated liver function tests | 1 | 7 | ||||
| Elevated LDH | 1 | |||||
| Rash | 2 | |||||
| *Nausea, vomiting, or both Numbers of individuals within each study undertaking valproate: 14 (Callaghan 1982), 22 (Sato 1982), 10 (Martinovic 1983), 19 (Coppola 2004), 23 (Huang 2009), 146 (Glauser 2013a). | ||||||
| Event | ||||
|---|---|---|---|---|
| Drowsiness | 1 | 5 | ||
| Tiredness | 2 | |||
| Nausea | 3 | 2 | 29* | |
| Vomiting | 3 | 29* | ||
| Increased appetite | 6 | |||
| Poor appetite | 1 | 10 | ||
| Diarrhoea | 9 | |||
| Dizziness | 1 | 10 | ||
| Headache | 2 | 23 | ||
| Leukopenia | 3 | |||
| Hiccups | 1 | |||
| Moodiness | 1 | |||
| Hyperactivity | 13 | |||
| Attention problems | 8 | |||
| Hostility | 4 | |||
| Concentration decreased | 6 | |||
| Personality change | 6 | |||
| Sleep problem | 11 | |||
| Depression | 4 | |||
| Slow process speed | 3 | |||
| Memory problem | 0 | |||
| Apathy | 4 | |||
| Fatigue | 26 | |||
| Rash | 6 | |||
| * Nausea, vomiting, or both Numbers of individuals within each study undertaking ethosuximide: 14 (Callaghan 1982), 23 (Sato 1982), 10 (Martinovic 1983), 154 (Glauser 2013a). | ||||
| Lamotrigine compared to valproate for absence seizures in children and adolescents | ||||||
| Patient or population: absence seizures in children and adolescents | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with valproate | Risk with lamotrigine | |||||
| Seizure freedom at 12 months | Study population | ‐ | 405 | ⊕⊕⊕⊝ | Higher proportion seizure free at 1 month in patients receiving valproate compared to those receiving lamotrigine (2 studies). No difference between valproate and lamotrigine for seizure freedom at 3 and 6 months (3 and 4 studies, respectively). Length of follow‐up in included studies: 12 months. | |
| see comment | see comment | |||||
| 80% or greater reduction in seizure frequency ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 50% or greater reduction in seizure frequency ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Normalisation of the EEG | Study population | RR 2.39 | 45 | ⊕⊝⊝⊝ | Length of follow‐up: 12 months. | |
| 273 per 1,000 | 652 per 1,000 | |||||
| Adverse effects (Table 1; Table 3) The most common adverse effects of treatment with lamotrigine were fatigue, and behavioural/psychiatric changes. The most common adverse effects of treatment with valproate were fatigue, nausea, vomiting, increased appetite with weight gain, behavioural/psychiatric changes (decreased concentration, personality change, hyperactivity, attention problems, hostility), and thrombocytopenia | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Most information comes from studies at low or unclear risk of bias; plausible bias is likely to seriously alter the results. | ||||||
| Event | ||||
|---|---|---|---|---|
| Abdominal pain | 5 | |||
| Headache | 2 | 2 | 14 | |
| Nausea | 3 | 2* | ||
| Vomiting | 2* | |||
| Poor appetite | 2 | 9 | ||
| Increased appetite | 1 | 10 | ||
| Diarrhoea | 2 | |||
| Dizziness | 3 | 5 | 5 | |
| Hyperkinesia | 2 | |||
| Hyperactivity | 12 | |||
| Attention problems | 11 | |||
| Hostility | 11 | |||
| Concentration decreased | 9 | |||
| Personality change | 10 | |||
| Sleep problem | 5 | |||
| Depression | 11 | |||
| Slow process speed | 7 | |||
| Memory problem | 8 | |||
| Apathy | 3 | |||
| Fatigue | 1 | 18 | ||
| Rash | 10 | 1 | 2 | 6 |
| Nervousness | 1 | |||
| Diplopia | 1 | |||
| *Nausea, vomiting, or both Numbers of individuals within each study undertaking lamotrigine: 15 (Frank 1999), 19 (Coppola 2004), 24 (Huang 2009), 146 (Glauser 2013a). | ||||
| Ethosuximide compared to lamotrigine for absence seizures in children and adolescents | ||||||
| Patient or population: absence seizures in children and adolescents | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with lamotrigine | Risk with ethosuximide | |||||
| Seizure freedom at 12 months | Study population | RR 0.47 | 300 | ⊕⊕⊕⊕ | Length of follow‐up: 12 months. | |
| 455 per 1,000 | 214 per 1,000 | |||||
| 80% or greater reduction in seizure frequency ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 50% or greater reduction in seizure frequency ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Normalisation of the EEG ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Adverse effects (Table 2; Table 3) Ethosuximide treatment was mostly associated with nausea, vomiting, and behavioural/psychiatric changes. The most common adverse effects of treatment with lamotrigine were fatigue, and behavioural/psychiatric changes. | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
Background
This is an updated version of the Cochrane Review previously published in 2019 (Brigo 2019).
Description of the condition
Absence seizures (AS) are brief epileptic seizures characterised by sudden loss of awareness. Depending on clinical features and electroencephalogram (EEG) findings, they are divided into typical AS, atypical AS, and AS with special features (Berg 2010; Tenney 2013). About 10% of seizures in children with epilepsy are typical AS. Typical AS are associated with an EEG showing regular generalised and symmetrical spike and slow wave complexes at a frequency of three cycles per second at the same time as the absence. Childhood seizure disorders are classified into syndromes, which take into account seizure types, age and EEG changes. Typical AS may be the only seizure type experienced by a child and this then constitutes either an epileptic syndrome called childhood absence epilepsy or juvenile absence epilepsy. However, AS may also be only one of multiple types of seizures, for example in juvenile myoclonic epilepsy where myoclonic and tonic‐clonic seizures occur as well as AS. Atypical AS are characterised by less abrupt onset and offset, longer duration, changes in muscular tone, and variable impairment of consciousness; they are associated with interictal 1.5‐2.5 Hz irregular, asymmetrical spike and wave complexes on the EEG, and with diffuse, irregular slow spike and wave as ictal pattern. The 2010 revised International League Against Epilepsy (ILAE) Report on Terminology and Classification has recently recognised two additional types of AS, which are associated with special features: myoclonic AS and eyelid myoclonia with absence (EMA) (Berg 2010). Seizures occurring in EMA are clinically associated with jerkings of the eyelids with upward eye‐deviation, which are usually triggered by eye closure; the ictal EEG shows 3‐6 Hz generalised polyspike and wave complexes, sometimes associated with occipital paroxysmal discharges.
Description of the intervention
Ethosuximide, valproic acid and lamotrigine are drugs commonly used for the treatment of children with AS. Ethosuximide was introduced into clinical practice in 1958, and is currently indicated only for the treatment of generalised AS; gastrointestinal side effects (nausea, vomiting, anorexia, and diarrhoea) occur in 4% to 29% of patients receiving ethosuximide (Shorvon 2010). Valproic acid is one of the most commonly prescribed antiepileptic drugs in the world, and thanks to its wide spectrum of activity, represents the drug of first choice for many types of epilepsy, including idiopathic generalised epilepsy; its risk of teratogenicity greatly limits its use in women of child‐bearing age (Shorvon 2010). Lamotrigine is a broad‐spectrum antiepileptic drug, which is used as add‐on or monotherapy of focal seizures and generalised seizures; it is generally effective and well tolerated, despite the risk of rash which can sometimes be severe, and complicated pharmacokinetics (Shorvon 2010).
How the intervention might work
The antiepileptic properties of ethosuximide and its efficacy against AS are due to its voltage‐dependent blockade of low‐threshold T‐ type calcium currents in the thalamus (Shorvon 2010). The mechanisms of action of valproate are various and not yet fully elucidated; it enhances inhibitory neurotransmission (mainly mediated by gamma‐Aminobutyric acid and glutamic acid), but it also reduces conductance at the voltage‐dependent sodium channel, as well as calcium (T) and potassium conductance (the latter mechanism may explain its efficacy against AS) (Shorvon 2010). Similar to carbamazepine or phenytoin, lamotrigine exerts its antiepileptic activity blocking voltage–dependent sodium channel conductance (Shorvon 2010).
Why it is important to do this review
Non‐systematic reviews have suggested that ethosuximide and sodium valproate are equally effective (Duncan 1995). Valproate is considered the drug of choice in juvenile myoclonic epilepsy (Chadwick 1987; Christe 1989), although there is little in the way of evidence from randomised controlled trials (RCTs) to support this. Lamotrigine used to be considered a second‐line drug, reserved for intractable AS (Duncan 1995), but its use has increased with time. It is especially valued in situations where sodium valproate leads to weight gain and also for women of childbearing age. The latter is due to fears of a higher rate of fetal abnormalities in pregnancies exposed to valproate (Moore 2000). Preliminary studies suggested that lamotrigine may become the first‐line drug in AS (Buoni 1999). This review aims to determine the best choice of anticonvulsant for children and adolescents with AS by reviewing the information available from RCTs.
Objectives
To review the evidence for the effects of ethosuximide, valproate and lamotrigine as treatments for children and adolescents with typical absence seizures (AS), when compared with placebo or each other.
Methods
Criteria for considering studies for this review
Types of studies
-
Randomised parallel group monotherapy or add‐on trials which include a comparison of any of the following in children or adolescents with typical AS: ethosuximide; sodium valproate; lamotrigine and placebo.
-
The studies should have used either adequate or quasi‐randomised methods (e.g. allocation by day of week).
-
Blinded and unblinded studies.
Types of participants
Children or adolescents (up to 16 years of age) with typical AS.
Types of interventions
Sodium valproate, ethosuximide or lamotrigine as monotherapy or add‐on treatment. These drugs may be compared with placebo or with one another.
Types of outcome measures
Primary outcomes
-
Proportion of participants seizure free at one, three, six, 12 and 18 months after randomisation.
-
Fifty per cent or greater reduction in the frequency of seizures.
-
Incidence of adverse effects.
Secondary outcomes
-
Normalisation of electroencephalogram (EEG) and/or negative hyperventilation test.
Search methods for identification of studies
Electronic searches
Searches were run for the original review in March 2003 and subsequent searches were run in March 2005, July 2007, November 2009, August 2011, March 2014, December 2015, September 2016, and May 2018.
For the latest update we searched:
-
the Cochrane Register of Studies (CRS Web, 22 September 2020) using the search strategy shown in Appendix 1;
-
MEDLINE (Ovid, 1946 to September 21, 2020) using the search strategy shown in Appendix 2.
CRS Web includes randomised or quasi‐randomised, controlled trials from PubMed, Embase, ClinicalTrials.gov, the World Health Organization International Clinical Trials Registry Platform (ICTRP), the Cochrane Central Register of Controlled Trials (CENTRAL), and the Specialized Registers of Cochrane Review Groups including Epilepsy.
There were no language restrictions.
Searching other resources
We contacted Sanofi Winthrop, Glaxo Wellcome (now GlaxoSmithKline) and Parke Davis (now Pfizer), manufacturers of sodium valproate, lamotrigine and ethosuximide, respectively. We also reviewed any references of identified studies and retrieved any relevant studies.
Data collection and analysis
Selection of studies
Three review authors (FB, SI, SL) independently assessed trials for inclusion and disagreements were resolved by discussion. The same review authors independently extracted data from trial reports.
Data extraction and management
We extracted the following data from the studies that met our inclusion criteria:
-
study design;
-
method of randomisation concealment;
-
method of blinding;
-
whether any participants had been excluded from reported analyses;
-
duration of treatment;
-
outcome measures;
-
participant data (total number of individuals allocated to each treatment group, age of participants, naive participants versus selected groups, individuals with other types of seizures co‐existing with typical absence seizures);
-
results (success rate and adverse effects).
Assessment of risk of bias in included studies
Two review authors (FB and SL) independently assessed risk of bias for each included trial using the Cochrane 'Risk of bias' tool (Higgins 2011), considering sequence generation, concealment of allocation, methods of blinding, incomplete outcome data, selective reporting, and other types of bias. A third party resolved disagreements in the assessment of the level of bias.
Measures of treatment effect
The data for our chosen outcomes are dichotomous and our preferred outcome statistic was the risk ratio (RR).
Unit of analysis issues
We planned to deal with any unit of analysis issues according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
We conducted an intention‐to‐treat analysis. Due to the small number of included studies no best‐case or worst‐case analysis was performed.
Assessment of heterogeneity
We assessed clinical heterogeneity by evaluating similarities and differences in the methodologies and outcomes measured in the included studies and by visually inspecting forest plots. We planned to assess statistical heterogeneity using the Chi² test and I² statistic (Higgins 2011) as follows: 0% to 40% might not be important, 30% to 60% may represent moderate heterogeneity, 50% to 90% may represent substantial heterogeneity, and 75% to 100% indicating considerable heterogeneity. We used a fixed‐effect model if we did not find statistically significant heterogeneity between the included studies. Otherwise, we planned to use a random‐effects model. However, despite our primary intention, due to insufficient information on outcomes and too high clinical and methodological heterogeneity, we were unable to perform any meta‐analyses.
Assessment of reporting biases
We sought all protocols from study authors to identify any discrepancies between protocol and trial methodology.
Data synthesis
Provided we thought it clinically appropriate, and no important heterogeneity was found, we planned to summarise results in a meta‐analysis. However, because of the methodological problems outlined below it was not possible to perform meta‐analysis of the data from the studies that fulfilled the inclusion criteria. The large difference in the length of follow‐up and timing of analysis was a particular problem. Further research could allow results to be pooled, leading to a quantitative rather than a qualitative summary of results.
Subgroup analysis and investigation of heterogeneity
We did not carry out any subgroup analysis or formal investigation of heterogeneity.
Sensitivity analysis
If we had found trial methodologies to be sufficiently distinct, we would have conducted sensitivity analyses to identify which factor(s) could have influenced the degree of heterogeneity.
Summary of findings and assessment of the certainty of the evidence
For the 2019 update, we presented the results concerning the outcomes of interest for which data were available from included studies in 'Summary of findings' tables, and we used GRADE (Guyatt 2008) quality assessment criteria to evaluate the certainty of evidence for the outcomes derived from the studies included in this review.
Results
Description of studies
Results of the search
See Figure 1.
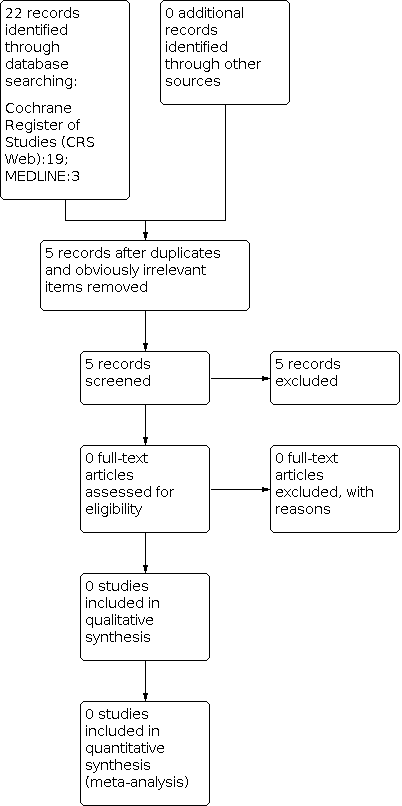
Study flow diagram (results refer only to the updated version of the review). The previous versions of the review (Posner 2003; Posner 2005a; Posner 2005b; Brigo 2017; Brigo 2019) included eight studies.
The previous versions of the review (Posner 2003; Posner 2005a; Posner 2005b; Brigo 2017; Brigo 2019) included eight studies.
The updated search strategy described yielded 22 results (19 Cochrane Register of Studies (CRS Web), three MEDLINE). After removing one duplicate and 16 obviously irrelevant items, we assessed five articles for possible inclusion and excluded all of them. Since the last version of this review, we have found no new studies. Below we provide details of the studies included in the previous versions of this review.
Included studies
Callaghan 1982
This was a randomised, parallel open study, which compared monotherapy with ethosuximide and sodium valproate. Ethosuximide was initially given at 250 mg/day and, whenever required, incremented by 250 mg to a maximum of 1500 mg/day. Valproate was started at 400 mg/day and, if deemed necessary, gradually incremented by 200 mg up to 2400 mg/day. Participants (total 28) had typical absence seizures (AS), were between four and 15 years, and were previously untreated. Follow‐up ranged from 18 months to four years. The report acknowledged support from Warner‐Lambert Pharmaceuticals, manufacturers of ethosuximide.
Sato 1982
This study used a complex response‐conditional design and recruited drug‐naive participants as well as participants already on treatment, with a total of 45 participants recruited. In the first phase of this trial; participants were randomised to receive either valproate (and placebo) or ethosuximide (and placebo) and followed up for six weeks. Participants responding to randomised treatment continued with the randomised drug for a further six weeks. Responders included previously untreated participants who became seizure free and participants who had been previously treated and had an 80% or greater reduction in AS frequency. Non‐responders and those with adverse effects were crossed over to the alternative treatment and followed up for a further six weeks. The age range of participants was three to 18 years. Apart from AS, some participants also had other types of seizures. The report does not specify if the AS were typical or atypical. Some of the participants were drug naive and some drug resistant. Participants of the study were selected from those who attended the epilepsy clinic at the Clinical Research Center, University of Virginia Hospital, USA. The work was supported by a contract from the Institute of Neurological and Communicative Disorders and Stroke (NINCDS).
Martinovic 1983
This was a parallel, open‐design study comparing ethosuximide and sodium valproate. Participants were between five and eight years old with a recent (less than six weeks) onset of seizures. All participants (total 20) had 'simple absences' and were followed up for one to two years. Six individuals did not co‐operate and were therefore not included in the analysis. No information about sponsorship by a pharmaceutical company is given.
Frank 1999
This was a double‐blind study using a 'responder enriched' design. Participants (total 29) had newly diagnosed typical AS and were aged between three to 15 years. Prior to randomisation, all participants received treatment with lamotrigine. After four weeks or more of treatment, participants who were seizure free and had a negative 24‐hour electroencephalogram (EEG) with hyperventilation, were randomised to either continue lamotrigine or to placebo and were followed up for four weeks. This study was sponsored by Glaxo Wellcome (now GlaxoSmithKline), makers of lamotrigine.
Coppola 2004
This was a randomised, parallel group unblinded study comparing lamotrigine and sodium valproate. All participants (n = 38) were drug naive, aged three to 13 years old with typical AS. The follow‐up time was 12 months. The primary outcome measure was total seizure freedom, measured at one, three and 12 months. This study was not sponsored by any commercial organisation.
Basu 2005
Results of this study were published as an abstract. We contacted the main author of this study via email three times (30 October and 4 November 2015, and 7 January 2016) asking for further information; we did not receive a reply. This was a randomised, open‐label, parallel group design comparing sodium valproate with lamotrigine used in monotherapy for treatment of typical AS (diagnosed clinically and by EEG support). Thirty patients were included (males 16; females 14 – aged between five and 14 years). Patients with other comorbidities were excluded. Fifteen patients were randomly allocated to receive valproate and 15 to receive lamotrigine. The follow‐up was 12 months. The primary outcome was seizure freedom and no EEG evidence of seizure. Drug dosages were not explicitly reported. The dosages were escalated according to the clinical response, starting from a low dose. Lamotrigine was titrated very slowly at two‐weekly intervals to avoid unwanted side effects (maximum 10 mg/kg/day). After one month of treatment nine patients (60%) receiving valproate and none (0%) receiving lamotrigine were seizure free. After three months, 11 patients (73.3%) in the sodium valproate and eight patients (53.3%) in the lamotrigine group receiving lamotrigine were seizure free. After 12 months, 12 patients (80%) receiving sodium valproate and 10 patients (66.6%) treated with lamotrigine were seizure free (P > 0.05). Minimal adverse events (not explicitly reported) were observed in 26.6% of patients treated with sodium valproate and in 20% of patients receiving lamotrigine. No dropouts were observed. No information about sponsorship by a pharmaceutical company was available.
This study (Huang 2009), compared valproate with lamotrigine monotherapy in drug‐naive children (n = 48, six to 10 years) with newly diagnosed childhood AS (typical seizures). Included patients were 17 males and 31 females (no detailed descriptions in each group, respectively). The follow‐up time was 12 months. The outcome measure was total seizure freedom, measured at one, three, six and 12 months. Complete normalisation of EEG with seizure freedom and occurrence of adverse effects were also considered. In the valproate group, sustained‐release tablets or oral solution were administered twice daily (totally 15 mg/kg per day); in case of persisting seizures after one week, the dose was increased to 20 mg/kg per day, twice daily (maximum dose daily 30 mg/kg). In case of persisting seizures despite a maximum dose of 30 mg/kg within a month, combination with lamotrigine 0.15 mg/kg daily to 2 mg to 5 mg/kg was administrated. In the lamotrigine group, patients received a starting dose of lamotrigine of 0.5 mg/kg daily, administered twice, increased to 0.15 mg/kg per two weeks. The daily maintenance dose was 2 mg to 5 mg/kg, and the maximum daily dose 10 mg/kg. In case of persisting seizures despite a maximum dose within a month, combination with valproate 10 mg/kg daily to 20 mg/kg was administrated. No information about sponsorship by a pharmaceutical company was available.
Glauser 2013a
This was a randomised, parallel double‐blind controlled trial comparing ethosuximide, lamotrigine and sodium valproate in children with newly diagnosed childhood absence epilepsy. The study design also included a partial cross‐over to open‐label (at treatment failure only) with subsequent follow‐up: participants reaching a treatment‐failure criterion in the double‐blind treatment phase were given the opportunity to enter into the open‐label phase, during which participants were randomised to one of the two other antiepileptic drugs. Participants (total 453 enrolled) had typical AS, were between seven months and 12 years 11 months, and were previously untreated. Among the 453 patients enrolled, seven were withdrawn, hence 446 participants were included in subsequent effectiveness analyses and 451 participants included in the safety analyses. Follow‐up was up to 12 months. Study drugs were titrated as tolerated in predetermined increments every one to two weeks over 16 weeks. Ethosuximide and valproic acid doses were incremented of 5 mg to 10 mg/kg/day at intervals of two weeks, whilst lamotrigine doses were incremented of 0.3 mg to 0.6 mg/kg/day at intervals of two weeks. The maximal target doses were ethosuximide 60 mg/kg/day or 2000 mg/day (whichever was lower), valproic acid 60 mg/kg/day or 3000 mg/day (whichever was lower), and lamotrigine 12 mg/kg/day or 600 mg/day (whichever was lower). The main effectiveness outcome was the freedom from treatment failure assessed 12 months after randomisation. Freedom from treatment failure was also assessed at 16 to 20 weeks. Treatment failure was defined as failure either due to lack of seizure control, or meeting safety exit criteria, or withdrawal from the study for any other reason. This study was not sponsored by any commercial organisation.
Excluded studies
For this update, we excluded five studies for the following reasons: three studies were commentaries, one study was a review, and one study wasn't relevant to our current review.
Risk of bias in included studies
See Figure 2; Figure 3; Characteristics of included studies.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Results of one study (Basu 2005) were published as an abstract. Despite several attempts to contact the research authors to obtain more information on methodological issues and risk of bias, we received no reply. Thus, for this study there is an unclear risk of bias.
Three of the included studies (Callaghan 1982; Sato 1982; Martinovic 1983) date back 30 years and there was an obvious difference in the quality of the reporting in comparison with the newer studies (Frank 1999; Coppola 2004; Huang 2009; Glauser 2013a).
Allocation
Only two of the studies described explicitly the methods of allocation concealment (Coppola 2004; Glauser 2013a).
Blinding
The studies reported by Sato (Sato 1982), Frank (Frank 1999), and Glauser (Glauser 2013a) were double‐blinded, whilst the studies reported by Martinovic (Martinovic 1983), Callaghan (Callaghan 1982), Coppola (Coppola 2004), and Huang (Huang 2009) were unblinded (high risk of bias). In two out of the three double‐blinded studies, placebo and active drugs were indistinguishable (Frank 1999; Glauser 2013a) and were considered to have a low risk of bias.
Incomplete outcome data
Five studies described losses to follow‐up or exclusions from analyses. Frank 1999 reports that one participant withdrew consent before treatment but after randomisation and that one participant did not comply but was included in the analysis. Martinovic 1983 reports that six of the initially recruited participants did not co‐operate and were not included in the analysis. Coppola 2004 reports loss of nine patients overall, all due to lack of efficacy, these patients exited the study at three months follow‐up; all randomised patients were included in the analysis. Huang 2009 reports that one patient in the valproate group was lost to follow‐up (no further specifications), whereas two patients in the lamotrigine group were withdrawn due to severe adverse effects (systemic anaphylaxis rash). Glauser 2013a reports that among the 453 patients enrolled, seven were withdrawn due to ineligibility at baseline, so that 446 participants were included in subsequent effectiveness analyses and 451 participants in safety analyses. Two reports (Callaghan 1982; Sato 1982) did not make an explicit statement that participants were not lost to follow‐up or excluded from analyses.
Selective reporting
Seven of the studies were assessed at low risk of bias (Callaghan 1982; Coppola 2004; Frank 1999; Glauser 2013a; Huang 2009; Martinovic 1983; Sato 1982); the remaining study was assessed at unclear risk of bias (Basu 2005).
Effects of interventions
See: Summary of findings 1 Ethosuximide compared to valproate for absence seizures in children and adolescents; Summary of findings 2 Lamotrigine compared to valproate for absence seizures in children and adolescents; Summary of findings 3 Ethosuximide compared to lamotrigine for absence seizures in children and adolescents
Lamotrigine versus placebo
We found one study (Frank 1999) comparing lamotrigine with placebo which recruited 29 participants. As outlined in Description of studies above, this trial used a responder‐enriched design where participants responding to lamotrigine during a pre‐randomisation baseline phase were randomised to continue lamotrigine or have it withdrawn. This trial therefore compares the effect of continuing versus withdrawing lamotrigine. The results were as follows:
-
in the initial open‐label dose‐escalation phase, 71% of the participants became seizure free on lamotrigine using a 24‐hour EEG/video telemetry recording;
-
in the placebo‐controlled phase 64% of the participants on lamotrigine remained seizure free versus 21% receiving placebo (P < 0.03).
Valproate versus placebo
We found no trials comparing valproate versus placebo.
Ethosuximide versus placebo
We found no trials comparing ethosuximide versus placebo.
Ethosuximide versus valproate
We found four studies comparing valproate with ethosuximide (Callaghan 1982; Sato 1982; Martinovic 1983; Glauser 2013a). Due to differences in study design, participants and length of follow‐up we did not think it appropriate to pool results in a meta‐analysis. For our chosen outcome 'seizure freedom', we were unable to extract data for this outcome at the time points we had specified (one, six and 18 months). Rather than not present any data for this outcome, we have summarised results for individual trials, where the proportion of participants seizure free during follow‐up was reported. Results for individual studies are presented below as well as in Analysis 1.1, Analysis 1.2 and Analysis 1.3.
Seizure freedom
The risk ratio (RR) estimates with 95% confidence intervals (CIs) for seizure freedom (RR < 1 favours ethosuximide) are (Analysis 1.1):
-
Callaghan 1982: RR 0.70 (95% CI 0.32 to 1.51); seizure freedom was observed in six out of 15 patients receiving valproate and in eight out of 14 patients receiving ethosuximide. One patient in the ethosuximide group was subsequently treated with valproate, but failed to respond to either single drug and did not improve when both drugs were used in combination. The outcomes of this patient on combined treatment were therefore counted twice ("no remission"), since the patient received both drugs.
-
Sato 1982: RR 1.93 (95% CI 0.87 to 4.25); seizure freedom was observed in six out of seven patients receiving valproate and in four out of nine patients receiving ethosuximide.
-
Martinovic 1983: RR 0.88 (95% CI 0.53 to 1.46); seizure freedom was observed in seven out of 10 patients receiving valproate and in eight out of 10 patients receiving ethosuximide.
-
Glauser 2013a: RR 0.96 (95% CI 0.75 to 1.24); seizure freedom was observed in 64 out of 146 patients receiving valproate and in 70 out of 154 patients receiving ethosuximide.
Hence, none of these trials found a difference for this outcome. Adopting the GRADE methodology, we assessed the certainty of the evidence for this outcome from these studies as moderate (summary of findings Table 1).
80% or greater reduction in seizure frequency
This outcome was only reported by Sato 1982, and the RR was 0.70 (95% CI 0.19 to 2.59, Analysis 1.2); 80% or greater reduction in seizure frequency was observed in three out of 15 patients receiving valproate and in four out of 14 patients receiving ethosuximide. Again, no difference was found, but the confidence interval is wide and equivalence cannot be inferred. Adopting the GRADE methodology, we assessed the certainty of the evidence for this outcome as very low (summary of findings Table 1).
50% or greater reduction in seizure frequency
This was reported for two trials (Analysis 1.3). In one trial (Martinovic 1983), all participants achieved this outcome (10/10 in the valproate and 10/10 in the ethosuximide group). For the other trial (Callaghan 1982) the RR was 1.02 (95% CI 0.70 to 1.48); 12 out of 15 patients receiving valproate and 11 out of 14 patients receiving ethosuximide experienced 50% or greater reduction in seizure frequency. Again, no difference is found, but the confidence interval is wide and equivalence cannot be inferred. In this study, one patient in the ethosuximide group was subsequently treated with valproate, but failed to respond to either single drug and did not improve when both drugs were used in combination. The outcomes of this patient on combined treatment were therefore counted twice ("no remission"). Adopting the GRADE methodology, we assessed the certainty of the evidence for this outcome as low (summary of findings Table 1).
Lamotrigine versus valproate
We found four studies comparing valproate with lamotrigine (Coppola 2004; Basu 2005; Huang 2009; Glauser 2013a). Due to differences in study design, participants and length of follow‐up, we did not think it appropriate to pool results in a meta‐analysis. For our chosen outcome 'seizure freedom', we were unable to extract data for this outcome at the time points we had specified (one, six and 18 months). Rather than not present any data for this outcome, we have summarised results for individual trials, where the proportion of participants seizures free during follow‐up was reported. Results for individual studies are presented below as well as in Analysis 2.1 and Analysis 2.2.
Seizure freedom at one month
This outcome was reported in two trials (Coppola 2004; Huang 2009;). One study (Coppola 2004) comparing valproate and lamotrigine head‐to‐head, recruited drug‐naive children with typical AS. The primary outcome measure was total seizure freedom and was assessed at one, three and 12 months. At one‐month follow‐up 52.6% of patients taking valproate (10 out of 19) were seizure free compared to only 5.3% of patients taking lamotrigine (one out of 19) (P = 0.004). The other study (Huang 2009) compared valproate with lamotrigine monotherapy in drug‐naive children with newly diagnosed childhood As (typical seizures). After one month of treatment 16/23 patients (74%) receiving valproate and 2/22 (41%) receiving lamotrigine were seizure free.
Seizure freedom at three months
This outcome was reported in three trials (Coppola 2004; Basu 2005; Huang 2009;). In the first study (Coppola 2004), at three months seizure freedom was observed in 12 out of 19 (63.1%) patients taking sodium valproate and in seven out of 19 (36.8%) patients taking lamotrigine (P = 0.19). In one study (Basu 2005) after three months, 11 patients out of 15 (73.3%) in the sodium valproate and eight patients out of 15 (53.3%) in the lamotrigine group receiving lamotrigine were seizure free. In the third study (Huang 2009), 17/23 patients (70%) receiving valproate and 9/22 (9%) receiving lamotrigine were seizure free at three months.
Seizure freedom at six months
Only one study (Huang 2009) reported data on this outcome: 17/24 patients (71%) receiving valproate and 12/24 (50%) receiving lamotrigine were seizure free at six months.
Seizure freedom at 12 months
This outcome was reported for four trials (Coppola 2004; Basu 2005; Huang 2009; Glauser 2013a). The RR estimates with 95% CIs for seizure freedom (RR < 1 favours lamotrigine) at 12 months are (Analysis 2.1):
-
Coppola 2004: 1.30 (95% CI 0.77 to 2.20); seizure freedom was observed in 13 out of 19 patients receiving valproate and in 10 out of 19 patients receiving lamotrigine.
-
Basu 2005: 1.20 (95% CI 0.77 to 1.86); seizure freedom was observed in 12 out of 15 patients receiving valproate and in 10 out of 15 patients receiving lamotrigine.
-
Huang 2009: 1.36 (95% CI 0.86 to 2.13); seizure freedom was observed in 17 out of 23 patients receiving valproate and in 12 out of 22 patients receiving lamotrigine.
-
Glauser 2013a: 2.06 (95% CI 1.44 to 2.97); seizure freedom was observed in 64 out of 146 patients receiving valproate and in 31 out of 146 patients receiving lamotrigine.
Hence, none of these trials found a difference for this outcome. However, confidence intervals are all wide and the possibility of important differences has not been excluded and equivalence cannot be inferred. Adopting the GRADE methodology, we assessed the certainty of the evidence on this outcome from included studies as moderate (summary of findings Table 2).
Normalisation of the EEG
Only one study (Huang 2009) explicitly reported data on this outcome. The proportion showing normal EEG at 12 months in the lamotrigine group (6/22, 27.3%) was significantly lower than that in the valproic acid group (15/23, 65.2%) (P < 0.05); RR = 2.39 (95% CI: 1.14 to 5.04; P = 0.0218). Adopting the GRADE methodology, we assessed the certainty of the evidence for this outcome as very low (summary of findings Table 2).
Ethosuximide versus lamotrigine
One study (Glauser 2013a), compared ethosuximide and lamotrigine in drug‐naive patients with childhood AS. The main effectiveness outcome was the freedom from treatment failure assessed 12 months after randomisation. Freedom from treatment failure was also assessed at 16 to 20 weeks, and in between 16 and 20 weeks and month 12. Treatment failure was defined as failure either due to lack of seizure control, or meeting safety exit criteria, or withdrawal from the study for any other reason. Seizure freedom at 12 months after randomisation was higher in patients taking ethosuximide (70/154, 45%) than in patients taking lamotrigine (31/146, 21%; P < 0.001). At 16 to 20 weeks, freedom from treatment failure was observed in 81/154 (53%) patients taking ethosuximide and 43/146 (29%) patients taking lamotrigine. Adopting the GRADE methodology, we assessed the certainty of the evidence for seizure freedom at 12 months as high (summary of findings Table 3).
Adverse effects related to the use of ethosuximide, sodium valproate or lamotrigine
This section and the related tables apply to all studies and comparisons.
The most common adverse effects of treatment with valproate reported by the studies assessing this drug (Callaghan 1982; Sato 1982; Martinovic 1983; Huang 2009; Glauser 2013a) were fatigue, nausea, vomiting, increased appetite with weight gain, behavioural/psychiatric changes (decreased concentration, personality change, hyperactivity, attention problems, hostility), and thrombocytopenia (Table 1).
Ethosuximide treatment was mostly associated with nausea, vomiting, and behavioural/psychiatric changes (Table 2).
The most common adverse effects of treatment with lamotrigine were fatigue, and behavioural/psychiatric changes (Table 3). In one lamotrigine study (Frank 1999), the most commonly reported adverse event was rash (reported on 11 occasions in 10 patients). However, only in one of the individuals was this thought to be related to lamotrigine. There were two serious adverse events during the treatment, but they were judged to be unrelated to treatment. In one study (Huang 2009), systemic anaphylaxis rash during lamotrigine treatment led to patients' withdrawal from the study. In the Glauser 2013a study, no side effects (including rash, reported in two patients taking valproate, six patients taking ethosuximide, and six patients taking lamotrigine) occurred more frequently in the lamotrigine cohort compared to the other treatment groups (valproate and ethosuximide). In this study, the frequency of treatment failures due to intolerable adverse events was significantly different among the treatment groups, with the largest proportion of proportion of adverse events in the valproic acid group (48/146, 33%) compared to the ethosuximide (38/154, 25%) and the lamotrigine (29/146, 20%) groups (P < 0.037).
Discussion
Summary of main results
Since the last version of this review was published, we have found no new studies. Hence we have made no changes to the conclusions of this update as presented in the last updated version of this review (Brigo 2019).
Despite absence seizures (AS) being a relatively common seizure type in children, we found only eight randomised controlled trials, seven of them recruiting 20 to 48 participants. Only the study of Glauser 2013a included a much larger sample.
One trial compared lamotrigine with placebo (Frank 1999), three compared ethosuximide with valproate (Callaghan 1982; Sato 1982; Martinovic 1983), three compared lamotrigine with valproate (Coppola 2004; Basu 2005; Huang 2009), and one compared ethosuximide, valproate, and lamotrigine (Glauser 2013a).
The trial comparing lamotrigine with placebo (Frank 1999), found that individuals becoming seizure free on lamotrigine, were more likely to remain seizure free if they were randomised to stay on lamotrigine rather than placebo. In essence, this trial assessed the effect of lamotrigine withdrawal. Although this trial finds evidence of an effect of lamotrigine on AS, it was of only four weeks duration, and the design is inadequate to inform clinical practice. Also, clinicians and people living with epilepsy are likely more concerned with how drugs compare with each other rather than with placebo.
Three studies (Coppola 2004; Basu 2005; Huang 2009) directly compared lamotrigine with the long‐established treatment for typical AS, sodium valproate. All three studies found both valproate and lamotrigine to be efficacious in the treatment of typical AS in children. However, in these studies, the study sample size was small (38, 30 and 48 patients, respectively), and estimates are therefore imprecise.
Most robust results are provided by the much larger study including three groups: valproic acid, lamotrigine and ethosuximide (Glauser 2013a). This study found that at 12 months, the freedom‐from‐failure rates for ethosuximide and valproic acid were similar and were higher than the rate for lamotrigine. The frequency of treatment failures due to lack of seizure control (P < 0.001) and intolerable adverse events (P < 0.037) was significantly different among the treatment groups. Almost two thirds of the 125 participants with treatment failure due to lack of seizure control were in the lamotrigine cohort. The largest subgroup (42%) of the 115 participants discontinuing due to adverse events was in the valproic acid group. Overall, this study demonstrates the superior effectiveness of ethosuximide and valproic acid compared to lamotrigine as initial monotherapy aimed to control seizures without intolerable adverse events in children with childhood absence epilepsy. Because of the higher rate of adverse events leading to drug discontinuation and the significant negative effects on attentional measures seen in the valproate cohort, the authors concluded that ethosuximide represents the optimal initial empirical monotherapy for childhood absence epilepsy. Notably, this study was the very first randomised controlled trial (RCT) to meet the International League Against Epilepsy (ILAE) criteria for class I evidence for childhood absence epilepsy (or for any type of generalised seizure in adults or children) (Glauser 2006). Consequently, ethosuximide and valproate were designed/designated as treatments with level A evidence in children with childhood absence epilepsy in the recent ILAE treatment guidelines (Glauser 2013b). Data on tolerability of valproate reported in the included studies are consistent with the general adverse‐effects profile of this drug. Adverse effects often seen with valproate treatment are dyspepsia, weight gain, tremor, transient hair loss and haematological abnormalities (Panayiotopoulos 2001). The occurrence of rash in patients receiving lamotrigine is a well‐known adverse event of this drug and its risk may be reduced by slow titration (Wang 2015).
Overall completeness and applicability of evidence
Evidence on the efficacy of ethosuximide, valproic acid and lamotrigine derive mostly from the large and high‐quality RCT conducted in the United States of America by Glauser 2013a. Absence seizures are due to a genetic predisposition and not likely to be influenced by different socioeconomic environments. However, data on efficacy from this RCT should be replicated by further studies in middle‐ and low‐income countries to evaluate the worldwide generalisability of these results.
Quality of the evidence
The description of important methodology was sometimes poor, and only two studies (Coppola 2004; Glauser 2013a) gave a description of allocation concealment. Three of the trials were explicitly reported as double‐blind (Sato 1982; Frank 1999; Glauser 2013a). In three of the trials there was no mention of losses to follow‐up or exclusions from analyses. The trials used a variety of methodologies; six were parallel trials (Callaghan 1982; Martinovic 1983; Coppola 2004; Basu 2005; Huang 2009; Glauser 2013a) and two used response conditional designs (Sato 1982; Martinovic 1983). The length of follow‐up ranged from four weeks to four years.
Using GRADE, we assessed the certainty of the evidence to be very low to high for outcomes for which data were available. The reasons for these judgements are outlined in the summary of findings Table 1; summary of findings Table 2; summary of findings Table 3. We assessed the study of Glauser 2013a as being at low risk of bias, and providing high certainty of the evidence for outcomes for which data were available. However, the certainty of the evidence provided by the other included studies was low, primarily due to risk of bias and imprecise results because of the small sample size. Hence, conclusions regarding the efficacy of ethosuximide, valproic acid and lamotrigine derive mostly from the large and high‐quality RCT by Glauser 2013a. Hence, we rated the overall certainty of the evidence for most outcomes to be moderate or high, although we downgraded the certainty of the evidence for outcomes for which data were obtained from small studies judged at unclear or high risk of bias to low or very low certainty.
Potential biases in the review process
We made every effort to identify all RCTs on the use of ethosuximide, valproic acid and lamotrigine for absence seizures through a comprehensive search of the literature, and it is unlikely that we failed to identify large relevant studies. However, despite our efforts, there remains the possibility that we have missed small studies published in the less accessible literature.
We also contacted drug companies and experts in the field to obtain information on ongoing trials or unpublished results.
Finally, we contacted authors to obtain information on missing data or on completion of study. Unavoidably, some of the authors of this review were familiar with most of the included trials before updating this review. However, data extraction was undertaken blind to the results of the prior version of the review.
Agreements and disagreements with other studies or reviews
The good efficacy profile of ethosuximide for the treatment of absence seizures as shown in Glauser 2013a confirms results of three other smaller studies that compared ethosuximide with valproate (Callaghan 1982; Sato 1982; Martinovic 1983); all of these three smaller studies reported a superior efficacy profile for ethosuximide over valproate with regards to seizure freedom (Callaghan 1982; Sato 1982; Martinovic 1983), although with wide confidence intervals due to small sample size. However, it is noteworthy to consider that ethosuximide does not suppress tonic‐clonic seizures (Berkovic 1993), and it has even been suggested that it can transform absences into grand mal seizures (Glauser 2002), although with contrasting data (Schmitt 2007). Hence, ethosuximide should probably be avoided in patients with AS and co‐existing generalised tonic‐clonic seizures.
Significance
There are no placebo‐controlled trials for ethosuximide or valproate, and hence no evidence from RCTs to support a specific effect on AS for either of these two drugs. Due to the differing methodologies used in the trials comparing ethosuximide, lamotrigine and valproate, we thought it inappropriate to undertake a meta‐analysis. Hence, guidance for practice from this review are based on a narrative comparison. Further trials with larger size than many of the studies currently included in this review are required. Further research could allow results to be pooled, leading to a quantitative rather than a qualitative summary of results. In summary, ethosuximide, lamotrigine and valproate are commonly used to treat children and adolescents with AS. We now have evidence from a recently conducted, high‐quality, large trial that ethosuximide and valproate have higher efficacy than lamotrigine as initial monotherapy in children and adolescents with AS. This study showed a higher rate of adverse events leading to drug discontinuation and significant negative effects on attentional measures in the valproate group. Consequently, with regards to both efficacy and tolerability, ethosuximide represents the optimal initial empirical monotherapy for children and adolescents with AS. However, the use of ethosuximide should be avoided in patients with AS and generalised tonic‐clonic seizures, as this drug is probably inefficacious on tonic‐clonic seizures.

Study flow diagram (results refer only to the updated version of the review). The previous versions of the review (Posner 2003; Posner 2005a; Posner 2005b; Brigo 2017; Brigo 2019) included eight studies.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1: Ethosuximide versus valproate, Outcome 1: Seizure free

Comparison 1: Ethosuximide versus valproate, Outcome 2: 80% or greater reduction in seizure frequency

Comparison 1: Ethosuximide versus valproate, Outcome 3: 50% or greater reduction in seizure frequency

Comparison 2: Lamotrigine versus valproate, Outcome 1: Seizure free

Comparison 2: Lamotrigine versus valproate, Outcome 2: Normalisation of the EEG

Comparison 3: Ethosuximide versus lamotrigine, Outcome 1: Seizure free at 12 months
| Ethosuximide compared to valproate for absence seizures in children and adolescents | ||||||
| Patient or population: absence seizures in children and adolescents | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with valproate | Risk with ethosuximide | |||||
| Seizure freedom at 12 months | Study population | ‐ | 365 | ⊕⊕⊕⊝ | None of the included trials found a difference for this outcome. Length of follow‐up in included studies: from 6 weeks to 4 years. | |
| see comment | see comment | |||||
| 80% or greater reduction in seizure frequency | Study population | RR 0.70 | 29 | ⊕⊝⊝⊝ | No difference was found, but the confidence interval is wide and equivalence cannot be inferred. Length of follow‐up: 6 weeks. | |
| 286 per 1,000 | 200 per 1,000 | |||||
| 50% or greater reduction in seizure frequency | Study population | ‐ | 49 | ⊕⊕⊝⊝ | No difference was found, but the confidence interval is wide and equivalence cannot be inferred. Length of follow‐up in included studies: from 1 to 4 years. | |
| see comment | see comment | |||||
| Normalisation of the EEG ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Adverse effects (Table 1; Table 2) Ethosuximide treatment was mostly associated with nausea, vomiting, and behavioural/psychiatric changes. The most common adverse effects of treatment with valproate were fatigue, nausea, vomiting, increased appetite with weight gain, behavioural/psychiatric changes (decreased concentration, personality change, hyperactivity, attention problems, hostility), and thrombocytopenia | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Most information is from studies at low or unclear risk of bias; plausible bias is likely to seriously alter the results. 2 Information is from a study with unclear and high risk of bias; plausible bias is likely to seriously alter the results. 3 Small number of patients included in this study (29) (see also footnote below). 4 Information is from two small studies with unclear or high risk of bias; plausible bias is likely to seriously alter the results. 5 Information is from two studies with small number of patients included. aIn this study, one patient in the ethosuximide group was subsequently treated with valproate, but failed to respond to either single drug and did not improve when both drugs were used in combination. The outcomes of this patient on combined treatment were therefore counted twice ("no remission"), since the patient received both drugs. | ||||||
| Event | ||||||
|---|---|---|---|---|---|---|
| Acute pancreatitis | 1 | |||||
| Obesity/Weight gain | 1 | 1 | 14 | |||
| Drowsiness | 4 | |||||
| Nausea | 5 | 3 | 12* | |||
| Vomiting | 1 | 2 | 12* | |||
| Decreased platelet numbers | 2 | 4 | ||||
| Increased appetite | 15 | |||||
| Poor appetite | 1 | 8 | ||||
| Diarrhoea | 1 | 7 | ||||
| Dizziness | 1 | 2 | ||||
| Hyperactivity | 23 | |||||
| Attention problems | 24 | |||||
| Hostility | 22 | |||||
| Concentration decreased | 18 | |||||
| Personality change | 17 | |||||
| Sleep problem | 17 | |||||
| Depression | 11 | |||||
| Slow process speed | 11 | |||||
| Memory problem | 10 | |||||
| Apathy | 9 | |||||
| Fatigue | 27 | |||||
| Headache | 1 | 18 | ||||
| Leukopenia | 2 | |||||
| Elevated liver function tests | 1 | 7 | ||||
| Elevated LDH | 1 | |||||
| Rash | 2 | |||||
| *Nausea, vomiting, or both Numbers of individuals within each study undertaking valproate: 14 (Callaghan 1982), 22 (Sato 1982), 10 (Martinovic 1983), 19 (Coppola 2004), 23 (Huang 2009), 146 (Glauser 2013a). | ||||||
| Event | ||||
|---|---|---|---|---|
| Drowsiness | 1 | 5 | ||
| Tiredness | 2 | |||
| Nausea | 3 | 2 | 29* | |
| Vomiting | 3 | 29* | ||
| Increased appetite | 6 | |||
| Poor appetite | 1 | 10 | ||
| Diarrhoea | 9 | |||
| Dizziness | 1 | 10 | ||
| Headache | 2 | 23 | ||
| Leukopenia | 3 | |||
| Hiccups | 1 | |||
| Moodiness | 1 | |||
| Hyperactivity | 13 | |||
| Attention problems | 8 | |||
| Hostility | 4 | |||
| Concentration decreased | 6 | |||
| Personality change | 6 | |||
| Sleep problem | 11 | |||
| Depression | 4 | |||
| Slow process speed | 3 | |||
| Memory problem | 0 | |||
| Apathy | 4 | |||
| Fatigue | 26 | |||
| Rash | 6 | |||
| * Nausea, vomiting, or both Numbers of individuals within each study undertaking ethosuximide: 14 (Callaghan 1982), 23 (Sato 1982), 10 (Martinovic 1983), 154 (Glauser 2013a). | ||||
| Lamotrigine compared to valproate for absence seizures in children and adolescents | ||||||
| Patient or population: absence seizures in children and adolescents | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with valproate | Risk with lamotrigine | |||||
| Seizure freedom at 12 months | Study population | ‐ | 405 | ⊕⊕⊕⊝ | Higher proportion seizure free at 1 month in patients receiving valproate compared to those receiving lamotrigine (2 studies). No difference between valproate and lamotrigine for seizure freedom at 3 and 6 months (3 and 4 studies, respectively). Length of follow‐up in included studies: 12 months. | |
| see comment | see comment | |||||
| 80% or greater reduction in seizure frequency ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 50% or greater reduction in seizure frequency ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Normalisation of the EEG | Study population | RR 2.39 | 45 | ⊕⊝⊝⊝ | Length of follow‐up: 12 months. | |
| 273 per 1,000 | 652 per 1,000 | |||||
| Adverse effects (Table 1; Table 3) The most common adverse effects of treatment with lamotrigine were fatigue, and behavioural/psychiatric changes. The most common adverse effects of treatment with valproate were fatigue, nausea, vomiting, increased appetite with weight gain, behavioural/psychiatric changes (decreased concentration, personality change, hyperactivity, attention problems, hostility), and thrombocytopenia | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Most information comes from studies at low or unclear risk of bias; plausible bias is likely to seriously alter the results. | ||||||
| Event | ||||
|---|---|---|---|---|
| Abdominal pain | 5 | |||
| Headache | 2 | 2 | 14 | |
| Nausea | 3 | 2* | ||
| Vomiting | 2* | |||
| Poor appetite | 2 | 9 | ||
| Increased appetite | 1 | 10 | ||
| Diarrhoea | 2 | |||
| Dizziness | 3 | 5 | 5 | |
| Hyperkinesia | 2 | |||
| Hyperactivity | 12 | |||
| Attention problems | 11 | |||
| Hostility | 11 | |||
| Concentration decreased | 9 | |||
| Personality change | 10 | |||
| Sleep problem | 5 | |||
| Depression | 11 | |||
| Slow process speed | 7 | |||
| Memory problem | 8 | |||
| Apathy | 3 | |||
| Fatigue | 1 | 18 | ||
| Rash | 10 | 1 | 2 | 6 |
| Nervousness | 1 | |||
| Diplopia | 1 | |||
| *Nausea, vomiting, or both Numbers of individuals within each study undertaking lamotrigine: 15 (Frank 1999), 19 (Coppola 2004), 24 (Huang 2009), 146 (Glauser 2013a). | ||||
| Ethosuximide compared to lamotrigine for absence seizures in children and adolescents | ||||||
| Patient or population: absence seizures in children and adolescents | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with lamotrigine | Risk with ethosuximide | |||||
| Seizure freedom at 12 months | Study population | RR 0.47 | 300 | ⊕⊕⊕⊕ | Length of follow‐up: 12 months. | |
| 455 per 1,000 | 214 per 1,000 | |||||
| 80% or greater reduction in seizure frequency ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 50% or greater reduction in seizure frequency ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Normalisation of the EEG ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Adverse effects (Table 2; Table 3) Ethosuximide treatment was mostly associated with nausea, vomiting, and behavioural/psychiatric changes. The most common adverse effects of treatment with lamotrigine were fatigue, and behavioural/psychiatric changes. | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Seizure free Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1.1 Drug naive | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.2 80% or greater reduction in seizure frequency Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.2.1 Previously treated | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3 50% or greater reduction in seizure frequency Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Seizure free Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1.1 Seizure free at 1 month | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1.2 Seizure free at 3 months | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1.3 Seizure freedom at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1.4 Seizure free at 12 months | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.2 Normalisation of the EEG Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Seizure free at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

