Bisfosfonati za osteoporozu kod osoba s cističnom fibrozom
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Study characteristics | ||
| Methods | Randomised controlled trial, parallel design. Trial duration 2 years. Single centre, university hospital, USA. | |
| Participants | Inclusion criteria: CF; 1 to 12 months post‐lung transplantation; ambulatory. Total participants: n = 34 (17 female). Control group: n = 18 (10 female); mean (SD) age 29.1 years (6.4 years). Groups similar in age, gender, baseline T‐scores, renal function, hospitalisation rates, immunosuppressant levels, change in lung function and BMI over study period. 13 in treatment group and 12 controls had baseline T‐scores < ‐2.5 at a minimum of 1 site; all others ‐1 < T < ‐2.5 at a minimum of 1 site. | |
| Interventions | Treatment group: intravenous pamidronate (30 mg every 3 months) plus oral vitamin D (800 IU/day) and oral calcium (1 g/day). Control group: oral vitamin D (800 IU/day) and oral calcium (1 g/day). | |
| Outcomes | Primary outcome
Secondary outcomes
| |
| Notes | 44 people with CF were eligible during the course of this study, 7 died immediately post‐operatively and were therefore not eligible for this trial. As outlined above, 3 people died during the course of the study before the first primary endpoint measurement. 34 people were included in the final analyses. Funding was provided by grants from the Cystic Fibrosis Foundation and the Verne S Caviness General Center for Clinical Research. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Blocks of four" design stated (stratified on basis of gender and severity of osteoporosis using spine Z score of ‐3.0), but actual method of randomisation is not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) | High risk | Person(s) responsible for participants care and participants were not blinded. Of outcome assessors, only the radiologist who interpreted the DXA scans was blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | It was described that 3 participants died during the course of the study before the first primary endpoint measurement (causes of death were 1 each from sepsis, acute respiratory distress syndrome and obliterative bronchiolitis). These participants were excluded from the final analysis of baseline characteristics and outcome data. However, it was not reported which treatment group they were in. |
| Selective reporting (reporting bias) | High risk | Serum and urine biochemical measurements that were measured at 2 days (only after first pamidronate infusion in intervention group) were not reported. |
| Other bias | Unclear risk | None identified. |
| Study characteristics | ||
| Methods | Randomised controlled trial; parallel design; double‐blind placebo‐controlled. Trial duration 1 year for primary outcome measure (trial was intended to be 2 years duration). Single centre, adult CF centre, USA. | |
| Participants | Inclusion criteria: CF; ambulatory; DXA showed a spine or femur T‐score of ‐1 or less. 101 participants consented to be screened, 86 qualified and 53 started protocol and were randomised. Total participants: n = 48 (23 female). Control group: n = 24 (14 female); mean (SD) age 27 years (9 years). At baseline, osteoporosis was found in 3 participants and osteopenia was present in 20 participants in both the treatment and control groups. | |
| Interventions | Treatment group: oral alendronate (10 mg daily) plus oral vitamin D (800 IU/day) and oral calcium carbonate (1000 mg/day). Control group: oral vitamin D (800 IU/day) and oral calcium carbonate (1000 mg/day). | |
| Outcomes | Primary outcome
Secondary outcomes
| |
| Notes | Funded by the US Food and Drug Administration, Merck and Co. Inc., the Clinical Nutrition Research Unit, the Verne S Caviness General Center for Clinical Research at University of North Carolina, the Cystic Fibrosis Foundation and the National Institute of Diabetes and Digestive and Kidney Diseases. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Blocks of four" design stated, but actual method of randomisation is not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) | Unclear risk | Described as "double‐blind". Participants: blinded. Clinicians or persons delivering treatment: unclear if clinicians involved in the study and clinicians managing the medical problems of the participants were all blinded. Outcome assessors: stated that the musculoskeletal radiologist who analysed baseline and end‐of‐study chest radiographs for fracture was blinded, not specifically stated that other outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | High risk | Withdrawals up to 6 months: 5 withdrawals in total, but not stated whether they were in treatment or control group, so 48 participants were evaluable. Reasons for dropping out: pregnancy (n = 1); diarrhoea and weight loss (n = 3); dysphagia (n = 1). The 3 participants with diarrhoea reported abdominal cramping, loss of appetite, and diarrhoea before the medications began that worsened during the study but persisted after the study medications were discontinued, 1 participant was on alendronate and 2 on placebo. Withdrawals between 6 and 12 months: 4 withdrawals from each group were described. Treatment group: 4 dropouts; reasons were: transplanted (n = 1); moved (n = 2); non‐compliance (n = 1). Withdrawals between 1 and 2 years: The primary endpoint measure was analysed in 40/53 (75%) participants, hence there is a risk of attrition bias. Stated that an intention‐to‐treat principle was used in the analyses of the treatment endpoints. |
| Selective reporting (reporting bias) | Low risk | Outcome measures that were described in the methods section were reported in the results section. |
| Other bias | High risk | Described that protocol was originally designed to be 2 years in length, but few participants were willing to consent to such a lengthy study, so protocol was revised to measure the primary endpoint at 12 months. |
| Study characteristics | ||
| Methods | 2 phases: 1. observational phase ‐ 12‐month open‐label trial; followed by 2. intervention phase ‐ 12‐month, parallel, double‐blind randomised, placebo‐controlled trial (included some participants from the observational phase dependent upon criteria as below). Multicentre: 10 CF regional centres in Italy. | |
| Participants | Observational phase
Total participants: n = 171 (84 female). Intervention phase
Total participants: n = 128 (63 female). | |
| Interventions | Observational phase (12 months) All participants:
Interventional phase (12 months)
| |
| Outcomes | Primary outcome
Secondary outcomes
| |
| Notes | Funded by Telethon Foundation (Italy). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The random allocation was undertaken centrally by a statistician using a computer‐generated list of random numbers. |
| Allocation concealment (selection bias) | Low risk | For each participant enrolled in the randomised phase, the centre of enrolment and participant code, age, and sex were sent to the co‐ordinating centre, which allocated the participant to the alendronate group (odd numbers) or placebo group (even numbers) and sent an unlabelled drug package for that participant. There were 10 centres involved, hence allocation would have been concealed. |
| Blinding (performance bias and detection bias) | Low risk | Participants, caregivers and the co‐ordinator were masked to treatment allocation until completion of the trial. The authors confirmed that outcome assessors (nuclear medicine physicians and radiologists) were also blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Every participant who was enrolled in the randomised phase of the study completed the study and their data were analysed and reported. There were no withdrawals and no participants lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Details of bone density and vertebral radiographs and biochemistry (bone turnover markers, serum calcium, PTH, 25OH vitamin D, 1,25 vitamin D and urinary calcium, phosphate) were reported. Details of other biochemistry were not reported in details but stated that "no relevant biochemical or haematological anomalies"). |
| Other bias | Unclear risk | There was a 12‐month observation phase in which all participants received counselling to adjust their dietary calcium intake to the recommended daily allowance, they were prescribed oral calcifediol (15 ug/day if bodyweight was under 20 kg; 25 ug/day if bodyweight was between 20 kg and 30 kg; and 35 ug/day if bodyweight was over 30 kg) and previous vitamin D supplementation (cholecalciferol 400 IU/day) was withdrawn (Bianchi 2013). The trial only included those participants in the intervention phase who did not have an increase in bone mineral apparent density by 5% or more after 12 months in the observational phase. If these participants were also included, it is possible the results of the antiresorptive intervention would have shown an even greater positive effect. |
| Study characteristics | ||
| Methods | Randomised, double‐blinded, placebo‐controlled trial; parallel design. Trial duration 6 months (originally intended for 12 months). Unclear if single or multicentre trial. | |
| Participants | Inclusion criteria: CF; osteopenia of the lumbar spine (T‐scores ‐1.0 to ‐2.5); serum 25‐hydroxyvitamin D levels ≥ 20 ng/ml prior to infusion. N = 40 planned for enrolment but only 5 enroled (3 in treatment group, gender split not provided) before study stopped by Data and Safety Monitoring Board (see notes). | |
| Interventions | Treatment group: intravenous zoledronate, 5 mg infusion administered on a single occasion over 20 minutes plus supplemental oral vitamin D (800 IU) and oral calcium (1000 mg) daily. Control group: supplemental oral vitamin D (800 IU) and oral calcium (1000 mg) daily. | |
| Outcomes |
| |
| Notes | The study was stopped by its Data and Safety Monitoring Board after 3 participants experienced dramatic musculoskeletal pain, 2 requiring emergency room assessment. Symptoms began 6 to 8 hours after infusion, peaked at 12 to 18 hours, and were characterised by severe chest and back pain. Along with musculoskeletal pain, 1 participant also experienced a fever of 104° F lasting for several hours and a rise in tumour necrosis factor‐α. Although the most severe symptoms resolved within 48 to 72 hours, participants reported continued arthralgias for up to a week. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but process not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) | Unclear risk | Described as "double‐blind". Participants: blinded. Not discussed if clinicians or persons delivering treatment and outcome assessors were both blinded. |
| Incomplete outcome data (attrition bias) | High risk | Based on interpretation of data, we have presumed that the 3 participants who had severe bone pain were the 3 in the treatment group. Clarification from the author was requested but not received. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only but outcome measures were described in the results. Did not report on fractures as an outcome however, which is curious for a study on bisphosphonate therapy to improve BMD, even to report that none occurred. |
| Other bias | High risk | The study was stopped by its Data and Safety Monitoring Board after 3 participants experienced dramatic musculoskeletal pain, 2 requiring emergency room assessment. Symptoms began 6 to 8 hours after infusion, peaked at 12 to 18 hours, and were characterised by severe chest and back pain. Along with musculoskeletal pain, 1 participant also experienced a fever of 104° F lasting for several hours and a rise in tumour necrosis factor‐α. Although the most severe symptoms resolved within 48 to 72 hours, participants reported continued arthralgias for up to a week. |
| Study characteristics | ||
| Methods | Randomised, double‐blinded, placebo‐controlled trial; parallel design. Trial duration 24 months. Multicentre: 2 sites, CF clinics, Australia. | |
| Participants | Inclusion criteria: CF (diagnosis previously made by sweat chloride test and an appropriate CF phenotype); ≥ 18 years; bone density T‐score < ‐1.5 in at least 1 of 3 sites (hip (femoral neck), lumbar spine 2 to 4 (L2 to L4) and distal forearm) in the month before trial commencement. Exclusion criteria: pre‐existing, symptomatic, fragility fractures; untreated hyperthyroidism, primary hyperparathyroidism or hypogonadism; bisphosphonate treatment in the 3 months before starting the study; serum calcium concentration below the lower limit of the laboratory normal range; serum creatinine concentration more than 1.5 times the upper limit of the laboratory normal range; serum ALT, ALP or bilirubin more than 3 times the upper limit of the laboratory normal range; on the waiting list for lung transplantation; pregnant or lactating; considered unlikely to complete the trial. Total participants: n = 22 (5 females). Age range over all: males 21 to 47 years, females 19 to 28 years. Treatment group: n = 10 (3 female); mean (SD) age 30.1 (2.2) years. Control group: n = 12 (2 females) mean (SD) age 28.6 (2.4) years. | |
| Interventions | Treatment group: intravenous zoledronic acid (zoledronate) in 100 ml of normal saline infused over 15 minutes every 3 months for 21 months (8 infusions in total). For 5 out of 63 doses, 4 mg zoledronate was administered, then dose reduced to 2 mg for subsequent doses (due to febrile reactions to the higher dose in several participants). Placebo group: 100 ml normal saline as above. All participants were prescribed calcium carbonate 600 mg and vitamin D2 1000 IU each twice daily at least 3 days before the first treatment infusion and continued throughout the trial. All participants were prescribed oral prednisolone 25 mg/day for 3 days starting on the morning of the first infusion; repeated with subsequent infusions if a reaction to the first infusion was thought likely. If there were side effects of the study infusion that were considered to be possibly due to the infusion during the first or any subsequent infusion, at the discretion of the investigator and participant, oral analgesia (paracetamol) was also administered for subsequent infusions. | |
| Outcomes |
| |
| Notes | Novartis Pharmaceuticals Pty Ltd, Australia partly funded this trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but process not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) | Unclear risk | Described as "double‐blind". Participants: blinded. Outcome assessors: DXA scans were performed and analysed by personnel blinded to treatment assignment. Not specifically discussed if clinicians or persons delivering treatment and other outcome assessors were all blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | Withdrawals described and equal across groups ‐ there were 3/10 in treatment group and 5/12 in control group. In the treatment group, 2 participants withdrew due to side effects, 1 due to psychiatric illness. In the placebo group, 1 participant was lost to follow‐up, 1 participant's BMD decreased to withdrawal threshold, 2 participants were poorly compliant to study protocols and in 1 participant, both of the latter 2 reasons were applicable. However, it was unclear which specific participants had BMD measurements available at each time point, particularly for forearm measures (fewer measurements compared with lumbar spine and femoral neck). |
| Selective reporting (reporting bias) | Low risk | Outcome measures that were described in the methods section were reported in the results section. |
| Other bias | Low risk | None identified. |
| Study characteristics | ||
| Methods | Randomised controlled trial; parallel design. Trial duration planned for 1 year, but was shortened to 6 months because of adverse events. Single centre, UK. | |
| Participants | Inclusion criteria: CF; no organ transplantation; 70% of all eligible participants in a longitudinal BMD study recruited after 1 year of follow‐up; no prior treatment with bone‐sparing agents; BMD Z score of ≦ ‐2 at lumbar spine, proximal femur or distal forearm. Total participants: n = 31 (9 female); mean (SD) age 26.1 (5.8) years; mean (SD) BMI 21.1 (2.7) kg/m2; mean (SD) FEV1 50.9 (20.3) % of predicted treatment. Groups similar with respect to age, initial BMD, bone biochemistry and respiratory disease severity. Treatment group: n = 15 (more females in this group but exact number not reported). Control group: n = 16. 3 participants did not complete the study (1 participant in the treatment group received a double lung transplant and 1 participant in each group died of respiratory failure). | |
| Interventions | Treatment group: intravenous pamidronate 30 mg every 3 months for 6 months (2 doses) plus oral calcium (1 g daily). Control group: oral calcium (1 g daily). All participants with pancreatic insufficiency (relevant to all except 1 in control group) continued long‐term oral vitamin D (900 IU/day). | |
| Outcomes |
| |
| Notes | Received funding from the Cystic Fibrosis Trust in the UK. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but process not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) | High risk | Not possible to blind as they did not give placebo infusions, so participants and clinicians would know who was in the treatment group and who in the control group. |
| Incomplete outcome data (attrition bias) | Low risk | Withdrawals were reported; 1 participant in each group died of respiratory failure and 1 participant in the treatment group underwent a double lung transplant. |
| Selective reporting (reporting bias) | Unclear risk | Outcome measures that were described in the methods section were reported in the results section. Did not report on fractures as an outcome however, which is curious for a study on bisphosphonate therapy to improve BMD, even to report that none occurred. |
| Other bias | High risk | Trial duration planned for 1 year, but was shortened to 6 months because of adverse events. |
| Study characteristics | ||
| Methods | Randomised, double‐blinded, placebo‐controlled trial; parallel design. Trial duration 24 months. Multicentre: 4 sites in UK, 1 site in Ireland. | |
| Participants | Inclusion criteria: CF (diagnosis on the basis of a positive sweat test or gene analysis and a consistent CF phenotype); >18 years, low BMD defined as lumbar spine, total hip or femoral neck BMD Z score < 1. Exclusion criteria: prescription of daily oral glucocorticoids for 6 weeks or more in the 12 months preceding the study; breastfeeding, pregnancy, desire to become pregnant within 3 years; listed for, or recipient of solid organ transplant; history of gastroscopy proven oesophageal abnormalities; renal impairment (elevated serum creatinine and an estimated creatinine clearance of 30 ml/min or less); hypocalcaemia; previous prescription of bone active drugs (bisphosphonates, hormone replacement therapy, raloxifene, calcitriol, calcitonin, teriparatide); biochemical evidence of vitamin D deficiency in the 12 months prior to the screening visit (25‐hydroxyvitamin D level < 10 ng/ml and PTH > 45 pg/ml); previous poor clinic attendance; previous poor adherence; pre‐terminal illness or other serious concomitant illness. Female participants of reproductive age were advised not to become pregnant for at least 12 months after study completion. Total participants: n = 36 (9 females). Treatment group: n = 17 (4 females); mean (SD) age 30.2 (12) years. Control group: n = 19 (5 females); mean (SD) age 27.8 (8.0) years. | |
| Interventions | Treatment group: once weekly oral risedronate 35 mg. Control group: once weekly identical placebo. Both groups were both prescribed Calcichew D3 Forte 2 tablets daily which provides 1000 mg calcium + 800 IU vitamin D3/day. Participants were advised to continue their standard multivitamin supplements. | |
| Outcomes |
| |
| Notes | Concomitant medications were recorded at study visits (3, 6, 12, 18 and 24 months). Pregnancy tests were performed in females at study visits (3, 6, 12, 18 and 24 months). Funded by unrestricted educational grants from Proctor & Gamble (Norwich, USA) and the Cystic Fibrosis Trust, with support to investigators from the UK National Institute of Health and Care Research. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated to risedronate or placebo using a computer program to minimise differences between groups in treatment centre, sex and baseline lumbar spine BMD. |
| Allocation concealment (selection bias) | Low risk | Only the study pharmacist had access to the treatment allocation. |
| Blinding (performance bias and detection bias) | Low risk | Identical placebo used. |
| Incomplete outcome data (attrition bias) | Low risk | Withdrawals are described in full and fairly equally spread across groups. Intervention group In first 12 months: 3 out of 17 in the oral risedronate group withdrew from the trial completely (due to bone pain) and 3 participants discontinued the medication (1 citing bone pain and the other 2 participants citing muscle aches or generalised pain) but remained in the trial for follow‐up. Between 12 and 24 months: 1 further participant withdrew, citing bone pain. At 24 months: 12 participants in the intervention group remained in trial with 9 still taking the drug. Placebo group Immediately after randomisation: 1 participant in the placebo group withdrew consent before taking the medication. Therefore, only 18 participants were commenced on placebo. In first 12 months: 2 participants in the placebo group withdrew consent and 1 participant had died. By 24 months: 3 participants in the placebo group had withdrawn consent and 4 participants had died, hence at 24 months, 12 participants remained in the control group. Primary outcome data included 12/17 of risedronate group (although only 9 still on drug); 12/19 placebo group. In the published abstract, it was not clear if the analysis included the 3 participants. |
| Selective reporting (reporting bias) | Low risk | Outcome measures that were described in the methods section were reported in the results section. |
| Other bias | Low risk | None identified. |
| Study characteristics | ||
| Methods | Randomised controlled trial; parallel design; open‐comparison. 12‐month duration. | |
| Participants | Inclusion criteria: CF, BMD Z score < ‐2 SD. Exclusion criteria: not detailed. Total participants: n = 20 (12 males, 8 females; age range 24 +/‐5 years). Treatment group: n = 10 (7 males, 3 females; age range 23.4 +/‐ 2.46 years). Control group: n = 10 (6 males, 4 females; age range 25.9 +/‐ 6.0 years). Groups similar BMI, FEV1, bone density, Z score at study onset. | |
| Interventions | Treatment group: alendronate/colecalciferol (70 mg/2800 ME) once a week + colecalciferol/calcium (200 ME/500 mg) twice a day. Control group: colecalciferol/calcium (400 ME/500 mg) twice a day. | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of how sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No mention of concealment. |
| Blinding (performance bias and detection bias) | High risk | Open comparison. |
| Incomplete outcome data (attrition bias) | Unclear risk | Does not explicitly mention whether or not there were withdrawals from the trial. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient detail in results. |
| Other bias | Unclear risk | No mention of funding or conflict of interest. |
| Study characteristics | ||
| Methods | Randomised, placebo‐controlled trial, parallel design. Trial duration: 12 months. Multicentre: 6 Canadian CF specialty clinics. | |
| Participants | Inclusion criteria: participants had CF confirmed by positive sweat test result or DNA acid analysis and a BMD T score of 1.0, as determined by DXA. Exclusion criteria: organ transplantation; endoscopy‐proven oesophagitis, gastritis, and ulceration; metabolic bone disorders; severe renal disease; use of systemic corticosteroids (dose 7.5 mg/day) or other drugs known to influence bone metabolism in the previous 6 months; osteomalacia and other documented contraindications. Total adults randomised: n = 56 (22 female). 9 withdrew, 47 completed trial. Treatment group: n = 27 (10 female); mean (SD) age 28.1 (7.7) years. 4 withdrew, 23 completed trial. Control group: n = 29 (12 female); mean (SD) age 30.9 (9.7) years. 5 withdrew, 24 completed trial. | |
| Interventions | Treatment group: oral alendronate (70 mg) once weekly for 12 months. Control group: placebo. Medication was taken while sitting upright and with water only on an empty stomach at least 30 minutes before first food or beverage of the day. In addition, all participants received 800 IU of vitamin D and 1000 mg of calcium (500 mg supplementation, 500 mg from diet) daily. | |
| Outcomes | In‐clinic assessments at baseline, 6 and 12 months; telephone follow‐up conducted by study staff at months 3 and 9.
Safety analyses included all vertebral fractures, osteoporosis‐related fractures, adverse reactions, and abnormal findings that had been detected through laboratory tests and physical examinations. Documentation for all adverse events were blinded and adjudicated by the external Data Safety Monitoring Committee. All adverse events were reported regardless of attribution to study medication. | |
| Notes | Participants who received at least 80% of the drug were classified as being adherent to the protocol. 5 participants completed the trial protocol but received sub‐optimal dosing (< 80% adherence; treatment group, 3 participants; control group, 2 participants). 1 of the participants in the treatment group missed > 50% of doses. Stopping and study withdrawal rules were monitored by an external Data Safety Monitoring Committee. During the trial, 3 participants in the treatment group used oral corticosteroids compared to none in the control group. Study funding provided by Merck Frosst Canada. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation code, stratified according to institution was prepared by an independent randomisation centre and block allocation was employed to ensure equitable distribution to each treatment group. |
| Allocation concealment (selection bias) | Low risk | The randomisation code was prepared by an independent randomisation centre and the medication treatment arm was concealed from all participants, central and local site coordinators, physicians, staff, and caregivers. |
| Blinding (performance bias and detection bias) | Low risk | Person(s) responsible for participants care, participants and outcome assessors were blinded to treatment group allocation. A medical physicist, who was blinded to the study treatment arm and study status, reviewed all DXA scans. Radiographs were sent to the central methods centre, and read independently by 2 radiologists who were blinded to the study treatment arm. Differences between radiologists were resolved by consensus. |
| Incomplete outcome data (attrition bias) | Low risk | All analyses were performed as intention‐to‐treat and included all available data. Withdrawals were described. Treatment group: 27 randomised, 4 withdrew (2 non‐compliance, 1 due to adverse event, 1 withdrew consent). 23 completed study. Control group: 29 randomised, 5 withdrew (2 non‐compliance, 2 due to adverse event, 1 lost to follow‐up). 24 completed study. |
| Selective reporting (reporting bias) | Low risk | Outcome measures that were described in the methods section were reported in the results section. It was reported that there were no differences in baseline CRP, 25‐hydroxyvitamin D, PTH or CTX levels between the risedronate group who experienced bone pain and those that did not (but data not shown). |
| Other bias | Low risk | None identified. |
ALP: alkaline phosphatase.
ALT: alanine aminotransferase.
BMD: bone mineral density.
BMI: body mass index.
CF: cystic fibrosis.
CRP: C‐reactive protein.
CTX: C‐terminal telopeptide.
DXA: dual‐energy x‐ray absorptiometry.
FEV1: forced expiratory volume in one second.
FVC: forced vital capacity.
IU: international units.
PTH: parathyroid hormone.
SD: standard deviation.
SF‐36v2: Medical Outcomes Study 36‐item short form, version 2.
SXA: single energy x‐ray absorptiometry.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Retrospective study, not randomised controlled trial. | |
| This trial was a prospective open design, not a randomised controlled trial. | |
| This trial assessed the effect of growth hormone on total‐body bone mineral content in pre‐pubertal children with CF. It did not assess the effect of bisphosphonates. | |
| Only examined side effect profile of bisphosphonates, did not assess treatment of disease. | |
| Funding withdrawn, study stopped before enrolling first participant. | |
| Single group assignment, not a randomised controlled trial. | |
| Retrospective study, not a randomised controlled trial. | |
| The trial assessed the effects of once weekly oral alendronate for 12 months on bone in children on glucocorticoid treatment. Of the 22 included children, only 1 had CF and this participant was in the placebo group. |
CF: cystic fibrosis.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Total fractures Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 1: Total fractures | ||||
| 1.1.1 12 months | 5 | 142 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.13, 2.50] |
| 1.1.2 24 months | 2 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.2 Non‐vertebral fractures Show forest plot | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 2: Non‐vertebral fractures | ||||
| 1.2.1 12 months | 4 | 95 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.11 [0.18, 25.35] |
| 1.2.2 24 months | 2 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.3 Vertebral fractures Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 3: Vertebral fractures | ||||
| 1.3.1 12 months | 5 | 142 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.02, 2.09] |
| 1.3.2 24 months | 2 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.4 % change in BMD, lumbar spine [DXA] Show forest plot | 8 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4 ![Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 4: % change in BMD, lumbar spine [DXA]](/cdsr/doi/10.1002/14651858.CD002010.pub5/media/CDSR/CD002010/image_n/nCD002010-CMP-001.04.svg) Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 4: % change in BMD, lumbar spine [DXA] | ||||
| 1.4.1 6 months | 4 | 101 | Mean Difference (IV, Fixed, 95% CI) | 4.61 [3.90, 5.32] |
| 1.4.2 12 months | 6 | 171 | Mean Difference (IV, Fixed, 95% CI) | 6.31 [5.39, 7.22] |
| 1.4.3 24 months | 2 | 43 | Mean Difference (IV, Fixed, 95% CI) | 5.49 [4.38, 6.60] |
| 1.5 % change in BMD, lumbar spine [DXA] (end of trial data only) Show forest plot | 8 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5 ![Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 5: % change in BMD, lumbar spine [DXA] (end of trial data only)](/cdsr/doi/10.1002/14651858.CD002010.pub5/media/CDSR/CD002010/image_n/nCD002010-CMP-001.05.svg) Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 5: % change in BMD, lumbar spine [DXA] (end of trial data only) | ||||
| 1.5.1 End of trial | 8 | 199 | Mean Difference (IV, Fixed, 95% CI) | 5.95 [5.17, 6.73] |
| 1.6 % change in BMD, total hip/femur [DXA] Show forest plot | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6 ![Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 6: % change in BMD, total hip/femur [DXA]](/cdsr/doi/10.1002/14651858.CD002010.pub5/media/CDSR/CD002010/image_n/nCD002010-CMP-001.06.svg) Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 6: % change in BMD, total hip/femur [DXA] | ||||
| 1.6.1 6 months | 3 | 96 | Mean Difference (IV, Fixed, 95% CI) | 3.58 [2.75, 4.40] |
| 1.6.2 12 months | 5 | 155 | Mean Difference (IV, Fixed, 95% CI) | 4.41 [3.44, 5.37] |
| 1.6.3 24 months | 2 | 42 | Mean Difference (IV, Fixed, 95% CI) | 6.49 [5.32, 7.66] |
| 1.7 % change in BMD, total hip/femur [DXA] (end of trial data only) Show forest plot | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.7 ![Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 7: % change in BMD, total hip/femur [DXA] (end of trial data only)](/cdsr/doi/10.1002/14651858.CD002010.pub5/media/CDSR/CD002010/image_n/nCD002010-CMP-001.07.svg) Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 7: % change in BMD, total hip/femur [DXA] (end of trial data only) | ||||
| 1.7.1 End of trial | 6 | 178 | Mean Difference (IV, Fixed, 95% CI) | 4.89 [4.06, 5.72] |
| 1.8 % change in BMD, distal radius [SXA] Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.8 ![Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 8: % change in BMD, distal radius [SXA]](/cdsr/doi/10.1002/14651858.CD002010.pub5/media/CDSR/CD002010/image_n/nCD002010-CMP-001.08.svg) Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 8: % change in BMD, distal radius [SXA] | ||||
| 1.8.1 6 months | 2 | 48 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.73, 0.78] |
| 1.8.2 12 months | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐0.30, 0.94] |
| 1.8.3 24 months | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 1.50 [0.41, 2.59] |
| 1.9 % change in BMD, distal radius [SXA] (end of trial data only) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.9 ![Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 9: % change in BMD, distal radius [SXA] (end of trial data only)](/cdsr/doi/10.1002/14651858.CD002010.pub5/media/CDSR/CD002010/image_n/nCD002010-CMP-001.09.svg) Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 9: % change in BMD, distal radius [SXA] (end of trial data only) | ||||
| 1.9.1 End of study | 2 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.74 [‐0.21, 1.70] |
| 1.10 % change in BMD, ultra distal radius [SXA] Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.10 ![Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 10: % change in BMD, ultra distal radius [SXA]](/cdsr/doi/10.1002/14651858.CD002010.pub5/media/CDSR/CD002010/image_n/nCD002010-CMP-001.10.svg) Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 10: % change in BMD, ultra distal radius [SXA] | ||||
| 1.10.1 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.11 QoL Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.11  Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 11: QoL | ||||
| 1.11.1 Physical component | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | 2.51 [‐1.38, 6.40] |
| 1.11.2 Mental component | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐5.93 [‐11.73, ‐0.13] |
| 1.12 Bone pain Show forest plot | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.12  Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 12: Bone pain | ||||
| 1.12.1 All routes of bisphosphonate administration | 7 | 206 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.49 [3.20, 22.56] |
| 1.12.2 Oral bisphosphonates | 4 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.98 [1.24, 20.09] |
| 1.12.3 Intravenous bisphosphonates | 3 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 14.17 [3.64, 55.17] |
| 1.13 Fever Show forest plot | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.13  Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 13: Fever | ||||
| 1.13.1 All routes of bisphosphonate administration | 5 | 118 | Odds Ratio (M‐H, Fixed, 95% CI) | 12.64 [2.31, 69.11] |
| 1.13.2 Oral bisphosphonates | 2 | 63 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.13.3 Intravenous bisphosphonates | 3 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 12.64 [2.31, 69.11] |
| 1.14 Withdrawals due to adverse events Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.14  Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 14: Withdrawals due to adverse events | ||||
| 1.14.1 6 months | 2 | 53 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.95 [0.14, 108.09] |
| 1.14.2 12 months | 5 | 177 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.07 [1.11, 14.90] |
| 1.14.3 24 months | 2 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 16.34 [1.98, 134.89] |
| 1.15 Withdrawals (total) Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.15  Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 15: Withdrawals (total) | ||||
| 1.15.1 6 months | 2 | 53 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.27, 11.60] |
| 1.15.2 12 months | 5 | 177 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.45, 2.28] |
| 1.15.3 24 months | 2 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.34, 2.69] |
| 1.16 Survival Show forest plot | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.16  Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 16: Survival | ||||
| 1.16.1 6 months | 2 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.05, 16.39] |
| 1.16.2 12 months | 4 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.98 [0.30, 29.84] |
| 1.16.3 24 months | 2 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.27 [0.43, 42.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Total fractures Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 1: Total fractures | ||||
| 2.1.1 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.2 Non‐vertebral fractures Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 2: Non‐vertebral fractures | ||||
| 2.2.1 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.3 Vertebral fractures Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 3: Vertebral fractures | ||||
| 2.3.1 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.4 % change in BMD, lumbar spine, DXA Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.4  Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 4: % change in BMD, lumbar spine, DXA | ||||
| 2.4.1 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.5 Bone or muscle pain Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.5  Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 5: Bone or muscle pain | ||||
| 2.5.1 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.6 Fever Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.6  Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 6: Fever | ||||
| 2.6.1 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.7 Gastrointestinal adverse events Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.7  Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 7: Gastrointestinal adverse events | ||||
| 2.7.1 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.8 Headache Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.8  Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 8: Headache | ||||
| 2.8.1 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.9 Any adverse event Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.9  Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 9: Any adverse event | ||||
| 2.9.1 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.10 Withdrawals, due to adverse events Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.10  Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 10: Withdrawals, due to adverse events | ||||
| 2.10.1 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.11 Withdrawals, total Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.11  Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 11: Withdrawals, total | ||||
| 2.11.1 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.12 Survival Show forest plot | 1 | 113 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| Analysis 2.12  Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 12: Survival | ||||
| 2.12.1 12 months | 1 | 113 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Total Fractures Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.1  Comparison 3: Bisphosphonates versus control (with lung transplantation) ‐ adults (18 years and over), Outcome 1: Total Fractures | ||||
| 3.1.1 24 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2 Non‐vertebral fractures Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.2  Comparison 3: Bisphosphonates versus control (with lung transplantation) ‐ adults (18 years and over), Outcome 2: Non‐vertebral fractures | ||||
| 3.2.1 24 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.3 Vertebral fractures Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.3  Comparison 3: Bisphosphonates versus control (with lung transplantation) ‐ adults (18 years and over), Outcome 3: Vertebral fractures | ||||
| 3.3.1 24 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.4 % change in BMD, lumbar spine, DXA Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.4  Comparison 3: Bisphosphonates versus control (with lung transplantation) ‐ adults (18 years and over), Outcome 4: % change in BMD, lumbar spine, DXA | ||||
| 3.4.1 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.5 % change in BMD, femur, DXA Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.5  Comparison 3: Bisphosphonates versus control (with lung transplantation) ‐ adults (18 years and over), Outcome 5: % change in BMD, femur, DXA | ||||
| 3.5.1 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.6 Bone pain Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.6  Comparison 3: Bisphosphonates versus control (with lung transplantation) ‐ adults (18 years and over), Outcome 6: Bone pain | ||||
| 3.6.1 24 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.7 Withdrawals, due to adverse events Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.7  Comparison 3: Bisphosphonates versus control (with lung transplantation) ‐ adults (18 years and over), Outcome 7: Withdrawals, due to adverse events | ||||
| 3.8 Withdrawals, total Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.8  Comparison 3: Bisphosphonates versus control (with lung transplantation) ‐ adults (18 years and over), Outcome 8: Withdrawals, total | ||||
| 3.8.1 24 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.9 Survival Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.9  Comparison 3: Bisphosphonates versus control (with lung transplantation) ‐ adults (18 years and over), Outcome 9: Survival | ||||
| 3.9.1 24 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
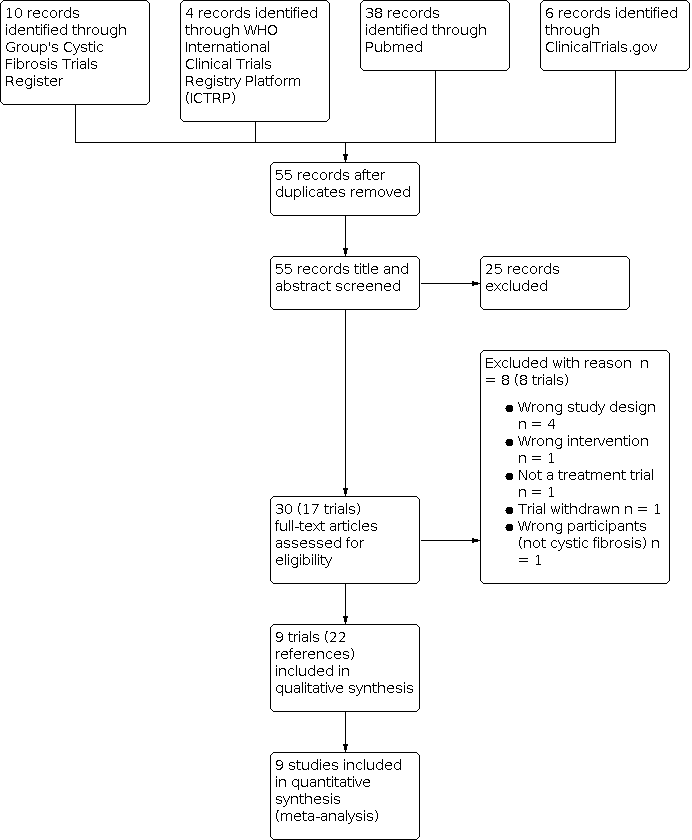
Selection process for this update.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison between bisphosphonates versus control (without lung transplantation), Outcome 1a Number of participants with any fracture.

Forest plot of comparison between bisphosphonates versus control (without lung transplantation), Outcome 1b i. Number of participants with non‐vertebral fractures.

Forest plot of comparison between bisphosphonates versus control (without lung transplantation), Outcome 1b ii. Number of participants with vertebral fractures.

Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 1: Total fractures

Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 2: Non‐vertebral fractures

Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 3: Vertebral fractures
![Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 4: % change in BMD, lumbar spine [DXA]](/es/cdsr/doi/10.1002/14651858.CD002010.pub5/media/CDSR/CD002010/image_n/nCD002010-CMP-001.04.svg)
Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 4: % change in BMD, lumbar spine [DXA]
![Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 5: % change in BMD, lumbar spine [DXA] (end of trial data only)](/es/cdsr/doi/10.1002/14651858.CD002010.pub5/media/CDSR/CD002010/image_n/nCD002010-CMP-001.05.svg)
Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 5: % change in BMD, lumbar spine [DXA] (end of trial data only)
![Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 6: % change in BMD, total hip/femur [DXA]](/es/cdsr/doi/10.1002/14651858.CD002010.pub5/media/CDSR/CD002010/image_n/nCD002010-CMP-001.06.svg)
Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 6: % change in BMD, total hip/femur [DXA]
![Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 7: % change in BMD, total hip/femur [DXA] (end of trial data only)](/es/cdsr/doi/10.1002/14651858.CD002010.pub5/media/CDSR/CD002010/image_n/nCD002010-CMP-001.07.svg)
Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 7: % change in BMD, total hip/femur [DXA] (end of trial data only)
![Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 8: % change in BMD, distal radius [SXA]](/es/cdsr/doi/10.1002/14651858.CD002010.pub5/media/CDSR/CD002010/image_n/nCD002010-CMP-001.08.svg)
Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 8: % change in BMD, distal radius [SXA]
![Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 9: % change in BMD, distal radius [SXA] (end of trial data only)](/es/cdsr/doi/10.1002/14651858.CD002010.pub5/media/CDSR/CD002010/image_n/nCD002010-CMP-001.09.svg)
Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 9: % change in BMD, distal radius [SXA] (end of trial data only)
![Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 10: % change in BMD, ultra distal radius [SXA]](/es/cdsr/doi/10.1002/14651858.CD002010.pub5/media/CDSR/CD002010/image_n/nCD002010-CMP-001.10.svg)
Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 10: % change in BMD, ultra distal radius [SXA]

Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 11: QoL

Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 12: Bone pain

Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 13: Fever

Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 14: Withdrawals due to adverse events

Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 15: Withdrawals (total)

Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 16: Survival

Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 1: Total fractures

Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 2: Non‐vertebral fractures

Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 3: Vertebral fractures

Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 4: % change in BMD, lumbar spine, DXA

Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 5: Bone or muscle pain

Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 6: Fever

Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 7: Gastrointestinal adverse events

Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 8: Headache

Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 9: Any adverse event

Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 10: Withdrawals, due to adverse events

Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 11: Withdrawals, total

Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 12: Survival

Comparison 3: Bisphosphonates versus control (with lung transplantation) ‐ adults (18 years and over), Outcome 1: Total Fractures

Comparison 3: Bisphosphonates versus control (with lung transplantation) ‐ adults (18 years and over), Outcome 2: Non‐vertebral fractures

Comparison 3: Bisphosphonates versus control (with lung transplantation) ‐ adults (18 years and over), Outcome 3: Vertebral fractures

Comparison 3: Bisphosphonates versus control (with lung transplantation) ‐ adults (18 years and over), Outcome 4: % change in BMD, lumbar spine, DXA

Comparison 3: Bisphosphonates versus control (with lung transplantation) ‐ adults (18 years and over), Outcome 5: % change in BMD, femur, DXA

Comparison 3: Bisphosphonates versus control (with lung transplantation) ‐ adults (18 years and over), Outcome 6: Bone pain

Comparison 3: Bisphosphonates versus control (with lung transplantation) ‐ adults (18 years and over), Outcome 7: Withdrawals, due to adverse events

Comparison 3: Bisphosphonates versus control (with lung transplantation) ‐ adults (18 years and over), Outcome 8: Withdrawals, total

Comparison 3: Bisphosphonates versus control (with lung transplantation) ‐ adults (18 years and over), Outcome 9: Survival
| Patient or population: adults with cystic fibrosis who have not had a lung transplant Settings: outpatients Comparison: placebo or no bisphosphonatesb | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Bisphosphonates | |||||
| New vertebral fractures
Follow‐up: 12 months | 42 per 1000 | 9 per 1000 (1 to 88) | OR 0.22 (0.02 to 2.09) | 142 (5) | ⊕⊝⊝⊝ very lowc,d |
|
| New non‐vertebral fractures
Follow‐up: 12 months | 21 per 1000 | 44 per 1000 (4 to 532) | OR 2.11 (0.18 to 25.35) | 95 (4) | ⊕⊝⊝⊝ very lowd,e |
|
| BMD: % change in BMD at the lumbar spine
Follow‐up: 12 months | The mean % change in BMD (lumbar spine) ranged across control groups from ‐1.8% to 0.7% | The mean % change in BMD in the intervention groups was 6.31% higher (5.39% higher to 7.22% higher) | NA | 171 (6) | ⊕⊕⊝⊝ lowf |
|
| BMD: % change in BMD at the hip or femur
Follow‐up: 12 months | The mean % change in BMD (hip or femur) ranged across control groups from ‐2.8% to ‐0.7% | The mean % change in BMD in the intervention groups was 4.41% higher (3.44% higher to 5.37% higher) | NA | 155 (5) | ⊕⊕⊝⊝ lowf |
|
| QoL: change in physical and mental component scores (SF36v2)
Follow‐up: 12 months | The change in mean (SD) SF36 physical score was ‐3.69 (8.33) in the control group | The mean change in SF36 physical score in the intervention group was 2.51 higher (1.38 lower to 6.40 higher) than in the control group | NA | 47 (1) | ⊕⊕⊝⊝ lowd,g | The results for the physical and mental components are heterogeneous
|
| The change in mean (SD) SF36 mental score was 3.26 (12.27) in the control group | The mean change in SF36 mental score in the intervention group was 5.93 lower (11.73 lower to 0.13 higher) than in the control group | |||||
| Adverse events: bone pain (all routes of bisphosphonate administration)
Follow‐up: 12 months | 38 per 1000 | 323 per 1000 (122 to 857) | OR 8.49 (3.20 to 22.56) | 206 (7) | ⊕⊕⊕⊝ moderateh | Bone pain Separating by route of administration did not change the results; both routes favoured control. Oral bisphosphonates: MD 4.98 (1.24 to 20.09) and IV bisphosphonates: MD 14.17 (3.64 to 55.17)
Fever Oral route: 2 trials of oral alendronate reported that none of the participants in either group experienced fever IV route: 3 trials showed that bisphosphonates were associated with a higher occurrence of fever OR 12.64 (2.31 to 69.11)
GI adverse effects Only measured in the oral bisphosphonates trials. At 12 months, 1 trial reported 3 occurrences of diarrhoea (1 in the bisphosphonate group and 2 in the placebo group) (Aris 2004) 1 trial reported no GI adverse effects in either group (Bianchi 2013) 1 trial reported 10 GI adverse events in the bisphosphonates group compared to 7 in the control group (Papaioannou 2008) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMD: bone mineral density; CI: confidence interval; GI: gastrointestinal; IV: intravenous; MD: mean difference; NA: not applicable; OR: odds ratio; QoL: quality of life; SD: standard deviation; SF36v2: medical outcomes study 36‐item short form. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
| aTrials used different types and formulations of bisphosphonates: IV zoledronate (Boyle 2005; Chapman 2009); IV pamidronate (Haworth 2001); oral alendronate (Aris 2004; Bianchi 2013; Krasovsky 2010; Papaioannou 2008); oral risedronate (Haworth 2011). | ||||||
| Patient or population: children with cystic fibrosis who have not had a lung transplant Settings: outpatients Intervention: oral alendronate plus oral calcifediol and RDA dietary calcium Comparison: oral placebo plus oral calcifediol and RDA dietary calcium | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Bisphosphonates | |||||
| New vertebral fractures
Follow‐up: 12 months | 54 per 1000 | 17 per 1000 (2 to 169) | OR 0.32 (0.03 to 3.13) | 113 (1) | ⊕⊕⊝⊝ lowa |
|
| New non‐vertebral fractures
Follow‐up: 12 months | 36 per 1000 | 7 per 1000 (1 to 145) | OR 0.19 (0.01 to 4.04) | 113 (1) | ⊕⊕⊝⊝ lowa |
|
| BMD: % change in BMD at the lumbar spine
Follow‐up: 12 months | The % change in BMD at the lumbar spine was higher in the treatment group than the control group, MD 14.50 (95% CI 12.91 to 16.09) | NA | 113 (1) | ⊕⊕⊝⊝ lowa |
| |
| BMD: % change in BMD at the hip or femur | This outcome was not reported |
| ||||
| QoL | This outcome was not reported |
| ||||
| Adverse events
Follow‐up: 12 months | There was no difference in bone or muscle pain, OR 3.00 (95% CI 0.12 to 75.22); fever, OR 3.00 (95% CI 0.12 to 75.22); or GI adverse events OR 0.67 (95% CI 0.20 to 2.26) | NA | 113 (1) | ⊕⊕⊝⊝ lowa |
| |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMD: bone mineral density; CI: confidence interval; GI: gastrointestinal; MD: mean difference; NA: not applicable; OR: odds ratio; QoL: quality of life; RDA: recommended daily allowance. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
| aDowngraded twice due to imprecision cause by small participant numbers and low event rates. | ||||||
| Patient or population: adults with cystic fibrosis who have had a lung transplant Settings: outpatients Intervention: bisphosphonates (IV pamidronate plus oral vitamin D and oral calcium) Comparison: oral vitamin D and oral calcium | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Bisphosphonates | |||||
| New vertebral fractures
Follow‐up: 12 months | This outcome was not reported at 12 months ‐ see comments | 1 study reported results at 24 months (Aris 2000) and found no difference in the number of participants with a vertebral fracture, OR 3.92 (95% CI 0.36 to 42.20) | ||||
| New non‐vertebral fractures
Follow‐up: 12 months | This outcome was not reported at 12 months ‐ see comments | 1 study reported results at 24 months (Aris 2000) and found no difference in the number of participants with a non‐vertebral fracture, OR 0.46 (95% CI 0.09 to 2.27) | ||||
| BMD: % change in BMD at the lumbar spine
Follow‐up: 12 months | This outcome was not reported at 12 months ‐ see comments | 1 study reported results at 24 months (Aris 2000) and found the % change in BMD was higher in the treatment group than the control group MD 6.20 (95% CI 4.28 to 8.12) | ||||
| BMD: % change in BMD at the hip or femur
Follow‐up: 12 months | This outcome was not reported at 12 months ‐ see comments | 1 study reported results at 24 months (Aris 2000) and found the % change in BMD was higher in the treatment group than the control group, MD 7.90 (95% CI 5.78 to 10.02). | ||||
| QoL | This outcome was not reported at any time point |
| ||||
| Adverse events
Follow‐up: 12 months | This outcome was not reported at 12 months ‐ see comment | 1 study reported results at 24 months (Aris 2000) and found none of the participants in either group experienced bone pain or fever | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMD: bone mineral density; CI: confidence interval; IV: intravenous; MD: mean difference; OR: odds ratio; QoL: quality of life. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Total fractures Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1.1 12 months | 5 | 142 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.13, 2.50] |
| 1.1.2 24 months | 2 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.2 Non‐vertebral fractures Show forest plot | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 12 months | 4 | 95 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.11 [0.18, 25.35] |
| 1.2.2 24 months | 2 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.3 Vertebral fractures Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.3.1 12 months | 5 | 142 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.02, 2.09] |
| 1.3.2 24 months | 2 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.4 % change in BMD, lumbar spine [DXA] Show forest plot | 8 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.4.1 6 months | 4 | 101 | Mean Difference (IV, Fixed, 95% CI) | 4.61 [3.90, 5.32] |
| 1.4.2 12 months | 6 | 171 | Mean Difference (IV, Fixed, 95% CI) | 6.31 [5.39, 7.22] |
| 1.4.3 24 months | 2 | 43 | Mean Difference (IV, Fixed, 95% CI) | 5.49 [4.38, 6.60] |
| 1.5 % change in BMD, lumbar spine [DXA] (end of trial data only) Show forest plot | 8 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.5.1 End of trial | 8 | 199 | Mean Difference (IV, Fixed, 95% CI) | 5.95 [5.17, 6.73] |
| 1.6 % change in BMD, total hip/femur [DXA] Show forest plot | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.6.1 6 months | 3 | 96 | Mean Difference (IV, Fixed, 95% CI) | 3.58 [2.75, 4.40] |
| 1.6.2 12 months | 5 | 155 | Mean Difference (IV, Fixed, 95% CI) | 4.41 [3.44, 5.37] |
| 1.6.3 24 months | 2 | 42 | Mean Difference (IV, Fixed, 95% CI) | 6.49 [5.32, 7.66] |
| 1.7 % change in BMD, total hip/femur [DXA] (end of trial data only) Show forest plot | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.7.1 End of trial | 6 | 178 | Mean Difference (IV, Fixed, 95% CI) | 4.89 [4.06, 5.72] |
| 1.8 % change in BMD, distal radius [SXA] Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.8.1 6 months | 2 | 48 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.73, 0.78] |
| 1.8.2 12 months | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐0.30, 0.94] |
| 1.8.3 24 months | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 1.50 [0.41, 2.59] |
| 1.9 % change in BMD, distal radius [SXA] (end of trial data only) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.9.1 End of study | 2 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.74 [‐0.21, 1.70] |
| 1.10 % change in BMD, ultra distal radius [SXA] Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.10.1 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.11 QoL Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.11.1 Physical component | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | 2.51 [‐1.38, 6.40] |
| 1.11.2 Mental component | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐5.93 [‐11.73, ‐0.13] |
| 1.12 Bone pain Show forest plot | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.12.1 All routes of bisphosphonate administration | 7 | 206 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.49 [3.20, 22.56] |
| 1.12.2 Oral bisphosphonates | 4 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.98 [1.24, 20.09] |
| 1.12.3 Intravenous bisphosphonates | 3 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 14.17 [3.64, 55.17] |
| 1.13 Fever Show forest plot | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.13.1 All routes of bisphosphonate administration | 5 | 118 | Odds Ratio (M‐H, Fixed, 95% CI) | 12.64 [2.31, 69.11] |
| 1.13.2 Oral bisphosphonates | 2 | 63 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.13.3 Intravenous bisphosphonates | 3 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 12.64 [2.31, 69.11] |
| 1.14 Withdrawals due to adverse events Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.14.1 6 months | 2 | 53 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.95 [0.14, 108.09] |
| 1.14.2 12 months | 5 | 177 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.07 [1.11, 14.90] |
| 1.14.3 24 months | 2 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 16.34 [1.98, 134.89] |
| 1.15 Withdrawals (total) Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.15.1 6 months | 2 | 53 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.27, 11.60] |
| 1.15.2 12 months | 5 | 177 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.45, 2.28] |
| 1.15.3 24 months | 2 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.34, 2.69] |
| 1.16 Survival Show forest plot | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.16.1 6 months | 2 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.05, 16.39] |
| 1.16.2 12 months | 4 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.98 [0.30, 29.84] |
| 1.16.3 24 months | 2 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.27 [0.43, 42.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Total fractures Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1.1 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.2 Non‐vertebral fractures Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.2.1 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.3 Vertebral fractures Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.3.1 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.4 % change in BMD, lumbar spine, DXA Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.4.1 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.5 Bone or muscle pain Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.5.1 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.6 Fever Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.6.1 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.7 Gastrointestinal adverse events Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.7.1 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.8 Headache Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.8.1 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.9 Any adverse event Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.9.1 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.10 Withdrawals, due to adverse events Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.10.1 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.11 Withdrawals, total Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.11.1 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.12 Survival Show forest plot | 1 | 113 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.12.1 12 months | 1 | 113 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Total Fractures Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1.1 24 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2 Non‐vertebral fractures Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2.1 24 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.3 Vertebral fractures Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.3.1 24 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.4 % change in BMD, lumbar spine, DXA Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.4.1 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.5 % change in BMD, femur, DXA Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.5.1 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.6 Bone pain Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.6.1 24 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.7 Withdrawals, due to adverse events Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.8 Withdrawals, total Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.8.1 24 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.9 Survival Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.9.1 24 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

