Corticosteroids for treating sepsis
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomized controlled trial with 2 parallel groups 19 centres | |
| Participants | Adults (n = 300) with vasopressor‐ and ventilator‐dependent septic shock Stratification according to cortisol response to 250 µg Synacthene into non‐responders (delta cortisol ≤ 9 µg/dL) and responders (> 9 µg/dL) | |
| Interventions |
Treatments have to be initiated within 8 hours from shock onset | |
| Outcomes | PRIMARY
SECONDARY
| |
| Notes | Study location: France | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding (performance bias and detection bias) | Low risk | Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Low risk | Access to study protocol excluding reporting bias |
| Other bias | Low risk | Full access to data excluding selection bias |
| Methods | Randomized controlled trial with 2 × 2 factorial design 11 centres | |
| Participants | Adults (n = 509) with vasopressor‐dependent septic shock | |
| Interventions |
Treatments have to be initiated within 24 hours from shock onset | |
| Outcomes | PRIMARY
SECONDARY
| |
| Notes | Study location: France | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Centralized randomization through a secured website |
| Blinding (performance bias and detection bias) | High risk | Participants: yes Care‐givers: no Data collectors: yes Outcome assessors: no Data analysts: yes |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Access to study protocol excluding reporting bias |
| Other bias | Low risk | Full access to data excluding selection bias |
| Methods | Randomized controlled trial 1 centre | |
| Participants | Adult (n = 75) with liver cirrhosis and septic shock | |
| Interventions |
| |
| Outcomes | Primary
Secondary
Outcomes were also analysed in relation to adrenal insufficiency | |
| Notes | Study location: Saudi Arabia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Use of sealed envelopes by pharmacists |
| Blinding (performance bias and detection bias) | Low risk | Pharmacists: no Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) | Unclear risk | Unexplained discrepancy between reported K‐M curves and number of deaths at 28 days in placebo arm |
| Selective reporting (reporting bias) | Low risk | Access to unpublished data |
| Other bias | High risk | Trial terminated prematurely after enrolment of 75 participants while planned sample size was 150 |
| Methods | Randomized controlled trial with 2 parallel groups 2 centres | |
| Participants | Adults (n = 41) with vasopressor‐ and ventilator‐dependent septic shock Stratification according to cortisol response to 250 µg Synacthene into non‐responders (delta cortisol ≤ 6 µg/dL) and responders (> 6 µg/dL) | |
| Interventions |
Treatments have to be initiated after 48 hours or longer from shock onset | |
| Outcomes | PRIMARY
SECONDARY
| |
| Notes | Study location: France | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Randomization list kept confidential by the pharmacist |
| Blinding (performance bias and detection bias) | Low risk | Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Low risk | Access to study protocol excluding reporting bias |
| Other bias | Low risk | Full access to data excluding selection bias |
| Methods | Randomized controlled trial with 2 parallel groups 19 centres | |
| Participants | Adults (n = 382) with sepsis (n = 234) or septic shock (n = 148) | |
| Interventions |
Treatments have to be initiated 2 hours from time entry criteria were met | |
| Outcomes | PRIMARY
| |
| Notes | Study location: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding (performance bias and detection bias) | Low risk | Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol to exclude reporting bias |
| Other bias | Low risk | No access to full data including screening log to exclude selection bias |
| Methods | Randomized controlled trial with 2 parallel groups 1 centre | |
| Participants | Adults (n = 40) with vasopressor‐ and ventilator‐dependent septic shock | |
| Interventions |
Treatments have to be initiated within 72 hours from shock onset | |
| Outcomes | PRIMARY
SECONDARY
| |
| Notes | Study location: Germany | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Adequate randomization |
| Blinding (performance bias and detection bias) | Low risk | Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Low risk | Access to study protocol excluding reporting bias |
| Other bias | Low risk | Access to full data including screening log |
| Methods | Randomized controlled trial with 2 parallel groups 1 centre | |
| Participants | Adults (n = 44) with vasopressor‐dependent septic shock | |
| Interventions |
Treatments have to be initiated after 72 hours or longer from shock onset | |
| Outcomes | PRIMARY
SECONDARY
| |
| Notes | Study location: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Randomization list was kept confidential by the pharmacist |
| Blinding (performance bias and detection bias) | Low risk | Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Low risk | Access to study protocol excluding reporting bias |
| Other bias | Low risk | Access to full data including screening log |
| Methods | Randomized controlled trial with 2 parallel groups 1 centre | |
| Participants | Adults (n = 29) with vasopressor‐dependent septic shock | |
| Interventions |
| |
| Outcomes |
| |
| Notes | Study location: Brazil | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Randomization list kept confidential by the pharmacist |
| Blinding (performance bias and detection bias) | Low risk | Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) | Unclear risk | Lost to follow‐up: none; 3 participants were withdrawn after next of kin refused to consent |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol to rule out reporting bias |
| Other bias | Unclear risk | No access to data to rule out selection bias |
| Methods | Randomized controlled trial with 2 parallel groups 6 centres | |
| Participants | Adults (n = 46) with severe community‐acquired pneumonia | |
| Interventions |
| |
| Outcomes | PRIMARY
SECONDARY
| |
| Notes | Study location: Italy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding (performance bias and detection bias) | Low risk | Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up: 2 at 60 days after randomization, all in the placebo group |
| Selective reporting (reporting bias) | High risk | Study was stopped prematurely for apparent benefit; no sample size was defined a priori, but study authors used the triangular test as a stopping rule, analysing the primary outcome after each 20 participants |
| Other bias | Low risk | Access to full data including screening log |
| Methods | Randomized controlled trial with 2 parallel groups 5 centres | |
| Participants | Adults (n = 194) and children (n = 135) with vasopressor‐dependent septic shock | |
| Interventions |
| |
| Outcomes | PRIMARY
SECONDARY
| |
| Notes | Study location: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not given |
| Allocation concealment (selection bias) | Unclear risk | Not given |
| Blinding (performance bias and detection bias) | Unclear risk | Participants: yes Care‐givers: yes Data collectors: unclear Outcome assessors: unclear Data analysts: unclear |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol to exclude reporting bias |
| Other bias | Unclear risk | No access to data to exclude selection bias |
| Methods | Randomized controlled trial with 2 parallel groups 4 centres | |
| Participants | Adults (n = 61) with septic shock on a maximal dose of vasopressin of up to 0.06 U/min | |
| Interventions |
| |
| Outcomes | PRIMARY
SECONDARY
| |
| Notes | Study location: United Kingdom | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers prepared by an independent statistician |
| Allocation concealment (selection bias) | Low risk | Randomization done via an online system |
| Blinding (performance bias and detection bias) | Low risk | Hydrocortisone and its placebo presented in indiscernible forms |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Low risk | Reported information matched published statistical plan |
| Other bias | Low risk | Access to unpublished information to exclude other risk of bias |
| Methods | Randomized controlled trial 1 centre | |
| Participants | Adults (n = 77) with septic shock | |
| Interventions |
| |
| Outcomes | PRIMARY
SECONDARY
| |
| Notes | Study location: China | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated in the manuscript |
| Allocation concealment (selection bias) | Unclear risk | Not stated in the manuscript |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated in the manuscript |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | No information |
| Methods | Randomized controlled trial with 2 parallel groups 1 centre | |
| Participants | Adults (n = 82) with septic shock and adrenal insufficiency | |
| Interventions |
| |
| Outcomes | PRIMARY
SECONDARY
| |
| Notes | Study location: South Korea | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Unclear risk | Not given |
| Blinding (performance bias and detection bias) | High risk | Participants: no Care‐givers: no Data collectors: no Outcome assessors: no Data analysts: no |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Unclear risk | No explicit information on plan analysis |
| Other bias | Unclear risk | No information |
| Methods | Randomized controlled trial with cross‐over design 1 centre | |
| Participants | Adults (n = 40) with vasopressor‐dependent septic shock | |
| Interventions |
All participants received hydrocortisone for 3 days preceded or followed by placebo for 3 days | |
| Outcomes | PRIMARY
SECONDARY
| |
| Notes | Study location: Germany | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Randomization list kept confidential by the pharmacist |
| Blinding (performance bias and detection bias) | Low risk | Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Low risk | Access to study protocol |
| Other bias | Low risk | Full access to data including screening log |
| Methods | Randomized controlled trial with parallel groups 1 centre | |
| Participants | Adults (n = 26) with ARDS and sepsis, including septic shock (n = 12) | |
| Interventions |
| |
| Outcomes | PRIMARY
SECONDARY
| |
| Notes | Study location: China | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Unclear risk | No explicit information in the manuscript |
| Blinding (performance bias and detection bias) | Unclear risk | No explicit information in the manuscript |
| Incomplete outcome data (attrition bias) | Unclear risk | No explicit information in the manuscript |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | No information |
| Methods | Randomized controlled trial 1 centre | |
| Participants | Adults (n = 75) with sepsis and septic shock | |
| Interventions |
| |
| Outcomes | PRIMARY
SECONDARY
| |
| Notes | Study location: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Randomization list kept confidential by the pharmacist |
| Blinding (performance bias and detection bias) | Low risk | Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) | High risk | 12 out of 87 randomly assigned participants were not analysed, and their follow‐up was not given |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol |
| Other bias | Unclear risk | No access to data to exclude selection bias |
| Methods | Randomized controlled trial (2:1 scheme) 5 centres | |
| Participants | Adults (n = 91) with early ARDS (≤ 72 hours from diagnosis of ARDS). 61 (67%) had sepsis or septic shock, and the primary author provided separate data for these participants Stratification according to cortisol response to 250 µg Synacthene into non‐responders (delta cortisol ≤ 9 µg/dL) and responders (> 9 µg/dL) | |
| Interventions |
| |
| Outcomes | PRIMARY
SECONDARY
| |
| Notes | If participant failed to improve on Lung Injury Score between day 7 and day 9, he/she received open‐label methylprednisolone at 2 mg/kg/d for unresolving ARDS Study location: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding (performance bias and detection bias) | Low risk | Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) | Low risk | Full access to data excluding any attrition bias |
| Selective reporting (reporting bias) | High risk | Study was stopped prematurely for efficacy |
| Other bias | Low risk | Full access to data including screening log |
| Methods | Randomized controlled trial with 2 parallel groups 2 centres | |
| Participants | Adults (n = 304) with confirmed community‐acquired pneumonia who presented to emergency departments | |
| Interventions |
| |
| Outcomes | PRIMARY
SECONDARY
| |
| Notes | Study location: The Netherlands | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Randomization list kept confidential by the pharmacist |
| Blinding (performance bias and detection bias) | Low risk | Pharmacist: no Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the study protocol are reported in the final analysis |
| Other bias | Low risk | Full access to study protocol |
| Methods | Randomized controlled trial with 2 parallel groups 1 centre | |
| Participants | Adults (n = 40) with vasopressor‐dependent septic shock | |
| Interventions |
| |
| Outcomes | PRIMARY
SECONDARY
| |
| Notes | Study location: Germany | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Randomization list kept confidential by the pharmacist |
| Blinding (performance bias and detection bias) | Low risk | Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) | Low risk | 7 of 48 randomly assigned participants were not analysed: 5 in the corticosteroid group and 2 in the placebo group. 4 of these 7 participants were lost to follow‐up, and 3 died (all in the steroid group) |
| Selective reporting (reporting bias) | Low risk | Access to study protocol excluding reporting bias |
| Other bias | Unclear risk | Full access to data including screening log |
| Methods | Randomized controlled trial (2:1 scheme) with 2 parallel groups 1 centre | |
| Participants | Adults (n = 27) with ARDS and hospital‐ or community‐acquired pneumonia | |
| Interventions |
| |
| Outcomes | PRIMARY
SECONDARY
| |
| Notes | Study location: Egypt | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No explicit information in the manuscript |
| Allocation concealment (selection bias) | Unclear risk | No explicit information in the manuscript |
| Blinding (performance bias and detection bias) | Unclear risk | No explicit information in the manuscript |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | No information |
| Methods | Randomized controlled trial with 2 parallel groups 1 centre | |
| Participants | Adults (n = 40) with sepsis and not receiving vasopressor support | |
| Interventions |
| |
| Outcomes | PRIMARY
SECONDARY
| |
| Notes | Study location: Italy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization list |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) | High risk | Participants: no Care‐givers: no Data collectors: no Outcome assessors: no Data analysts: no |
| Incomplete outcome data (attrition bias) | Low risk | 12 of 52 participants dropped out of the study: 6 in the control group and 6 in the corticosteroid group; contact with the primary author permitted completion of follow‐up for all 12 participants |
| Selective reporting (reporting bias) | Low risk | Access to study protocol excluding any reporting bias |
| Other bias | Low risk | Full access to data including screening log |
| Methods | Randomized controlled trial 3 centres | |
| Participants | Adults (n = 80) admitted to ICU with community‐acquired pneumonia and sepsis | |
| Interventions |
| |
| Outcomes | PRIMARY
SECONDARY
| |
| Notes | Study location: Egypt | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information in the manuscript |
| Allocation concealment (selection bias) | Unclear risk | No information in the manuscript |
| Blinding (performance bias and detection bias) | Unclear risk | No information in the manuscript |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Unclear risk | No information in the manuscript |
| Other bias | Unclear risk | No information in the manuscript |
| Methods | Randomized controlled trial with 3 parallel groups 1 centre | |
| Participants | Adults (n = 172) with septic shock with positive blood culture | |
| Interventions |
Treatments might have been repeated once after 4 hours and had to be initiated at the time of diagnosis | |
| Outcomes | PRIMARY
SECONDARY
| |
| Notes | Study location: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomized card system |
| Allocation concealment (selection bias) | High risk | Unsealed envelopes |
| Blinding (performance bias and detection bias) | Unclear risk | Participants: yes Care‐givers: unclear Data collectors: unclear Outcome assessors: unclear Data analysts: unclear |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol |
| Other bias | Unclear risk | No data to exclude selection bias |
| Methods | Randomized controlled trial 2 centres | |
| Participants | African children (n = 72; 1 to 16 years of age) with sepsis or septic shock | |
| Interventions |
Treatments had to be initiated 5 to 10 minutes before first dose of antibiotic | |
| Outcomes | PRIMARY
SECONDARY
| |
| Notes | Study location: USA, Kenya and Nigeria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not given |
| Allocation concealment (selection bias) | Unclear risk | Unclear; not reported |
| Blinding (performance bias and detection bias) | Low risk | Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol |
| Other bias | Unclear risk | No data to exclude selection bias |
| Methods | Randomized controlled trial with 2 parallel groups 1 centre | |
| Participants | Adults (n = 213) with severe community‐acquired pneumonia | |
| Interventions |
| |
| Outcomes | PRIMARY
SECONDARY
| |
| Notes | Study location: The Netherlands | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Randomization list kept confidential by the pharmacist |
| Blinding (performance bias and detection bias) | Low risk | Pharmacist: no Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in study protocol are reported in final analysis |
| Other bias | Unclear risk | No access to full protocol |
| Methods | Randomized controlled trial with 3 parallel groups 2 centres | |
| Participants | Adults (n = 59) with vasopressor‐dependent septic shock | |
| Interventions |
Treatments might have been repeated once after 4 hours if shock persisted and had to be initiated at time of diagnosis | |
| Outcomes | PRIMARY
SECONDARY
| |
| Notes | Study location: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | High risk | At 1 centre, not clear how randomization list was kept confidential |
| Blinding (performance bias and detection bias) | High risk | Participants: yes at 1 centre, no at the other Care‐givers: yes at 1 centre, no at the other Data collectors: yes at 1 centre, no at the other Outcome assessors: yes at 1 centre, no at the other Data analysts: unclear University of Miami Research Committee did not allow study to be performed in a double‐blind manner, nor that participants received placebo |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol |
| Other bias | Unclear risk | No data to exclude selection bias |
| Methods | Randomized controlled trial with 2 parallel groups 52 centres | |
| Participants | Adults (n = 499) with septic shock | |
| Interventions |
| |
| Outcomes | PRIMARY
SECONDARY
| |
| Notes | Study locations: Europe and Israel | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding (performance bias and detection bias) | Low risk | Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) | High risk | Lost to follow‐up: none; 1 participant withdrew his consent Data for serious adverse events reported for only 466 of 499 participants, and analysis of these outcomes was performed per‐protocol, not by intent‐to‐treat |
| Selective reporting (reporting bias) | Low risk | Access to study protocol to confirm absence of reporting bias |
| Other bias | High risk | Only 500 participants included; expected sample size 800 participants |
| Methods | Randomized controlled trial with 2 parallel groups 1 centre | |
| Participants | Adults (n = 28) with septic shock and adrenal insufficiency | |
| Interventions |
| |
| Outcomes | PRIMARY
SECONDARY
| |
| Notes | Study location: India | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Randomization list kept confidential by the local pharmacist |
| Blinding (performance bias and detection bias) | Low risk | Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) | Unclear risk | Lost to follow‐up: unknown |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol |
| Other bias | Unclear risk | No data to exclude selection bias |
| Methods | Randomized controlled trial with 2 parallel groups 3 centres | |
| Participants | Adults (n = 61) with both severe CAP and high inflammatory response, defined as levels of C‐reactive protein > 15 mg/dL on admission | |
| Interventions |
| |
| Outcomes | PRIMARY
SECONDARY
| |
| Notes | Study location: Spain | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Randomization list kept confidential by the pharmacist |
| Blinding (performance bias and detection bias) | Low risk | Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Low risk | Access to full protocol and unpublished information |
| Other bias | Low risk | Access to full protocol and unpublished information |
| Methods | Randomized controlled trial on 2 parallel groups 1 centre | |
| Participants | Children (n = 38; 2 months to 12 years of age) with septic shock unresponsive to fluid therapy alone | |
| Interventions |
| |
| Outcomes | PRIMARY
SECONDARY
| |
| Notes | Study location: India | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No explicit information in the manuscript |
| Allocation concealment (selection bias) | Unclear risk | No explicit information in the manuscript |
| Blinding (performance bias and detection bias) | High risk | Open label |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | No information |
| Methods | Randomized controlled trial 10 centres | |
| Participants | Adults (n = 223) with sepsis or septic shock (n = 100) | |
| Interventions |
Treatment had to be initiated within 2 hours | |
| Outcomes | PRIMARY
SECONDARY
| |
| Notes | Study location: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding (performance bias and detection bias) | Low risk | Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol |
| Other bias | Unclear risk | No data to exclude selection bias |
| Methods | Randomized controlled trial 1 centre | |
| Participants | Adults (n = 40) with sepsis (n = 14), severe sepsis (n = 17) and septic shock (n = 9) | |
| Interventions |
| |
| Outcomes | PRIMARY
SECONDARY
| |
| Notes | Study location: Turkey | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Randomization list kept confidential by the pharmacist |
| Blinding (performance bias and detection bias) | Low risk | Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Unclear risk | No access to protocol |
| Other bias | Unclear risk | No data to exclude selection bias |
| Methods | Randomized controlled trial on 2 parallel groups 1 centre | |
| Participants | Adults (n = 55) with sepsis or septic shock | |
| Interventions |
| |
| Outcomes | PRIMARY
SECONDARY
Outcomes were also assessed in relation to adrenal insufficiency | |
| Notes | Study location: Turkey | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers used |
| Allocation concealment (selection bias) | Low risk | Randomization list kept by the pharmacist |
| Blinding (performance bias and detection bias) | Low risk | Pharmacist: no Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | No information |
Abbreviations:
APACHE II: Acute Physiology and Chronic Health Evaluation II.
ARDS: acute respiratory distress syndrome.
CAP: community acquired pneumonia.
FiO2: fractional inspired oxygen.
ICU: intensive care unit.
LIS: Lung Injury Scale score.
MOD: multiple organ dysfunction.
PaO2: arterial oxygen tension.
SOFA: sequential organ failure assessment
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Mixed population of critically ill patients; separate data on septic shock not available | |
| Patients with acute streptococcal infection This trial investigated effects of hydrocortisone on fever, anti‐streptolysin titers and onset of rheumatic fever. No data are reported for analysis of the various outcomes considered in this systematic review | |
| Only acute effects (within 1 hour) of methylprednisolone and/or naloxone on haemodynamic data were available; no data for any of the outcomes considered in this systematic review were reported | |
| In this study, participants were randomly assigned to receive hydrocortisone or its placebo for 24 hours only. Then, treatment with open‐labelled hydrocortisone was given at physicians' discretion. This study was aimed at exploring effects of hydrocortisone on immune cell function | |
| This study was not a randomized trial. Investigators did not describe how participants were allocated to experimental treatment | |
| This study was not a randomized trial. Participants were allocated to experimental treatment according to their hospital number | |
| Mixed population of critically ill patients; separate data on septic shock not available | |
| This trial included participants with late acute respiratory distress syndrome phase ‐ not those with septic shock | |
| This study included participants with community‐acquired pneumonia and explicitly excluded patients with sepsis, those needing admission to the intensive care unit and those requiring mechanical ventilation | |
| Study published only as an abstract; no contact with study authors was possible; incomplete information on primary and secondary outcomes | |
| Study published only as an abstract; no contact with study authors was possible; incomplete information on primary and secondary outcomes | |
| This study included severely burned patients without sepsis | |
| This study was not a randomized trial. Participants were allocated to experimental treatment according to their hospital numbers | |
| Mixed population of critically ill patients Separate data on septic shock not available |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | STEP |
| Methods | Multi‐centre. randomized, placebo‐controlled, 2‐parallel‐group study |
| Participants | 800 adult patients hospitalized with community‐acquired pneumonia |
| Interventions | Prednisone 50 mg per day for 7 days Placebo |
| Outcomes | PRIMARY
SECONDARY
|
| Starting date | December 2009 |
| Contact information | Mirjam Christ‐Crain; [email protected] |
| Notes |
| Trial name or title | VANISH |
| Methods | Multi‐centre, factorial (2 × 2), randomized, double‐blind, placebo‐controlled trial |
| Participants | 412 adult patients who require vasopressors for management of sepsis despite fluid resuscitation. In this trial, hydrocortisone or its placebo will be initiated only when participants will require the maximum dose of vasopressin or norepinephrine as defined in the protocol |
| Interventions |
Hydrocrotisone phosphate (50 mg, i.e. 0.5 mL) will be administered by intravenous injection 6‐hourly for 5 days, then tapered to 0.5 mL every 12 hours for days 6 to 8, 0.5 mL every 24 hours for days 9 to 11, then stopped |
| Outcomes | PRIMARY
SECONDARY
|
| Starting date | February 2013 |
| Contact information | Anthony Gordon; [email protected] |
| Notes | EudraCT 2011‐005363‐24; ISRCTN20769191 |
| Trial name or title | 6‐Methylprednisolone for multiple organ dysfunction syndrome |
| Methods | Randomized, double‐blind, placebo‐controlled 2‐parallel‐group study |
| Participants | Adults with persistent multiple organ dysfunction |
| Interventions | Intravenous administration of 6‐methylprednisolone or placebo for 32 days Loading dose of 160 mg followed by IV bolus q6 of 40 mg from day 1 to 14, 20 mg from day 15 to 21, 10 mg from day 22 to 28, 5 mg on days 29 and 30 and 2.5 mg on days 31 and 32 |
| Outcomes | PRIMARY
|
| Starting date | 01/08/2005 |
| Contact information | Miguel Sanchez; [email protected] |
| Notes | This trial has been halted for low recruitment rate and lack of funding |
| Trial name or title | Hydrocortisone versus hydrocortisone plus fludrocortisone for treatment of adrenal insufficiency in sepsis |
| Methods | Treatment, randomized, single‐blind, placebo‐controlled, parallel‐assignment efficacy study |
| Participants | Adults with sepsis and positive corticotropin test (basal cortisol ≤ 34 µg/dL and delta cortisol ≤ 9 µg/dL |
| Interventions | Hydrocortisone vs hydrocortisone plus fludrocortisone |
| Outcomes | 28‐Day mortality |
| Starting date | September 2006 |
| Contact information | Contact: John A. Bethea, PharmD 304‐388‐6260
Contact: Carol A. Morreale, PharmD 304‐388‐3767
|
| Notes | This study has never started to recruit patients |
| Trial name or title | Steroids in patients with early ARDS |
| Methods | Randomized, double‐blind, placebo‐controlled, 2‐parallel‐group safety/efficacy study |
| Participants | Adults with ARDS < 72 hours |
| Interventions | Low‐dose methylprednisolone vs placebo |
| Outcomes | PRIMARY
|
| Starting date | February 2008 |
| Contact information | Massimo Antonelli; [email protected] |
| Notes | This study has never started to recruit patients |
| Trial name or title | Activated protein C and corticosteroids for human septic shock (APROCCHS) |
| Methods | Randomized, double‐blind, placebo‐controlled trial ‐ 2 × 2 factorial design |
| Participants | Adults with septic shock |
| Interventions |
|
| Outcomes | 90‐Day mortality |
| Starting date | April 2008 |
| Contact information | Djillali Annane; telephone: 331 47 10 77 87; [email protected] |
| Notes |
| Trial name or title | Hydrocortisone for prevention of septic shock |
| Methods | Randomized, double‐blind, placebo‐controlled, 2‐parallel‐group efficacy study |
| Participants | Sepsis |
| Interventions | Hydrocortisone vs placebo |
| Outcomes | PRIMARY
|
| Starting date | 01/06/2008 |
| Contact information | Didier Keh; [email protected] |
| Notes |
| Trial name or title | Evaluation of corticosteroid therapy in childhood severe sepsis: a randomized pilot study |
| Methods | Randomized, open‐label, uncontrolled, 2‐parallel‐group study |
| Participants | Children with sepsis |
| Interventions | Hydrocortisone |
| Outcomes | PRIMARY
|
| Starting date | 01/04/2008 |
| Contact information | Saul N Faust; [email protected] |
| Notes |
| Trial name or title | ADRENAL |
| Methods | Multi‐centre, randomized, controlled, 2‐parallel‐group study |
| Participants | 3800 ICU adults with septic shock |
| Interventions | Hydrocortisone Placebo |
| Outcomes | PRIMARY
SECONDARY
|
| Starting date | February 2013 |
| Contact information | Bala Venkatesh; [email protected] |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 28‐Day all‐cause mortality Show forest plot | 27 | 3176 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.76, 1.00] |
| Analysis 1.1  Comparison 1 Steroids versus control, Outcome 1 28‐Day all‐cause mortality. | ||||
| 2 All‐cause mortality by subgroup based on mortality rate Show forest plot | 20 | 2570 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.86, 1.06] |
| Analysis 1.2 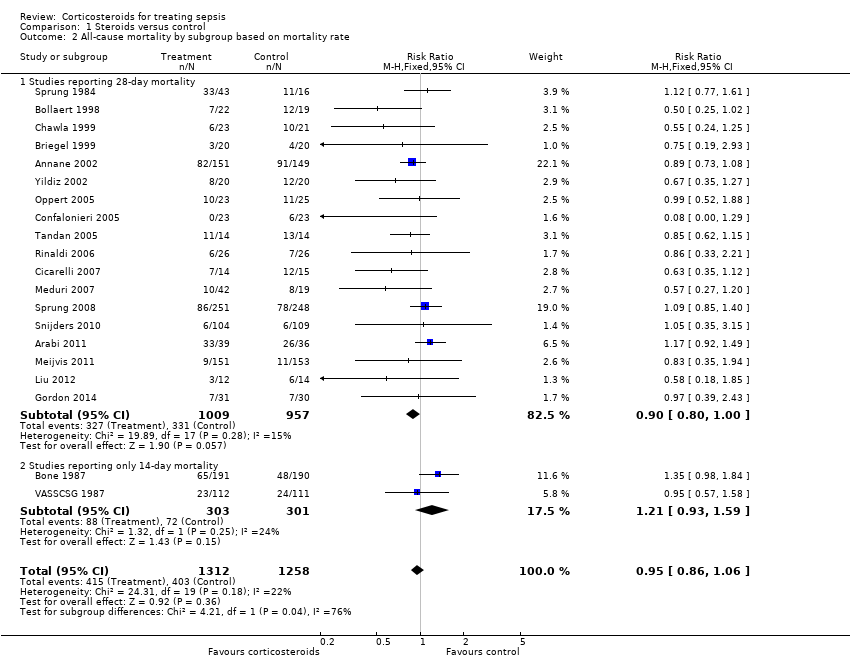 Comparison 1 Steroids versus control, Outcome 2 All‐cause mortality by subgroup based on mortality rate. | ||||
| 2.1 Studies reporting 28‐day mortality | 18 | 1966 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.80, 1.00] |
| 2.2 Studies reporting only 14‐day mortality | 2 | 604 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.93, 1.59] |
| 3 28‐Day all‐cause mortality by subgroups based on methodological quality Show forest plot | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Steroids versus control, Outcome 3 28‐Day all‐cause mortality by subgroups based on methodological quality. | ||||
| 3.1 Adequate generation of allocation sequence | 19 | 2342 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.86, 1.10] |
| 3.2 Adequate allocation concealment | 18 | 2283 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.84, 1.09] |
| 3.3 Blinded trials | 18 | 2259 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.84, 1.08] |
| 4 28‐Day all‐cause mortality by subgroups based on treatment dose/duration Show forest plot | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4 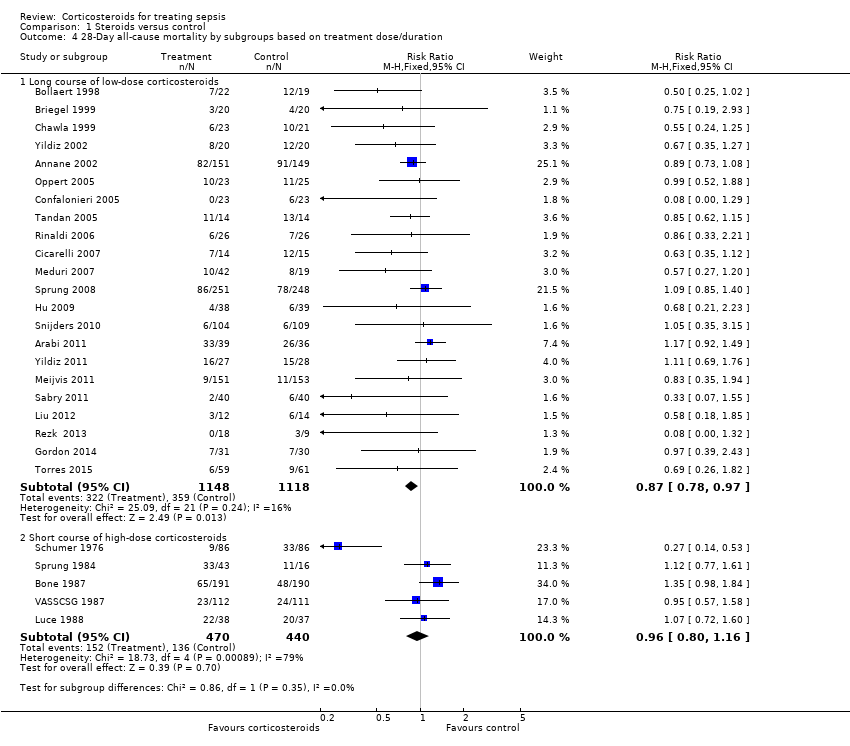 Comparison 1 Steroids versus control, Outcome 4 28‐Day all‐cause mortality by subgroups based on treatment dose/duration. | ||||
| 4.1 Long course of low‐dose corticosteroids | 22 | 2266 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.78, 0.97] |
| 4.2 Short course of high‐dose corticosteroids | 5 | 910 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.80, 1.16] |
| 5 28‐Day all‐cause mortality by subgroups based on targeted population Show forest plot | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Steroids versus control, Outcome 5 28‐Day all‐cause mortality by subgroups based on targeted population. | ||||
| 5.1 Sepsis | 6 | 826 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.91, 1.34] |
| 5.2 Septic shock only | 12 | 1444 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.78, 0.99] |
| 5.3 Sepsis and ARDS | 3 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.25, 0.85] |
| 5.4 Sepsis and community‐acquired pneumonia | 5 | 763 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.38, 1.02] |
| 6 28‐Day mortality in participants with critical illness‐related corticosteroid insufficiency Show forest plot | 8 | 583 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.76, 1.02] |
| Analysis 1.6  Comparison 1 Steroids versus control, Outcome 6 28‐Day mortality in participants with critical illness‐related corticosteroid insufficiency. | ||||
| 7 Intensive care unit mortality Show forest plot | 13 | 1463 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.68, 1.00] |
| Analysis 1.7  Comparison 1 Steroids versus control, Outcome 7 Intensive care unit mortality. | ||||
| 8 Hospital mortality Show forest plot | 17 | 2014 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.73, 0.98] |
| Analysis 1.8  Comparison 1 Steroids versus control, Outcome 8 Hospital mortality. | ||||
| 8.1 Long course of low‐dose corticosteroids | 14 | 1708 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.82, 1.01] |
| 8.2 Short course of high‐dose corticosteroids | 3 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.33, 1.60] |
| 9 Number of participants with shock reversal at day 7 Show forest plot | 12 | 1561 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.14, 1.51] |
| Analysis 1.9  Comparison 1 Steroids versus control, Outcome 9 Number of participants with shock reversal at day 7. | ||||
| 9.1 Shock reversal at day 7 in trials on long course of low‐dose corticosteroids | 10 | 1258 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [1.22, 1.46] |
| 9.2 Shock reversal at day 7 in trials on short course of high‐dose corticosteroids | 2 | 303 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.64, 1.79] |
| 10 Number of participants with shock reversal at 28 days Show forest plot | 7 | 1013 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [1.02, 1.21] |
| Analysis 1.10 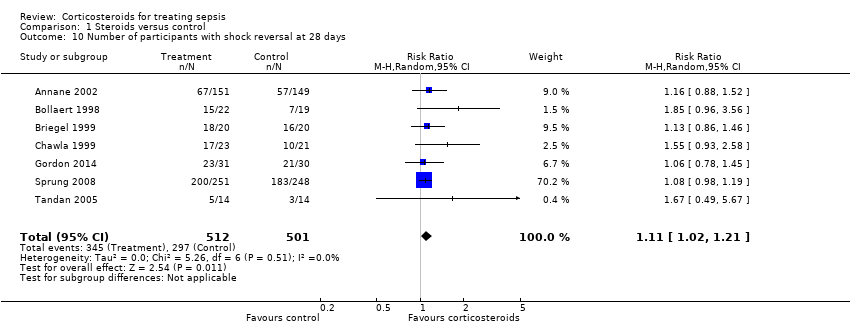 Comparison 1 Steroids versus control, Outcome 10 Number of participants with shock reversal at 28 days. | ||||
| 11 SOFA score at day 7 Show forest plot | 8 | 1132 | Mean Difference (IV, Random, 95% CI) | ‐1.53 [‐2.04, ‐1.03] |
| Analysis 1.11 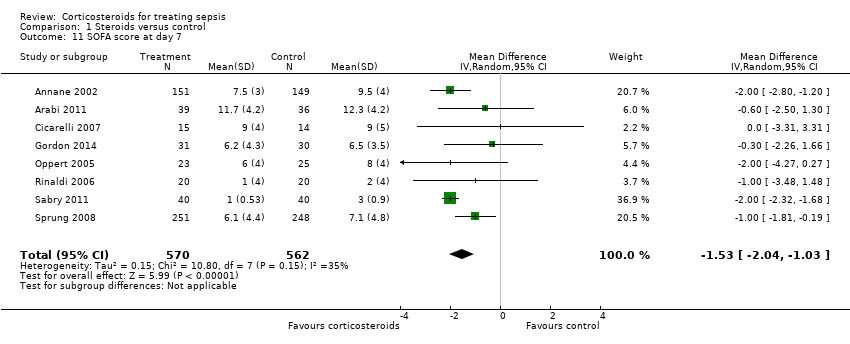 Comparison 1 Steroids versus control, Outcome 11 SOFA score at day 7. | ||||
| 12 Length of ICU stay for all participants Show forest plot | 12 | 1384 | Mean Difference (IV, Random, 95% CI) | ‐1.68 [‐3.27, ‐0.09] |
| Analysis 1.12  Comparison 1 Steroids versus control, Outcome 12 Length of ICU stay for all participants. | ||||
| 13 Length of ICU stay for survivors Show forest plot | 10 | 778 | Mean Difference (IV, Fixed, 95% CI) | ‐2.19 [‐3.93, ‐0.46] |
| Analysis 1.13  Comparison 1 Steroids versus control, Outcome 13 Length of ICU stay for survivors. | ||||
| 14 Length of hospital stay for all participants Show forest plot | 12 | 1802 | Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐2.55, 0.61] |
| Analysis 1.14 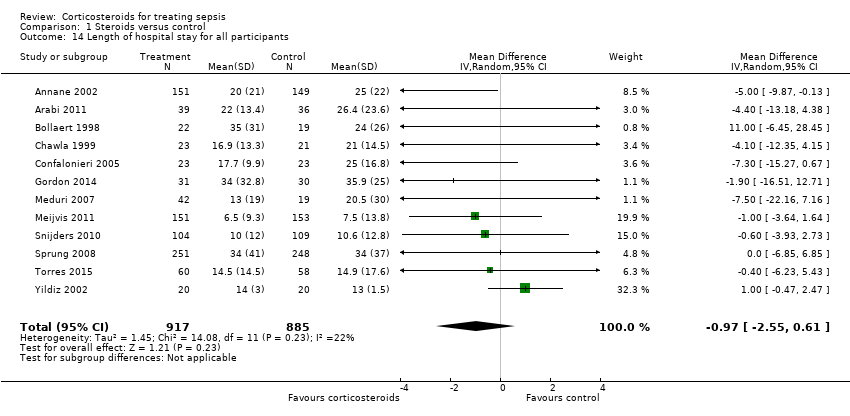 Comparison 1 Steroids versus control, Outcome 14 Length of hospital stay for all participants. | ||||
| 15 Length of hospital stay for survivors Show forest plot | 9 | 710 | Mean Difference (IV, Random, 95% CI) | ‐4.11 [‐8.50, 0.28] |
| Analysis 1.15  Comparison 1 Steroids versus control, Outcome 15 Length of hospital stay for survivors. | ||||
| 16 Number of participants with adverse events Show forest plot | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.16  Comparison 1 Steroids versus control, Outcome 16 Number of participants with adverse events. | ||||
| 16.1 Gastroduodenal bleeding | 19 | 2382 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.92, 1.67] |
| 16.2 Superinfections | 19 | 2567 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.87, 1.20] |
| 16.3 Hyperglycaemia | 13 | 2081 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.16, 1.37] |
| 16.4 Hypernatraemia | 3 | 805 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.28, 2.09] |
| 16.5 Neuromuscular weakness | 3 | 811 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.21, 1.88] |
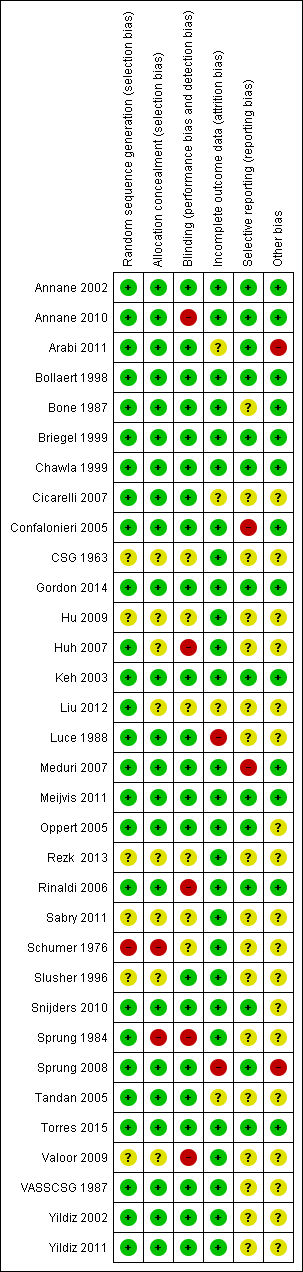
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Figure represents the results from meta‐regression of log of risk ratio of dying and log of the dose of corticosteroids given at day 1 and expressed as equivalent mg of hydrocortisone. Estimates from each study are represented by circles. Circle sizes depend on the precision of each estimate (the inverse of its within‐study variance), which is the weight given to each study in the fixed‐effect model.
Meta‐regression included 26 trials. The trial by Schummer et al was not included.
REML estimate of between‐study variance tau2 = .01078.
% residual variation due to heterogeneity: I2 res = 5.07%
Proportion of between‐study variance explained Adj R2 = 11.16%

Figure represents results from meta‐regression of log of risk ratio of dying and log of cumulated dose of corticosteroids expressed as equivalent mg of hydrocortisone. Estimates from each study are represented by circles. Circle sizes depend on the precision of each estimate (the inverse of its within‐study variance), which is the weight given to each study in the fixed‐effect model.
Meta‐regression included 26 trials. The trial by Schummer et al was not included.
REML estimate of between‐study variance tau2 = .01183
% residual variation due to heterogeneity I2 res = 6.99%
Proportion of between‐study variance explained Adj R2 = 2.49%
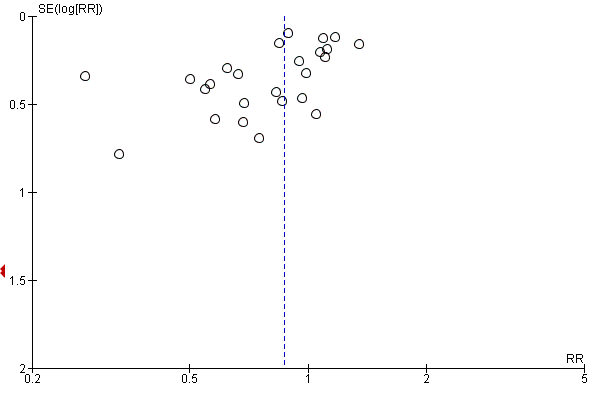
Funnel plot of comparison: 1 Steroids versus control, outcome: 1.1 28‐Day all‐cause mortality.

Contour‐enhanced funnel plot
Log of risk ratio for 28‐day mortality is plotted against its standard error

Comparison 1 Steroids versus control, Outcome 1 28‐Day all‐cause mortality.
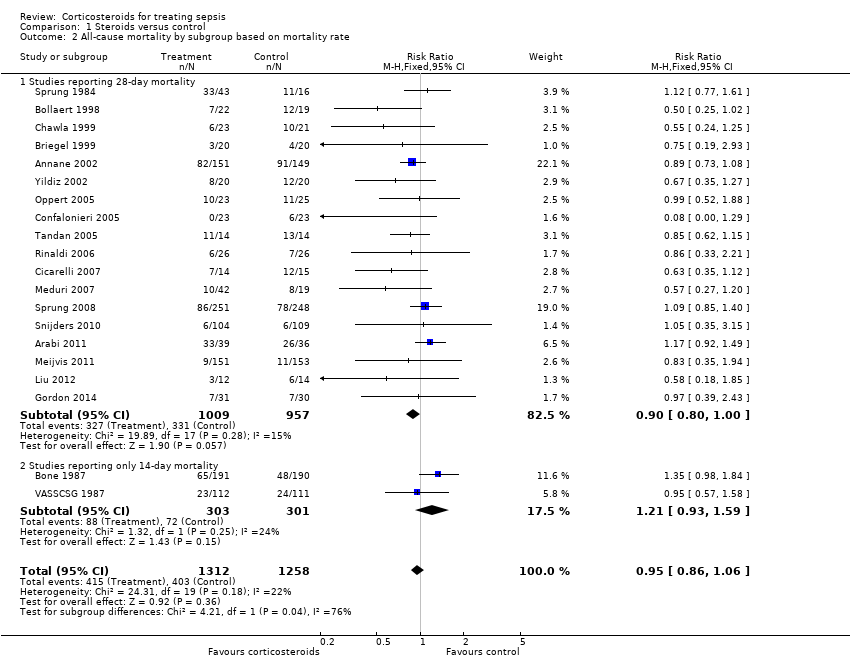
Comparison 1 Steroids versus control, Outcome 2 All‐cause mortality by subgroup based on mortality rate.

Comparison 1 Steroids versus control, Outcome 3 28‐Day all‐cause mortality by subgroups based on methodological quality.

Comparison 1 Steroids versus control, Outcome 4 28‐Day all‐cause mortality by subgroups based on treatment dose/duration.

Comparison 1 Steroids versus control, Outcome 5 28‐Day all‐cause mortality by subgroups based on targeted population.
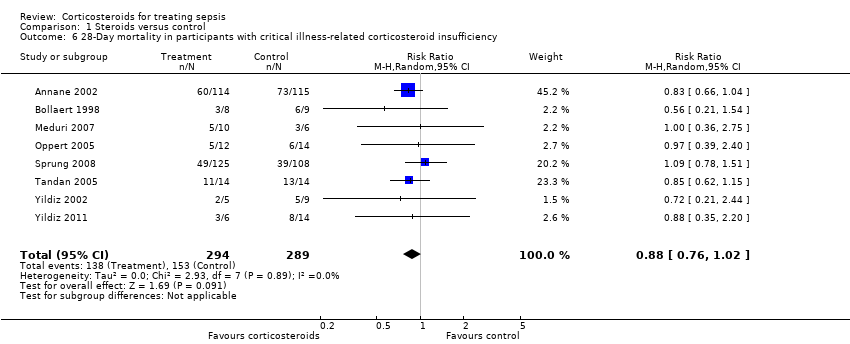
Comparison 1 Steroids versus control, Outcome 6 28‐Day mortality in participants with critical illness‐related corticosteroid insufficiency.
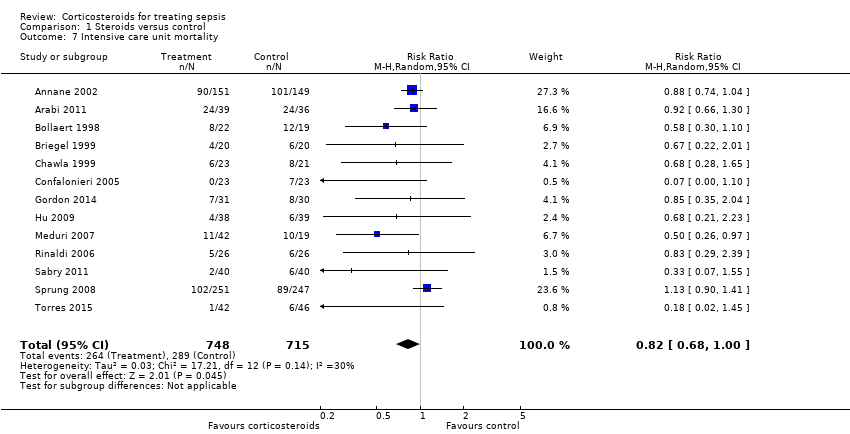
Comparison 1 Steroids versus control, Outcome 7 Intensive care unit mortality.
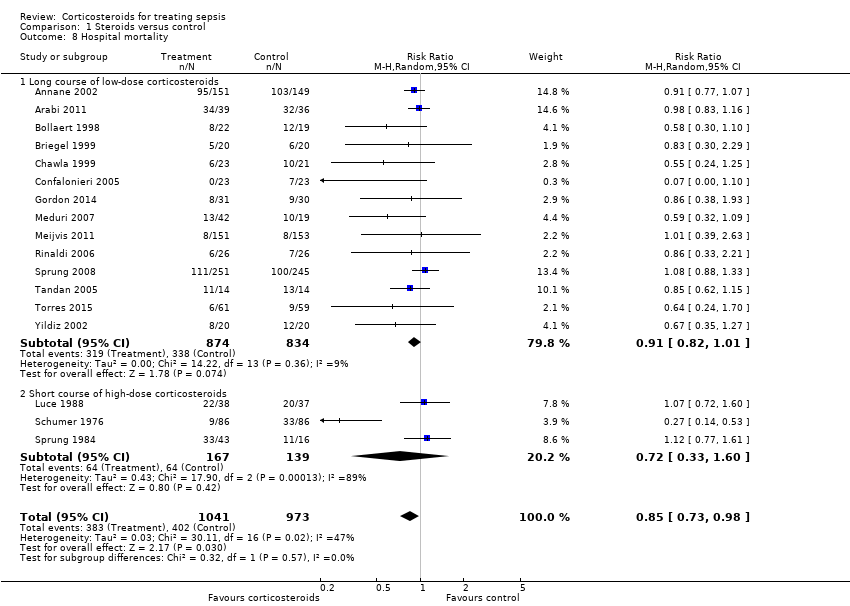
Comparison 1 Steroids versus control, Outcome 8 Hospital mortality.

Comparison 1 Steroids versus control, Outcome 9 Number of participants with shock reversal at day 7.

Comparison 1 Steroids versus control, Outcome 10 Number of participants with shock reversal at 28 days.

Comparison 1 Steroids versus control, Outcome 11 SOFA score at day 7.
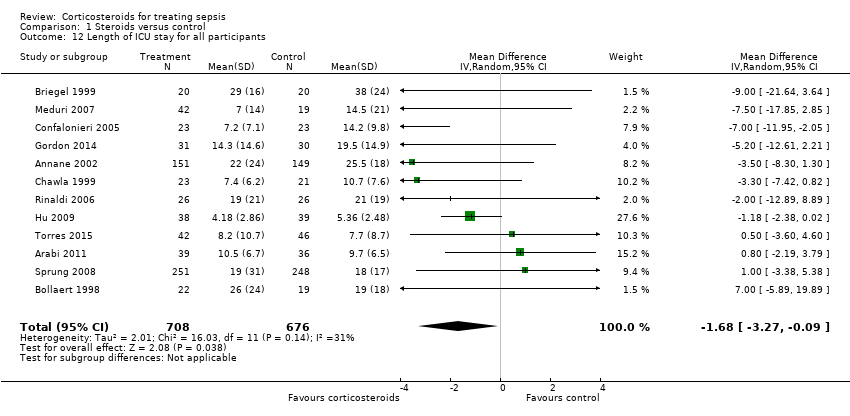
Comparison 1 Steroids versus control, Outcome 12 Length of ICU stay for all participants.

Comparison 1 Steroids versus control, Outcome 13 Length of ICU stay for survivors.

Comparison 1 Steroids versus control, Outcome 14 Length of hospital stay for all participants.
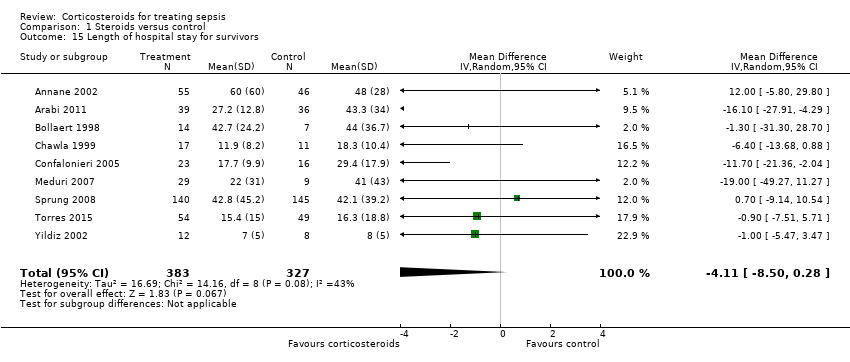
Comparison 1 Steroids versus control, Outcome 15 Length of hospital stay for survivors.

Comparison 1 Steroids versus control, Outcome 16 Number of participants with adverse events.
| Steroids versus control for treating sepsis | ||||||
| Patient or population: patients with sepsis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Steroids vs control | |||||
| 28‐Day all‐cause mortality | Study population | RR 0.87 | 3176 | ⊕⊕⊝⊝ | Trials were conducted over a period from 1976 to 2015. Differences in participant management and in the definition of sepsis were substantial <BR/>18 trial | |
| 318 per 1000 | 276 per 1000 | |||||
| 28‐Day all‐cause mortality by subgroups based on treatment dose/duration ‐ long course of low‐dose corticosteroids | Study population | RR 0.87 | 2266 | ⊕⊕⊕⊝ | Meta‐regression analysis also showed evidence of a dose response: Low doses were associated with better treatment response | |
| 321 per 1000 | 279 per 1000 | |||||
| Hospital mortality | Study population | RR 0.85 | 2014 | ⊕⊕⊕⊝ | Low doses of corticosteroids were associated with better treatment response | |
| 413 per 1000 | 351 per 1000 | |||||
| Number of participants with shock reversal at day 7 | Study population | RR 1.31 | 1561 | ⊕⊕⊕⊕ | Low doses of corticosteroids were associated with better treatment response | |
| 523 per 1000 | 685 per 1000 | |||||
| SOFA score at day 7 | Mean SOFA score at day 7 in intervention groups was | 1132 | ⊕⊕⊕⊕ | Observed reduction in SOFA score was of a magnitude that exceeded any reduction seen with any other treatment for sepsis | ||
| Length of ICU stay for survivors | Mean length of ICU stay for survivors in intervention groups was | 778 | ⊕⊕⊕⊕ | Observed reduction in length of ICU stay was of a magnitude that exceeded any reduction seen with any other treatment for sepsis | ||
| Length of hospital stay for survivors | Mean length of hospital stay for survivors in intervention groups was | 710 | ⊕⊕⊕⊝ | Observed reduction in length of hospital stay was of a magnitude that exceeded any reduction seen with any other treatment for sepsis | ||
| Number of participants with adverse events ‐ superinfections | Study population | RR 1.02 | 2567 | ⊕⊕⊕⊕ | One large trial suggested increased risk of new sepsis with corticosteroids | |
| 161 per 1000 | 164 per 1000 | |||||
| Number of participants with adverse events ‐ hyperglycaemia | Study population | RR 1.26 | 2081 | ⊕⊕⊕⊕ | One trial suggested that risk of hyperglycaemia was lower when corticosteroids were given as a continuous perfusion than when they were given as an intravenous bolus | |
| 348 per 1000 | 438 per 1000 | |||||
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aQuality of evidence was downgraded by 1 point owing to some inconsistency; 1 of the 2 largest trials showed no survival benefit | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 28‐Day all‐cause mortality Show forest plot | 27 | 3176 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.76, 1.00] |
| 2 All‐cause mortality by subgroup based on mortality rate Show forest plot | 20 | 2570 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.86, 1.06] |
| 2.1 Studies reporting 28‐day mortality | 18 | 1966 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.80, 1.00] |
| 2.2 Studies reporting only 14‐day mortality | 2 | 604 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.93, 1.59] |
| 3 28‐Day all‐cause mortality by subgroups based on methodological quality Show forest plot | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Adequate generation of allocation sequence | 19 | 2342 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.86, 1.10] |
| 3.2 Adequate allocation concealment | 18 | 2283 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.84, 1.09] |
| 3.3 Blinded trials | 18 | 2259 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.84, 1.08] |
| 4 28‐Day all‐cause mortality by subgroups based on treatment dose/duration Show forest plot | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Long course of low‐dose corticosteroids | 22 | 2266 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.78, 0.97] |
| 4.2 Short course of high‐dose corticosteroids | 5 | 910 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.80, 1.16] |
| 5 28‐Day all‐cause mortality by subgroups based on targeted population Show forest plot | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Sepsis | 6 | 826 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.91, 1.34] |
| 5.2 Septic shock only | 12 | 1444 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.78, 0.99] |
| 5.3 Sepsis and ARDS | 3 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.25, 0.85] |
| 5.4 Sepsis and community‐acquired pneumonia | 5 | 763 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.38, 1.02] |
| 6 28‐Day mortality in participants with critical illness‐related corticosteroid insufficiency Show forest plot | 8 | 583 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.76, 1.02] |
| 7 Intensive care unit mortality Show forest plot | 13 | 1463 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.68, 1.00] |
| 8 Hospital mortality Show forest plot | 17 | 2014 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.73, 0.98] |
| 8.1 Long course of low‐dose corticosteroids | 14 | 1708 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.82, 1.01] |
| 8.2 Short course of high‐dose corticosteroids | 3 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.33, 1.60] |
| 9 Number of participants with shock reversal at day 7 Show forest plot | 12 | 1561 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.14, 1.51] |
| 9.1 Shock reversal at day 7 in trials on long course of low‐dose corticosteroids | 10 | 1258 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [1.22, 1.46] |
| 9.2 Shock reversal at day 7 in trials on short course of high‐dose corticosteroids | 2 | 303 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.64, 1.79] |
| 10 Number of participants with shock reversal at 28 days Show forest plot | 7 | 1013 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [1.02, 1.21] |
| 11 SOFA score at day 7 Show forest plot | 8 | 1132 | Mean Difference (IV, Random, 95% CI) | ‐1.53 [‐2.04, ‐1.03] |
| 12 Length of ICU stay for all participants Show forest plot | 12 | 1384 | Mean Difference (IV, Random, 95% CI) | ‐1.68 [‐3.27, ‐0.09] |
| 13 Length of ICU stay for survivors Show forest plot | 10 | 778 | Mean Difference (IV, Fixed, 95% CI) | ‐2.19 [‐3.93, ‐0.46] |
| 14 Length of hospital stay for all participants Show forest plot | 12 | 1802 | Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐2.55, 0.61] |
| 15 Length of hospital stay for survivors Show forest plot | 9 | 710 | Mean Difference (IV, Random, 95% CI) | ‐4.11 [‐8.50, 0.28] |
| 16 Number of participants with adverse events Show forest plot | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 Gastroduodenal bleeding | 19 | 2382 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.92, 1.67] |
| 16.2 Superinfections | 19 | 2567 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.87, 1.20] |
| 16.3 Hyperglycaemia | 13 | 2081 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.16, 1.37] |
| 16.4 Hypernatraemia | 3 | 805 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.28, 2.09] |
| 16.5 Neuromuscular weakness | 3 | 811 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.21, 1.88] |


