Síntomas neurológicos transitorios (SNT) después de la anestesia espinal con lidocaína versus otros anestésicos locales en pacientes adultos quirúrgicos: un metanálisis en red
Resumen
Antecedentes
La anestesia espinal se ha implicado como una de las posibles causas de complicaciones neurológicas después de los procedimientos quirúrgicos. Esta afección dolorosa, que se produce durante el período postoperatorio inmediato, se denomina síntomas neurológicos transitorios (SNT) y se observa habitualmente después del uso de la lidocaína espinal. Se necesitan alternativas a la lidocaína que puedan proporcionar una anestesia de alta calidad sin el desarrollo de SNT. Esta revisión se publicó originalmente en 2005 y se actualizó por última vez en 2009.
Objetivos
Determinar la frecuencia de los SNT después de la anestesia espinal con lidocaína y compararla con otros tipos de anestésicos locales mediante un metanálisis de todas las comparaciones pareadas y un metanálisis en red (MAR) para clasificar las intervenciones.
Métodos de búsqueda
Se realizaron búsquedas en CENTRAL, MEDLINE, Elsevier Embase y LILACS el 25 de noviembre 2018. Se realizaron búsquedas en los registros de ensayos clínicos y búsquedas manuales en las listas de referencias de los ensayos y los artículos de revisión.
Criterios de selección
Se incluyeron los ensayos controlados aleatorizados y cuasialeatorizados que compararon la frecuencia de SNT después de la anestesia espinal con lidocaína con otros anestésicos locales. Los estudios debían tener dos o más brazos que utilizaran anestésicos locales diferentes (independientemente de la concentración y la baricidad de la solución) para la anestesia espinal en la preparación para la cirugía.
Se incluyeron adultos que recibieron anestesia espinal y todas los participantes embarazadas se consideraron como un subgrupo. El período de seguimiento para los SNT fue de al menos 24 horas.
Obtención y análisis de los datos
Cuatro autores de la revisión de forma independiente evaluaron los estudios para su inclusión. Tres autores de la revisión de forma independiente evaluaron la calidad de los estudios relevantes y extrajeron los datos de los estudios incluidos. Se realizó un metanálisis para todas las comparaciones pareadas de los anestésicos locales, así como un MAR.
Se utilizó una ponderación de la varianza inversa para las estadísticas resumen y un modelo de efectos aleatorios, ya que se esperaba heterogeneidad metodológica y clínica entre los estudios incluidos, lo que dio lugar a tamaños del efecto variables entre los estudios de las comparaciones pareadas. El MAR utilizó todos los estudios incluidos sobre la base de un enfoque teórico gráfico dentro de un marco frecuentista. Finalmente, los tratamientos en comparación se calificaron según las puntuaciones P.
Resultados principales
El análisis incluyó 24 ensayos que informaron sobre 2226 participantes, de los cuales 239 desarrollaron SNT. Dos estudios están en espera de clasificación y uno está en curso. La mayoría de los estudios tuvieron riesgo de sesgo incierto a alto.
El MAR incluyó 24 estudios y ocho anestésicos locales diferentes; el número de comparaciones pareadas fue 32 y el número de comparaciones pareadas diferentes fue 11. Este análisis mostró que, en comparación con la lidocaína, el riesgo relativo (RR) de SNT fue menor para la bupivacaína, la levobupivacaína, la prilocaína, la procaína y la ropivacaína, con RR en el rango de 0,10 a 0,23; mientras que la 2‐cloroprocaína y la mepivacaína no difirieron en cuanto al RR de desarrollar SNT en comparación con la lidocaína.
El metanálisis pareado mostró que, en comparación con la lidocaína, la mayoría de los anestésicos locales se asociaron con un menor riesgo de desarrollar SNT (excepto la 2‐cloroprocaína y la mepivacaína) (bupivacaína): RR 0,16; intervalo de confianza (IC) del 95%: 0,09 a 0,28; 12 estudios; evidencia de calidad moderada; 2‐cloroprocaína: RR 0,09; IC del 95%: 0,01 a 1,51; dos estudios; evidencia de calidad baja; levobupivacaína: RR 0,13; IC del 95%: 0,02 a 0,69; dos estudios; evidencia de calidad baja; mepivacaína: RR 1,01; IC del 95%: 0,18 a 5,82; cuatro estudios; evidencia de calidad muy baja; prilocaína: RR 0,18; IC del 95%: 0,07 a 0,49; cuatro estudios; evidencia de calidad moderada; procaína: RR 0,14; IC del 95%: 0,04 a 0,52; dos estudios; evidencia de calidad moderada; ropivacaína: RR 0,10; IC del 95%: 0,01 a 0,78; dos estudios; evidencia de calidad baja).
No fue posible realizar los análisis de subgrupos previstos debido al bajo número de eventos de SNT.
Conclusiones de los autores
Los resultados del MAR y del metanálisis pareado indican que el riesgo de desarrollar SNT después de la anestesia espinal es menor cuando se utiliza bupivacaína, levobupivacaína, prilocaína, procaína y ropivacaína, en comparación con la lidocaína. La administración de 2‐cloroprocaína y mepivacaína tuvo un riesgo similar a la lidocaína en cuanto a desarrollar SNT después de la anestesia espinal.
Se debe informar a los pacientes sobre los SNT como un posible efecto adverso de la anestesia local con lidocaína, y la elección del agente anestésico se debe basar en el contexto clínico específico y parámetros como la duración esperada del procedimiento y la calidad de la anestesia.
Debido a la evidencia de calidad muy baja a moderada (GRADE), se requieren estudios de investigación futuros en este campo para evaluar alternativas a la lidocaína que puedan proporcionar una anestesia de alta calidad sin desarrollar SNT. Los dos estudios en espera de clasificación y un estudio en curso pueden alterar las conclusiones de la revisión una vez evaluados.
PICO
Resumen en términos sencillos
Ocurrencia de síntomas neurológicos transitorios después de la anestesia espinal con lidocaína versus otros anestésicos locales en adultos sometidos a cirugía
Pregunta de la revisión
Se intentó determinar si los síntomas neurológicos transitorios (SNT) ocurren con mayor frecuencia después de la recuperación de la anestesia espinal con lidocaína que con otros anestésicos locales en adultos. Los síntomas son un dolor de leve a intenso en los glúteos y las piernas que puede durar varios días. También se buscaron las alteraciones sensoriales o motoras de mayor duración causadas por el daño nervioso por los anestésicos locales, conocidas como complicaciones neurológicas.
Antecedentes
El dolor leve en la zona lumbar es una queja frecuente después de la anestesia espinal (en la que se inyecta un anestésico local en la columna vertebral en lugar de utilizar un anestésico general en todo el cuerpo). Los pacientes también pueden experimentar dolor de cabeza y presión arterial baja. Los SNT son diferentes. Aparecen unas pocas horas hasta 24 horas después de la anestesia espinal y pueden durar hasta dos a cinco días.
La lidocaína (un anestésico local) aún se utiliza para la anestesia espinal debido a su corta duración de acción única, el bloqueo intenso, la recuperación rápida y su idoneidad para la cirugía ambulatoria, pero se necesitan alternativas.
Esta revisión se publicó originalmente en 2005 y se actualizó en 2009.
Características de los estudios
Se incluyeron todos los estudios aleatorizados y cuasialeatorizados que compararon la frecuencia de SNT y las complicaciones neurológicas después de la anestesia espinal con lidocaína, en comparación con otros anestésicos locales. Los ensayos aleatorizados comparan dos o más tratamientos en los que los tratamientos se les asignan a los participantes de una manera aleatorizada que los organizadores del estudio no pueden predecir. Los estudios cuasialeatorizados son similares pero no son verdaderamente aleatorizados; tienen mayores probabilidades de que el organizador del estudio pueda predecir qué tratamiento reciben los participantes (p.ej. basado en la fecha de nacimiento o en el orden en el que se reclutaron las personas).
La evidencia está actualizada hasta el 25 de noviembre 2018.
Resultados clave
Se incluyeron 24 ensayos que informaron sobre 2226 participantes, 239 de los cuales desarrollaron SNT. No hubo evidencias de que los SNT se asociaran con alguna enfermedad neurológica específica y los síntomas desaparecieron espontáneamente al quinto día postoperatorio. El riesgo de desarrollar SNT con lidocaína para la anestesia espinal aumentó en comparación con la bupivacaína, la prilocaína o la procaína; y fue similar en comparación con la 2‐cloroprocaína y la mepivacaína.
Específicamente, cuando los anestésicos locales alternativos se compararon directamente con la lidocaína, el riesgo de desarrollar SNT se redujo entre el 82% y el 90% cuando se utilizó bupivacaína, levobupivacaína, prilocaína, procaína y ropivacaína en lugar de lidocaína. No hubo diferencias claras en los SNT entre la lidocaína y la 2‐cloroprocaína o la mepivacaína. En el caso de la 2‐cloroprocaína, los SNT ocurrieron en un solo estudio y los resultados variaron enormemente para el pequeño número de participantes. Los síntomas dolorosos se detuvieron al quinto día postoperatorio en todos los participantes. Entre las mujeres embarazadas sometidas a cirugía, solo 3/310 mujeres desarrollaron SNT; no fue posible establecer conclusiones sobre si los síntomas fueron más probables con la lidocaína.
Los autores de la revisión utilizaron el método estadístico del metanálisis en red para comparar los diferentes anestésicos locales. Este análisis mostró de forma similar que el riesgo de SNT fue inferior para la bupivacaína, la levobupivacaína, la prilocaína, la procaína y la ropivacaína, mientras que la 2‐cloroprocaína y la mepivacaína no difirieron en cuanto al riesgo de SNT, en comparación con la lidocaína.
Calidad de la evidencia
Debido a la calidad muy baja a moderada de la evidencia entre los estudios actualmente disponibles, se necesitan estudios de investigación futuros en este campo para evaluar las alternativas a la lidocaína que pueden proporcionar una anestesia de alta calidad sin el desarrollo de SNT.
Conclusión
La lidocaína ha sido el fármaco de elección para inducir la anestesia espinal en la cirugía ambulatoria debido a su rápido inicio de acción, su intenso bloqueo nervioso y su corta duración de acción. La presente revisión muestra que la lidocaína posee mayores probabilidades de causar síntomas neurológicos transitorios que la bupivacaína, la prilocaína y la procaína. Sin embargo, estos fármacos producen efectos anestésicos locales más prolongados y, por lo tanto, no son deseables para los pacientes ambulatorios.
Los resultados indican que la 2‐cloroprocaína podría ser una alternativa viable a la lidocaína para la cirugía ambulatoria de corta duración y los procedimientos obstétricos, ya que este anestésico local tiene un rápido inicio de acción, se metaboliza rápidamente y tiene baja toxicidad. Sin embargo, esta conclusión se basa en solo dos estudios y en evidencia de calidad baja.
Authors' conclusions
Summary of findings
| Risk of transient neurological symptoms (TNS) with spinal lidocaine compared to other local anaesthetics in adults undergoing surgerya | ||||||
| Patient or population: adult undergoing surgery Settings: hospital or ambulatory surgery setting (Belgium, Brazil, Canada, Denmark, Egypt, Finland, Iran, Italy, Lebanon, Nepal, the Netherlands, Norway, Spain, Switzerland, Turkey, USA) Intervention: spinal lidocaine Comparison: other local anaesthetics as indicated | ||||||
| Outcomes | Anticipated absolute effectsb (95% CI) | Relative effect (95 CI) | № of participants | Quality of the evidence | Comments | |
| Risk with lidocaine | Risk difference with the other local anaesthetic | |||||
| Presence of any TNS –lidocaine vs bupivacaine Follow‐up: range 1–30 days | 210 per 1000 | 176 fewer per 1000 (191 fewer to 151 fewer) | RR 0.16 (0.09 to 0.28) | 1220 (12 RCTs) | ⊕⊕⊕⊝ Moderatec | Bupivacaine probably reduced the risk of TNS compared to lidocaine. |
| Presence of any TNS – lidocaine vs 2‐chloroprocaine Follow‐up: range 1–7 days | 106 per 1000 | 97 fewer per 1000 (105 fewer to 54 more) | RR 0.09 (0.01 to 1.51) | 94 (2 RCTs) | ⊕⊕⊝⊝ Lowc,d | 2‐chloroprocaine may have resulted in no difference in the risk of TNS compared to lidocaine. |
| Presence of any TNS – lidocaine vs levobupivacaine Follow‐up: range 2–7 days | 183 per 1000 | 159 fewer per 1000 (180 fewer to 57 fewer) | RR 0.13 (0.02 to 0.69) | 120 (2 RCTs) | ⊕⊕⊝⊝ | Levobupivacaine may have reduced the risk of TNS compared to lidocaine. |
| Presence of any TNS – lidocaine vs mepivacaine Follow‐up: range 1–5 days | 95 per 1000 | 1 more per 1000 (78 fewer to 457 more) | RR 1.01 (0.18 to 5.82) | 274 (4 RCTs) | ⊕⊝⊝⊝ Very lowd,e,f | Mepivacaine may have resulted in no difference in the risk of TNS compared to lidocaine but the evidence was very uncertain. |
| Presence of any TNS – lidocaine vs prilocaine Follow‐up: range 1–5 days | 127 per 1000 | 104 fewer per 1000 (118 fewer to 65 fewer) | RR 0.18 (0.07 to 0.49) | 429 (4 RCTs) | ⊕⊕⊕⊝ Moderatec | Prilocaine probably reduced the risk of TNS compared to lidocaine. |
| Presence of any TNS – lidocaine vs procaine Follow‐up: range 2–3 days | 292 per 1000 | 251 fewer per 1000 (281 fewer to 140 fewer) | RR 0.14 (0.04 to 0.52) | 130 (2 RCTs) | ⊕⊕⊕⊝ Moderatef | Procaine probably reduced the risk of TNS compared to lidocaine. |
| Presence of any TNS – lidocaine vs ropivacaine Follow‐up: range 2–7 days | 200 per 1000 | 180 fewer per 1000 (198 fewer to 44 fewer) | RR 0.10 (0.01 to 0.78) | 90 (2 RCTs) | ⊕⊕⊝⊝ Lowc,e | Ropivacaine may have reduced the risk of TNS compared to lidocaine. |
| CI: confidence interval; TNS: transient neurological symptoms; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| a'Summary of findings' table is based on pair‐wise meta‐analysis (Figure 1). Results of the network meta‐analysis are presented in Table 2; Table 3; Table 4 and Figure 2; Figure 3; Figure 4; Figure 5; Figure 6; Figure 7. | ||||||
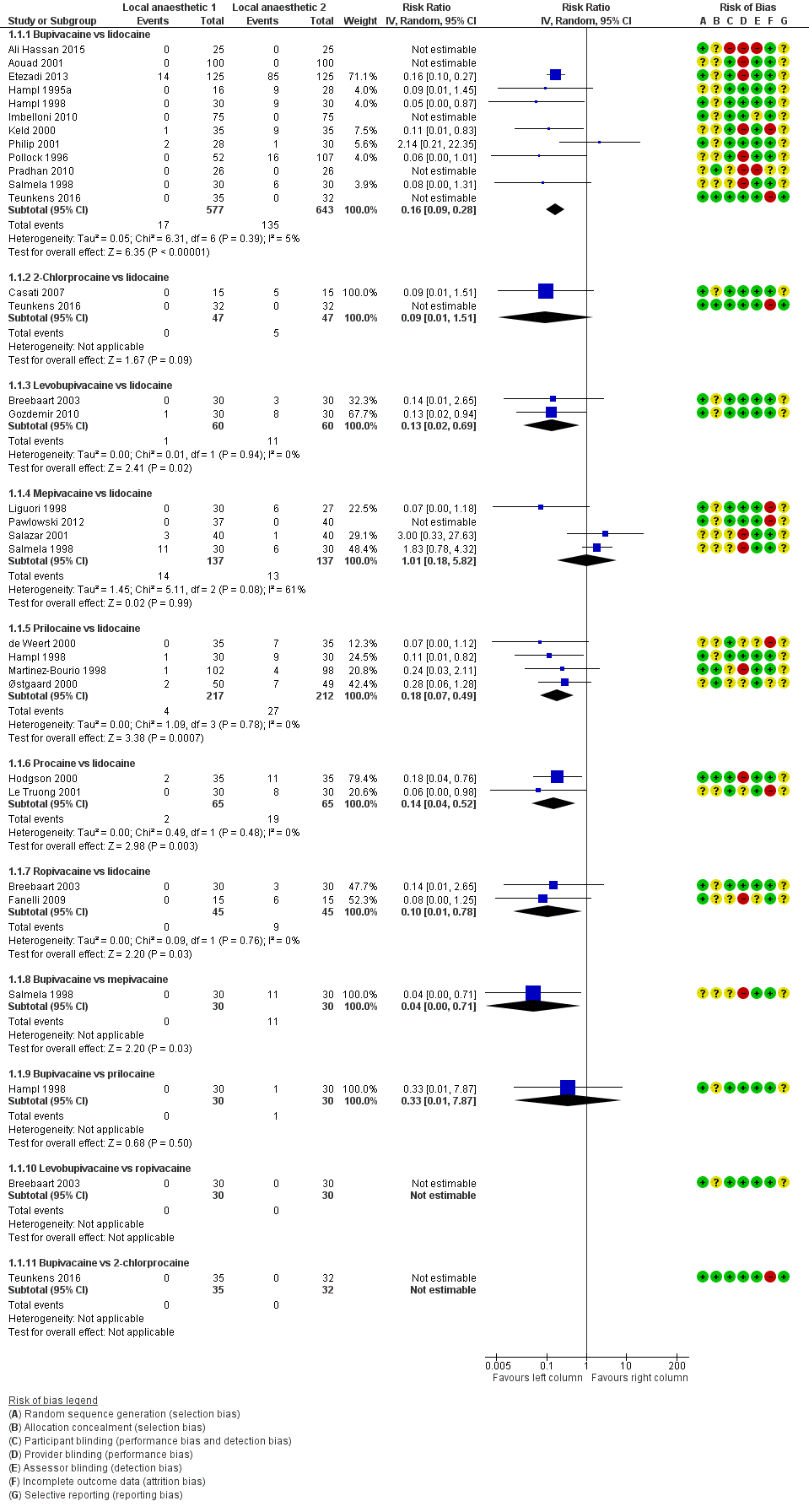
Forest plot of comparison: 1 Lidocaine versus other local anaesthetic, outcome: 1.1 Transient neurological symptoms.

Network meta‐analysis plot of interactions among included studies displayed for a random‐effects, risk ratio model, regarding the risk of transient neurological symptoms following spinal anaesthesia with lidocaine versus other local anaesthetics in adults undergoing surgery. Each node represents an individual local anaesthetic. The node size is proportional to the number of studies.
The node colours are determined by the individual study 'Risk of bias' assessment (green: no concerns; yellow: some concerns; red: major concerns).
The width of the edges is proportional to the inverse variance of the effect size. The edge colours reflect the average risk of bias. bupi: bupivacaine; chloro: 2‐chloroprocaine; levo: levobupivacaine; lido: lidocaine; mepi: mepivacaine; prilo: prilocaine; pro: procaine; ropi: ropivacaine.

Risk of bias (RoB) assessment among studies included in the network meta‐analysis with direct effect estimation is displayed, regarding the risk of transient neurological symptoms following spinal anaesthesia with lidocaine versus other local anaesthetics in adults undergoing surgery. Direct RoB was determined by the average RoB assigned to each particular network interaction. bupi: bupivacaine; chloro: 2‐chloroprocaine; levo: levobupivacaine; lido: lidocaine; mepi: mepivacaine; prilo: prilocaine; pro: procaine; ropi: ropivacaine.

Contributions of indirect and mixed effects in the network meta‐analysis, regarding the risk of transient neurological symptoms following spinal anaesthesia with lidocaine versus other local anaesthetics in adults undergoing surgery. bupi: bupivacaine; chloro: 2‐chloroprocaine; levo: levobupivacaine; lido: lidocaine; mepi: mepivacaine; NMA: network meta‐analysis; prilo: prilocaine; pro: procaine; ropi: ropivacaine

Analysis of imprecision among studies included in the network meta‐analysis, regarding the risk of transient neurological symptoms following spinal anaesthesia with lidocaine versus other local anaesthetics in adults undergoing surgery. bupi: bupivacaine; chloro: 2‐chloroprocaine; levo: levobupivacaine; lido: lidocaine; mepi: mepivacaine; prilo: prilocaine; pro: procaine; ropi: ropivacaine.
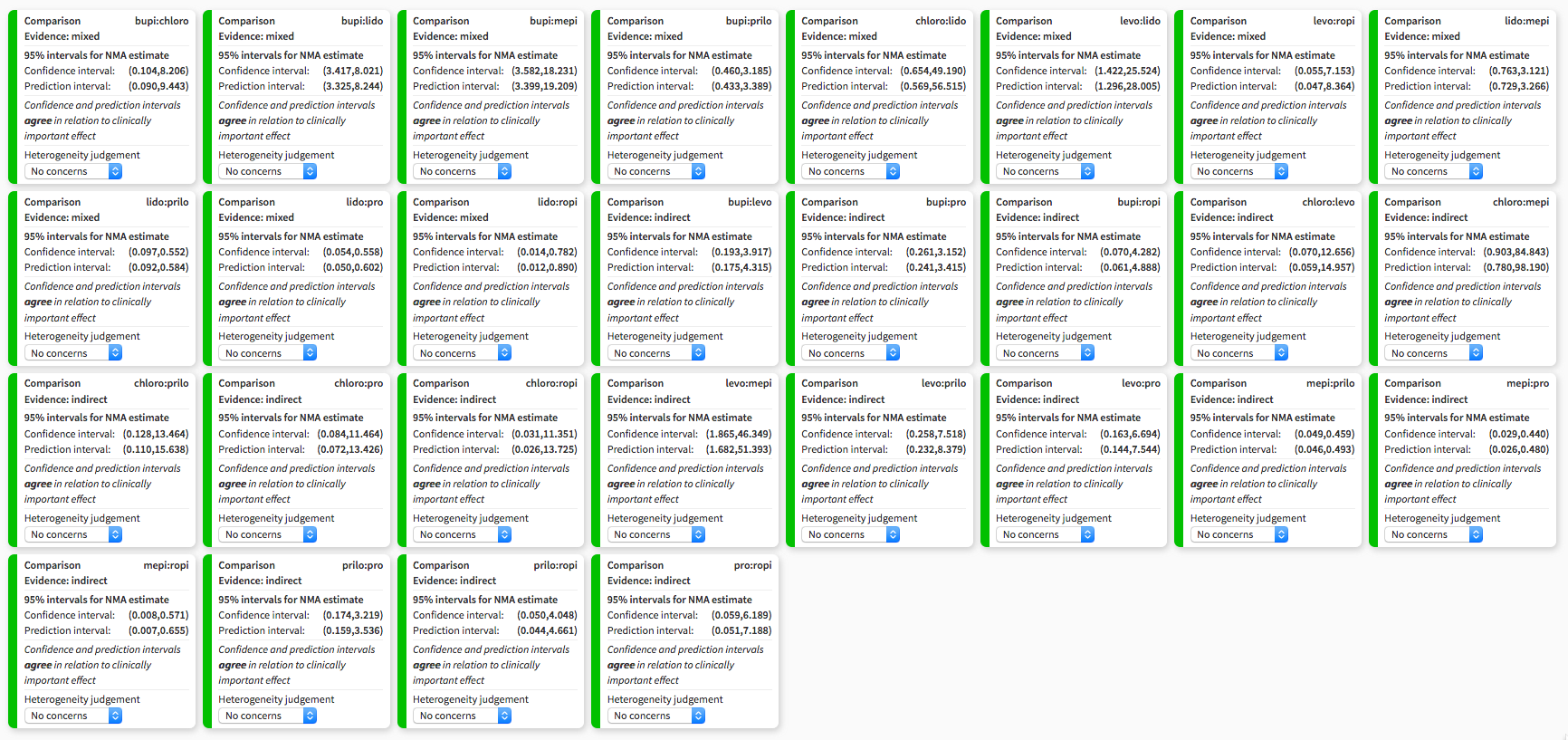
Heterogeneity assessment in network meta‐analysis displaying confidence and prediction intervals, regarding the risk of transient neurological symptoms following spinal anaesthesia with lidocaine versus other local anaesthetics in adults undergoing surgery. bupi: bupivacaine; chloro: 2‐chloroprocaine; levo: levobupivacaine; lido: lidocaine; mepi: mepivacaine; prilo: prilocaine; pro: procaine; ropi: ropivacaine.

Summary of risk of bias assigned to studies included in the network meta‐analysis across six domains: study limitations, imprecision, heterogeneity, incoherence, indirectness, publication bias, regarding the risk of transient neurological symptoms following spinal anaesthesia with lidocaine versus other local anaesthetics in adults undergoing surgery. Output was created with CINeMA software. bupi: bupivacaine; chloro: 2‐chloroprocaine; levo: levobupivacaine; lido: lidocaine; mepi: mepivacaine; prilo: prilocaine; pro: procaine; ropi: ropivacaine.
Background
Description of the condition
August Bier performed the first spinal anaesthesia in 1898 using cocaine, which was the first known local anaesthetic (Bier 1899). Cocaine was soon replaced by another less toxic local anaesthetic, amylocaine. Other local anaesthetics were gradually introduced: procaine, 2‐chloroprocaine, dibucaine, lidocaine, tetracaine, mepivacaine, prilocaine, bupivacaine, and finally ropivacaine and levobupivacaine. Lidocaine, procaine, tetracaine, mepivacaine, dibucaine, and bupivacaine are still used for spinal anaesthesia (Axelrod 1998; Hiller 1997; Holmdahl 1998; Iselin‐Chaves 1996; Masuda 1998; Tagariello 1998). Spinal anaesthesia allows patients to avoid the undesirable effects of general anaesthetic drugs (Doleman 2018; Miller 2018), and may reduce the likelihood of patients having long‐term pain after surgery (Weinstein 2018). However, spinal anaesthesia does have problems, including postdural puncture headache (Aravelo‐Rodriguez 2017).
The increase in day‐case surgery has generated a need for a local anaesthetic with a quick onset and short duration of action that allows for a speedy recovery and early discharge. So far, this profile is fulfilled only by lidocaine (Liu 1998).
Intrathecally (spinally) administered local anaesthetics cause reversible blockade of nerve impulse conduction in the affected nerve roots. Experiments on animals have shown that all tested local anaesthetics have potentially neurotoxic effects that are dependent on the dosage used and the duration of exposure (Li 1985). All local anaesthetics can cause permanent nerve damage when administered in a high concentration or when applied over a long period of time.
However, retrospective and prospective surveys and databases dealing with postoperative outcomes have shown that serious and permanent neurological complications after spinal anaesthesia are rare events (Corbey 1998; Dahlgren 1995; Freedman 1998; Noble 1971; Phillips 1969; Renck 1995; Tarkkila 1991; Vandam 1955). Reported cases of such permanent neurological deficits involve all known local anaesthetics (Auroy 1997; Green 1961; Kane 1981; Sumi 1996; Vandam 1960). 2‐Chloroprocaine is an example of a local anaesthetic that has been used for spinal anaesthesia since 1952 (Foldes 1952), especially for obstetric epidural anaesthesia because of its rapid onset of action, quick metabolism, and low toxicity (Winnie 2001). In the early 1980s reports about permanent neurological deficits in eight people who inadvertently received high doses of 2‐chloroprocaine intrathecally were published (Moore 1982; Reisner 1980). Although these sequelae were probably due to the combination of low pH and the presence of the antioxidant bisulfite, the use of 2‐chloroprocaine was then abandoned.
From the beginning of 1990, a number of cases were published reporting cauda equina syndrome that was related to the introduction of a microcatheter technique for continuous spinal anaesthesia with hyperbaric 5% lidocaine (the drug of choice) (Rigler 1991; Schell 1991).
In 1993, a new adverse effect, transient neurological toxicity, was described in people recovering from single injection spinal anaesthesia with lidocaine (Schneider 1993). In the following years, new names for this condition appeared in the literature including transient radicular irritation (TRI) (Hampl 1995a) and transient neurological symptoms (TNS) (Hampl 1998).
The symptoms of TNS can appear in a few hours or within up to 24 hours; that is, well after full recovery (return of sensory and motor function) has been made from uneventful spinal anaesthesia. These symptoms consist of pain originating in the gluteal region and radiating to both lower extremities, in the absence of abnormal neurological examination or imaging (Gerancher 1997; Pollock 2000; Tarkkila 1995).
Lower back pain is different from pain experienced in the buttocks and lower extremities after recovery from spinal anaesthesia, which has been characterized as 'transient neurological symptoms'; this also shows no evidence of localized nerve damage. Studies with different concentrations and doses of lidocaine have shown that the risk of TNS was not dose‐ or concentration‐dependent (Freedman 1998; Hampl 1996; Pollock 1999; Tong 2003). All forms of lidocaine have been associated with TNS: hyperbaric (Tong 2003); isobaric (Hampl 1996); and when diluted with cerebrospinal fluid (Pollock 1999). The cause of this painful condition is still unknown and none of the speculations on its origin have been substantiated. The term 'transient neurological symptoms' implies neurological pathology. Failing identification of the pathogenesis of TNS, there should be consideration given to choosing a neutral descriptive term which does not imply particular causation.
Description of the intervention
Spinal anaesthesia consists of using a fine needle to locate the fluid‐filled subarachnoid space around the spinal cord and to inject local anaesthetics before surgery. In some circumstances, spinal anaesthesia is a valuable alternative to general anaesthesia. For short procedures and procedures conducted in ambulatory settings, a rapid, short‐acting anaesthesia may be beneficial. The chosen medication and dose can affect the quality, duration and potential adverse effects of spinal anaesthesia. Consequently, all these factors are important when choosing a specific technique.
Lidocaine is an effective agent for inducing spinal anaesthesia but also known to be associated with TNS. There are many alternative agents that may be associated with a lower incidence of this adverse event.
How the intervention might work
TNS has been interpreted as a sign of possible neurotoxicity of local anaesthetics (Casati 1998; Douglas 1995; Hiller 1997; Lynch 1997). However, as the pathogenesis of TNS remains unknown, potential explanations as to why lidocaine may be associated with a higher incidence of TNS than other local anaesthetics remain speculative.
Why it is important to do this review
Previous versions of this review have suggested that lidocaine is more likely to cause TNS than many alternative agents. In this update, we sought further data published since 2009 to confirm or modify our previous findings. We also added a network meta‐analysis (NMA), which allows us to perform direct and indirect comparisons between all types of interventions, which may help clarify which specific local anaesthetics present the lower risk of TNS, by ranking these in terms of risk of TNS.
Objectives
To determine the frequency of TNS after spinal anaesthesia with lidocaine and compare it with other types of local anaesthetics by performing a meta‐analysis for all pair‐wise comparisons, and conducting network meta‐analysis (NMA) to rank interventions.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomized controlled trials (RCTs), and quasi‐RCTs (i.e. in which participants were allocated to treatment or control groups in a non‐random way such as alternate allocation, allocation by day of the week, odd‐even study numbers), that were published in full, regardless of blinding.
The included studies could have included any type of surgery, any spinal needle size, and any patient positioning after administration of the intrathecal local anaesthetics.
Types of participants
We included all adults who received spinal anaesthesia. The follow‐up of these participants was at least 24 hours and longer for participants who developed TNS. We chose this time interval because the symptoms of TNS appear within 24 hours after spinal anaesthesia (Aouad 2001).
Types of interventions
The included studies had to have two or more arms that used a distinct local anaesthetic (irrespective of the dose, concentration, and baricity of the solution) for spinal anaesthesia in preparation for surgery.
We excluded studies dealing with meperidine as a sole intrathecal agent, or combinations of local anaesthetics and opioids. We also excluded studies in which spinal anaesthesia was combined with epidural analgesia to restrict our analysis to intrathecal injection of pure local anaesthetics. This approach was meant to support the clinical and methodological comparability across all direct comparisons in the whole network.
Types of outcome measures
Primary outcomes
-
Presence of any transient neurological symptoms (TNS), defined as pain originating in the gluteal region and radiating to both lower extremities and appearing within up to 24 hours after full recovery (return of sensory and motor function) has been made from uneventful and non‐complicated spinal anaesthesia.
Secondary outcomes
-
Postoperative neurological symptoms (sensory deficits including numbness and weakness) which lasted longer than 24 hours after onset of spinal anaesthesia and which did not exist before the anaesthetic.
-
Postoperative neurological signs (motor deficits including weakness in a radicular distribution) which lasted longer than 24 hours after onset of spinal anaesthesia and which did not exist before the anaesthetic.
Search methods for identification of studies
Electronic searches
We searched for studies with systematic and sensitive search strategies as outlined in Chapter 6.4 of the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011). We applied no restrictions to language or publication status.
We searched the following databases:
-
Cochrane Central Register of Controlled Trials Register (CENTRAL; the Cochrane Library, 2018, Issue 9);
-
MEDLINE ALL, OvidSP (1966 to 25 November 2018);
-
Elsevier Embase (1980 to 25 November 2018);
-
LILACS (25 November 2018).
We developed a subject‐specific search strategy in MEDLINE and modified it appropriately for the other databases. This search strategy allowed retrieval of trials of any two local anaesthetics for spinal anaesthesia. Where appropriate, we used the search strategy recommended in the Cochrane Handbook for Systematic Reviews of Interventions for identifying RCTs (Lefebvre 2011). See Appendix 1; Appendix 2; Appendix 3; Appendix 4 for the search strategies.
Searching other resources
We searched ClinicalTrials.gov Registry of Clinical Trials by National Institutes of Health (NIH) and the World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp/en/) on 25 November 2018.
We scanned the reference lists and citations of relevant studies and reviews for further references to trials. When necessary, we contacted trial authors for additional information.
Data collection and analysis
Selection of studies
Four review authors (PF, EMT, NLP, and JAB) assessed the search results, and excluded irrelevant reports. They independently examined studies for eligibility without blinding of study authors, institutions, journal of publication, and results. We retrieved and read the full text of potentially relevant studies and decided which studies to include. We listed excluded studies in the Characteristics of excluded studies table. We resolved disagreements by discussion.
Data extraction and management
Four review authors (PF, EMT, NLP, and JAB) recorded and documented the following information from the included studies in the Characteristics of included studies table: experimental design characteristics; number of participants; demographics; country of investigation; treatment groups; concentration and volume of the local anaesthetic used; duration of the follow‐up period; and spinal needle size and shape. We regarded TNS, sensory deficits, or motor deficits as three separate outcomes.
We independently extracted data using a standard form and agreed on the data before entry into Review Manager 5 (Review Manager 2014). We resolved any discrepancies by discussion and internal correspondence. The data collection form very closely resembled the form used in the previous versions, already assessed for usability.
Assessment of risk of bias in included studies
Four review authors (PF, EMT, NLP, and JAB) independently and without blinding assessed the risk of bias. We resolved disagreements by discussion and internal correspondence.
We assessed the risk of bias for the domains: random sequence generation, allocation concealment, blinding of participants, blinding of personnel, blinding of assessors, incomplete outcome data, and selective reporting. Study level judgements are presented in the 'Risk of bias' table of the Characteristics of included studies table (Appendix 6), and were made as described in the Cochrane Handbook for Systematic Reviews of Interventions, Section 8.5 (Higgins 2011).
We summarized the overall risk of bias for each study depending on the judgements for the domains:
-
low risk of bias: low risk of bias for all key domains;
-
unclear risk of bias: unclear risk of bias for one or more key domains;
-
high risk of bias: high risk bias for one or more key domains.
Measures of treatment effect
We used the risk ratio (RR) as the effect estimate. We estimated the pair‐wise relative treatment effects of the competing interventions in each included study using the RR with 95% confidence intervals (95% CI).
Unit of analysis issues
We included multi‐arm studies in the data set as a series of two‐arm comparisons. In the pair‐wise direct comparison meta‐analyses, no overall summary statistic was estimated across all interventions. In the NMA, the standard error of each two‐arm comparison within a multi‐arm study was adjusted by a method proposed by Rücker and Schwarzer (Rücker 2012; Rücker 2014), that uses back‐calculated standard errors in the weighted least squares estimator to reflect the within‐study correlation.
We did not find nor include cluster randomized trials. As each participant received a single surgical procedure, there were no trials with a crossover design.
Dealing with missing data
For the trials where dropouts were reported but without mention of their outcomes, we contacted the authors and included the missing data, where possible, to reduce the number of excluded participants.
Assessment of heterogeneity
Clinical and methodological diversity always occur between different studies, making heterogeneity inevitable (Higgins 2011). We performed meta‐analysis only in the case of low‐ to moderate‐clinical heterogeneity, defined as similar positioning for the procedure (lithotomy versus supine), and shape and size of the spinal needle. This heterogeneity or diversity reflects differences in potential effect modifiers such as patient mix or agent dose. We assessed statistical heterogeneity as outlined below.
Measures and tests for pair‐wise meta‐analysis heterogeneity
We assessed statistical heterogeneity by the Q test, the I2 statistic, and a comparison of the between‐trial variance (tau2) of the effect estimates for each pair‐wise comparison. We used a Chi2 test to examine the Q statistic for evidence of heterogeneity. The I2 statistic was interpreted following the guidelines suggested by Higgins 2011:
-
0% to 40% might not be important;
-
30% to 60% may represent moderate heterogeneity;
-
50% to 90% may represent substantial heterogeneity;
-
75% to 100% represents considerable heterogeneity.
Assessment of statistical inconsistency
Inconsistency is the statistical manifestation of intransitivity and occurs when the direct and indirect estimates in a network of treatments do not agree. This is analogous to the distinction between clinical/methodological heterogeneity and statistical heterogeneity in the pair‐wise meta‐analysis (Cipriani 2013). Consistency within, and between, designs were assessed by decomposition of Cochran's Q statistic. The consistency of direct and indirect treatment comparisons used back‐calculation methods (Dias 2010), as implemented in the netsplit function. If necessary, we examined net heat plots (Krahn 2013).
Assessment of transitivity across treatment comparisons
The main assumption underlying indirect and mixed comparison is that there are no important differences between trials comparing different local anaesthetics other than the agents given. As an example, consider the comparison of three agents A, B, and C with the effect sizes labelled AB, AC, and BC in studies reporting A versus C and B versus C. Under the assumption of transitivity, then ABindirect = ACdirect – BCdirect.
We assessed the assumption of transitivity by comparing the distribution of potential effect modifiers across the different pair‐wise comparisons. Variation in the spinal dose of local anaesthetic among studies was considered acceptable for combining. We also considered varied surgical procedures and varied populations as suitable for combining.
We assumed that participants who fulfilled the inclusion criteria and received these interventions would have been equally eligible to be randomized to any local anaesthetic in a hypothetical joint RCT of all local anaesthetics (Chaimani 2017). We assumed that this met the requirement of transitivity for an NMA (Caldwell 2005; Salanti 2012).
Assessment of reporting biases
Reporting bias occurs when the dissemination of research findings is influenced by the nature and direction of results. We constructed and examined funnel plots for asymmetry to detect reporting biases. We performed statistical tests of funnel plot asymmetry as necessary.
Data synthesis
We followed guidance on NMA in the PRISMA extension (Hutton 2015), and documents developed by the Cochrane Comparing Multiple Interventions Methods Group (methods.cochrane.org/cmi/about‐us).
Methods for direct treatment comparisons
We conducted pair‐wise meta‐analyses for all comparisons of local anaesthetics. We assumed a random‐effects model for all data syntheses. We used an inverse variance weighting for summary statistics and random‐effects models as we expected methodological and clinical heterogeneity across the included studies resulting in varying effect sizes between studies of pair‐wise comparisons. We reported summary statistics as point estimates with 95% CIs; and determined summary statistics to indicate a difference if 95% CIs did not cross the line of identity. We used Review Manager 5, for data synthesis, statistical analysis, and creation of forest plots (Review Manager 2014).
Methods for indirect and mixed comparisons
We performed a random‐effects NMA for the primary outcome of TNS using all included studies based on a graph theoretical approach (Rücker 2012), within a frequentist framework in the R statistical platform (R 2018), using the package netmeta (Rücker 2016). Spinal anaesthesia with lidocaine was the reference group due to its known association with TNS (see: Description of the intervention). The geometry of the network was displayed using graph theory as implemented in the netgraph function of netmeta.
For display of specific treatments in the NMA, abbreviations were used: bupi: bupivacaine; chloro: 2‐chloroprocaine; levo: levobupivacaine; lido: lidocaine; mepi: mepivacaine; prilo: prilocaine; pro: procaine, and ropi: ropivacaine. Nodes in the network structure represented individual local anaesthetics.
Relative treatment ranking
For the primary outcome of TNS, we ranked the competing treatments by P scores using the netrank function in netmeta. P scores allow ranking of treatments on a continuous 0 (worst) to 1 (better) scale and are derived from the P values of all pair‐wise comparisons. P scores are a frequentist analogue and numerically similar to the Bayesian Surface Under the Cumulative Ranking curve (SUCRA) values (Rücker 2015). The P score of treatment is the mean certainty that it is better than another treatment.
Subgroup analysis and investigation of heterogeneity
We were unable to perform the preplanned subgroup analyses included participant positioning (lithotomy versus supine), the shape and size of the spinal needle, and pregnancy because of the available amount of data.
Sensitivity analysis
We performed no sensitivity analyses. Regarding the missing data, assuming that these data were missing at random, we intended to analyze only the available data (i.e. ignoring the missing data); and to evaluate how sensitive results were to reasonable changes in the assumptions that were made.
'Summary of findings' table and GRADE
Methods for evaluating the quality of evidence of a NMA have been proposed by Puhan and colleagues (Puhan 2014), and Salanti and colleagues (Salanti 2014). The quality of the body of evidence is assessed by the domains of study limitations, indirectness, inconsistency (heterogeneity, incoherence), imprecision, and risk of publication bias.
For the evaluation of the quality of evidence derived from NMA, we used a web app online tool denoted Confidence in Network Meta‐Analysis (version 0.6) (CINeMA 2018; CINeMA). It implements proposals by Salanti 2014 and uses the netmeta 2013 package for calculations. According to the GRADE for NMA guidelines, we assessed the risk of bias for each treatment comparison separately.
Results
Description of studies
Results of the search
The results of the search are outlined in Figure 8.

Study selection flow diagram corresponding to the last search update, up to November 2018.
The electronic database searches yielded 2002 citations. No additional records were found through other sources. After we removed duplicates, 1264 unique citations remained. We excluded 1185 citations on the basis of title and abstract.
We considered 76 full‐text studies dealing with neurological complications and spinal anaesthesia with lidocaine. Of these, we excluded 52 studies for the reasons cited in the Characteristics of excluded studies table. There are two studies awaiting classification (see Characteristics of studies awaiting classification table), and one ongoing study (see Characteristics of ongoing studies table).
Included studies
See Characteristics of included studies table.
Interventions
We included 24 RCTs in the final analysis and respective NMA. The characteristics of these studies can be found in Table 1. One RCT was identified in the first review update (Casati 2007). Eight were identified in the current update (Ali Hassan 2015; Etezadi 2013; Pradhan 2010; Teunkens 2016; de Santiago 2010; Imbelloni 2008a; Imbelloni 2008b; Yazicioglu 2013). Twenty‐one studies satisfied the inclusion criterion of comparing lidocaine as one treatment arm with different local anaesthetics in the second treatment arm. The local anaesthetic in this second arm consisted of bupivacaine in 12 studies (Ali Hassan 2015; Aouad 2001; Etezadi 2013; Hampl 1995a; Hampl 1998; Keld 2000; Philip 2001; Pollock 1996; Pradhan 2010; Salmela 1998; de Santiago 2010; Yazicioglu 2013); prilocaine in four studies (de Weert 2000; Hampl 1998; Martinez‐Bourio 1998; Salmela 1998); mepivacaine in three studies (Liguori 1998; Salazar 2001; Salmela 1998); procaine in two studies (Hodgson 2000; Le Truong 2001); ropivacaine and levobupivacaine in one study (Breebaart 2003), and 2‐chloroprocaine in one study (Casati 2007). There were five studies with more than two treatment arms (Breebaart 2003; Hampl 1998; Pollock 1996; Salmela 1998; Teunkens 2016). Hampl 1998 used two concentrations of lidocaine and, as the outcome of interest is not dependent on the concentration (Freedman 1998; Hampl 1996; Pollock 1999; Tong 2003), we pooled the results of the two groups. Four studies had three treatment groups: two alternative local anaesthetics in addition to lidocaine (Breebaart 2003; Pollock 1996; Salmela 1998; Teunkens 2016). In one study, a control group with participants receiving general anaesthesia was not taken into consideration for statistical analysis (Hampl 1995a).
| Study ID | TNS #/N (%) | Pain score (0–10) | TNS duration | Therapy |
| 0/100 (0) | — | — | — | |
| 3/30 (10) | Not tallied | 1 day | Not described | |
| 5/15 (33) | Not tallied | Up to 7 days | NSAIDs | |
| 7/35 (20) | Day 1 mean VPS 5.3 (range 2–8) | Maximum duration 3 days | Not described | |
| 85/135 (63) | Mean VAS 6–7 | Maximum duration 5 days | NSAIDs | |
| 6/15 (40) | Not tallied | Resolved within 7 days | Not described | |
| 8/30 (27) | Median VPS 3 (range 1–6) | Resolved within 7 days | Not described | |
| 9/28 (32) | Not tallied | Maximum duration 4 days | Not described | |
| 9/30 (30) | Mean maximum VAS 3.75 | Maximum duration 2 days | Not described | |
| 0/25 (0) | — | — | — | |
| 11/35 (31) | Mean VPS 5 | Mean duration 2 days | Not described | |
| 0/75 (0) | — | — | — | |
| 9/35 (26) | Mean VPS 3.5 (range 2–8) | Maximum duration 4 days | Not described | |
| 8/30 (27) | Not tallied | Unspecified | Not described | |
| 6/27 (22) | Not tallied | Maximum duration 5 days | NSAIDs | |
| 4/98 (4) | Not tallied | Maximum duration 10 days | NSAIDs | |
| 7/49 (14) | VPS range 5–9.5 | Maximum duration 3 days | Not described | |
| 0/40 (0) | — | — | — | |
| 1/30 (3) | Maximum VAS 3 | Maximum duration 2 days | Not described | |
| 16/107 (15) | Mean VPS 6.2 (range 1–9) | Maximum duration 4 days | NSAIDs and opioids | |
| 0/26 (0) | — | — | — | |
| 1/40 (3) | Maximum VAS 9–10 | Maximum duration 1 day | NSAIDs | |
| 6/30 (20) | Moderate pain | Maximum duration 1 day | NSAIDs and opioids | |
| 0/32 (0) | — | — | — |
N: number of participants; NSAIDs: non‐steroidal anti‐inflammatory drugs; TNS: transient neurological symptoms; VAS: visual analogue scale; VPS: verbal pain scale.
Countries and other differences between the studies
Four studies were conducted in the USA; three each in Scandinavia and Spain; two each in Brazil, Switzerland, and Belgium; and one each in Canada, Egypt, Iran, Italy, Lebanon, Nepal, the Netherlands, and Turkey. Three studies differed from the rest because the participants were pregnant women (Aouad 2001; Philip 2001; Pradhan 2010). All studies investigated the frequency of TNS; two reported no events in any of the studied arms (Aouad 2001; Teunkens 2016). Seven studies had no events in the 'other treatment' arm (Breebaart 2003; Casati 2007; de Weert 2000; Hampl 1995a; Le Truong 2001; Liguori 1998; Pollock 1996). Six RCTs included subgroups of participants exposed to lithotomy or the supine position and pencil point or sharp spinal needles (Etezadi 2013; Le Truong 2001; Martinez‐Bourio 1998; Pollock 1996; Salmela 1998; Østgaard 2000). In the remaining RCTs, most participants underwent surgery in the supine position, and each study used only one type of spinal needle (sharp or pencil point, 27 G or larger).
Outcomes
All included studies investigated the risk of TNS, and no study was set up to investigate sensory and motor deficits after spinal anaesthesia. The follow‐up period was at least 24 hours, and all participants with TNS were followed longer than 24 hours, until recovery. The interviewer who assessed the occurrence of TNS was blinded as to which treatment group the participant belonged to in all but one RCT (de Weert 2000; in which blinding was unclear). In nine of the studies the interview was done by telephone (Breebaart 2003; Casati 2007; Hodgson 2000; Keld 2000; Liguori 1998; Martinez‐Bourio 1998; Pollock 1996; Imbelloni 2008a; Imbelloni 2008b), and in the remaining studies by direct contact with the participant, except for Le Truong 2001 (where it was unclear).
Excluded studies
We excluded 52 studies after full‐text review. The reasons for exclusion included: a combination of local anaesthetics with other medications; no relevant comparison; and absence of lidocaine in any compared group or presence of lidocaine in all the groups.
Additional details are provided for 31 of these 52 studies. See Characteristics of excluded studies table for further details.
Studies awaiting classification
There are two studies awaiting classification (Frisch 2018; Gozdemir 2016); see: Characteristics of studies awaiting classification table.
Ongoing studies
We identified one ongoing study (NCT02818894); see: Characteristics of ongoing studies table. This ongoing study will compare lidocaine to bupivacaine spinal anaesthesia in people having a total hip arthroplasty. The objective of this study is to compare the effect of two spinal anaesthesia treatments on TNS.
Risk of bias in included studies
See the 'Risk of bias' graph (Figure 9), 'Risk of bias' summary Figure 10, and 'Risk of bias' tables in the Characteristics of included studies table for more details.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
The overall quality of the included RCTs based on risk of bias assessment ranged from unclear to high. All of the studies were randomized; however, 10 studies did not specify the method of randomization (referring to a random number table, computer‐generated random number sequence, tossing coin, etc.) and were thus considered to have an unclear risk of bias with regard to randomization of participants (Aouad 2001; de Weert 2000; Hampl 1995a; Keld 2000; Le Truong 2001; Pollock 1996; Pradhan 2010; Salazar 2001; Salmela 1998; Østgaard 2000).
Allocation
None of the included studies showed high risk of bias. Five studies specified adequate concealment of allocation (Hodgson 2000; Martinez‐Bourio 1998; Pradhan 2010; Teunkens 2016; Østgaard 2000). Nine authors described allocation concealment consisting of participant assignment being determined by sealed envelopes or coded envelopes (Aouad 2001; Casati 2007; de Weert 2000; Etezadi 2013; Fanelli 2009; Gozdemir 2010; Imbelloni 2010; Pollock 1996; Salmela 1998). However, because these descriptions did not specify "sequentially numbered, sealed, opaque envelopes," the risk of bias was unclear. Ten studies did not describe allocation concealment, presenting an unclear risk of bias (Ali Hassan 2015; Breebaart 2003; Hampl 1995a; Hampl 1998; Keld 2000; Le Truong 2001; Liguori 1998; Pawlowski 2012; Philip 2001; Salazar 2001).
Blinding
Nine studies had a complete blinding procedure (participant, provider, and assessor) (Breebaart 2003; Casati 2007; Gozdemir 2010; Hampl 1995a; Hampl 1998; Liguori 1998; Pawlowski 2012; Philip 2001; Teunkens 2016). Six studies had blinding of participant and assessor (Aouad 2001; Etezadi 2013; Hodgson 2000; Keld 2000; Le Truong 2001; Pollock 1996). Blinding was unclear, inadequate, or not performed in eight trials (Ali Hassan 2015; de Weert 2000; Fanelli 2009; Martinez‐Bourio 1998; Pradhan 2010; Salazar 2001; Østgaard 2000; Salmela 1996).
Incomplete outcome data
Three studies reported outcomes for dropouts (Martinez‐Bourio 1998; Philip 2001; Pollock 1996). Six RCTs with dropouts failed to report outcomes (de Weert 2000: one participant; Keld 2000: one participant; Le Truong 2001: six participants; Liguori 1998: three participants; Pawlowski 2012: seven participants; Teunkens 2016: seven participants). For two studies dropout information was unclear (Pradhan 2010; Østgaard 2000); see the Characteristics of included studies table for further details.
Selective reporting
Only one study stated whether all predefined or clinically relevant and reasonably expected outcomes were recorded and fully reported (Teunkens 2016). All of the other studies were reported in such a way that there was unclear risk of bias concerning selective reporting.
Other potential sources of bias
None noted.
Effects of interventions
We included 24 studies with 2253 enrolled participants in the NMA (Table 1; Table 2). Reported outcomes were available for 2226 participants. There were 27 (1.65%) dropouts, or missing or not reported outcomes. Due to these numbers, we did not perform any related sensitivity analysis.
| Treatment | RR | 95% CI | 95% PI |
| bupi | 0.19 | 0.12 to 0.29a | 0.12 to 0.30 |
| chloro | 0.18 | 0.02 to 1.53 | 0.02 to 1.75 |
| levo | 0.17 | 0.04 to 0.70a | 0.04 to 0.77 |
| mepi | 1.54 | 0.76 to 3.12 | 0.73 to 3.27 |
| prilo | 0.23 | 0.10 to 0.55a | 0.09 to 0.58 |
| pro | 0.17 | 0.05 to 0.56a | 0.05 to 0.60 |
| ropi | 0.10 | 0.01 to 0.78a | 0.01 to 0.89 |
bupi: bupivacaine; chloro: 2‐chloroprocaine; CI: confidence Interval; levo: levobupivacaine; lido: lidocaine; mepi: mepivacaine; PI: prediction interval; prilo: prilocaine; pro: procaine, ropi: ropivacaine.
aNull hypothesis of no difference rejected.
In 13 RCTs, we found no events of TNS in the comparator treatment group (Ali Hassan 2015; Aouad 2001; Breebaart 2003; Casati 2007; de Weert 2000; Hampl 1995a; Le Truong 2001; Liguori 1998; Pollock 1996; Pradhan 2010; Teunkens 2016; de Santiago 2010; Yazicioglu 2013). In six of these 13 RCTs, there were no cases of TNS (Ali Hassan 2015; Aouad 2001; Pradhan 2010; Teunkens 2016; de Santiago 2010; Yazicioglu 2013).
Pair‐wise meta‐analysis of lidocaine versus alternative local anaesthetic agents
Rate of development of transient neurological symptoms
Figure 1 summarizes our findings for all pair‐wise comparisons between lidocaine and individual alternative agents. A summary RR comparing all alternative local anaesthetic agents to lidocaine was not estimated.
In total, 201/1097 (18%) participants who received lidocaine developed TNS (occurrence of TNS by study is presented in Table 1).
The RR for the development of TNS was lower for bupivacaine (RR 0.16, 95% CI 0.09 to 0.28; I2 = 5%; studies = 12, participants = 1220; moderate‐quality evidence), levobupivacaine (RR 0.13, 95% CI 0.02 to 0.69; I2 = 0%; studies = 2, participants = 120; low‐quality evidence), prilocaine (RR 0.18, 95% CI 0.07 to 0.49; I2 = 0%; studies = 4, participants = 429; moderate‐quality evidence), procaine (RR 0.14, 95% CI 0.04 to 0.52; I2 = 0%; studies = 2, participants = 130; moderate‐quality evidence), and ropivacaine (RR 0.10, 95% CI 0.01 to 0.78; I2 = 0%; studies = 2, participants = 90; low‐quality evidence) (Analysis 1.1).
The RR was not different for 2‐chloroprocaine (RR 0.09, 95% CI 0.01 to 1.51; I2 = 0%; studies = 2, participants = 94; low‐quality evidence), and mepivacaine (RR 1.01, 95% CI 0.18 to 5.82; I2 = 61%; studies = 4, participants = 274; very low‐quality evidence).
Postoperative neurological symptoms (sensory deficits including numbness and weakness)
There were no reports of ongoing sensory changes for the duration of follow‐up in any of these trials.
Postoperative neurological signs (motor deficits including weakness in a radicular distribution)
There were no reports of ongoing motor changes for the duration of follow‐up in any of these trials.
Network meta‐analysis
See Table 3.
| Comparison | k | pro | NMA | 95% CI | Direct | 95% CI | Indirect | 95% CI | RoR | 95% CI | z | P value |
| bupi:chloro | 1 | 0.32 | 1.08 | 0.12 to 9.63 | 0.92 | 0.02 to 44.90 | 1.17 | 0.08 to 1.6e+01 | 0.78 | 0.01 to 8.6e+01 | –0.10 | — |
| bupi:levo | 0 | 0 | 1.15 | 0.26 to 5.18 | — | — | 1.15 | 0.26 to 5.2e+00 | — | — | — | — |
| bupi:lido | 12 | 1.00 | 0.19 | 0.12 to 0.29 | 0.19 | 0.13 to 0.30 | 0.00 | 0.00 to 1.8e+01 | 42.58 | 0.01 to 1.7e+05 | 0.89 | 0.38 |
| bupi:mepi | 1 | 0.09 | 0.12 | 0.05 to 0.28 | 0.04 | 0.00 to 0.71 | 0.14 | 0.06 to 3.2e‐01 | 0.32 | 0.02 to 5.9e+00 | –0.77 | 0.44 |
| bupi:prilo | 1 | 0.09 | 0.83 | 0.31 to 2.17 | 0.33 | 0.01 to 7.87 | 0.91 | 0.33 to 2.5e+00 | 0.37 | 0.01 to 1.0e+01 | –0.59 | 0.55 |
| bupi:pro | 0 | 0 | 1.10 | 0.32 to 3.83 | — | — | 1.10 | 0.32 to 3.8e+00 | — | — | — | — |
| bupi:ropi | 0 | 0 | 1.83 | 0.23 to 14.32 | — | — | 1.83 | 0.23 to 1.4e+01 | — | — | — | — |
| chloro:levo | 0 | 0 | 1.06 | 0.08 to 14.27 | — | — | 1.06 | 0.08 to 1.4e+01 | — | — | — | — |
| chloro:lido | 2 | 0.90 | 0.18 | 0.02 to 1.53 | 0.21 | 0.02 to 2.02 | 0.04 | 0.00 to 3.8e+01 | 4.88 | 0.00 to 6.3e+03 | 0.43 | 0.66 |
| chloro:mepi | 0 | 0 | 0.11 | 0.01 to 1.11 | — | — | 0.11 | 0.01 to 1.1e+00 | — | — | — | — |
| chloro:prilo | 0 | 0 | 0.76 | 0.07 to 7.82 | — | — | 0.76 | 0.07 to 7.8e+00 | — | — | — | — |
| chloro:pro | 0 | 0 | 1.02 | 0.09 to 11.87 | — | — | 1.02 | 0.09 to 1.2e+01 | — | — | — | — |
| chloro:ropi | 0 | 0 | 1.69 | 0.09 to 32.35 | — | — | 1.69 | 0.09 to 3.2e+01 | — | — | — | — |
| levo:lido | 2 | 1.00 | 0.17 | 0.04 to 0.70 | 0.17 | 0.04 to 0.71 | 0.00 | 0.00 to 1.3e+12 | 266.30 | 0.00 to 5.8e+17 | 0.31 | 0.76 |
| levo:mepi | 0 | 0 | 0.11 | 0.02 to 0.54 | — | — | 0.11 | 0.02 to 5.4e‐01 | — | — | — | — |
| levo:prilo | 0 | 0 | 0.72 | 0.13 to 3.87 | — | — | 0.72 | 0.13 to 3.9e+00 | — | — | — | — |
| levo:pro | 0 | 0 | 0.96 | 0.15 to 6.15 | — | — | 0.96 | 0.15 to 6.2e+00 | — | — | — | — |
| levo:ropi | 1 | 0.39 | 1.59 | 0.14 to 18.08 | 1.00 | 0.02 to 48.82 | 2.14 | 0.09 to 4.8e+01 | 0.47 | 0.00 to 6.8e+01 | –0.30 | 0.76 |
| mepi:lido | 4 | 0.97 | 1.54 | 0.76 to 3.12 | 1.47 | 0.72 to 3.01 | 6.85 | 0.12 to 4.0e+02 | 0.22 | 0.00 to 1.3e+01 | –0.73 | 0.47 |
| prilo:lido | 4 | 1.00 | 0.23 | 0.10 to 0.55 | 0.23 | 0.10 to 0.55 | 55983.54 | 0.00 to 4.5e+16 | 0.00 | 0.00 to 3.3e+06 | –0.89 | 0.37 |
| prop:lido | 2 | 1.00 | 0.17 | 0.05 to 0.56 | 0.17 | 0.05 to 0.56 | — | — | — | — | — | — |
| ropi:lido | 2 | 1.00 | 0.10 | 0.01 to 0.78 | 0.10 | 0.01 to 0.78 | 0.91 | 0.00 to 4.9e+12 | 0.11 | 0.00 to 6.5e+11 | –0.15 | 0.88 |
| mepi:prilo | 0 | 0 | 6.67 | 2.18 to 20.44 | — | — | 6.67 | 2.18 to 2.0e+01 | — | — | — | — |
| mepi:pro | 0 | 0 | 8.91 | 2.27 to 34.91 | — | — | 8.91 | 2.27 to 3.5e+01 | — | — | — | — |
| mepi:ropi | 0 | 0 | 14.78 | 1.75 to 124.74 | — | — | 14.78 | 1.75 to 1.2e+02 | — | — | — | — |
| prilo:pro | 0 | 0 | 1.34 | 0.31 to 5.74 | — | — | 1.34 | 0.31 to 5.7e+00 | — | — | — | — |
| prilo:ropi | 0 | 0 | 2.21 | 0.25 to 19.86 | — | — | 2.21 | 0.25 to 2.0e+01 | — | — | — | — |
| prop:ropi | 0 | 0 | 1.66 | 0.16 to 17.03 | — | — | 1.66 | 0.16 to 1.7e+01 | — | — | — | — |
bupi: bupivacaine; chloro: 2‐chloroprocaine; k: number of studies providing direct evidence; levo: levobupivacaine; lido: lidocaine; mepi: mepivacaine; NA: not available; NMA: network meta‐analysis; pro: direct evidence proportion; prilo: prilocaine; pro: procaine, ropi: ropivacaine; RoR: ratio of ratios (direct versus indirect).
All RoRs cross the identity line.
The NMA included 24 studies. These studies included eight different treatments. The total number of pair‐wise comparisons was 32, which included 11 unique comparisons. Compared to lidocaine, the RR of TNS was lower for bupivacaine, levobupivacaine, prilocaine, procaine, and ropivacaine. The RRs were in the range of 0.10 to 0.23 (Table 2). The RR for 2‐chloroprocaine was also small (0.18), but the 95% CIs were wide (0.02 to 1.53). The RR for mepivacaine was greater than 1 suggesting an increased risk for TNS, but the 95% CIs were wide (0.76 to 3.12). The tau2 was 0 and the I2 statistic was 0%; this is consistent with no heterogeneity and no inconsistency. The decomposition of Cochran's Q statistic revealed no heterogeneity within or between designs. P values were greater than 0.3 (Table 4). The splitting of the contribution of direct and indirect evidence did not demonstrate inconsistency; the 95% CI of the ratio of ratios (direct versus indirect) crossed the line of identity for all comparisons (Table 3).
| Q | df | P value | |
| Total | 18.4 | 21 | 0.6232 |
| Within designs | 14.8 | 13 | 0.3209 |
| Between designs | 3.6 | 8 | 0.8897 |
df: degrees of freedom; Q: Cochran's Q heterogeneity statistic.
A meta‐analysis interaction plot for the 24 included studies showing the geometry of network was generated by using CINeMA tool (Figure 2).
Finally, we ranked the competing treatments by P scores, in terms of risk of developing TNS after spinal anaesthesia. There was a clear separation in the ranking of treatments (Table 5). The P scores of lidocaine and mepivacaine were low and very low. The P scores of the other treatments were 0.5 or above.
| Treatment | P score |
| ropi | 0.772 |
| levo | 0.657 |
| pro | 0.647 |
| chloro | 0.624 |
| bupi | 0.610 |
| prilo | 0.528 |
| lido | 0.138 |
| mepi | 0.022 |
bupi: bupivacaine; chloro: 2‐chloroprocaine; levo: levobupivacaine; lido: lidocaine; mepi: mepivacaine; prilo: prilocaine; pro: procaine, ropi: ropivacaine.
Subgroup analysis
We did not perform subgroup analysis based on participant position (lithotomy versus supine) or the shape and size of the spinal needle as we could not derive systematic data from the 24 included RCTs. Most participants were operated on in the supine position and most used pencil‐point needles (in most cases 25 G). A subgroup analysis was not possible due to this lack of data.
A possible subgroup analysis concerned the effect of pregnancy on TNS. Three studies comparing bupivacaine versus lidocaine (caesarean delivery: Aouad 2001; Pradhan 2010; postpartum tubal ligation: Philip 2001), had only three events (lidocaine one, bupivacaine two) among the 310 participants. Considering the low event rate, we did not perform subgroup analysis.
Missing data
Only one study stated clearly that all predefined or clinically relevant and reasonably expected outcomes were recorded and fully reported (Teunkens 2016). The other studies were reported with an unclear risk of selective reporting, although none mentioned any missing data. After contacting the authors, we were left with 27 (1.65%) dropouts.
Sensitivity analysis
An intention‐to‐treat analysis was possible for 14/20 studies. Because of the small number of missing outcomes, we did not attempt to impute optimistic and pessimistic missing outcomes for the other five studies in a sensitivity analysis.
Summary of findings and GRADE
The CINeMA web app created a series of tables and plots for the quality of evidence of an NMA. Study limitations were determined by the average risk of bias after each investigator independently assigned their assessment of direct risk of bias (Figure 3). The NMA‐generated risk of bias assessment for indirect and mixed‐effects included in the interaction analysis is available in Figure 4. A large proportion of studied interactions showed some or major concern in terms of study limitations.
To assess imprecision, an RR outside of the range of 0.909 to 1.1 was considered clinically important. Each component table within Figure 5 shows the imprecision judgement for each pair‐wise comparison. In the 28 pair‐wise comparisons with either mixed or indirect evidence, there were major concerns for imprecision in 18.
Among included studies in the NMA, there were no concerns across studies in terms of heterogeneity (Figure 6), or incoherence (Chi2 = 3.599, degrees of freedom = 8, P = 0.891). All studies were considered to directly report TNS and thus indirectness was assigned a judgement of low concern. Similarly, publication bias was rated of low concern.
An aggregated chart summarizing all studies included in the NMA across the domains is available in Figure 7. For example, the mixed evidence for bupivacaine versus lidocaine has some concerns for study limitations, but no concerns for another domain.
Discussion
Summary of main results
The main clinical question addressed by this review is whether local anaesthetics used for spinal anaesthesia caused symptoms of TNS less frequently than lidocaine.
We included 24 trials of mostly low‐ to moderate‐quality evidence (GRADE), reporting on 2226 participants of whom 239 developed TNS, in the analysis. Included studies mostly had unclear to high risk of bias. Compared with lidocaine, most local anaesthetics were associated with a reduced risk of TNS development (with the exception of 2‐chloroprocaine and mepivacaine) (bupivacaine: RR 0.16, 95% CI 0.09 to 0.28; 12 studies; moderate‐quality evidence; 2‐chloroprocaine: RR 0.09, 95% CI 0.01 to 1.51; 2 studies; low‐quality evidence; levobupivacaine: RR 0.13, 95% CI 0.02 to 0.69; 2 studies; low‐quality evidence; mepivacaine: RR 1.01, 95% CI 0.18 to 5.82; 4 studies; very low‐quality evidence; prilocaine: RR 0.18, 95% CI 0.07 to 0.49; 4 studies; moderate‐quality evidence; procaine: RR 0.14, 95% CI 0.04 to 0.52; 2 studies; moderate‐quality evidence; ropivacaine: RR 0.10, 95% CI 0.01 to 0.78; 2 studies; low‐quality evidence).
These data, with additional explanations, are found in the summary of findings Table for the main comparison. Approximately one in five participants who received spinal anaesthesia with lidocaine developed TNS.
The NMA included 24 studies. These studies assessed eight different local anaesthetics. The number of pair‐wise comparisons was 32 and the number of unique pair‐wise comparisons was 11. This analysis showed that, compared to lidocaine, the RRs of TNS development were lower for bupivacaine, levobupivacaine, prilocaine, procaine, and ropivacaine with RRs in the range of 0.10 to 0.23 while 2‐chloroprocaine and mepivacaine did not differ.
While summary of findings Table for the main comparison for this review focused on the pair‐wise meta‐analysis results, results from the NMA can be found in Table 2 (results of NMA), Table 3, Table 4, and Table 5 (treatment ranking), as well as in Figure 2 (network structure), Figure 3 (risk of bias assessments), Figure 4, Figure 5, Figure 6, and Figure 7. Updates of this review will consider contemporary practices for presenting a 'Summary of findings' table for NMA results.
Overall completeness and applicability of evidence
How much does TNS influence patients' level of satisfaction and their rehabilitation? In one multicentre RCT, 20% of 453 participants who received spinal anaesthesia with lidocaine for short urological procedures developed TNS (Tong 2003). People with TNS had higher pain scores, used more analgesics postoperatively, and experienced higher degrees of functional impairment during the two postoperative days than those who did not develop TNS. Satisfaction was higher among people without TNS (96%) than in people with TNS (89%). However, the proportion of participants who stated they would accept future spinal anaesthesia was the same (95%). It seems that the transitory pain and functional impairment are not of such degree that they have a negative influence on the patients' decisions to receive spinal anaesthesia in the future. In contrast, in one epidemiological study of 1863 participants, 30% of the 104 participants who developed TNS after intrathecal lidocaine rated their pain as severe (Freedman 1998).
Quality of the evidence
The present review showed that bupivacaine, prilocaine, and procaine are less likely to cause TNSs than lidocaine, but the quality of evidence was low to moderate based on GRADE assessments.
The quality of evidence comparing the risk of TNS following bupivacaine spinal anaesthesia compared with lidocaine was moderate, due to multiple studies in which blinding was inadequate and two studies that reported eight participants lost to follow‐up (Ali Hassan 2015; Aouad 2001; Etezadi 2013; Keld 2000; Pollock 1996; Pradhan 2010; Salmela 1998; Teunkens 2016). Low‐quality of evidence supported levobupivacaine as causing less TNS than lidocaine; the quality of evidence was downgraded for relatively small sample sizes, a rare event of interest, and wide CIs (Breebaart 2003; Gozdemir 2010). Moderate‐quality evidence also supported prilocaine when compared with lidocaine; in this case, the quality of evidence was decreased for concerns about blinding, participants lost to follow‐up, and pervasive unclear risk of bias.
The quality of evidence comparing 2‐chloroprocaine, procaine, and ropivacaine with lidocaine was low. In the case of 2‐chloroprocaine, only one study noted the event of interest (TNS), limiting the quality with small sample size and imprecision (Casati 2007; Teunkens 2016). The studies comparing procaine and ropivacaine with lidocaine were limited by relatively small sample sizes, a rare event of interest, wide CIs, and inadequate blinding, as well as participants lost to follow‐up (Breebaart 2003; Fanelli 2009; Hodgson 2000; Le Truong 2001).
The quality of evidence comparing mepivacaine with lidocaine presents an interesting problem. These studies had similar limitations in terms of inadequate blinding, participants lost to follow‐up, and wide CIs (Liguori 1998; Pawlowski 2012; Salazar 2001; Salmela 1998). Furthermore, the studies demonstrated a small overlap in CIs and different directions of effect, with the CI including the null hypothesis. These data may represent heterogeneity or no difference in effect. See summary of findings Table for the main comparison for further details.
Potential biases in the review process
The consistent magnitude of the risk ratio reduction observed when local anaesthetics such as bupivacaine, levobupivacaine, prilocaine, procaine, and ropivacaine are used, compared to lidocaine, is a strength of the present review.
However, the limitations were numerous. The events were so rare that no randomized trial relating to this complication has been performed. In the patient population contained in this review, the total number of participants was only 2226. Only 239 developed TNS and no prolonged neurological sequelae were reported. Within the review, 13 of the included RCTs found no events of TNS in the treatment comparator arm, and six of these studies found no cases of TNS in either treatment arm. No specific corrections were made for studies with no events in either arm or for studies with no events in one or more arm, which may have introduced bias in the estimation of the treatment effect. The lack of responsiveness of many primary study authors to requests for information must be taken into account. These rare events can be found in the literature as case reports, retrospective and prospective surveys, or as reports from databases for registration of postoperative complications. Nevertheless, as the chosen primary outcome definitions were dichotomous (presence or absence of the complications), no study was excluded depending on alternative outcome definitions or clinical heterogeneity (or both), prioritization of data from multiple time points, the definition of subgroups, use of adjusted as opposed to unadjusted data, or outcome surrogacy.
Consequently, we did not detect any marginal decisions around the inclusion or exclusion of studies or use and analysis of data which could have impacted on the findings of the review.
There were no relevant departures from the protocol (Zaric 2003) (or last published version of the review, Zaric 2009) as a potential source of bias (see Differences between protocol and review).
Finally, we attempted to conduct a comprehensive search for studies, but the fact that two studies have not yet been incorporated (see Characteristics of studies awaiting classification) may be a source of potential bias. The results of these studies may impact the conclusions of this review in the future.
Agreements and disagreements with other studies or reviews
There have been no previous published systematic reviews including short‐acting local anaesthetics (such as prilocaine or 2‐chloroprocaine), rendering conclusions of other reports mostly speculative regarding the implications of these agents in the development of TNS.

Forest plot of comparison: 1 Lidocaine versus other local anaesthetic, outcome: 1.1 Transient neurological symptoms.

Network meta‐analysis plot of interactions among included studies displayed for a random‐effects, risk ratio model, regarding the risk of transient neurological symptoms following spinal anaesthesia with lidocaine versus other local anaesthetics in adults undergoing surgery. Each node represents an individual local anaesthetic. The node size is proportional to the number of studies.
The node colours are determined by the individual study 'Risk of bias' assessment (green: no concerns; yellow: some concerns; red: major concerns).
The width of the edges is proportional to the inverse variance of the effect size. The edge colours reflect the average risk of bias. bupi: bupivacaine; chloro: 2‐chloroprocaine; levo: levobupivacaine; lido: lidocaine; mepi: mepivacaine; prilo: prilocaine; pro: procaine; ropi: ropivacaine.

Risk of bias (RoB) assessment among studies included in the network meta‐analysis with direct effect estimation is displayed, regarding the risk of transient neurological symptoms following spinal anaesthesia with lidocaine versus other local anaesthetics in adults undergoing surgery. Direct RoB was determined by the average RoB assigned to each particular network interaction. bupi: bupivacaine; chloro: 2‐chloroprocaine; levo: levobupivacaine; lido: lidocaine; mepi: mepivacaine; prilo: prilocaine; pro: procaine; ropi: ropivacaine.

Contributions of indirect and mixed effects in the network meta‐analysis, regarding the risk of transient neurological symptoms following spinal anaesthesia with lidocaine versus other local anaesthetics in adults undergoing surgery. bupi: bupivacaine; chloro: 2‐chloroprocaine; levo: levobupivacaine; lido: lidocaine; mepi: mepivacaine; NMA: network meta‐analysis; prilo: prilocaine; pro: procaine; ropi: ropivacaine

Analysis of imprecision among studies included in the network meta‐analysis, regarding the risk of transient neurological symptoms following spinal anaesthesia with lidocaine versus other local anaesthetics in adults undergoing surgery. bupi: bupivacaine; chloro: 2‐chloroprocaine; levo: levobupivacaine; lido: lidocaine; mepi: mepivacaine; prilo: prilocaine; pro: procaine; ropi: ropivacaine.
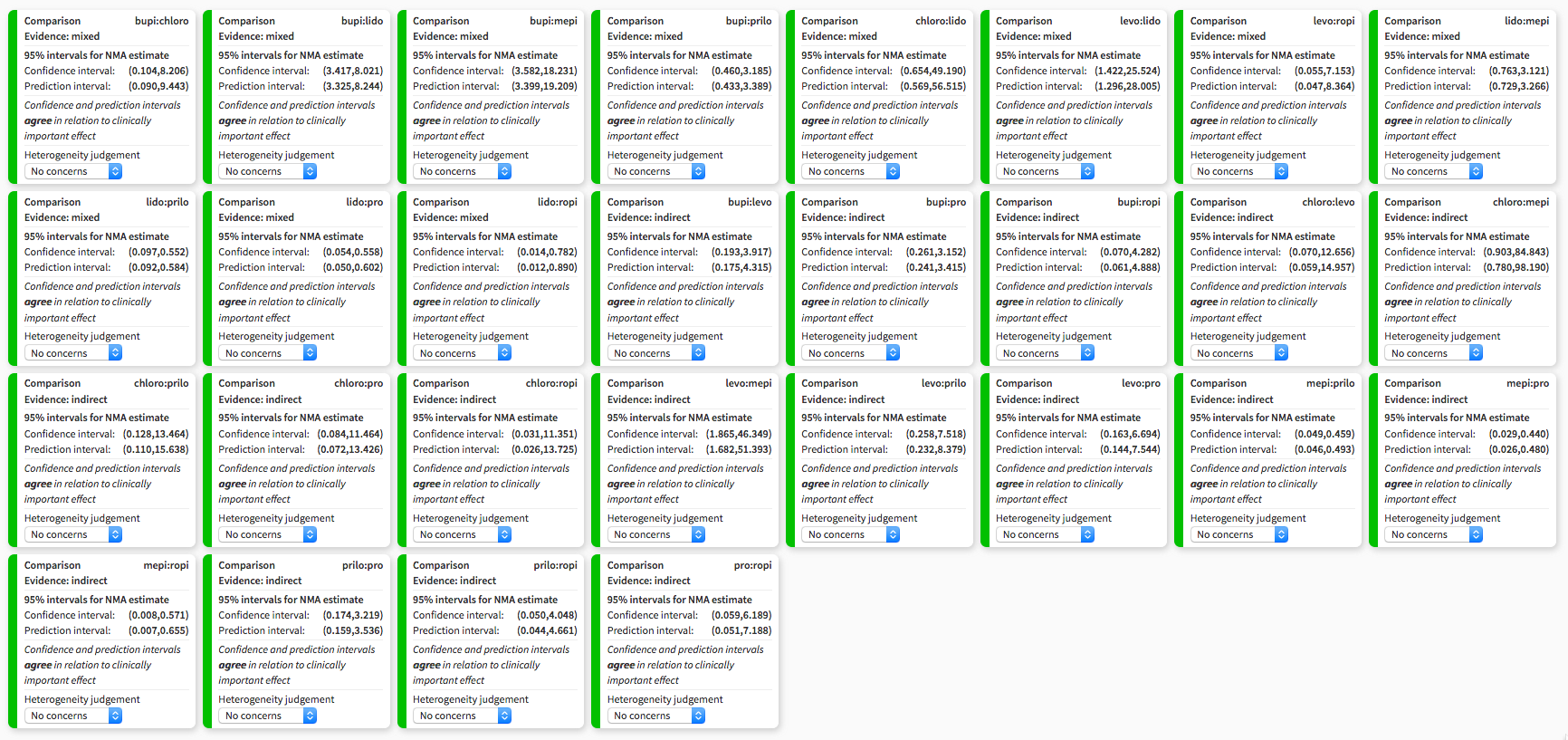
Heterogeneity assessment in network meta‐analysis displaying confidence and prediction intervals, regarding the risk of transient neurological symptoms following spinal anaesthesia with lidocaine versus other local anaesthetics in adults undergoing surgery. bupi: bupivacaine; chloro: 2‐chloroprocaine; levo: levobupivacaine; lido: lidocaine; mepi: mepivacaine; prilo: prilocaine; pro: procaine; ropi: ropivacaine.

Summary of risk of bias assigned to studies included in the network meta‐analysis across six domains: study limitations, imprecision, heterogeneity, incoherence, indirectness, publication bias, regarding the risk of transient neurological symptoms following spinal anaesthesia with lidocaine versus other local anaesthetics in adults undergoing surgery. Output was created with CINeMA software. bupi: bupivacaine; chloro: 2‐chloroprocaine; levo: levobupivacaine; lido: lidocaine; mepi: mepivacaine; prilo: prilocaine; pro: procaine; ropi: ropivacaine.

Study selection flow diagram corresponding to the last search update, up to November 2018.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
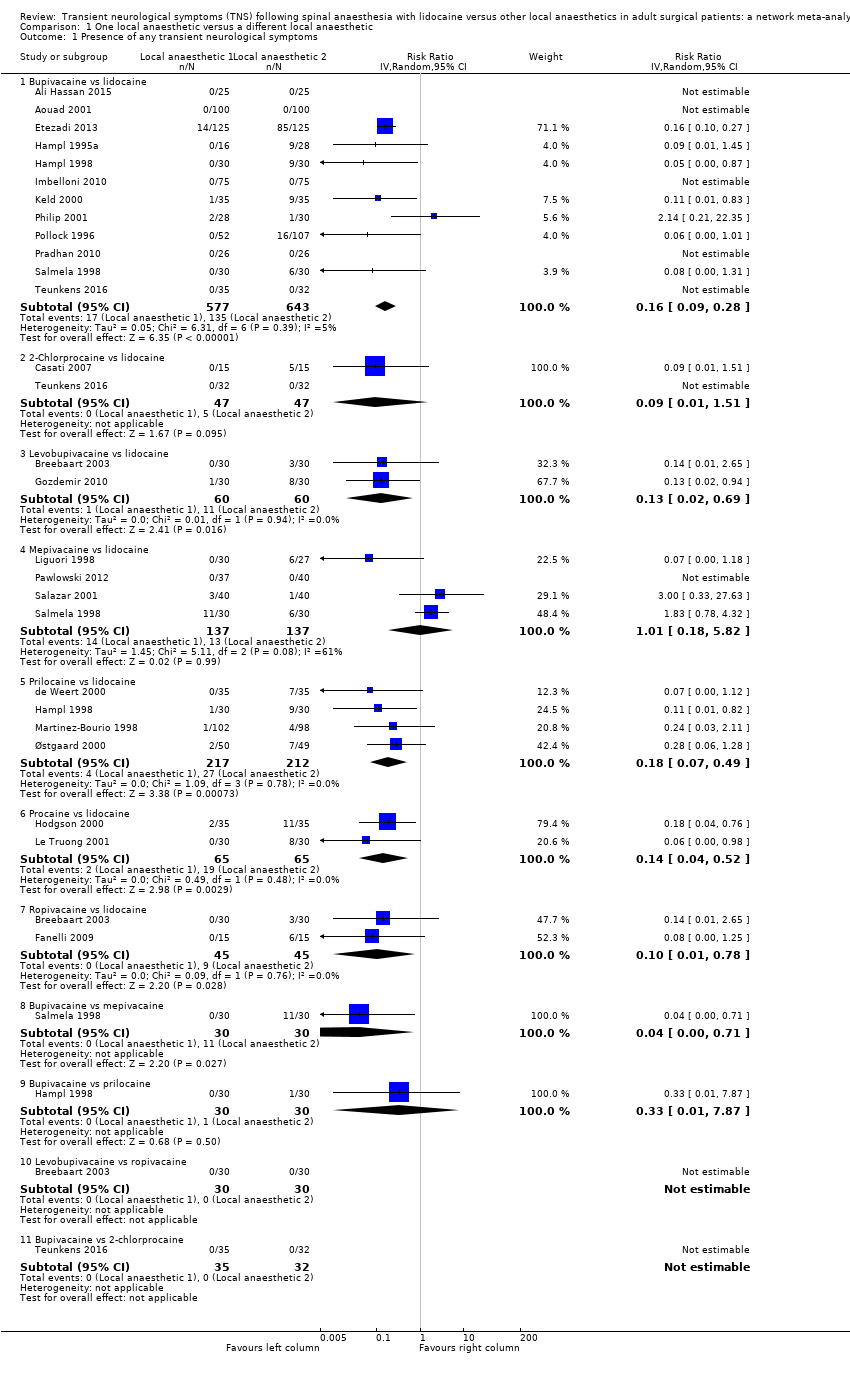
Comparison 1 One local anaesthetic versus a different local anaesthetic, Outcome 1 Presence of any transient neurological symptoms.
| Risk of transient neurological symptoms (TNS) with spinal lidocaine compared to other local anaesthetics in adults undergoing surgerya | ||||||
| Patient or population: adult undergoing surgery Settings: hospital or ambulatory surgery setting (Belgium, Brazil, Canada, Denmark, Egypt, Finland, Iran, Italy, Lebanon, Nepal, the Netherlands, Norway, Spain, Switzerland, Turkey, USA) Intervention: spinal lidocaine Comparison: other local anaesthetics as indicated | ||||||
| Outcomes | Anticipated absolute effectsb (95% CI) | Relative effect (95 CI) | № of participants | Quality of the evidence | Comments | |
| Risk with lidocaine | Risk difference with the other local anaesthetic | |||||
| Presence of any TNS –lidocaine vs bupivacaine Follow‐up: range 1–30 days | 210 per 1000 | 176 fewer per 1000 (191 fewer to 151 fewer) | RR 0.16 (0.09 to 0.28) | 1220 (12 RCTs) | ⊕⊕⊕⊝ Moderatec | Bupivacaine probably reduced the risk of TNS compared to lidocaine. |
| Presence of any TNS – lidocaine vs 2‐chloroprocaine Follow‐up: range 1–7 days | 106 per 1000 | 97 fewer per 1000 (105 fewer to 54 more) | RR 0.09 (0.01 to 1.51) | 94 (2 RCTs) | ⊕⊕⊝⊝ Lowc,d | 2‐chloroprocaine may have resulted in no difference in the risk of TNS compared to lidocaine. |
| Presence of any TNS – lidocaine vs levobupivacaine Follow‐up: range 2–7 days | 183 per 1000 | 159 fewer per 1000 (180 fewer to 57 fewer) | RR 0.13 (0.02 to 0.69) | 120 (2 RCTs) | ⊕⊕⊝⊝ | Levobupivacaine may have reduced the risk of TNS compared to lidocaine. |
| Presence of any TNS – lidocaine vs mepivacaine Follow‐up: range 1–5 days | 95 per 1000 | 1 more per 1000 (78 fewer to 457 more) | RR 1.01 (0.18 to 5.82) | 274 (4 RCTs) | ⊕⊝⊝⊝ Very lowd,e,f | Mepivacaine may have resulted in no difference in the risk of TNS compared to lidocaine but the evidence was very uncertain. |
| Presence of any TNS – lidocaine vs prilocaine Follow‐up: range 1–5 days | 127 per 1000 | 104 fewer per 1000 (118 fewer to 65 fewer) | RR 0.18 (0.07 to 0.49) | 429 (4 RCTs) | ⊕⊕⊕⊝ Moderatec | Prilocaine probably reduced the risk of TNS compared to lidocaine. |
| Presence of any TNS – lidocaine vs procaine Follow‐up: range 2–3 days | 292 per 1000 | 251 fewer per 1000 (281 fewer to 140 fewer) | RR 0.14 (0.04 to 0.52) | 130 (2 RCTs) | ⊕⊕⊕⊝ Moderatef | Procaine probably reduced the risk of TNS compared to lidocaine. |
| Presence of any TNS – lidocaine vs ropivacaine Follow‐up: range 2–7 days | 200 per 1000 | 180 fewer per 1000 (198 fewer to 44 fewer) | RR 0.10 (0.01 to 0.78) | 90 (2 RCTs) | ⊕⊕⊝⊝ Lowc,e | Ropivacaine may have reduced the risk of TNS compared to lidocaine. |
| CI: confidence interval; TNS: transient neurological symptoms; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| a'Summary of findings' table is based on pair‐wise meta‐analysis (Figure 1). Results of the network meta‐analysis are presented in Table 2; Table 3; Table 4 and Figure 2; Figure 3; Figure 4; Figure 5; Figure 6; Figure 7. | ||||||
| Study ID | TNS #/N (%) | Pain score (0–10) | TNS duration | Therapy |
| 0/100 (0) | — | — | — | |
| 3/30 (10) | Not tallied | 1 day | Not described | |
| 5/15 (33) | Not tallied | Up to 7 days | NSAIDs | |
| 7/35 (20) | Day 1 mean VPS 5.3 (range 2–8) | Maximum duration 3 days | Not described | |
| 85/135 (63) | Mean VAS 6–7 | Maximum duration 5 days | NSAIDs | |
| 6/15 (40) | Not tallied | Resolved within 7 days | Not described | |
| 8/30 (27) | Median VPS 3 (range 1–6) | Resolved within 7 days | Not described | |
| 9/28 (32) | Not tallied | Maximum duration 4 days | Not described | |
| 9/30 (30) | Mean maximum VAS 3.75 | Maximum duration 2 days | Not described | |
| 0/25 (0) | — | — | — | |
| 11/35 (31) | Mean VPS 5 | Mean duration 2 days | Not described | |
| 0/75 (0) | — | — | — | |
| 9/35 (26) | Mean VPS 3.5 (range 2–8) | Maximum duration 4 days | Not described | |
| 8/30 (27) | Not tallied | Unspecified | Not described | |
| 6/27 (22) | Not tallied | Maximum duration 5 days | NSAIDs | |
| 4/98 (4) | Not tallied | Maximum duration 10 days | NSAIDs | |
| 7/49 (14) | VPS range 5–9.5 | Maximum duration 3 days | Not described | |
| 0/40 (0) | — | — | — | |
| 1/30 (3) | Maximum VAS 3 | Maximum duration 2 days | Not described | |
| 16/107 (15) | Mean VPS 6.2 (range 1–9) | Maximum duration 4 days | NSAIDs and opioids | |
| 0/26 (0) | — | — | — | |
| 1/40 (3) | Maximum VAS 9–10 | Maximum duration 1 day | NSAIDs | |
| 6/30 (20) | Moderate pain | Maximum duration 1 day | NSAIDs and opioids | |
| 0/32 (0) | — | — | — | |
| N: number of participants; NSAIDs: non‐steroidal anti‐inflammatory drugs; TNS: transient neurological symptoms; VAS: visual analogue scale; VPS: verbal pain scale. | ||||
| Treatment | RR | 95% CI | 95% PI |
| bupi | 0.19 | 0.12 to 0.29a | 0.12 to 0.30 |
| chloro | 0.18 | 0.02 to 1.53 | 0.02 to 1.75 |
| levo | 0.17 | 0.04 to 0.70a | 0.04 to 0.77 |
| mepi | 1.54 | 0.76 to 3.12 | 0.73 to 3.27 |
| prilo | 0.23 | 0.10 to 0.55a | 0.09 to 0.58 |
| pro | 0.17 | 0.05 to 0.56a | 0.05 to 0.60 |
| ropi | 0.10 | 0.01 to 0.78a | 0.01 to 0.89 |
| bupi: bupivacaine; chloro: 2‐chloroprocaine; CI: confidence Interval; levo: levobupivacaine; lido: lidocaine; mepi: mepivacaine; PI: prediction interval; prilo: prilocaine; pro: procaine, ropi: ropivacaine. | |||
| Comparison | k | pro | NMA | 95% CI | Direct | 95% CI | Indirect | 95% CI | RoR | 95% CI | z | P value |
| bupi:chloro | 1 | 0.32 | 1.08 | 0.12 to 9.63 | 0.92 | 0.02 to 44.90 | 1.17 | 0.08 to 1.6e+01 | 0.78 | 0.01 to 8.6e+01 | –0.10 | — |
| bupi:levo | 0 | 0 | 1.15 | 0.26 to 5.18 | — | — | 1.15 | 0.26 to 5.2e+00 | — | — | — | — |
| bupi:lido | 12 | 1.00 | 0.19 | 0.12 to 0.29 | 0.19 | 0.13 to 0.30 | 0.00 | 0.00 to 1.8e+01 | 42.58 | 0.01 to 1.7e+05 | 0.89 | 0.38 |
| bupi:mepi | 1 | 0.09 | 0.12 | 0.05 to 0.28 | 0.04 | 0.00 to 0.71 | 0.14 | 0.06 to 3.2e‐01 | 0.32 | 0.02 to 5.9e+00 | –0.77 | 0.44 |
| bupi:prilo | 1 | 0.09 | 0.83 | 0.31 to 2.17 | 0.33 | 0.01 to 7.87 | 0.91 | 0.33 to 2.5e+00 | 0.37 | 0.01 to 1.0e+01 | –0.59 | 0.55 |
| bupi:pro | 0 | 0 | 1.10 | 0.32 to 3.83 | — | — | 1.10 | 0.32 to 3.8e+00 | — | — | — | — |
| bupi:ropi | 0 | 0 | 1.83 | 0.23 to 14.32 | — | — | 1.83 | 0.23 to 1.4e+01 | — | — | — | — |
| chloro:levo | 0 | 0 | 1.06 | 0.08 to 14.27 | — | — | 1.06 | 0.08 to 1.4e+01 | — | — | — | — |
| chloro:lido | 2 | 0.90 | 0.18 | 0.02 to 1.53 | 0.21 | 0.02 to 2.02 | 0.04 | 0.00 to 3.8e+01 | 4.88 | 0.00 to 6.3e+03 | 0.43 | 0.66 |
| chloro:mepi | 0 | 0 | 0.11 | 0.01 to 1.11 | — | — | 0.11 | 0.01 to 1.1e+00 | — | — | — | — |
| chloro:prilo | 0 | 0 | 0.76 | 0.07 to 7.82 | — | — | 0.76 | 0.07 to 7.8e+00 | — | — | — | — |
| chloro:pro | 0 | 0 | 1.02 | 0.09 to 11.87 | — | — | 1.02 | 0.09 to 1.2e+01 | — | — | — | — |
| chloro:ropi | 0 | 0 | 1.69 | 0.09 to 32.35 | — | — | 1.69 | 0.09 to 3.2e+01 | — | — | — | — |
| levo:lido | 2 | 1.00 | 0.17 | 0.04 to 0.70 | 0.17 | 0.04 to 0.71 | 0.00 | 0.00 to 1.3e+12 | 266.30 | 0.00 to 5.8e+17 | 0.31 | 0.76 |
| levo:mepi | 0 | 0 | 0.11 | 0.02 to 0.54 | — | — | 0.11 | 0.02 to 5.4e‐01 | — | — | — | — |
| levo:prilo | 0 | 0 | 0.72 | 0.13 to 3.87 | — | — | 0.72 | 0.13 to 3.9e+00 | — | — | — | — |
| levo:pro | 0 | 0 | 0.96 | 0.15 to 6.15 | — | — | 0.96 | 0.15 to 6.2e+00 | — | — | — | — |
| levo:ropi | 1 | 0.39 | 1.59 | 0.14 to 18.08 | 1.00 | 0.02 to 48.82 | 2.14 | 0.09 to 4.8e+01 | 0.47 | 0.00 to 6.8e+01 | –0.30 | 0.76 |
| mepi:lido | 4 | 0.97 | 1.54 | 0.76 to 3.12 | 1.47 | 0.72 to 3.01 | 6.85 | 0.12 to 4.0e+02 | 0.22 | 0.00 to 1.3e+01 | –0.73 | 0.47 |
| prilo:lido | 4 | 1.00 | 0.23 | 0.10 to 0.55 | 0.23 | 0.10 to 0.55 | 55983.54 | 0.00 to 4.5e+16 | 0.00 | 0.00 to 3.3e+06 | –0.89 | 0.37 |
| prop:lido | 2 | 1.00 | 0.17 | 0.05 to 0.56 | 0.17 | 0.05 to 0.56 | — | — | — | — | — | — |
| ropi:lido | 2 | 1.00 | 0.10 | 0.01 to 0.78 | 0.10 | 0.01 to 0.78 | 0.91 | 0.00 to 4.9e+12 | 0.11 | 0.00 to 6.5e+11 | –0.15 | 0.88 |
| mepi:prilo | 0 | 0 | 6.67 | 2.18 to 20.44 | — | — | 6.67 | 2.18 to 2.0e+01 | — | — | — | — |
| mepi:pro | 0 | 0 | 8.91 | 2.27 to 34.91 | — | — | 8.91 | 2.27 to 3.5e+01 | — | — | — | — |
| mepi:ropi | 0 | 0 | 14.78 | 1.75 to 124.74 | — | — | 14.78 | 1.75 to 1.2e+02 | — | — | — | — |
| prilo:pro | 0 | 0 | 1.34 | 0.31 to 5.74 | — | — | 1.34 | 0.31 to 5.7e+00 | — | — | — | — |
| prilo:ropi | 0 | 0 | 2.21 | 0.25 to 19.86 | — | — | 2.21 | 0.25 to 2.0e+01 | — | — | — | — |
| prop:ropi | 0 | 0 | 1.66 | 0.16 to 17.03 | — | — | 1.66 | 0.16 to 1.7e+01 | — | — | — | — |
| bupi: bupivacaine; chloro: 2‐chloroprocaine; k: number of studies providing direct evidence; levo: levobupivacaine; lido: lidocaine; mepi: mepivacaine; NA: not available; NMA: network meta‐analysis; pro: direct evidence proportion; prilo: prilocaine; pro: procaine, ropi: ropivacaine; RoR: ratio of ratios (direct versus indirect). | ||||||||||||
| Q | df | P value | |
| Total | 18.4 | 21 | 0.6232 |
| Within designs | 14.8 | 13 | 0.3209 |
| Between designs | 3.6 | 8 | 0.8897 |
| df: degrees of freedom; Q: Cochran's Q heterogeneity statistic. | |||
| Treatment | P score |
| ropi | 0.772 |
| levo | 0.657 |
| pro | 0.647 |
| chloro | 0.624 |
| bupi | 0.610 |
| prilo | 0.528 |
| lido | 0.138 |
| mepi | 0.022 |
| bupi: bupivacaine; chloro: 2‐chloroprocaine; levo: levobupivacaine; lido: lidocaine; mepi: mepivacaine; prilo: prilocaine; pro: procaine, ropi: ropivacaine. | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Presence of any transient neurological symptoms Show forest plot | 24 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Bupivacaine vs lidocaine | 12 | 1220 | Risk Ratio (IV, Random, 95% CI) | 0.16 [0.09, 0.28] |
| 1.2 2‐Chlorprocaine vs lidocaine | 2 | 94 | Risk Ratio (IV, Random, 95% CI) | 0.09 [0.01, 1.51] |
| 1.3 Levobupivacaine vs lidocaine | 2 | 120 | Risk Ratio (IV, Random, 95% CI) | 0.13 [0.02, 0.69] |
| 1.4 Mepivacaine vs lidocaine | 4 | 274 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.18, 5.82] |
| 1.5 Prilocaine vs lidocaine | 4 | 429 | Risk Ratio (IV, Random, 95% CI) | 0.18 [0.07, 0.49] |
| 1.6 Procaine vs lidocaine | 2 | 130 | Risk Ratio (IV, Random, 95% CI) | 0.14 [0.04, 0.52] |
| 1.7 Ropivacaine vs lidocaine | 2 | 90 | Risk Ratio (IV, Random, 95% CI) | 0.10 [0.01, 0.78] |
| 1.8 Bupivacaine vs mepivacaine | 1 | 60 | Risk Ratio (IV, Random, 95% CI) | 0.04 [0.00, 0.71] |
| 1.9 Bupivacaine vs prilocaine | 1 | 60 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.01, 7.87] |
| 1.10 Levobupivacaine vs ropivacaine | 1 | 60 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.11 Bupivacaine vs 2‐chlorprocaine | 1 | 67 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |

