Bisfosfonati za osteoporozu kod osoba s cističnom fibrozom
Appendices
Appendix 1. Additional electronic search strategies
Strategies for current version of this review
| Database | Search strategy | Date last searched |
|---|---|---|
| PubMed (all years to date of search) | #1 cystic fibrosis OR mucoviscidosis OR mucoviscidose #2 Biphosphonate OR Diphosphonate OR alendronate OR pamidronate OR etidronate OR Etidronic OR clodronate OR clodronic OR tiludronate OR olpadronate OR incadronate OR risedronate OR risedronic OR zoledronate OR zolendronic OR elodronate OR ibandronate #3 #1 AND #2 #4 randomized controlled trial [pt] #5 controlled clinical trial [pt] #6 randomized [tiab] #7 placebo [tiab] #8 drug therapy [sh] #9 randomly [tiab] #10 trial [tiab] #11 groups [tiab] #12 #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 #13 animals [mh] NOT humans [mh] #14 #12 NOT #13 #15 #3 AND #14
Note: lines #4 to #14 is the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐maximising version (2008 revision); PubMed format (training.cochrane.org/handbook/version-6/chapter-4-tech-suppl, page 60) | 5 May 2022 |
| ClinicalTrials.gov | [Advanced search]
Condition or disease: cystic fibrosis OR mucoviscidosis OR mucoviscidose
Other terms: Biphosphonate OR Diphosphonate OR alendronate OR pamidronate OR etidronate OR Etidronic OR clodronate OR clodronic OR tiludronate OR olpadronate OR incadronate OR risedronate OR risedronic OR zoledronate OR zolendronic OR elodronate OR ibandronate
Study type: Interventional Studies (Clinical Trials) | 5 May 2022 |
| WHO International Clinical Trials Registry Platform (ICTRP) | [Advanced search]
Condition: cystic fibrosis OR mucoviscidosis OR mucoviscidose
Intervention: Biphosphonate OR Diphosphonate OR alendronate OR pamidronate OR etidronate OR Etidronic OR clodronate OR clodronic OR tiludronate OR olpadronate OR incadronate OR risedronate OR risedronic OR zoledronate OR zolendronic OR elodronate OR ibandronate
Recruitment status: All | 5 May 2022 |
Strategies for previous versions of this review
| Database | Search strategy | Date last searched |
|---|---|---|
| PubMed (all years up to date) | zoledronate AND cystic fibrosis OR "Diphosphonates" [Mesh] AND "Cystic Fibrosis" [Mesh] | 14 January 2014 |
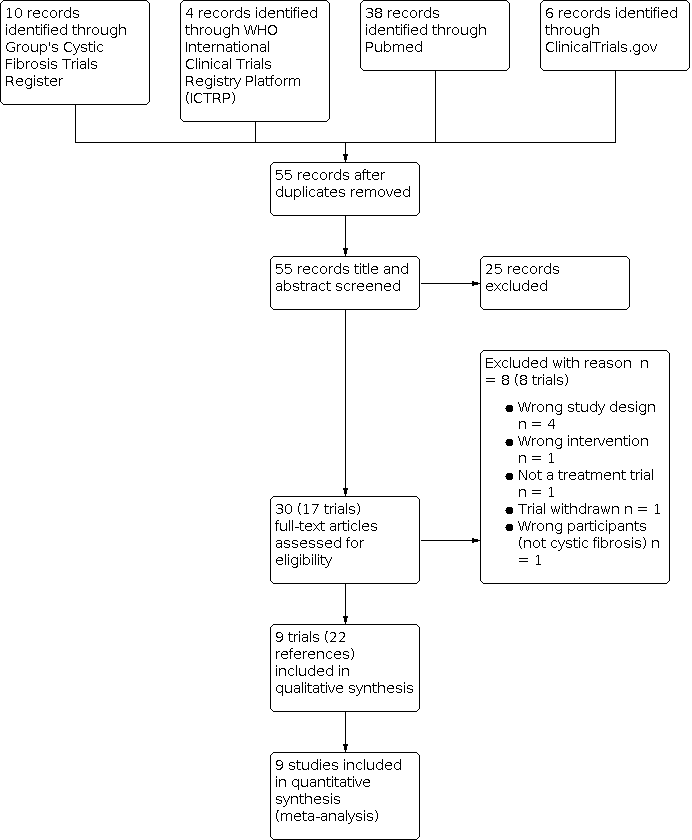
Selection process for this update.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison between bisphosphonates versus control (without lung transplantation), Outcome 1a Number of participants with any fracture.

Forest plot of comparison between bisphosphonates versus control (without lung transplantation), Outcome 1b i. Number of participants with non‐vertebral fractures.

Forest plot of comparison between bisphosphonates versus control (without lung transplantation), Outcome 1b ii. Number of participants with vertebral fractures.

Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 1: Total fractures

Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 2: Non‐vertebral fractures

Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 3: Vertebral fractures
![Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 4: % change in BMD, lumbar spine [DXA]](/es/cdsr/doi/10.1002/14651858.CD002010.pub5/media/CDSR/CD002010/image_n/nCD002010-CMP-001.04.svg)
Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 4: % change in BMD, lumbar spine [DXA]
![Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 5: % change in BMD, lumbar spine [DXA] (end of trial data only)](/es/cdsr/doi/10.1002/14651858.CD002010.pub5/media/CDSR/CD002010/image_n/nCD002010-CMP-001.05.svg)
Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 5: % change in BMD, lumbar spine [DXA] (end of trial data only)
![Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 6: % change in BMD, total hip/femur [DXA]](/es/cdsr/doi/10.1002/14651858.CD002010.pub5/media/CDSR/CD002010/image_n/nCD002010-CMP-001.06.svg)
Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 6: % change in BMD, total hip/femur [DXA]
![Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 7: % change in BMD, total hip/femur [DXA] (end of trial data only)](/es/cdsr/doi/10.1002/14651858.CD002010.pub5/media/CDSR/CD002010/image_n/nCD002010-CMP-001.07.svg)
Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 7: % change in BMD, total hip/femur [DXA] (end of trial data only)
![Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 8: % change in BMD, distal radius [SXA]](/es/cdsr/doi/10.1002/14651858.CD002010.pub5/media/CDSR/CD002010/image_n/nCD002010-CMP-001.08.svg)
Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 8: % change in BMD, distal radius [SXA]
![Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 9: % change in BMD, distal radius [SXA] (end of trial data only)](/es/cdsr/doi/10.1002/14651858.CD002010.pub5/media/CDSR/CD002010/image_n/nCD002010-CMP-001.09.svg)
Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 9: % change in BMD, distal radius [SXA] (end of trial data only)
![Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 10: % change in BMD, ultra distal radius [SXA]](/es/cdsr/doi/10.1002/14651858.CD002010.pub5/media/CDSR/CD002010/image_n/nCD002010-CMP-001.10.svg)
Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 10: % change in BMD, ultra distal radius [SXA]

Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 11: QoL

Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 12: Bone pain

Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 13: Fever

Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 14: Withdrawals due to adverse events

Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 15: Withdrawals (total)

Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 16: Survival

Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 1: Total fractures

Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 2: Non‐vertebral fractures

Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 3: Vertebral fractures

Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 4: % change in BMD, lumbar spine, DXA

Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 5: Bone or muscle pain

Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 6: Fever

Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 7: Gastrointestinal adverse events

Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 8: Headache

Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 9: Any adverse event

Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 10: Withdrawals, due to adverse events

Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 11: Withdrawals, total

Comparison 2: Bisphosphonates versus control (without lung transplantation) ‐ children up to 18 years of age, Outcome 12: Survival

Comparison 3: Bisphosphonates versus control (with lung transplantation) ‐ adults (18 years and over), Outcome 1: Total Fractures

Comparison 3: Bisphosphonates versus control (with lung transplantation) ‐ adults (18 years and over), Outcome 2: Non‐vertebral fractures

Comparison 3: Bisphosphonates versus control (with lung transplantation) ‐ adults (18 years and over), Outcome 3: Vertebral fractures

Comparison 3: Bisphosphonates versus control (with lung transplantation) ‐ adults (18 years and over), Outcome 4: % change in BMD, lumbar spine, DXA

Comparison 3: Bisphosphonates versus control (with lung transplantation) ‐ adults (18 years and over), Outcome 5: % change in BMD, femur, DXA

Comparison 3: Bisphosphonates versus control (with lung transplantation) ‐ adults (18 years and over), Outcome 6: Bone pain

Comparison 3: Bisphosphonates versus control (with lung transplantation) ‐ adults (18 years and over), Outcome 7: Withdrawals, due to adverse events

Comparison 3: Bisphosphonates versus control (with lung transplantation) ‐ adults (18 years and over), Outcome 8: Withdrawals, total

Comparison 3: Bisphosphonates versus control (with lung transplantation) ‐ adults (18 years and over), Outcome 9: Survival
| Patient or population: adults with cystic fibrosis who have not had a lung transplant Settings: outpatients Comparison: placebo or no bisphosphonatesb | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Bisphosphonates | |||||
| New vertebral fractures
Follow‐up: 12 months | 42 per 1000 | 9 per 1000 (1 to 88) | OR 0.22 (0.02 to 2.09) | 142 (5) | ⊕⊝⊝⊝ very lowc,d |
|
| New non‐vertebral fractures
Follow‐up: 12 months | 21 per 1000 | 44 per 1000 (4 to 532) | OR 2.11 (0.18 to 25.35) | 95 (4) | ⊕⊝⊝⊝ very lowd,e |
|
| BMD: % change in BMD at the lumbar spine
Follow‐up: 12 months | The mean % change in BMD (lumbar spine) ranged across control groups from ‐1.8% to 0.7% | The mean % change in BMD in the intervention groups was 6.31% higher (5.39% higher to 7.22% higher) | NA | 171 (6) | ⊕⊕⊝⊝ lowf |
|
| BMD: % change in BMD at the hip or femur
Follow‐up: 12 months | The mean % change in BMD (hip or femur) ranged across control groups from ‐2.8% to ‐0.7% | The mean % change in BMD in the intervention groups was 4.41% higher (3.44% higher to 5.37% higher) | NA | 155 (5) | ⊕⊕⊝⊝ lowf |
|
| QoL: change in physical and mental component scores (SF36v2)
Follow‐up: 12 months | The change in mean (SD) SF36 physical score was ‐3.69 (8.33) in the control group | The mean change in SF36 physical score in the intervention group was 2.51 higher (1.38 lower to 6.40 higher) than in the control group | NA | 47 (1) | ⊕⊕⊝⊝ lowd,g | The results for the physical and mental components are heterogeneous
|
| The change in mean (SD) SF36 mental score was 3.26 (12.27) in the control group | The mean change in SF36 mental score in the intervention group was 5.93 lower (11.73 lower to 0.13 higher) than in the control group | |||||
| Adverse events: bone pain (all routes of bisphosphonate administration)
Follow‐up: 12 months | 38 per 1000 | 323 per 1000 (122 to 857) | OR 8.49 (3.20 to 22.56) | 206 (7) | ⊕⊕⊕⊝ moderateh | Bone pain Separating by route of administration did not change the results; both routes favoured control. Oral bisphosphonates: MD 4.98 (1.24 to 20.09) and IV bisphosphonates: MD 14.17 (3.64 to 55.17)
Fever Oral route: 2 trials of oral alendronate reported that none of the participants in either group experienced fever IV route: 3 trials showed that bisphosphonates were associated with a higher occurrence of fever OR 12.64 (2.31 to 69.11)
GI adverse effects Only measured in the oral bisphosphonates trials. At 12 months, 1 trial reported 3 occurrences of diarrhoea (1 in the bisphosphonate group and 2 in the placebo group) (Aris 2004) 1 trial reported no GI adverse effects in either group (Bianchi 2013) 1 trial reported 10 GI adverse events in the bisphosphonates group compared to 7 in the control group (Papaioannou 2008) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMD: bone mineral density; CI: confidence interval; GI: gastrointestinal; IV: intravenous; MD: mean difference; NA: not applicable; OR: odds ratio; QoL: quality of life; SD: standard deviation; SF36v2: medical outcomes study 36‐item short form. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
| aTrials used different types and formulations of bisphosphonates: IV zoledronate (Boyle 2005; Chapman 2009); IV pamidronate (Haworth 2001); oral alendronate (Aris 2004; Bianchi 2013; Krasovsky 2010; Papaioannou 2008); oral risedronate (Haworth 2011). | ||||||
| Patient or population: children with cystic fibrosis who have not had a lung transplant Settings: outpatients Intervention: oral alendronate plus oral calcifediol and RDA dietary calcium Comparison: oral placebo plus oral calcifediol and RDA dietary calcium | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Bisphosphonates | |||||
| New vertebral fractures
Follow‐up: 12 months | 54 per 1000 | 17 per 1000 (2 to 169) | OR 0.32 (0.03 to 3.13) | 113 (1) | ⊕⊕⊝⊝ lowa |
|
| New non‐vertebral fractures
Follow‐up: 12 months | 36 per 1000 | 7 per 1000 (1 to 145) | OR 0.19 (0.01 to 4.04) | 113 (1) | ⊕⊕⊝⊝ lowa |
|
| BMD: % change in BMD at the lumbar spine
Follow‐up: 12 months | The % change in BMD at the lumbar spine was higher in the treatment group than the control group, MD 14.50 (95% CI 12.91 to 16.09) | NA | 113 (1) | ⊕⊕⊝⊝ lowa |
| |
| BMD: % change in BMD at the hip or femur | This outcome was not reported |
| ||||
| QoL | This outcome was not reported |
| ||||
| Adverse events
Follow‐up: 12 months | There was no difference in bone or muscle pain, OR 3.00 (95% CI 0.12 to 75.22); fever, OR 3.00 (95% CI 0.12 to 75.22); or GI adverse events OR 0.67 (95% CI 0.20 to 2.26) | NA | 113 (1) | ⊕⊕⊝⊝ lowa |
| |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMD: bone mineral density; CI: confidence interval; GI: gastrointestinal; MD: mean difference; NA: not applicable; OR: odds ratio; QoL: quality of life; RDA: recommended daily allowance. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
| aDowngraded twice due to imprecision cause by small participant numbers and low event rates. | ||||||
| Patient or population: adults with cystic fibrosis who have had a lung transplant Settings: outpatients Intervention: bisphosphonates (IV pamidronate plus oral vitamin D and oral calcium) Comparison: oral vitamin D and oral calcium | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Bisphosphonates | |||||
| New vertebral fractures
Follow‐up: 12 months | This outcome was not reported at 12 months ‐ see comments | 1 study reported results at 24 months (Aris 2000) and found no difference in the number of participants with a vertebral fracture, OR 3.92 (95% CI 0.36 to 42.20) | ||||
| New non‐vertebral fractures
Follow‐up: 12 months | This outcome was not reported at 12 months ‐ see comments | 1 study reported results at 24 months (Aris 2000) and found no difference in the number of participants with a non‐vertebral fracture, OR 0.46 (95% CI 0.09 to 2.27) | ||||
| BMD: % change in BMD at the lumbar spine
Follow‐up: 12 months | This outcome was not reported at 12 months ‐ see comments | 1 study reported results at 24 months (Aris 2000) and found the % change in BMD was higher in the treatment group than the control group MD 6.20 (95% CI 4.28 to 8.12) | ||||
| BMD: % change in BMD at the hip or femur
Follow‐up: 12 months | This outcome was not reported at 12 months ‐ see comments | 1 study reported results at 24 months (Aris 2000) and found the % change in BMD was higher in the treatment group than the control group, MD 7.90 (95% CI 5.78 to 10.02). | ||||
| QoL | This outcome was not reported at any time point |
| ||||
| Adverse events
Follow‐up: 12 months | This outcome was not reported at 12 months ‐ see comment | 1 study reported results at 24 months (Aris 2000) and found none of the participants in either group experienced bone pain or fever | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMD: bone mineral density; CI: confidence interval; IV: intravenous; MD: mean difference; OR: odds ratio; QoL: quality of life. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Total fractures Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1.1 12 months | 5 | 142 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.13, 2.50] |
| 1.1.2 24 months | 2 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.2 Non‐vertebral fractures Show forest plot | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 12 months | 4 | 95 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.11 [0.18, 25.35] |
| 1.2.2 24 months | 2 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.3 Vertebral fractures Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.3.1 12 months | 5 | 142 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.02, 2.09] |
| 1.3.2 24 months | 2 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.4 % change in BMD, lumbar spine [DXA] Show forest plot | 8 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.4.1 6 months | 4 | 101 | Mean Difference (IV, Fixed, 95% CI) | 4.61 [3.90, 5.32] |
| 1.4.2 12 months | 6 | 171 | Mean Difference (IV, Fixed, 95% CI) | 6.31 [5.39, 7.22] |
| 1.4.3 24 months | 2 | 43 | Mean Difference (IV, Fixed, 95% CI) | 5.49 [4.38, 6.60] |
| 1.5 % change in BMD, lumbar spine [DXA] (end of trial data only) Show forest plot | 8 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.5.1 End of trial | 8 | 199 | Mean Difference (IV, Fixed, 95% CI) | 5.95 [5.17, 6.73] |
| 1.6 % change in BMD, total hip/femur [DXA] Show forest plot | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.6.1 6 months | 3 | 96 | Mean Difference (IV, Fixed, 95% CI) | 3.58 [2.75, 4.40] |
| 1.6.2 12 months | 5 | 155 | Mean Difference (IV, Fixed, 95% CI) | 4.41 [3.44, 5.37] |
| 1.6.3 24 months | 2 | 42 | Mean Difference (IV, Fixed, 95% CI) | 6.49 [5.32, 7.66] |
| 1.7 % change in BMD, total hip/femur [DXA] (end of trial data only) Show forest plot | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.7.1 End of trial | 6 | 178 | Mean Difference (IV, Fixed, 95% CI) | 4.89 [4.06, 5.72] |
| 1.8 % change in BMD, distal radius [SXA] Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.8.1 6 months | 2 | 48 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.73, 0.78] |
| 1.8.2 12 months | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐0.30, 0.94] |
| 1.8.3 24 months | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 1.50 [0.41, 2.59] |
| 1.9 % change in BMD, distal radius [SXA] (end of trial data only) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.9.1 End of study | 2 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.74 [‐0.21, 1.70] |
| 1.10 % change in BMD, ultra distal radius [SXA] Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.10.1 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.11 QoL Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.11.1 Physical component | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | 2.51 [‐1.38, 6.40] |
| 1.11.2 Mental component | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐5.93 [‐11.73, ‐0.13] |
| 1.12 Bone pain Show forest plot | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.12.1 All routes of bisphosphonate administration | 7 | 206 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.49 [3.20, 22.56] |
| 1.12.2 Oral bisphosphonates | 4 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.98 [1.24, 20.09] |
| 1.12.3 Intravenous bisphosphonates | 3 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 14.17 [3.64, 55.17] |
| 1.13 Fever Show forest plot | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.13.1 All routes of bisphosphonate administration | 5 | 118 | Odds Ratio (M‐H, Fixed, 95% CI) | 12.64 [2.31, 69.11] |
| 1.13.2 Oral bisphosphonates | 2 | 63 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.13.3 Intravenous bisphosphonates | 3 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 12.64 [2.31, 69.11] |
| 1.14 Withdrawals due to adverse events Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.14.1 6 months | 2 | 53 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.95 [0.14, 108.09] |
| 1.14.2 12 months | 5 | 177 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.07 [1.11, 14.90] |
| 1.14.3 24 months | 2 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 16.34 [1.98, 134.89] |
| 1.15 Withdrawals (total) Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.15.1 6 months | 2 | 53 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.27, 11.60] |
| 1.15.2 12 months | 5 | 177 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.45, 2.28] |
| 1.15.3 24 months | 2 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.34, 2.69] |
| 1.16 Survival Show forest plot | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.16.1 6 months | 2 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.05, 16.39] |
| 1.16.2 12 months | 4 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.98 [0.30, 29.84] |
| 1.16.3 24 months | 2 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.27 [0.43, 42.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Total fractures Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1.1 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.2 Non‐vertebral fractures Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.2.1 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.3 Vertebral fractures Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.3.1 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.4 % change in BMD, lumbar spine, DXA Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.4.1 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.5 Bone or muscle pain Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.5.1 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.6 Fever Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.6.1 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.7 Gastrointestinal adverse events Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.7.1 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.8 Headache Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.8.1 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.9 Any adverse event Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.9.1 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.10 Withdrawals, due to adverse events Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.10.1 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.11 Withdrawals, total Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.11.1 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.12 Survival Show forest plot | 1 | 113 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.12.1 12 months | 1 | 113 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Total Fractures Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1.1 24 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2 Non‐vertebral fractures Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2.1 24 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.3 Vertebral fractures Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.3.1 24 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.4 % change in BMD, lumbar spine, DXA Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.4.1 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.5 % change in BMD, femur, DXA Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.5.1 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.6 Bone pain Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.6.1 24 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.7 Withdrawals, due to adverse events Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.8 Withdrawals, total Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.8.1 24 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.9 Survival Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.9.1 24 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

