Etosuksymid, walproinian sodu lub lamotrygina w leczeniu padaczki z napadami nieświadomości u dzieci i młodzieży
Appendices
Appendix 1. Cochrane Epilepsy Group Specialized Register search strategy
#1 valproic or valproate or Epilim
#2 MeSH DESCRIPTOR Valproic Acid Explode All
#3 ethosuximide or Zarontin
#4 MeSH DESCRIPTOR Ethosuximide Explode All
#5 lamotrigine or Lamictal
#6 #1 OR #2 OR #3 OR #4 OR #5
#7 MeSH DESCRIPTOR Epilepsy, Absence Explode All
#8 absence adj1 (epilep* or seizure*)
#9 "petit mal"
#10 #7 OR #8 OR #9
#11 #6 AND #10
#12 INREGISTER AND >15/12/2015:CRSCREATED
#13 #11 AND #12
Appendix 2. CENTRAL via CRSO search strategy
#1 MESH DESCRIPTOR Valproic Acid EXPLODE ALL TREES
#2 convulex OR depacon OR depakene OR depakine OR depakote OR dpa OR epilim OR epival OR stavzor OR valproat* OR valproic OR vpa
#3 #1 OR #2
#4 MESH DESCRIPTOR Ethosuximide EXPLODE ALL TREES
#5 ethosuximide OR zarontin
#6 #4 OR #5
#7 epilepax OR lamictal OR lamotrigin*
#8 #3 OR #6 OR #7
#9 MESH DESCRIPTOR Epilepsy, Absence EXPLODE ALL TREES
#10 (absence NEXT seizure*):TI,AB,KY
#11 (absence NEXT epilep*):TI,AB,KY
#12 (petit mal):TI,AB,KY
#13 #9 OR #10 OR #11 OR #12
#14 #8 AND #13
#15 15/12/2015 TO 01/09/2016:CD
#16 #14 AND #15
Appendix 3. MEDLINE search strategy
This search strategy is based on the Cochrane Highly Sensitive Search Strategy for identifying randomized trials (Lefebvre 2011).
1. (randomized controlled trial or controlled clinical trial or pragmatic clinical trial).pt. or (randomi?ed or placebo or randomly).ab.
2. clinical trials as topic.sh.
3. trial.ti.
4. 1 or 2 or 3
5. exp animals/ not humans.sh.
6. 4 not 5
7. (valproic or valproate or Epilim).tw.
8. *Valproic Acid/
9. (ethosuximide or Zarontin).tw.
10. *Ethosuximide/
11. (lamotrigine or Lamictal).tw.
12. 7 or 8 or 9 or 10 or 11
13. exp Epilepsy, Absence/
14. (absence adj1 (epilep$ or seizure$)).tw.
15. petit mal.tw.
16. 13 or 14 or 15
17. 6 and 12 and 16
18. remove duplicates from 17
19. limit 18 to ed=20151215‐20160901
Appendix 4. ClinicalTrials.gov search strategy
Condition: absence seizures OR absence epilepsy
Intervention: Ethosuximide OR sodium valproate OR lamotrigine
First received from 12/17/2015 to 09/01/2016
Appendix 5. WHO International Clinical Trials Registry Platform (ICTRP) search strategy
Condition: absence seizures OR absence epilepsy
Intervention: Ethosuximide OR sodium valproate OR lamotrigine
Date of registration between 17/12/2015 and 01/09/2016
Appendix 6. SCOPUS search strategy
(((TITLE‐ABS‐KEY(valproic or valproate or Epilim)) OR (TITLE‐ABS‐KEY(ethosuximide or Zarontin)) OR (TITLE‐ABS‐KEY(lamotrigine or Lamictal))) AND ((TITLE‐ABS‐KEY(absence W/1 (epilep* or seizure*))) OR (TITLE‐ABS‐KEY("petit mal"))) AND (TITLE((randomiz* OR randomis* OR controlled OR placebo OR blind* OR unblind* OR "parallel group" OR crossover OR "cross over" OR cluster OR "head to head") PRE/2 (trial OR method OR procedure OR study)) OR ABS((randomiz* OR randomis* OR controlled OR placebo OR blind* OR unblind* OR "parallel group" OR crossover OR "cross over" OR cluster OR "head to head") PRE/2 (trial OR method OR procedure OR study)))) AND (PUBYEAR > 2003)
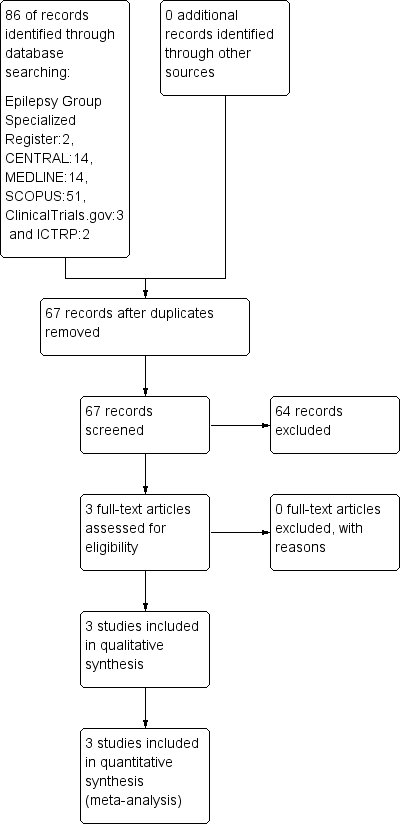
Study flow diagram (results refer only to the updated version of the review).
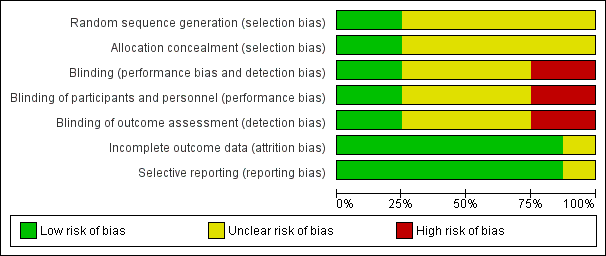
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
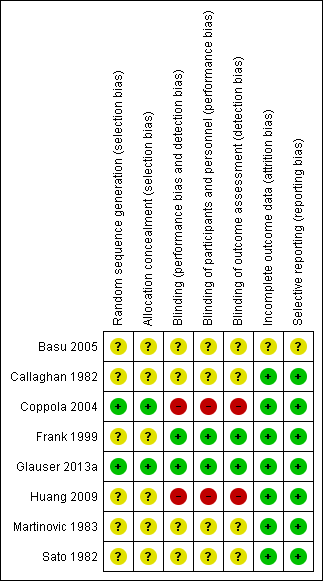
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
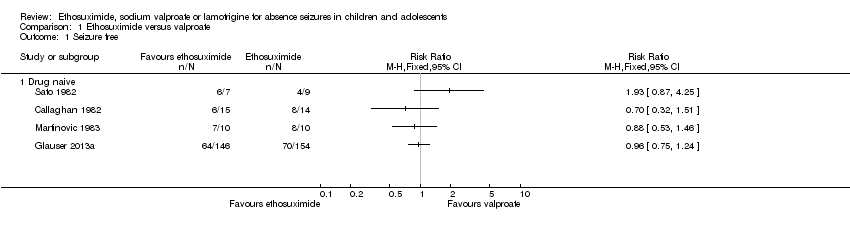
Comparison 1 Ethosuximide versus valproate, Outcome 1 Seizure free.
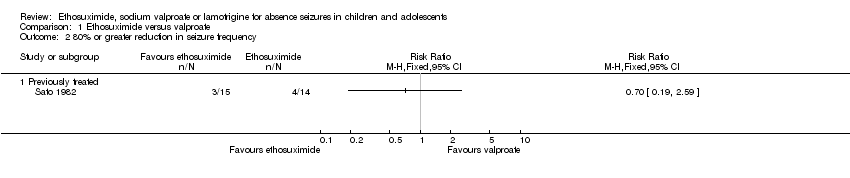
Comparison 1 Ethosuximide versus valproate, Outcome 2 80% or greater reduction in seizure frequency.

Comparison 1 Ethosuximide versus valproate, Outcome 3 50% or greater reduction in seizure frequency.

Comparison 2 Lamotrigine versus valproate, Outcome 1 Seizure free.
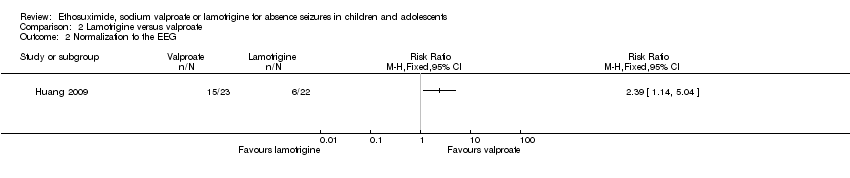
Comparison 2 Lamotrigine versus valproate, Outcome 2 Normalization fo the EEG.
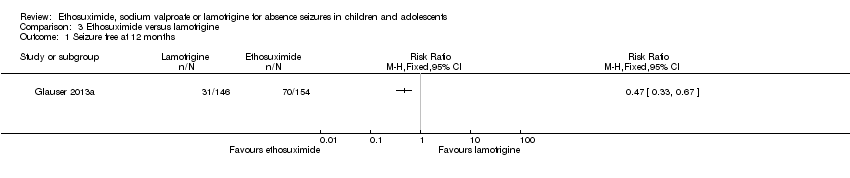
Comparison 3 Ethosuximide versus lamotrigine, Outcome 1 Seizure free at 12 months.
| Event | Callaghan 1982 | Sato 1982 | Martinovic 1983 | Coppola 2004 | Huang 2009 | Glauser 2013 |
| Acute pancreatitis | 1 | |||||
| Obesity/Weight gain | 1 | 1 | 14 | |||
| Drowsiness | 4 | |||||
| Nausea | 5 | 3 | 12* | |||
| Vomiting | 1 | 2 | 12* | |||
| Decreased platelet numbers | 2 | 4 | ||||
| Increased appetite | 15 | |||||
| Poor appetite | 1 | 8 | ||||
| Diarrhoea | 1 | 7 | ||||
| Dizziness | 1 | 2 | ||||
| Hyperactivity | 23 | |||||
| Attention problems | 24 | |||||
| Hostility | 22 | |||||
| Concentration decreased | 18 | |||||
| Personality change | 17 | |||||
| Sleep problem | 17 | |||||
| Depression | 11 | |||||
| Slow process speed | 11 | |||||
| Memory problem | 10 | |||||
| Apathy | 9 | |||||
| Fatigue | 27 | |||||
| Headache | 1 | 18 | ||||
| Leukopenia | 2 | |||||
| Elevated liver function tests | 1 | 7 | ||||
| Elevated LDH | 1 | |||||
| Rash | 2 | |||||
| * Nausea, vomiting, or both Numbers of individuals within each study undertaking valproate: 14 (Callaghan 1982), 22 (Sato 1982), 10 (Martinovic 1983), 19 (Coppola 2004), 23 (Huang 2009), 146 (Glauser 2013a). | ||||||
| Event | Callaghan 1982 | Sato 1982 | Martinovic 1983 | Glauser 2013 |
| Drowsiness | 1 | 5 | ||
| Tiredness | 2 | |||
| Nausea | 3 | 2 | 29* | |
| Vomiting | 3 | 29* | ||
| Increased appetite | 6 | |||
| Poor appetite | 1 | 10 | ||
| Diarrhoea | 9 | |||
| Dizziness | 1 | 10 | ||
| Headache | 2 | 23 | ||
| Leukopenia | 3 | |||
| Hiccups | 1 | |||
| Moodiness | 1 | |||
| Hyperactivity | 13 | |||
| Attention problems | 8 | |||
| Hostility | 4 | |||
| Concentration decreased | 6 | |||
| Personality change | 6 | |||
| Sleep problem | 11 | |||
| Depression | 4 | |||
| Slow process speed | 3 | |||
| Memory problem | 0 | |||
| Apathy | 4 | |||
| Fatigue | 26 | |||
| Rash | 6 | |||
| * Nausea, vomiting, or both Numbers of individuals within each study undertaking ethosuximide: 14 (Callaghan 1982), 23 (Sato 1982), 10 (Martinovic 1983), 154 (Glauser 2013a). | ||||
| Event | Frank 1999 | Coppola 2004 | Huang 2009 | Glauser 2013 |
| Abdominal pain | 5 | |||
| Headache | 2 | 2 | 14 | |
| Nausea | 3 | 2* | ||
| Vomiting | 2* | |||
| Poor appetite | 2 | 9 | ||
| Increased appetite | 1 | 10 | ||
| Diarrhoea | 2 | |||
| Dizziness | 3 | 5 | 5 | |
| Hyperkinesia | 2 | |||
| Hyperactivity | 12 | |||
| Attention problems | 11 | |||
| Hostility | 11 | |||
| Concentration decreased | 9 | |||
| Personality change | 10 | |||
| Sleep problem | 5 | |||
| Depression | 11 | |||
| Slow process speed | 7 | |||
| Memory problem | 8 | |||
| Apathy | 3 | |||
| Fatigue | 1 | 18 | ||
| Rash | 10 | 1 | 2 | 6 |
| Nervousness | 1 | |||
| Diplopia | 1 | |||
| * Nausea, vomiting, or both Numbers of individuals within each study undertaking lamotrigine: 15 (Frank 1999), 19 (Coppola 2004), 24 (Huang 2009), 146 (Glauser 2013a). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure free Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Drug naive | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 80% or greater reduction in seizure frequency Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Previously treated | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 50% or greater reduction in seizure frequency Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure free Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Seizure free at 1 month | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.42 [2.77, 25.59] | |
| 1.2 Seizure free at 3 months | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.16, 2.31] | |
| 1.3 Seizure freedom at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.88, 2.28] | |
| 1.4 Seizure free at 12 months | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.32, 2.11] | |
| 2 Normalization fo the EEG Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure free at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

