Metilxantinas para las exacerbaciones de la enfermedad pulmonar obstructiva crónica
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Type: Parallel group. | |
| Participants | Setting: Medical ward. | |
| Interventions | Experimental: longacting oral theophylline (Neulin‐24, 3M) 200mg or greater titrated to serum theo level of 10‐20 mg/L | |
| Outcomes | Analysed: Change in FEV1 at 3 days, length‐of‐stay, change in symptom scores, adverse effects. Reported: Change in FVC, Sa02 | |
| Notes | Likelihood of COPD: Stringent spirometry criteria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
| Methods | Type: Parallel group. | |
| Participants | Setting: ED/medical walk‐in. | |
| Interventions | Experimental: IV aminophylline 0‐6mg/kg load (based on prior theo use), 0.5mg/kg maintenance infusion for level of 72‐94 umol/L (abstract lists 72‐83 umol/L). | |
| Outcomes | Analysed: Change in FEV1, dyspnea index, adverse effects. | |
| Notes | Likelihood of COPD: Stringent spirometry criteria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
| Methods | Type: Parallel group. | |
| Participants | Setting: ED. | |
| Interventions | Experimental: IV aminophylline 2.8‐5.6mg/kg over 1 hour (based on prior theophylline exposure). | |
| Outcomes | Analysed: Change in FEV1 at 2 hours, returns to ED in one week. | |
| Notes | Likelihood of COPD: Clinical diagnosis of chronic bronchitis, baseline FEV1 0.8 L. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
| Methods | Type: Parallel group. | |
| Participants | Setting: ED. | |
| Interventions | Experimental: intravenous aminophylline 5.6mg/kg over 20 minutes, then 0.9mg/kg constant infusion. | |
| Outcomes | Analysed: Change in FEV1, PEFR, hospitalizations, return to ED in 3 days, adverse effects | |
| Notes | Likelihood of COPD: No prior PFT data; likely some misclassification with asthma since asthma/COPD subgroups established post hoc. Hospitalization decision made by non‐investigator with prespecified criteria. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not randomised | |
| Stable COPD | |
| Relevant endpoints not reported | |
| Not randomised | |
| Crossover design not appropriate for assessment of treatment of exacerbations | |
| Stable COPD | |
| Stable COPD | |
| Asthma | |
| No placebo group | |
| No placebo group | |
| Stable COPD | |
| Asthma | |
| Not randomised | |
| Unclear if randomised; stabilised exacerbation | |
| Stable COPD | |
| Not randomised | |
| Stable COPD | |
| No intervention | |
| Stable COPD | |
| Stable COPD | |
| Stable COPD | |
| Stable COPD | |
| Stable COPD | |
| Stable COPD | |
| Stable COPD | |
| No placebo group |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in FEV1 (ml) at 2 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Effect of methylxanthines on FEV1, Outcome 1 Change in FEV1 (ml) at 2 hours. | ||||
| 2 Change in FEV1 (ml) at 3 days Show forest plot | 2 | 78 | Mean Difference (IV, Fixed, 95% CI) | 101.13 [25.61, 176.65] |
| Analysis 1.2  Comparison 1 Effect of methylxanthines on FEV1, Outcome 2 Change in FEV1 (ml) at 3 days. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Admissions among emergency department patients Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Effect of methylxanthines on clinical endpoints, Outcome 1 Admissions among emergency department patients. | ||||
| 2 Emergency department returns within one week Show forest plot | 2 | 91 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.45, 5.15] |
| Analysis 2.2  Comparison 2 Effect of methylxanthines on clinical endpoints, Outcome 2 Emergency department returns within one week. | ||||
| 3 Difference in hospital length‐of‐stay (days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 Effect of methylxanthines on clinical endpoints, Outcome 3 Difference in hospital length‐of‐stay (days). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion with improvement in symptom score within hours Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Effect of methylxanthines on symptom scores, Outcome 1 Proportion with improvement in symptom score within hours. | ||||
| 2 Change in symptoms score at 3 days Show forest plot | 2 | 78 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.36 [‐5.11, 2.40] |
| Analysis 3.2  Comparison 3 Effect of methylxanthines on symptom scores, Outcome 2 Change in symptoms score at 3 days. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Effect of methyl‐xanthines on nausea/vomiting Show forest plot | 3 | 117 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.62 [1.70, 12.56] |
| Analysis 4.1  Comparison 4 Adverse effects, Outcome 1 Effect of methyl‐xanthines on nausea/vomiting. | ||||
| 2 Effect of methylxanthines on tremor Show forest plot | 3 | 117 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.73, 4.56] |
| Analysis 4.2  Comparison 4 Adverse effects, Outcome 2 Effect of methylxanthines on tremor. | ||||
| 3 Effect of methylxanthines on palpitations/arrhythmias Show forest plot | 2 | 89 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.14 [0.87, 19.61] |
| Analysis 4.3  Comparison 4 Adverse effects, Outcome 3 Effect of methylxanthines on palpitations/arrhythmias. | ||||

Comparison 1 Effect of methylxanthines on FEV1, Outcome 1 Change in FEV1 (ml) at 2 hours.

Comparison 1 Effect of methylxanthines on FEV1, Outcome 2 Change in FEV1 (ml) at 3 days.
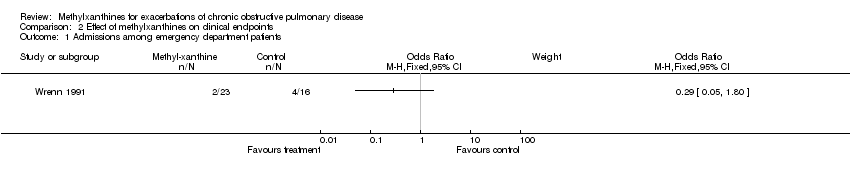
Comparison 2 Effect of methylxanthines on clinical endpoints, Outcome 1 Admissions among emergency department patients.

Comparison 2 Effect of methylxanthines on clinical endpoints, Outcome 2 Emergency department returns within one week.

Comparison 2 Effect of methylxanthines on clinical endpoints, Outcome 3 Difference in hospital length‐of‐stay (days).
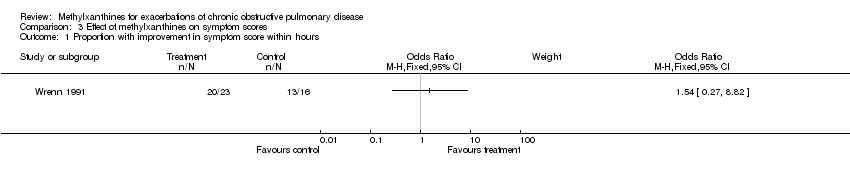
Comparison 3 Effect of methylxanthines on symptom scores, Outcome 1 Proportion with improvement in symptom score within hours.

Comparison 3 Effect of methylxanthines on symptom scores, Outcome 2 Change in symptoms score at 3 days.

Comparison 4 Adverse effects, Outcome 1 Effect of methyl‐xanthines on nausea/vomiting.

Comparison 4 Adverse effects, Outcome 2 Effect of methylxanthines on tremor.

Comparison 4 Adverse effects, Outcome 3 Effect of methylxanthines on palpitations/arrhythmias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in FEV1 (ml) at 2 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2 Change in FEV1 (ml) at 3 days Show forest plot | 2 | 78 | Mean Difference (IV, Fixed, 95% CI) | 101.13 [25.61, 176.65] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Admissions among emergency department patients Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Emergency department returns within one week Show forest plot | 2 | 91 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.45, 5.15] |
| 3 Difference in hospital length‐of‐stay (days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion with improvement in symptom score within hours Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Change in symptoms score at 3 days Show forest plot | 2 | 78 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.36 [‐5.11, 2.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Effect of methyl‐xanthines on nausea/vomiting Show forest plot | 3 | 117 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.62 [1.70, 12.56] |
| 2 Effect of methylxanthines on tremor Show forest plot | 3 | 117 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.73, 4.56] |
| 3 Effect of methylxanthines on palpitations/arrhythmias Show forest plot | 2 | 89 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.14 [0.87, 19.61] |

