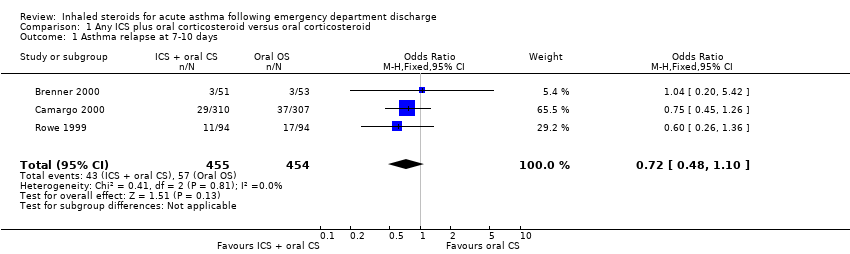
| 1 Asthma relapse at 7‐10 days Show forest plot | 3 | 909 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.48, 1.10] |
|
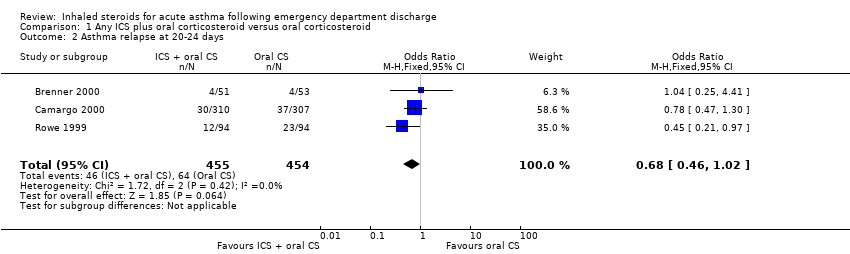
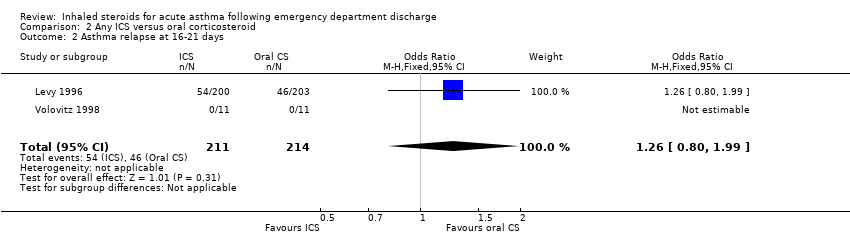
| 2 Asthma relapse at 20‐24 days Show forest plot | 3 | 909 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.46, 1.02] |
|
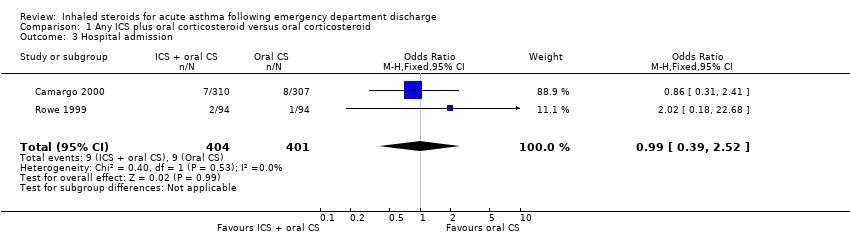
| 3 Hospital admission Show forest plot | 2 | 805 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.39, 2.52] |
|
| 4 Beta2‐agonist use at 7‐10 days Show forest plot | 3 | 672 | Mean Difference (IV, Fixed, 95% CI) | 0.51 [‐0.44, 1.47] |
|
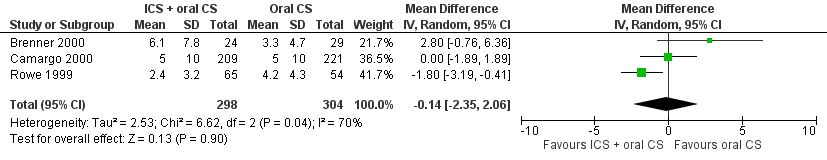
| 5 Beta2‐agonist use at 20‐24 days Show forest plot | 3 | 602 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐2.35, 2.06] |
|
| 6 PEF at 7‐10 days Show forest plot | 2 | 205 | Mean Difference (IV, Fixed, 95% CI) | ‐0.88 [‐28.49, 26.72] |
|
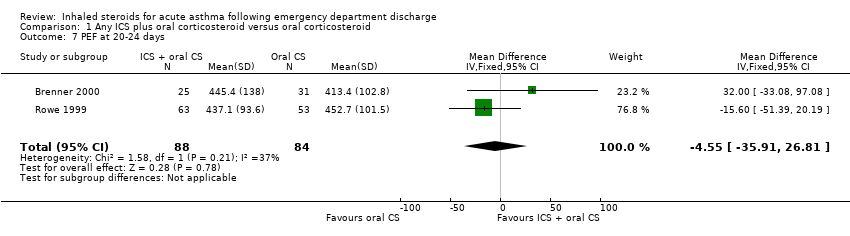
| 7 PEF at 20‐24 days Show forest plot | 2 | 172 | Mean Difference (IV, Fixed, 95% CI) | ‐4.55 [‐35.91, 26.81] |
|
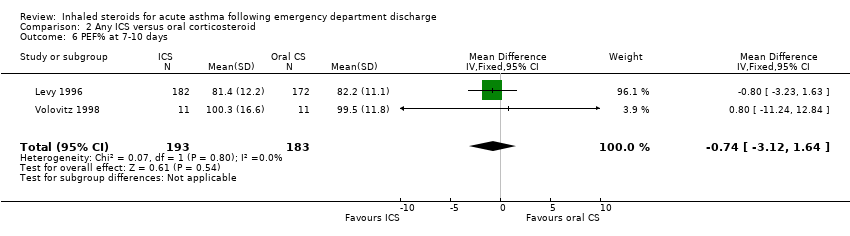
| 8 PEF% at 7‐10 days Show forest plot | 2 | 206 | Mean Difference (IV, Random, 95% CI) | ‐1.79 [‐11.04, 7.46] |
|
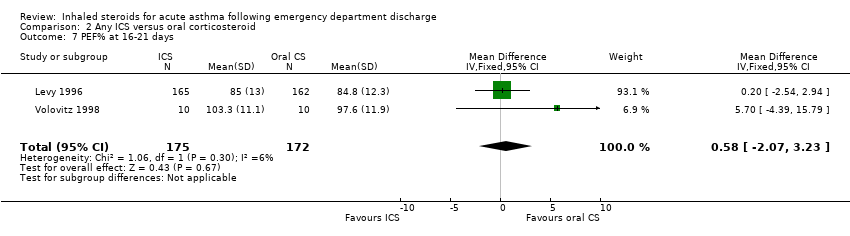
| 9 PEF% at 20‐24 days Show forest plot | 2 | 172 | Mean Difference (IV, Random, 95% CI) | ‐2.34 [‐9.44, 4.77] |
|
| 10 Quality of life at 7‐10 days Show forest plot | 2 | 613 | Mean Difference (IV, Fixed, 95% CI) | 0.19 [‐0.01, 0.39] |
|
| 11 Quality of life at 20‐24 days Show forest plot | 2 | 559 | Mean Difference (IV, Random, 95% CI) | 0.33 [‐0.36, 1.01] |
|
| 12 Cough at 7‐10 days Show forest plot | 2 | 620 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.57, 0.07] |
|
| 13 Cough at 20‐24 days Show forest plot | 2 | 571 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐1.25, 0.31] |
|
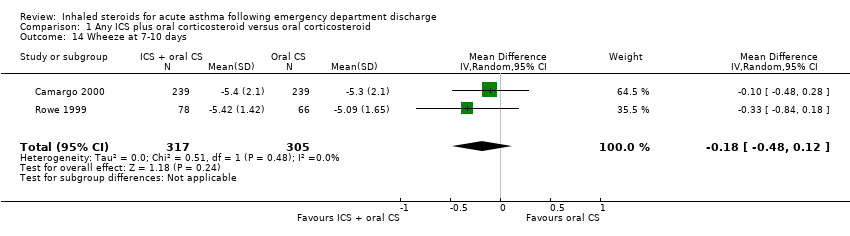
| 14 Wheeze at 7‐10 days Show forest plot | 2 | 622 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.48, 0.12] |
|
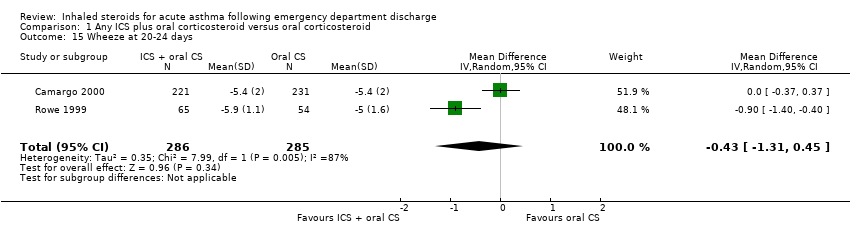
| 15 Wheeze at 20‐24 days Show forest plot | 2 | 571 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐1.31, 0.45] |
|
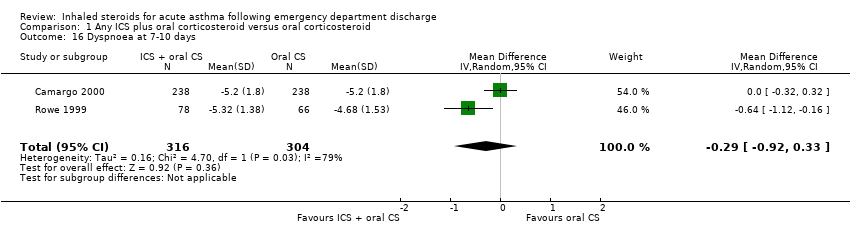
| 16 Dyspnoea at 7‐10 days Show forest plot | 2 | 620 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.92, 0.33] |
|
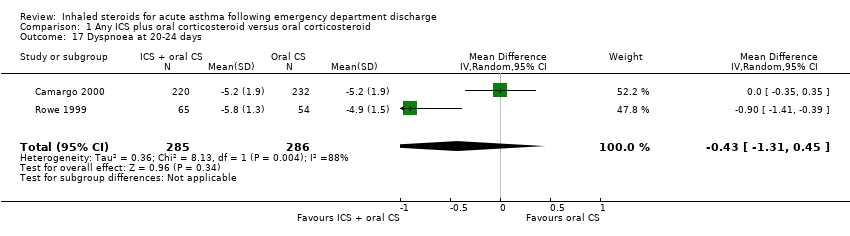
| 17 Dyspnoea at 20‐24 days Show forest plot | 2 | 571 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐1.31, 0.45] |
|
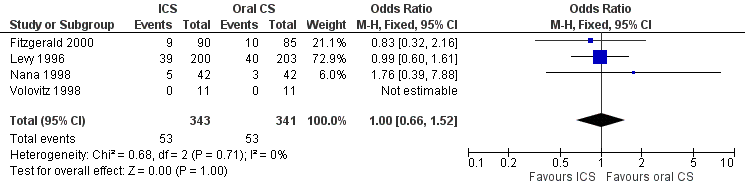
| 18 Hoarseness at 7‐10 days Show forest plot | 2 | 612 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.53, 1.46] |
|
| 19 Hoarseness at 20‐24 days Show forest plot | 2 | 596 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.36, 1.01] |
|
| 20 Sore throat at 7‐10 days Show forest plot | 2 | 612 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.43, 1.24] |
|
| 21 Sore throat at 20‐24 days Show forest plot | 2 | 596 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.35, 1.16] |
|
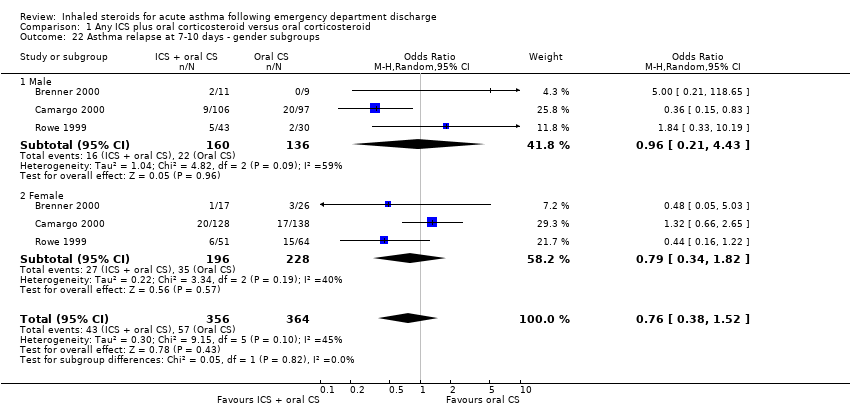
| 22 Asthma relapse at 7‐10 days ‐ gender subgroups Show forest plot | 3 | 720 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.38, 1.52] |
|
| 22.1 Male | 3 | 296 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.21, 4.43] |
| 22.2 Female | 3 | 424 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.34, 1.82] |
| 23 Asthma relapse at 20‐24 days ‐ gender subgroups Show forest plot | 3 | 761 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.37, 1.35] |
|
| 23.1 Male | 3 | 315 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.22, 1.62] |
| 23.2 Female | 3 | 446 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.30, 1.99] |
| 24 Asthma relapse at 7‐10 days; patients lost to follow‐up excluded Show forest plot | 3 | 725 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.47, 1.10] |
|
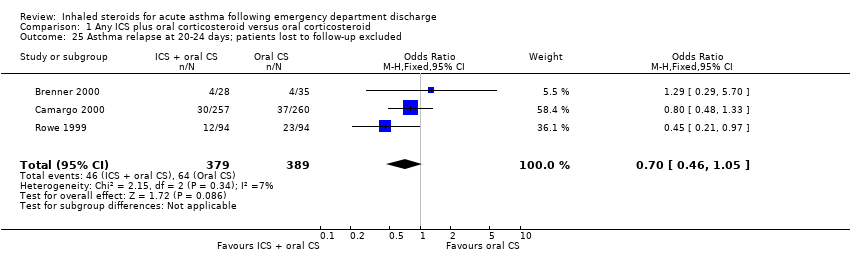
| 25 Asthma relapse at 20‐24 days; patients lost to follow‐up excluded Show forest plot | 3 | 768 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.46, 1.05] |
|