Surfactante para el síndrome de aspiración de meconio en recién nacidos a término y prematuros tardíos
Información
- DOI:
- https://doi.org/10.1002/14651858.CD002054.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 14 diciembre 2014see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Neonatología
- Copyright:
-
- Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Amr I El Shahed: Updated the search for articles, extracted data, reviewed results, and wrote the text of the review.
Peter Dargaville: Performed the original search for articles, extracted data, reviewed results, and edited the text of the review.
Arne Ohlsson: Updated the search for articles, extracted data, reviewed results, and wrote the text of the review.
Roger F. Soll: Performed the original search for articles, extracted data, reviewed results, and edited the text of the updated review
The November 2014 update was conducted centrally by the Cochrane Neonatal Review Group staff (Yolanda Brosseau, Diane Haughton, and Roger Soll). This 2014 update was reviewed, revised and approved by Amr I El Shahed and Arne Ohlsson.
Sources of support
Internal sources
-
Department of Paediatrics, Mount Sinai Hospital, Toronto, Canada.
External sources
-
The Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN275201100016C, USA.
Declarations of interest
Dr R. Soll has acted as a consultant and invited speaker for several of the pharmaceutical companies which manufacture or distribute surfactant preparations (Abbott Laboratories, Ross Laboratories, Chiesi Pharmaceuticals, Dey Laboratories, Burroughs Wellcome) but has not done so for over 6 years. Dr P. Dargaville has received support for basic science research in surfactant lavage studies from Abbott Australasia. Neither Dr El Shahed nor Dr Ohlsson have conflicts of interest to disclose.
Acknowledgements
For the original review we would like to thank Ms Elizabeth Zola, PharmD, for providing diagnosis‐specific data from the Survanta in Term Infants Study Group. .
For the 2007 update of this review we thank Ms Elizabeth Uleryk, Chief Librarian, the Hospital for Sick Children, Toronto, Ontario, Canada, for developing the search strategy for the literature retrieval. We also thank Dr Andres Maturana for providing us with unpublished data from his study, previously published in abstract form only
For the current update, Ms Yolanda R Brosseau, Managing Editor, Cochrane Neonatal Review Group and Ms Colleen M Ovelman, Trial Search Co‐ordinator and Webmaster, Cochrane Neonatal Review Group, conducted the literature searches in November 2014. One of the authors (AO) conducted searches of clinical trails registries in July 2013 and this was updated again in November 2014.
Version history
| Published | Title | Stage | Authors | Version |
| 2014 Dec 14 | Surfactant for meconium aspiration syndrome in term and late preterm infants | Review | Amr I El Shahed, Peter A Dargaville, Arne Ohlsson, Roger Soll | |
| 2007 Jul 18 | Surfactant for meconium aspiration syndrome in full term/near term infants | Review | Amr I El Shahed, Peter A. Dargaville, Arne Ohlsson, Roger Soll | |
| 2000 Apr 24 | Surfactant for meconium aspiration syndrome in full term infants | Review | Roger F Soll, Peter A. Dargaville | |
Differences between protocol and review
Additional outcomes sought for the update in 2014:
1. Death or chronic lung disease at 28 days
2. Death or chronic lung disease at 36 weeks postmenstrual age
3. Neurodevelopmental follow‐up
However, these outcomes were not available in included studies.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans; Infant, Newborn;
PICO

Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 1 Mortality.

Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 2 Treatment with ECMO.

Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 3 Pneumothorax.

Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 4 Pulmonary interstitial emphysema.

Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 5 Air leaks (pneumothorax, pneumomediastinum, interstitial emphysema).
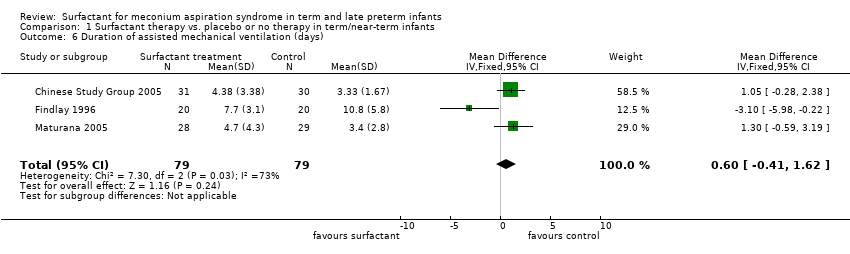
Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 6 Duration of assisted mechanical ventilation (days).

Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 7 Duration of supplemental oxygen (days).

Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 8 Need for supplemental oxygen at discharge.
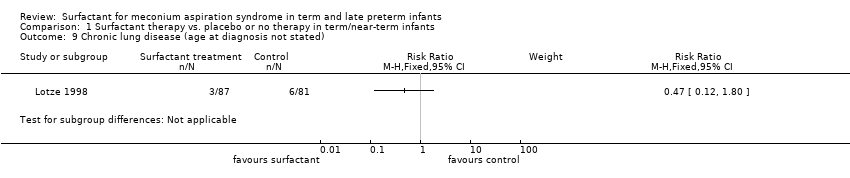
Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 9 Chronic lung disease (age at diagnosis not stated).

Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 10 Intraventricular haemorrhage (any grade).

Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 11 Severe intraventricular haemorrhage.

Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 12 Duration of hospital stay (days).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 4 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.41, 2.39] |
| 2 Treatment with ECMO Show forest plot | 2 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.46, 0.91] |
| 3 Pneumothorax Show forest plot | 3 | 269 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.08, 0.05] |
| 4 Pulmonary interstitial emphysema Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5 Air leaks (pneumothorax, pneumomediastinum, interstitial emphysema) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6 Duration of assisted mechanical ventilation (days) Show forest plot | 3 | 158 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.41, 1.62] |
| 7 Duration of supplemental oxygen (days) Show forest plot | 2 | 97 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐2.83, 3.64] |
| 8 Need for supplemental oxygen at discharge Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9 Chronic lung disease (age at diagnosis not stated) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10 Intraventricular haemorrhage (any grade) Show forest plot | 2 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.31, 1.46] |
| 11 Severe intraventricular haemorrhage Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12 Duration of hospital stay (days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |

