Síntomas neurológicos transitorios (SNT) después de la anestesia espinal con lidocaína versus otros anestésicos locales en pacientes adultos quirúrgicos: un metanálisis en red
Información
- DOI:
- https://doi.org/10.1002/14651858.CD003006.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 01 diciembre 2019see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Anestesia
- Copyright:
-
- Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Undertaking manual searches: all authors.
Screening search results: all authors.
Screening retrieved papers against inclusion criteria: all authors.
Appraising quality of papers: all authors.
Abstracting data from papers: all authors.
Data management for the review: all authors.
Entering data into Review Manager 5 (Review Manager 2014): all authors.
Review Manager 5 statistical data (Review Manager 2014): all authors.
Interpretation of data: all authors.
Statistical inferences and network meta‐analysis: NLP.
Writing the review: all authors.
Guarantor for the review: PF.
Person responsible for reading and checking review before submission: PF.
Sources of support
Internal sources
-
EMT, USA.
The author has no source of internal support to declare.
-
JAB, Croatia.
The author has no source of internal support to declare.
-
NLP, USA.
The author has no source of internal support to declare.
-
PF, UK.
The author has no source of internal support to declare.
External sources
-
EMT, USA.
The author has no source of external support to declare.
-
JAB, Croatia.
The author has no source of external support to declare.
-
PF, UK.
The author has no source of external support to declare.
-
NLP, USA.
The author has no source of external support to declare.
Declarations of interest
PF: none.
JAB: none.
EMT: taught a 'safe sedation simulation' course to doctors and nurses as a consultant for Applied Medical Visualizations.
NLP: is a tenured professor (University of Utah) and has no conflicts of interest regarding the topic of this review. He has received payment for the development of educational presentations (Barash, Cullen, Toelting Clinical Anaesthesia 8th Edition) and provided consultancy (St Marks Hospital, Salt Lake City, UT; JB3 Bioscience Inc, Salt Lake City, UT; Elute, Salt Lake City, UT) on topics unrelated to the current review. He has received financial supplements to attend Cochrane meetings. He also has stocks and shares in companies who have no interests in the topic of this review (TIAA‐CREF, Fidelity, Vanguard, USAA, Morgan Stanley).
Acknowledgements
April 2019: we would like to thank Mike Bennett (Content Editor), Cathal Walsh (Statistical Editor), Joanne Guay (Peer Reviewer), Janet Wale (Consumer Editor), Janne Vendt (Information Specialist), Teo Quay (Managing Editor), and Andrew Smith (Co‐ordinating Editor) for their help and editorial advice during the preparation of this systematic review. We also would like to thank Jane Cracknell (Managing Editor at the time of the review) for her help and advice. Finally, we thank Dimitris Mavridis who commented on an earlier draft.
November 2008: we would like to thank Prof Michael Bennett (Content Editor) and Dr Helen Worthington (Statistical and Co‐ordinating Editor Cochrane Oral Health Group) for editing our updated review (Zaric 2009).
We would like to acknowledge the contribution of Yodying Punjasawadwong and Christian Christiansen to our original review (Zaric 2005). Dr Punjasawadwong helped with the literature search, evaluation, and construction of tables. Dr Christiansen helped with the evaluation of literature and revision of the text.
We would also like to thank Dr Peter Choi and Dr Helen Worthington for editing our original review (Zaric 2005), and acknowledge and thank Dr Janet Wale for her consumer synopsis. Her efforts greatly contributed to the editorial process of the review.
Version history
| Published | Title | Stage | Authors | Version |
| 2019 Dec 01 | Transient neurological symptoms (TNS) following spinal anaesthesia with lidocaine versus other local anaesthetics in adult surgical patients: a network meta‐analysis | Review | Patrice Forget, Josip A Borovac, Elizabeth M Thackeray, Nathan L Pace | |
| 2009 Apr 15 | Transient neurologic symptoms (TNS) following spinal anaesthesia with lidocaine versus other local anaesthetics | Review | Dusanka Zaric, Nathan Leon Pace | |
| 2005 Oct 19 | Transient neurologic symptoms (TNS) following spinal anaesthesia with lidocaine versus other local anaesthetics | Review | Dusanka DZ Zaric, Christian CC Christiansen, Nathan Leon Pace, Yodying Punjasawadwong | |
| 2003 Apr 22 | Transient neurologic symptoms (TNS) following spinal anaesthesia with lidocaine versus other local anaesthetics | Review | Dusanka DZ Zaric, Christian CC Christiansen, Nathan Leon Pace, Yodying YP Punjasawadwong | |
Differences between protocol and review
We made the following changes to the published review (Zaric 2009).
-
Changed the title to: Transient neurological symptoms (TNS) following spinal anaesthesia with lidocaine versus other local anaesthetics in adult surgical patients.
-
Changed authors to: Forget P, Borovac JA, Thackeray EM, Pace NL.
-
Updated the inclusion criteria for studies to: two or more treatment arms that used a distinct local anaesthetic (irrespective of the concentration and baricity of the solution) for spinal anaesthesia in preparation for surgery.
-
Added network meta‐analysis.
-
Incorporated GRADE assessments.
-
Updated the review according to the MECIR standards.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO

Forest plot of comparison: 1 Lidocaine versus other local anaesthetic, outcome: 1.1 Transient neurological symptoms.

Network meta‐analysis plot of interactions among included studies displayed for a random‐effects, risk ratio model, regarding the risk of transient neurological symptoms following spinal anaesthesia with lidocaine versus other local anaesthetics in adults undergoing surgery. Each node represents an individual local anaesthetic. The node size is proportional to the number of studies.
The node colours are determined by the individual study 'Risk of bias' assessment (green: no concerns; yellow: some concerns; red: major concerns).
The width of the edges is proportional to the inverse variance of the effect size. The edge colours reflect the average risk of bias. bupi: bupivacaine; chloro: 2‐chloroprocaine; levo: levobupivacaine; lido: lidocaine; mepi: mepivacaine; prilo: prilocaine; pro: procaine; ropi: ropivacaine.

Risk of bias (RoB) assessment among studies included in the network meta‐analysis with direct effect estimation is displayed, regarding the risk of transient neurological symptoms following spinal anaesthesia with lidocaine versus other local anaesthetics in adults undergoing surgery. Direct RoB was determined by the average RoB assigned to each particular network interaction. bupi: bupivacaine; chloro: 2‐chloroprocaine; levo: levobupivacaine; lido: lidocaine; mepi: mepivacaine; prilo: prilocaine; pro: procaine; ropi: ropivacaine.

Contributions of indirect and mixed effects in the network meta‐analysis, regarding the risk of transient neurological symptoms following spinal anaesthesia with lidocaine versus other local anaesthetics in adults undergoing surgery. bupi: bupivacaine; chloro: 2‐chloroprocaine; levo: levobupivacaine; lido: lidocaine; mepi: mepivacaine; NMA: network meta‐analysis; prilo: prilocaine; pro: procaine; ropi: ropivacaine

Analysis of imprecision among studies included in the network meta‐analysis, regarding the risk of transient neurological symptoms following spinal anaesthesia with lidocaine versus other local anaesthetics in adults undergoing surgery. bupi: bupivacaine; chloro: 2‐chloroprocaine; levo: levobupivacaine; lido: lidocaine; mepi: mepivacaine; prilo: prilocaine; pro: procaine; ropi: ropivacaine.
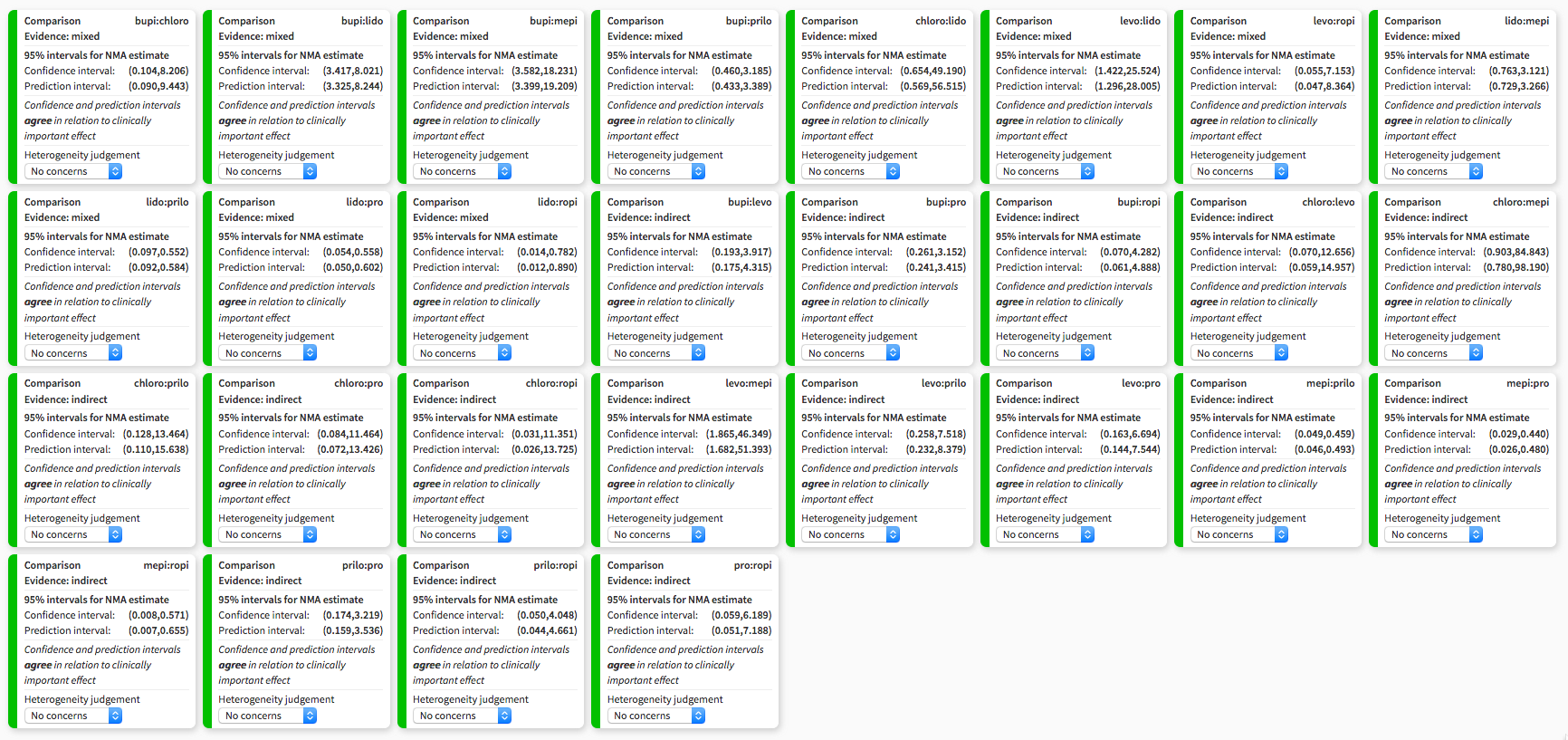
Heterogeneity assessment in network meta‐analysis displaying confidence and prediction intervals, regarding the risk of transient neurological symptoms following spinal anaesthesia with lidocaine versus other local anaesthetics in adults undergoing surgery. bupi: bupivacaine; chloro: 2‐chloroprocaine; levo: levobupivacaine; lido: lidocaine; mepi: mepivacaine; prilo: prilocaine; pro: procaine; ropi: ropivacaine.

Summary of risk of bias assigned to studies included in the network meta‐analysis across six domains: study limitations, imprecision, heterogeneity, incoherence, indirectness, publication bias, regarding the risk of transient neurological symptoms following spinal anaesthesia with lidocaine versus other local anaesthetics in adults undergoing surgery. Output was created with CINeMA software. bupi: bupivacaine; chloro: 2‐chloroprocaine; levo: levobupivacaine; lido: lidocaine; mepi: mepivacaine; prilo: prilocaine; pro: procaine; ropi: ropivacaine.

Study selection flow diagram corresponding to the last search update, up to November 2018.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
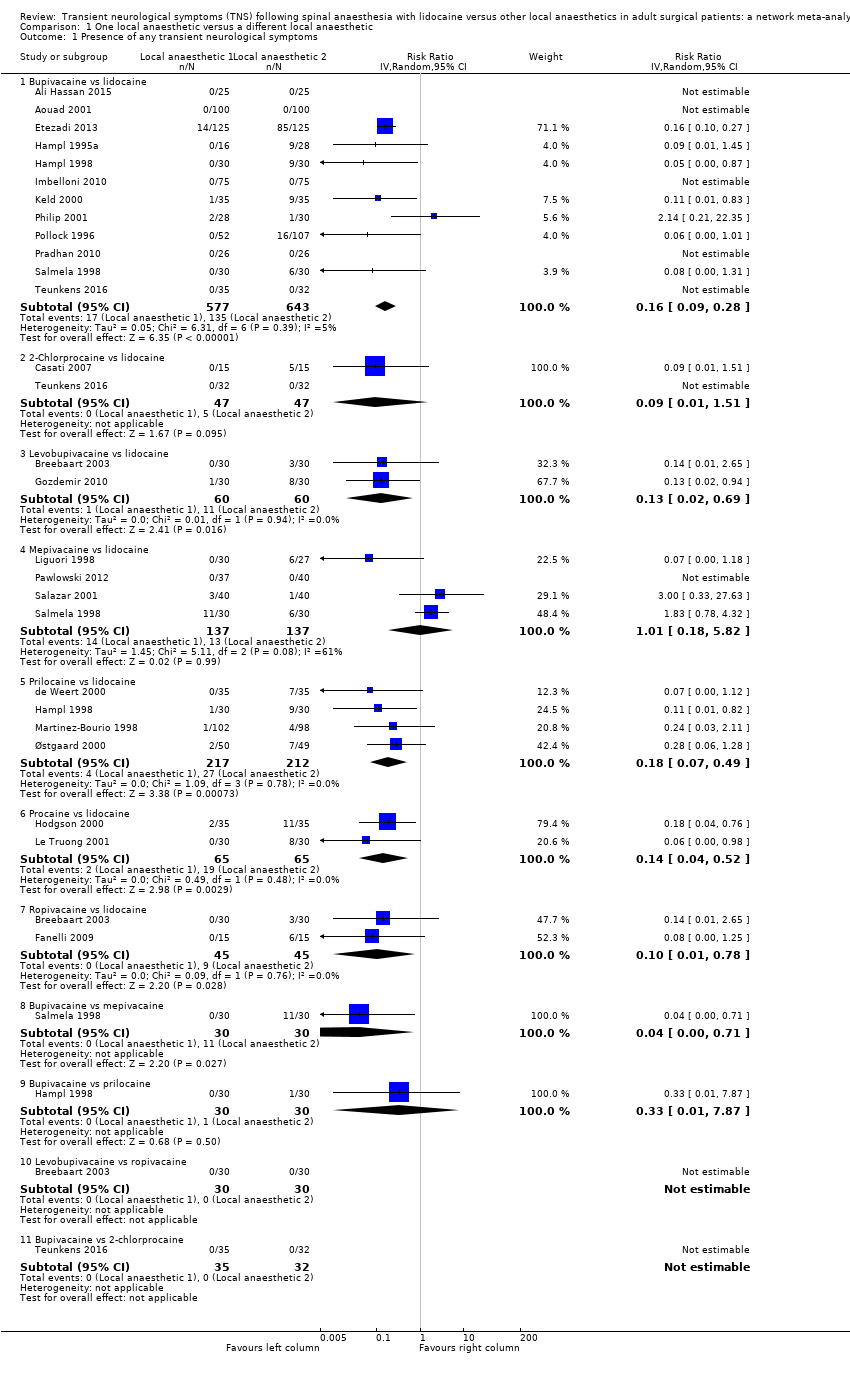
Comparison 1 One local anaesthetic versus a different local anaesthetic, Outcome 1 Presence of any transient neurological symptoms.
| Risk of transient neurological symptoms (TNS) with spinal lidocaine compared to other local anaesthetics in adults undergoing surgerya | ||||||
| Patient or population: adult undergoing surgery Settings: hospital or ambulatory surgery setting (Belgium, Brazil, Canada, Denmark, Egypt, Finland, Iran, Italy, Lebanon, Nepal, the Netherlands, Norway, Spain, Switzerland, Turkey, USA) Intervention: spinal lidocaine Comparison: other local anaesthetics as indicated | ||||||
| Outcomes | Anticipated absolute effectsb (95% CI) | Relative effect (95 CI) | № of participants | Quality of the evidence | Comments | |
| Risk with lidocaine | Risk difference with the other local anaesthetic | |||||
| Presence of any TNS –lidocaine vs bupivacaine Follow‐up: range 1–30 days | 210 per 1000 | 176 fewer per 1000 (191 fewer to 151 fewer) | RR 0.16 (0.09 to 0.28) | 1220 (12 RCTs) | ⊕⊕⊕⊝ Moderatec | Bupivacaine probably reduced the risk of TNS compared to lidocaine. |
| Presence of any TNS – lidocaine vs 2‐chloroprocaine Follow‐up: range 1–7 days | 106 per 1000 | 97 fewer per 1000 (105 fewer to 54 more) | RR 0.09 (0.01 to 1.51) | 94 (2 RCTs) | ⊕⊕⊝⊝ Lowc,d | 2‐chloroprocaine may have resulted in no difference in the risk of TNS compared to lidocaine. |
| Presence of any TNS – lidocaine vs levobupivacaine Follow‐up: range 2–7 days | 183 per 1000 | 159 fewer per 1000 (180 fewer to 57 fewer) | RR 0.13 (0.02 to 0.69) | 120 (2 RCTs) | ⊕⊕⊝⊝ | Levobupivacaine may have reduced the risk of TNS compared to lidocaine. |
| Presence of any TNS – lidocaine vs mepivacaine Follow‐up: range 1–5 days | 95 per 1000 | 1 more per 1000 (78 fewer to 457 more) | RR 1.01 (0.18 to 5.82) | 274 (4 RCTs) | ⊕⊝⊝⊝ Very lowd,e,f | Mepivacaine may have resulted in no difference in the risk of TNS compared to lidocaine but the evidence was very uncertain. |
| Presence of any TNS – lidocaine vs prilocaine Follow‐up: range 1–5 days | 127 per 1000 | 104 fewer per 1000 (118 fewer to 65 fewer) | RR 0.18 (0.07 to 0.49) | 429 (4 RCTs) | ⊕⊕⊕⊝ Moderatec | Prilocaine probably reduced the risk of TNS compared to lidocaine. |
| Presence of any TNS – lidocaine vs procaine Follow‐up: range 2–3 days | 292 per 1000 | 251 fewer per 1000 (281 fewer to 140 fewer) | RR 0.14 (0.04 to 0.52) | 130 (2 RCTs) | ⊕⊕⊕⊝ Moderatef | Procaine probably reduced the risk of TNS compared to lidocaine. |
| Presence of any TNS – lidocaine vs ropivacaine Follow‐up: range 2–7 days | 200 per 1000 | 180 fewer per 1000 (198 fewer to 44 fewer) | RR 0.10 (0.01 to 0.78) | 90 (2 RCTs) | ⊕⊕⊝⊝ Lowc,e | Ropivacaine may have reduced the risk of TNS compared to lidocaine. |
| CI: confidence interval; TNS: transient neurological symptoms; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| a'Summary of findings' table is based on pair‐wise meta‐analysis (Figure 1). Results of the network meta‐analysis are presented in Table 2; Table 3; Table 4 and Figure 2; Figure 3; Figure 4; Figure 5; Figure 6; Figure 7. | ||||||
| Study ID | TNS #/N (%) | Pain score (0–10) | TNS duration | Therapy |
| 0/100 (0) | — | — | — | |
| 3/30 (10) | Not tallied | 1 day | Not described | |
| 5/15 (33) | Not tallied | Up to 7 days | NSAIDs | |
| 7/35 (20) | Day 1 mean VPS 5.3 (range 2–8) | Maximum duration 3 days | Not described | |
| 85/135 (63) | Mean VAS 6–7 | Maximum duration 5 days | NSAIDs | |
| 6/15 (40) | Not tallied | Resolved within 7 days | Not described | |
| 8/30 (27) | Median VPS 3 (range 1–6) | Resolved within 7 days | Not described | |
| 9/28 (32) | Not tallied | Maximum duration 4 days | Not described | |
| 9/30 (30) | Mean maximum VAS 3.75 | Maximum duration 2 days | Not described | |
| 0/25 (0) | — | — | — | |
| 11/35 (31) | Mean VPS 5 | Mean duration 2 days | Not described | |
| 0/75 (0) | — | — | — | |
| 9/35 (26) | Mean VPS 3.5 (range 2–8) | Maximum duration 4 days | Not described | |
| 8/30 (27) | Not tallied | Unspecified | Not described | |
| 6/27 (22) | Not tallied | Maximum duration 5 days | NSAIDs | |
| 4/98 (4) | Not tallied | Maximum duration 10 days | NSAIDs | |
| 7/49 (14) | VPS range 5–9.5 | Maximum duration 3 days | Not described | |
| 0/40 (0) | — | — | — | |
| 1/30 (3) | Maximum VAS 3 | Maximum duration 2 days | Not described | |
| 16/107 (15) | Mean VPS 6.2 (range 1–9) | Maximum duration 4 days | NSAIDs and opioids | |
| 0/26 (0) | — | — | — | |
| 1/40 (3) | Maximum VAS 9–10 | Maximum duration 1 day | NSAIDs | |
| 6/30 (20) | Moderate pain | Maximum duration 1 day | NSAIDs and opioids | |
| 0/32 (0) | — | — | — | |
| N: number of participants; NSAIDs: non‐steroidal anti‐inflammatory drugs; TNS: transient neurological symptoms; VAS: visual analogue scale; VPS: verbal pain scale. | ||||
| Treatment | RR | 95% CI | 95% PI |
| bupi | 0.19 | 0.12 to 0.29a | 0.12 to 0.30 |
| chloro | 0.18 | 0.02 to 1.53 | 0.02 to 1.75 |
| levo | 0.17 | 0.04 to 0.70a | 0.04 to 0.77 |
| mepi | 1.54 | 0.76 to 3.12 | 0.73 to 3.27 |
| prilo | 0.23 | 0.10 to 0.55a | 0.09 to 0.58 |
| pro | 0.17 | 0.05 to 0.56a | 0.05 to 0.60 |
| ropi | 0.10 | 0.01 to 0.78a | 0.01 to 0.89 |
| bupi: bupivacaine; chloro: 2‐chloroprocaine; CI: confidence Interval; levo: levobupivacaine; lido: lidocaine; mepi: mepivacaine; PI: prediction interval; prilo: prilocaine; pro: procaine, ropi: ropivacaine. | |||
| Comparison | k | pro | NMA | 95% CI | Direct | 95% CI | Indirect | 95% CI | RoR | 95% CI | z | P value |
| bupi:chloro | 1 | 0.32 | 1.08 | 0.12 to 9.63 | 0.92 | 0.02 to 44.90 | 1.17 | 0.08 to 1.6e+01 | 0.78 | 0.01 to 8.6e+01 | –0.10 | — |
| bupi:levo | 0 | 0 | 1.15 | 0.26 to 5.18 | — | — | 1.15 | 0.26 to 5.2e+00 | — | — | — | — |
| bupi:lido | 12 | 1.00 | 0.19 | 0.12 to 0.29 | 0.19 | 0.13 to 0.30 | 0.00 | 0.00 to 1.8e+01 | 42.58 | 0.01 to 1.7e+05 | 0.89 | 0.38 |
| bupi:mepi | 1 | 0.09 | 0.12 | 0.05 to 0.28 | 0.04 | 0.00 to 0.71 | 0.14 | 0.06 to 3.2e‐01 | 0.32 | 0.02 to 5.9e+00 | –0.77 | 0.44 |
| bupi:prilo | 1 | 0.09 | 0.83 | 0.31 to 2.17 | 0.33 | 0.01 to 7.87 | 0.91 | 0.33 to 2.5e+00 | 0.37 | 0.01 to 1.0e+01 | –0.59 | 0.55 |
| bupi:pro | 0 | 0 | 1.10 | 0.32 to 3.83 | — | — | 1.10 | 0.32 to 3.8e+00 | — | — | — | — |
| bupi:ropi | 0 | 0 | 1.83 | 0.23 to 14.32 | — | — | 1.83 | 0.23 to 1.4e+01 | — | — | — | — |
| chloro:levo | 0 | 0 | 1.06 | 0.08 to 14.27 | — | — | 1.06 | 0.08 to 1.4e+01 | — | — | — | — |
| chloro:lido | 2 | 0.90 | 0.18 | 0.02 to 1.53 | 0.21 | 0.02 to 2.02 | 0.04 | 0.00 to 3.8e+01 | 4.88 | 0.00 to 6.3e+03 | 0.43 | 0.66 |
| chloro:mepi | 0 | 0 | 0.11 | 0.01 to 1.11 | — | — | 0.11 | 0.01 to 1.1e+00 | — | — | — | — |
| chloro:prilo | 0 | 0 | 0.76 | 0.07 to 7.82 | — | — | 0.76 | 0.07 to 7.8e+00 | — | — | — | — |
| chloro:pro | 0 | 0 | 1.02 | 0.09 to 11.87 | — | — | 1.02 | 0.09 to 1.2e+01 | — | — | — | — |
| chloro:ropi | 0 | 0 | 1.69 | 0.09 to 32.35 | — | — | 1.69 | 0.09 to 3.2e+01 | — | — | — | — |
| levo:lido | 2 | 1.00 | 0.17 | 0.04 to 0.70 | 0.17 | 0.04 to 0.71 | 0.00 | 0.00 to 1.3e+12 | 266.30 | 0.00 to 5.8e+17 | 0.31 | 0.76 |
| levo:mepi | 0 | 0 | 0.11 | 0.02 to 0.54 | — | — | 0.11 | 0.02 to 5.4e‐01 | — | — | — | — |
| levo:prilo | 0 | 0 | 0.72 | 0.13 to 3.87 | — | — | 0.72 | 0.13 to 3.9e+00 | — | — | — | — |
| levo:pro | 0 | 0 | 0.96 | 0.15 to 6.15 | — | — | 0.96 | 0.15 to 6.2e+00 | — | — | — | — |
| levo:ropi | 1 | 0.39 | 1.59 | 0.14 to 18.08 | 1.00 | 0.02 to 48.82 | 2.14 | 0.09 to 4.8e+01 | 0.47 | 0.00 to 6.8e+01 | –0.30 | 0.76 |
| mepi:lido | 4 | 0.97 | 1.54 | 0.76 to 3.12 | 1.47 | 0.72 to 3.01 | 6.85 | 0.12 to 4.0e+02 | 0.22 | 0.00 to 1.3e+01 | –0.73 | 0.47 |
| prilo:lido | 4 | 1.00 | 0.23 | 0.10 to 0.55 | 0.23 | 0.10 to 0.55 | 55983.54 | 0.00 to 4.5e+16 | 0.00 | 0.00 to 3.3e+06 | –0.89 | 0.37 |
| prop:lido | 2 | 1.00 | 0.17 | 0.05 to 0.56 | 0.17 | 0.05 to 0.56 | — | — | — | — | — | — |
| ropi:lido | 2 | 1.00 | 0.10 | 0.01 to 0.78 | 0.10 | 0.01 to 0.78 | 0.91 | 0.00 to 4.9e+12 | 0.11 | 0.00 to 6.5e+11 | –0.15 | 0.88 |
| mepi:prilo | 0 | 0 | 6.67 | 2.18 to 20.44 | — | — | 6.67 | 2.18 to 2.0e+01 | — | — | — | — |
| mepi:pro | 0 | 0 | 8.91 | 2.27 to 34.91 | — | — | 8.91 | 2.27 to 3.5e+01 | — | — | — | — |
| mepi:ropi | 0 | 0 | 14.78 | 1.75 to 124.74 | — | — | 14.78 | 1.75 to 1.2e+02 | — | — | — | — |
| prilo:pro | 0 | 0 | 1.34 | 0.31 to 5.74 | — | — | 1.34 | 0.31 to 5.7e+00 | — | — | — | — |
| prilo:ropi | 0 | 0 | 2.21 | 0.25 to 19.86 | — | — | 2.21 | 0.25 to 2.0e+01 | — | — | — | — |
| prop:ropi | 0 | 0 | 1.66 | 0.16 to 17.03 | — | — | 1.66 | 0.16 to 1.7e+01 | — | — | — | — |
| bupi: bupivacaine; chloro: 2‐chloroprocaine; k: number of studies providing direct evidence; levo: levobupivacaine; lido: lidocaine; mepi: mepivacaine; NA: not available; NMA: network meta‐analysis; pro: direct evidence proportion; prilo: prilocaine; pro: procaine, ropi: ropivacaine; RoR: ratio of ratios (direct versus indirect). | ||||||||||||
| Q | df | P value | |
| Total | 18.4 | 21 | 0.6232 |
| Within designs | 14.8 | 13 | 0.3209 |
| Between designs | 3.6 | 8 | 0.8897 |
| df: degrees of freedom; Q: Cochran's Q heterogeneity statistic. | |||
| Treatment | P score |
| ropi | 0.772 |
| levo | 0.657 |
| pro | 0.647 |
| chloro | 0.624 |
| bupi | 0.610 |
| prilo | 0.528 |
| lido | 0.138 |
| mepi | 0.022 |
| bupi: bupivacaine; chloro: 2‐chloroprocaine; levo: levobupivacaine; lido: lidocaine; mepi: mepivacaine; prilo: prilocaine; pro: procaine, ropi: ropivacaine. | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Presence of any transient neurological symptoms Show forest plot | 24 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Bupivacaine vs lidocaine | 12 | 1220 | Risk Ratio (IV, Random, 95% CI) | 0.16 [0.09, 0.28] |
| 1.2 2‐Chlorprocaine vs lidocaine | 2 | 94 | Risk Ratio (IV, Random, 95% CI) | 0.09 [0.01, 1.51] |
| 1.3 Levobupivacaine vs lidocaine | 2 | 120 | Risk Ratio (IV, Random, 95% CI) | 0.13 [0.02, 0.69] |
| 1.4 Mepivacaine vs lidocaine | 4 | 274 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.18, 5.82] |
| 1.5 Prilocaine vs lidocaine | 4 | 429 | Risk Ratio (IV, Random, 95% CI) | 0.18 [0.07, 0.49] |
| 1.6 Procaine vs lidocaine | 2 | 130 | Risk Ratio (IV, Random, 95% CI) | 0.14 [0.04, 0.52] |
| 1.7 Ropivacaine vs lidocaine | 2 | 90 | Risk Ratio (IV, Random, 95% CI) | 0.10 [0.01, 0.78] |
| 1.8 Bupivacaine vs mepivacaine | 1 | 60 | Risk Ratio (IV, Random, 95% CI) | 0.04 [0.00, 0.71] |
| 1.9 Bupivacaine vs prilocaine | 1 | 60 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.01, 7.87] |
| 1.10 Levobupivacaine vs ropivacaine | 1 | 60 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.11 Bupivacaine vs 2‐chlorprocaine | 1 | 67 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |

