Síntomas neurológicos transitorios (SNT) después de la anestesia espinal con lidocaína versus otros anestésicos locales
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomization: | |
| Participants | Country: Lebanon. | |
| Interventions | Drug 1: 5% lido, hyperbaric, fixed dose (1.5ml). | |
| Outcomes | TNS at 1 day. | |
| Notes | Follow‐up duration : 1.3 days. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Randomization: | |
| Participants | Country: Belgium | |
| Interventions | Drug 1: 2% lido, isobaric, fixed dose (3ml). | |
| Outcomes | TNS at 2 day. | |
| Notes | Follow‐up duration: 2 days. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomization: yes. | |
| Participants | Country: the Netherlands. | |
| Interventions | Drug 1: 2% lido, isobaric, fixed dose (4 ml). | |
| Outcomes | TNS at 1 day. | |
| Notes | Follow‐up duration: 1‐4 days. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomization: yes. | |
| Participants | Country : Switzerland. | |
| Interventions | Drug 1: 5% lido, hyperbaric, fixed dose (1.5 ml). | |
| Outcomes | TNS at 1 day. | |
| Notes | Follow‐up duration: 1‐4 days. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomization: yes. | |
| Participants | Country: Switzerland. | |
| Interventions | Drug 1: 2% lido, hyperbaric, fixed dose (2.5 ml). | |
| Outcomes | TNS at 1 day. | |
| Notes | Follow‐up duration: 1‐5 days. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomization: yes. | |
| Participants | Country: USA. | |
| Interventions | Drug 1: 5% lido, hyperbaric, fixed dose (1 ml). | |
| Outcomes | TNS at 1 day. | |
| Notes | Follow‐up duration: 3 days. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomization: yes. | |
| Participants | Country: Denmark. | |
| Interventions | Drug 1: 5% lido, hyperbaric, fixed dose (2 ml). | |
| Outcomes | TNS at 1 day. | |
| Notes | Follow‐up duration 1‐3 days. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Randomization: yes. | |
| Participants | Country: Canada. | |
| Interventions | Drug 1: 5% lido, hyperbaric, fixed dose (2 ml). | |
| Outcomes | TNS at 2 days. | |
| Notes | Follow‐up duration: 2 days. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Randomization: yes. | |
| Participants | Country: USA. | |
| Interventions | Drug 1: 2% lido, baricity unclear, fixed dose (3 ml). | |
| Outcomes | TNS at 1‐2 days. | |
| Notes | Follow‐up duration: 2‐5 days. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Randomization: yes. | |
| Participants | Country: Spain. | |
| Interventions | Drug 1: 5% lido, hyperbaric, variable dose. | |
| Outcomes | TNS at 1‐2 days. | |
| Notes | Follow‐up duration: 3‐5 days. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomization: yes. | |
| Participants | Country: USA | |
| Interventions | Drug 1: 5% lido, hyperbaric, variable dose. | |
| Outcomes | TNS at 1 day. | |
| Notes | Follow‐up duration: 3 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomization: yes. | |
| Participants | Country: USA. | |
| Interventions | Drug 1: 2%, 5% lido; hyperbaric, isobaric; fixed dose (1.2, 3.0, 1.5, 3.75 ml). | |
| Outcomes | TNS at 3 days. | |
| Notes | Follow‐up duration: 3‐14 days. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomization: yes. | |
| Participants | Country: Spain. | |
| Interventions | Drug 1: 2% lido, isobaric, variable dose. | |
| Outcomes | TNS at 1 day. | |
| Notes | Follow‐up duration: 1+ days. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Randomization: yes. | |
| Participants | Country: Finland | |
| Interventions | Drug 1: 5% lido, hyperbaric, variable dose. | |
| Outcomes | TNS at 1 day. | |
| Notes | Follow‐up duration: 1 day. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomization: yes. | |
| Participants | Country: Norway | |
| Interventions | Drug 1: 2% lido, isobaric, fixed dose (4 ml). | |
| Outcomes | TNS at 1 day. | |
| Notes | Follow‐up duration: 1 day. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
ASA: American Society of Anesthesiologist Physical Status Score (I, II, III, IV).
bupi: bupivacaine; levo: levobupivacaine; lido: lidocaine; mepi: mepivacaine; prilo: prilocaine; pro: procaine; ropi: ropivacaine.
NA: not available.
TNS: Transient Neurologic Symptoms.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Fentanyl was used together with lidocaine. No arm with another local anaesthetic. | |
| No lidocaine, only one arm with procaine | |
| Continuous spinal anaesthesia with lidocaine 5% . No follow‐up, no mention of TNS | |
| Volunteers ‐ no surgery ‐ excluded. | |
| No lidocaine. Bupivacaine 0.1%, 0.15% and 0.2%. Volume: | |
| Nonrandomized study | |
| Only lidocaine in two different concentrations: 5% vs. 2%. No difference in the incidence of TNS (8 of 25 vs. 10 of 25 with 2% lidocaine). Reduction in concentration did not reduce the risk of TNS. | |
| Case history: one patient with TNS after 1% Lidocaine 40 mg. Full recovery. | |
| Only one arm with lidocaine: second arm with general anaesthesia. Even patients who receive only general anaesthesia can develop TNS. | |
| Only lidocaine: three arms with 1% lidocaine in different volumes: 4, 6 and 8 ml ‐ no cases of TNS. | |
| Swedish Pharmacological Insurance reported six cases of cauda equina syndrome between 1993‐1997. Five cases after single spinal injection of lidocaine 5% and one case after repeated injection. Lidocaine doses was 60‐100 mg. All cases sustained permanent neurological deficits. Recommendation: use plain 2% lidocaine and no more than 60 mg. | |
| 1.5% lidocaine is as effective as 5% for patients undergoing hernia operation. No mention of TNS. | |
| Nonrandomized study : 4 of 1045 patients that received lidocaine 3% 45 mg (for anorectal surgery) had TNS. | |
| Intrathecal meperidine 0.3 mg/kg was added to lidocaine to prolong postoperative analgesia. No mention of TNS. | |
| No lidocaine. Mepivacaine 1.5% (60 mg) and 2% (80 mg) was used in 60 patients. Follow‐up 24 h: no cases of TNS. | |
| Only lidocaine was used: three arms with 0.5%, 1% and 2% lidocaine: incidence of TNS was not concentration‐dependant (20 out of 109 patients). | |
| Only one arm with Lidocaine 5%. 13 out of 44 urological patients had signs of TNS. | |
| 3 cases of TNS after spinal mepivacaine. | |
| Only lidocaine was used: two arms with 80 mg lidocaine: 1% (N = 218) and 5 % hyperbaric lidocaine (N = 235): incidence of TNS was not concentration‐dependant (21% vs. 18%). | |
| Nonrandomized study | |
| No lidocaine. |
N ‐ numbers
PTT ‐ patients
TNS ‐Transient neurologic symptoms
Vs ‐ versus
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Transient Neurologic Symptoms Show forest plot | 15 | 1437 | Risk Ratio (M‐H, Random, 95% CI) | 4.47 [2.17, 9.20] |
| Analysis 1.1  Comparison 1 Lidocane versus other local anaesthetic, Outcome 1 Transient Neurologic Symptoms. | ||||
| 1.1 Bupivacaine | 7 | 621 | Risk Ratio (M‐H, Random, 95% CI) | 6.65 [2.05, 21.56] |
| 1.2 Mepivacaine | 3 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.15, 7.45] |
| 1.3 Prilocaine | 4 | 414 | Risk Ratio (M‐H, Random, 95% CI) | 5.62 [2.07, 15.23] |
| 1.4 Procaine | 2 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 6.94 [1.94, 24.86] |
| 1.5 Ropivacaine | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 5.81 [0.25, 134.73] |
| 1.6 Levobupivacine | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 9.69 [0.49, 189.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Transient Neurologic Symptoms Show forest plot | 13 | 1255 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.16 [4.02, 12.75] |
| Analysis 2.1  Comparison 2 Lidocaine versus other local anaesthetic (excluding mepivacaine), Outcome 1 Transient Neurologic Symptoms. | ||||
| 1.1 Bupivacaine | 7 | 621 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.60 [3.00, 19.30] |
| 1.2 Prilocaine | 4 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.14 [2.31, 16.32] |
| 1.3 Procaine | 2 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.8 [2.19, 27.77] |
| 1.4 Ropivacaine | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.81 [0.25, 134.73] |
| 1.5 Levobupivacaine | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.69 [0.49, 189.93] |

Comparison 1 Lidocane versus other local anaesthetic, Outcome 1 Transient Neurologic Symptoms.
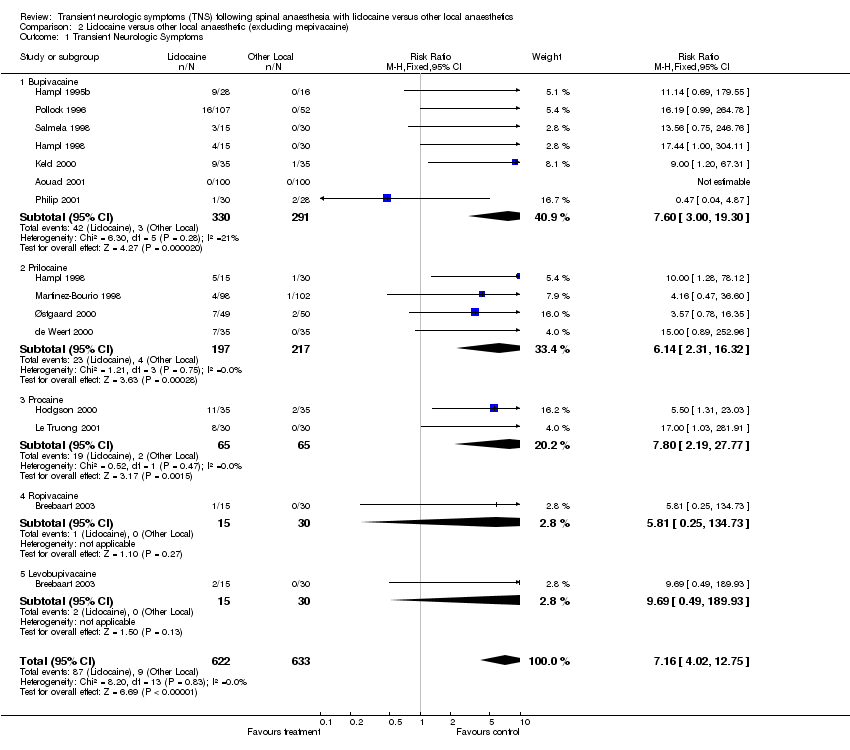
Comparison 2 Lidocaine versus other local anaesthetic (excluding mepivacaine), Outcome 1 Transient Neurologic Symptoms.
| Study ID | TNS #/N (%) | Pain score (0‐10) | TNS duration | Therapy |
| Aouad 2001 | 0 (0) | |||
| de Weert 2000 | 7/35 (20) | Day 1 mean VPS = 5.3 (range 2‐8) | Maximum duration 3 days | Not described |
| Hampl 1995b | 9/28 (32) | Not tallied | Maximum duration 4 days | Not described |
| Hampl 1998 | 9/30 (30) | Mean maximum VAS = 3.75 | Maximum duration 2 days | Not described |
| Hodgson 2000 | 11/35 (31) | Mean VPS = 5 | Mean duration 2 days | Not described |
| Keld 2000 | 9/35 (26) | Mean VPS = 3.5 (range 2‐8) | Maximum duration 4 days | Not described |
| Liguori 1998 | 6/27 (22) | Not tallied | Maximum duration 5 days | NSAIDs |
| Le Truong | 8/30 (27) | Not tallied | Unspecified | Not described |
| Martinez‐Bourio 1998 | 4/98 (4) | Not tallied | Maximum duration 10 days | NSAIDs |
| Østgaard 2000 | 7/49 (14) | VPS range 5‐9.5 | Maximum duration 3 days | Not described |
| Philip 2001 | 1/30 (3) | Maximum VAS = 3 | Maximum duration 2 days | Not described |
| Pollock 1996 | 16/107 (15) | Mean VPS = 6.2 (range 1‐9) | Maximum duration 4 days | NSAIDs and opioids |
| Salazar 2001 | 1/40 (3) | Maximum VAS = 9‐10 | Maximum duration 1 day | NSAIDs |
| Salmela 1998 | 6/30 (20) | Moderate pain | Maximum duration 1 day | NSAIDs and opioids |
| Breebaart 2003 | 3/30 (10) | Not tallied | 1 day | Not described |
| Key to abbreviations: | VPS: Verbal Pain Scale | VAS: Visual Analogue Scale |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Transient Neurologic Symptoms Show forest plot | 15 | 1437 | Risk Ratio (M‐H, Random, 95% CI) | 4.47 [2.17, 9.20] |
| 1.1 Bupivacaine | 7 | 621 | Risk Ratio (M‐H, Random, 95% CI) | 6.65 [2.05, 21.56] |
| 1.2 Mepivacaine | 3 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.15, 7.45] |
| 1.3 Prilocaine | 4 | 414 | Risk Ratio (M‐H, Random, 95% CI) | 5.62 [2.07, 15.23] |
| 1.4 Procaine | 2 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 6.94 [1.94, 24.86] |
| 1.5 Ropivacaine | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 5.81 [0.25, 134.73] |
| 1.6 Levobupivacine | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 9.69 [0.49, 189.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Transient Neurologic Symptoms Show forest plot | 13 | 1255 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.16 [4.02, 12.75] |
| 1.1 Bupivacaine | 7 | 621 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.60 [3.00, 19.30] |
| 1.2 Prilocaine | 4 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.14 [2.31, 16.32] |
| 1.3 Procaine | 2 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.8 [2.19, 27.77] |
| 1.4 Ropivacaine | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.81 [0.25, 134.73] |
| 1.5 Levobupivacaine | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.69 [0.49, 189.93] |

