Ethosuximide, sodium valproate or lamotrigine for absence seizures in children and adolescents
Information
- DOI:
- https://doi.org/10.1002/14651858.CD003032.pub5Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 21 January 2021see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Epilepsy Group
- Copyright:
-
- Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
Data were extracted by Francesco Brigo, Stanley C. Igwe and Simona Lattanzi. Analyses were undertaken by Francesco Brigo. Text of the final review was written by Francesco Brigo and Stanley C. Igwe.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK
Declarations of interest
FB received travel support from LusoFarmaco.
SI: none known.
SL received speaker's or consultancy fees from Eisai, UCB Pharma, and GW Pharmaceuticals, and served on the advisory board for GW Pharmaceuticals.
Acknowledgements
We wish to acknowledge the hard and valuable work that went in to the original version of the review by Ewa Posner, Khalid Mohamed and Tony Marson.
This review update was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Epilepsy Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health and Social Care.
Version history
| Published | Title | Stage | Authors | Version |
| 2021 Jan 21 | Ethosuximide, sodium valproate or lamotrigine for absence seizures in children and adolescents | Review | Francesco Brigo, Stanley C Igwe, Simona Lattanzi | |
| 2019 Feb 08 | Ethosuximide, sodium valproate or lamotrigine for absence seizures in children and adolescents | Review | Francesco Brigo, Stanley C Igwe, Simona Lattanzi | |
| 2017 Feb 14 | Ethosuximide, sodium valproate or lamotrigine for absence seizures in children and adolescents | Review | Francesco Brigo, Stanley C Igwe | |
| 2005 Oct 19 | Ethosuximide, sodium valproate or lamotrigine for absence seizures in children and adolescents | Review | Ewa B Posner, Khalid K Mohamed, Anthony G Marson | |
| 2003 Jul 21 | Ethosuximide, sodium valproate or lamotrigine for absence seizures in children and adolescents | Review | Ewa B Posner, Khalid K Mohamed, A G Marson, Tony G Marson | |
Differences between protocol and review
Compared to the protocol originally describing the methods for the review, when updating the review we performed a more comprehensive assessment of bias, focusing on the following methodological issues and risk of bias: random sequence generation (selection bias); allocation concealment (selection bias); blinding (performance bias and detection bias); blinding of participants and personnel (performance bias); blinding of outcome assessment (detection bias); incomplete outcome data (attrition bias); and selective reporting (reporting bias).
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Anticonvulsants [adverse effects, *therapeutic use];
- Epilepsy, Absence [*drug therapy, prevention & control];
- Ethosuximide [adverse effects, *therapeutic use];
- Lamotrigine [adverse effects, *therapeutic use];
- Randomized Controlled Trials as Topic;
- Seizures [prevention & control];
- Treatment Failure;
- Valproic Acid [adverse effects, *therapeutic use];
Medical Subject Headings Check Words
Adolescent; Child; Female; Humans; Male;
PICOs
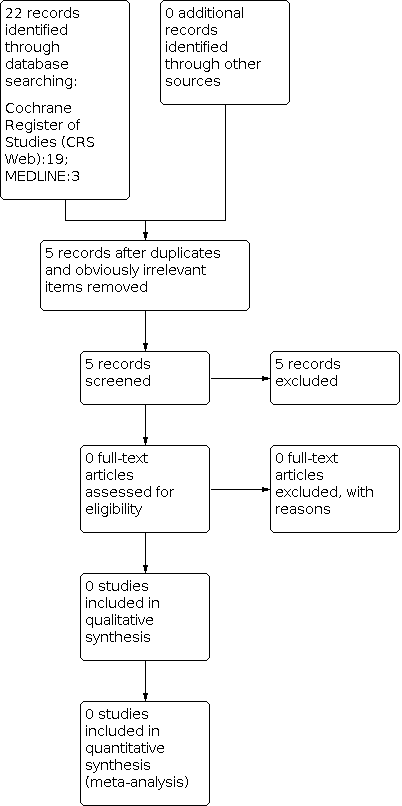
Study flow diagram (results refer only to the updated version of the review). The previous versions of the review (Posner 2003; Posner 2005a; Posner 2005b; Brigo 2017; Brigo 2019) included eight studies.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1: Ethosuximide versus valproate, Outcome 1: Seizure free

Comparison 1: Ethosuximide versus valproate, Outcome 2: 80% or greater reduction in seizure frequency

Comparison 1: Ethosuximide versus valproate, Outcome 3: 50% or greater reduction in seizure frequency

Comparison 2: Lamotrigine versus valproate, Outcome 1: Seizure free

Comparison 2: Lamotrigine versus valproate, Outcome 2: Normalisation of the EEG

Comparison 3: Ethosuximide versus lamotrigine, Outcome 1: Seizure free at 12 months
| Ethosuximide compared to valproate for absence seizures in children and adolescents | ||||||
| Patient or population: absence seizures in children and adolescents | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with valproate | Risk with ethosuximide | |||||
| Seizure freedom at 12 months | Study population | ‐ | 365 | ⊕⊕⊕⊝ | None of the included trials found a difference for this outcome. Length of follow‐up in included studies: from 6 weeks to 4 years. | |
| see comment | see comment | |||||
| 80% or greater reduction in seizure frequency | Study population | RR 0.70 | 29 | ⊕⊝⊝⊝ | No difference was found, but the confidence interval is wide and equivalence cannot be inferred. Length of follow‐up: 6 weeks. | |
| 286 per 1,000 | 200 per 1,000 | |||||
| 50% or greater reduction in seizure frequency | Study population | ‐ | 49 | ⊕⊕⊝⊝ | No difference was found, but the confidence interval is wide and equivalence cannot be inferred. Length of follow‐up in included studies: from 1 to 4 years. | |
| see comment | see comment | |||||
| Normalisation of the EEG ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Adverse effects (Table 1; Table 2) Ethosuximide treatment was mostly associated with nausea, vomiting, and behavioural/psychiatric changes. The most common adverse effects of treatment with valproate were fatigue, nausea, vomiting, increased appetite with weight gain, behavioural/psychiatric changes (decreased concentration, personality change, hyperactivity, attention problems, hostility), and thrombocytopenia | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Most information is from studies at low or unclear risk of bias; plausible bias is likely to seriously alter the results. 2 Information is from a study with unclear and high risk of bias; plausible bias is likely to seriously alter the results. 3 Small number of patients included in this study (29) (see also footnote below). 4 Information is from two small studies with unclear or high risk of bias; plausible bias is likely to seriously alter the results. 5 Information is from two studies with small number of patients included. aIn this study, one patient in the ethosuximide group was subsequently treated with valproate, but failed to respond to either single drug and did not improve when both drugs were used in combination. The outcomes of this patient on combined treatment were therefore counted twice ("no remission"), since the patient received both drugs. | ||||||
| Event | ||||||
|---|---|---|---|---|---|---|
| Acute pancreatitis | 1 | |||||
| Obesity/Weight gain | 1 | 1 | 14 | |||
| Drowsiness | 4 | |||||
| Nausea | 5 | 3 | 12* | |||
| Vomiting | 1 | 2 | 12* | |||
| Decreased platelet numbers | 2 | 4 | ||||
| Increased appetite | 15 | |||||
| Poor appetite | 1 | 8 | ||||
| Diarrhoea | 1 | 7 | ||||
| Dizziness | 1 | 2 | ||||
| Hyperactivity | 23 | |||||
| Attention problems | 24 | |||||
| Hostility | 22 | |||||
| Concentration decreased | 18 | |||||
| Personality change | 17 | |||||
| Sleep problem | 17 | |||||
| Depression | 11 | |||||
| Slow process speed | 11 | |||||
| Memory problem | 10 | |||||
| Apathy | 9 | |||||
| Fatigue | 27 | |||||
| Headache | 1 | 18 | ||||
| Leukopenia | 2 | |||||
| Elevated liver function tests | 1 | 7 | ||||
| Elevated LDH | 1 | |||||
| Rash | 2 | |||||
| *Nausea, vomiting, or both Numbers of individuals within each study undertaking valproate: 14 (Callaghan 1982), 22 (Sato 1982), 10 (Martinovic 1983), 19 (Coppola 2004), 23 (Huang 2009), 146 (Glauser 2013a). | ||||||
| Event | ||||
|---|---|---|---|---|
| Drowsiness | 1 | 5 | ||
| Tiredness | 2 | |||
| Nausea | 3 | 2 | 29* | |
| Vomiting | 3 | 29* | ||
| Increased appetite | 6 | |||
| Poor appetite | 1 | 10 | ||
| Diarrhoea | 9 | |||
| Dizziness | 1 | 10 | ||
| Headache | 2 | 23 | ||
| Leukopenia | 3 | |||
| Hiccups | 1 | |||
| Moodiness | 1 | |||
| Hyperactivity | 13 | |||
| Attention problems | 8 | |||
| Hostility | 4 | |||
| Concentration decreased | 6 | |||
| Personality change | 6 | |||
| Sleep problem | 11 | |||
| Depression | 4 | |||
| Slow process speed | 3 | |||
| Memory problem | 0 | |||
| Apathy | 4 | |||
| Fatigue | 26 | |||
| Rash | 6 | |||
| * Nausea, vomiting, or both Numbers of individuals within each study undertaking ethosuximide: 14 (Callaghan 1982), 23 (Sato 1982), 10 (Martinovic 1983), 154 (Glauser 2013a). | ||||
| Lamotrigine compared to valproate for absence seizures in children and adolescents | ||||||
| Patient or population: absence seizures in children and adolescents | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with valproate | Risk with lamotrigine | |||||
| Seizure freedom at 12 months | Study population | ‐ | 405 | ⊕⊕⊕⊝ | Higher proportion seizure free at 1 month in patients receiving valproate compared to those receiving lamotrigine (2 studies). No difference between valproate and lamotrigine for seizure freedom at 3 and 6 months (3 and 4 studies, respectively). Length of follow‐up in included studies: 12 months. | |
| see comment | see comment | |||||
| 80% or greater reduction in seizure frequency ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 50% or greater reduction in seizure frequency ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Normalisation of the EEG | Study population | RR 2.39 | 45 | ⊕⊝⊝⊝ | Length of follow‐up: 12 months. | |
| 273 per 1,000 | 652 per 1,000 | |||||
| Adverse effects (Table 1; Table 3) The most common adverse effects of treatment with lamotrigine were fatigue, and behavioural/psychiatric changes. The most common adverse effects of treatment with valproate were fatigue, nausea, vomiting, increased appetite with weight gain, behavioural/psychiatric changes (decreased concentration, personality change, hyperactivity, attention problems, hostility), and thrombocytopenia | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Most information comes from studies at low or unclear risk of bias; plausible bias is likely to seriously alter the results. | ||||||
| Event | ||||
|---|---|---|---|---|
| Abdominal pain | 5 | |||
| Headache | 2 | 2 | 14 | |
| Nausea | 3 | 2* | ||
| Vomiting | 2* | |||
| Poor appetite | 2 | 9 | ||
| Increased appetite | 1 | 10 | ||
| Diarrhoea | 2 | |||
| Dizziness | 3 | 5 | 5 | |
| Hyperkinesia | 2 | |||
| Hyperactivity | 12 | |||
| Attention problems | 11 | |||
| Hostility | 11 | |||
| Concentration decreased | 9 | |||
| Personality change | 10 | |||
| Sleep problem | 5 | |||
| Depression | 11 | |||
| Slow process speed | 7 | |||
| Memory problem | 8 | |||
| Apathy | 3 | |||
| Fatigue | 1 | 18 | ||
| Rash | 10 | 1 | 2 | 6 |
| Nervousness | 1 | |||
| Diplopia | 1 | |||
| *Nausea, vomiting, or both Numbers of individuals within each study undertaking lamotrigine: 15 (Frank 1999), 19 (Coppola 2004), 24 (Huang 2009), 146 (Glauser 2013a). | ||||
| Ethosuximide compared to lamotrigine for absence seizures in children and adolescents | ||||||
| Patient or population: absence seizures in children and adolescents | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with lamotrigine | Risk with ethosuximide | |||||
| Seizure freedom at 12 months | Study population | RR 0.47 | 300 | ⊕⊕⊕⊕ | Length of follow‐up: 12 months. | |
| 455 per 1,000 | 214 per 1,000 | |||||
| 80% or greater reduction in seizure frequency ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 50% or greater reduction in seizure frequency ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Normalisation of the EEG ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Adverse effects (Table 2; Table 3) Ethosuximide treatment was mostly associated with nausea, vomiting, and behavioural/psychiatric changes. The most common adverse effects of treatment with lamotrigine were fatigue, and behavioural/psychiatric changes. | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Seizure free Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1.1 Drug naive | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.2 80% or greater reduction in seizure frequency Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.2.1 Previously treated | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3 50% or greater reduction in seizure frequency Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Seizure free Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1.1 Seizure free at 1 month | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1.2 Seizure free at 3 months | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1.3 Seizure freedom at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1.4 Seizure free at 12 months | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.2 Normalisation of the EEG Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Seizure free at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

