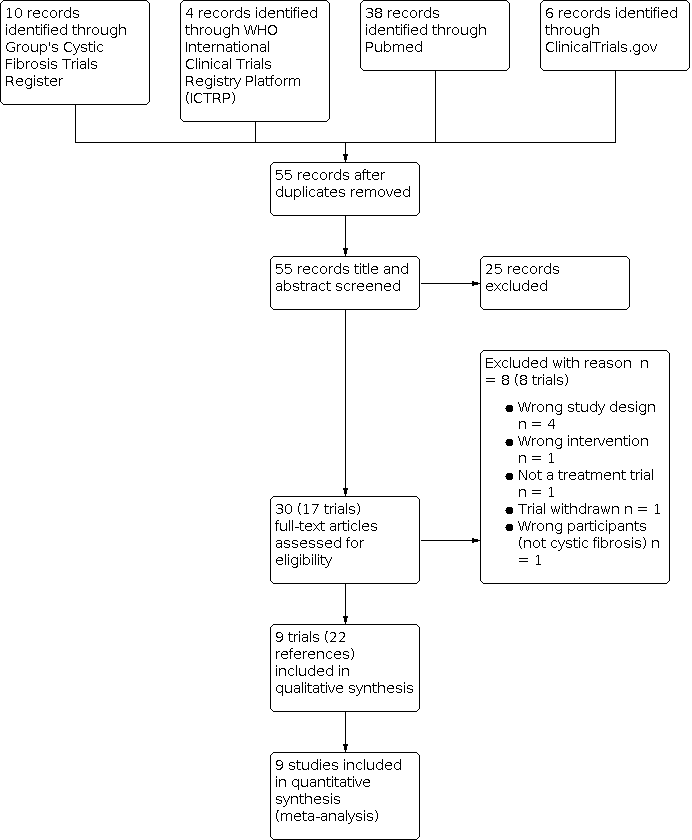
| 1.1 Total fractures Show forest plot | 6 | | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 1.1.1 12 months | 5 | 142 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.13, 2.50] |
| 1.1.2 24 months | 2 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.2 Non‐vertebral fractures Show forest plot | 5 | | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 1.2.1 12 months | 4 | 95 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.11 [0.18, 25.35] |
| 1.2.2 24 months | 2 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.3 Vertebral fractures Show forest plot | 6 | | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 1.3.1 12 months | 5 | 142 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.02, 2.09] |
| 1.3.2 24 months | 2 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.4 % change in BMD, lumbar spine [DXA] Show forest plot | 8 | | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
|
| 1.4.1 6 months | 4 | 101 | Mean Difference (IV, Fixed, 95% CI) | 4.61 [3.90, 5.32] |
| 1.4.2 12 months | 6 | 171 | Mean Difference (IV, Fixed, 95% CI) | 6.31 [5.39, 7.22] |
| 1.4.3 24 months | 2 | 43 | Mean Difference (IV, Fixed, 95% CI) | 5.49 [4.38, 6.60] |
| 1.5 % change in BMD, lumbar spine [DXA] (end of trial data only) Show forest plot | 8 | | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
|
| 1.5.1 End of trial | 8 | 199 | Mean Difference (IV, Fixed, 95% CI) | 5.95 [5.17, 6.73] |
| 1.6 % change in BMD, total hip/femur [DXA] Show forest plot | 6 | | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
|
| 1.6.1 6 months | 3 | 96 | Mean Difference (IV, Fixed, 95% CI) | 3.58 [2.75, 4.40] |
| 1.6.2 12 months | 5 | 155 | Mean Difference (IV, Fixed, 95% CI) | 4.41 [3.44, 5.37] |
| 1.6.3 24 months | 2 | 42 | Mean Difference (IV, Fixed, 95% CI) | 6.49 [5.32, 7.66] |
| 1.7 % change in BMD, total hip/femur [DXA] (end of trial data only) Show forest plot | 6 | | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
|
| 1.7.1 End of trial | 6 | 178 | Mean Difference (IV, Fixed, 95% CI) | 4.89 [4.06, 5.72] |
| 1.8 % change in BMD, distal radius [SXA] Show forest plot | 2 | | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
|
| 1.8.1 6 months | 2 | 48 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.73, 0.78] |
| 1.8.2 12 months | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐0.30, 0.94] |
| 1.8.3 24 months | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 1.50 [0.41, 2.59] |
| 1.9 % change in BMD, distal radius [SXA] (end of trial data only) Show forest plot | 2 | | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
|
| 1.9.1 End of study | 2 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.74 [‐0.21, 1.70] |
| 1.10 % change in BMD, ultra distal radius [SXA] Show forest plot | 1 | | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
|
| 1.10.1 6 months | 1 | | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
| 1.11 QoL Show forest plot | 1 | | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
|
| 1.11.1 Physical component | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | 2.51 [‐1.38, 6.40] |
| 1.11.2 Mental component | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐5.93 [‐11.73, ‐0.13] |
| 1.12 Bone pain Show forest plot | 7 | | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 1.12.1 All routes of bisphosphonate administration | 7 | 206 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.49 [3.20, 22.56] |
| 1.12.2 Oral bisphosphonates | 4 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.98 [1.24, 20.09] |
| 1.12.3 Intravenous bisphosphonates | 3 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 14.17 [3.64, 55.17] |
| 1.13 Fever Show forest plot | 5 | | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 1.13.1 All routes of bisphosphonate administration | 5 | 118 | Odds Ratio (M‐H, Fixed, 95% CI) | 12.64 [2.31, 69.11] |
| 1.13.2 Oral bisphosphonates | 2 | 63 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.13.3 Intravenous bisphosphonates | 3 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 12.64 [2.31, 69.11] |
| 1.14 Withdrawals due to adverse events Show forest plot | 6 | | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 1.14.1 6 months | 2 | 53 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.95 [0.14, 108.09] |
| 1.14.2 12 months | 5 | 177 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.07 [1.11, 14.90] |
| 1.14.3 24 months | 2 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 16.34 [1.98, 134.89] |
| 1.15 Withdrawals (total) Show forest plot | 6 | | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 1.15.1 6 months | 2 | 53 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.27, 11.60] |
| 1.15.2 12 months | 5 | 177 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.45, 2.28] |
| 1.15.3 24 months | 2 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.34, 2.69] |
| 1.16 Survival Show forest plot | 7 | | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 1.16.1 6 months | 2 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.05, 16.39] |
| 1.16.2 12 months | 4 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.98 [0.30, 29.84] |
| 1.16.3 24 months | 2 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.27 [0.43, 42.63] |









![Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 4: % change in BMD, lumbar spine [DXA]](/es/cdsr/doi/10.1002/14651858.CD002010.pub5/media/CDSR/CD002010/image_n/nCD002010-CMP-001.04.svg)
![Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 5: % change in BMD, lumbar spine [DXA] (end of trial data only)](/es/cdsr/doi/10.1002/14651858.CD002010.pub5/media/CDSR/CD002010/image_n/nCD002010-CMP-001.05.svg)
![Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 6: % change in BMD, total hip/femur [DXA]](/es/cdsr/doi/10.1002/14651858.CD002010.pub5/media/CDSR/CD002010/image_n/nCD002010-CMP-001.06.svg)
![Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 7: % change in BMD, total hip/femur [DXA] (end of trial data only)](/es/cdsr/doi/10.1002/14651858.CD002010.pub5/media/CDSR/CD002010/image_n/nCD002010-CMP-001.07.svg)
![Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 8: % change in BMD, distal radius [SXA]](/es/cdsr/doi/10.1002/14651858.CD002010.pub5/media/CDSR/CD002010/image_n/nCD002010-CMP-001.08.svg)
![Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 9: % change in BMD, distal radius [SXA] (end of trial data only)](/es/cdsr/doi/10.1002/14651858.CD002010.pub5/media/CDSR/CD002010/image_n/nCD002010-CMP-001.09.svg)
![Comparison 1: Bisphosphonates versus control (without lung transplantation) ‐ adults (18 years and over), Outcome 10: % change in BMD, ultra distal radius [SXA]](/es/cdsr/doi/10.1002/14651858.CD002010.pub5/media/CDSR/CD002010/image_n/nCD002010-CMP-001.10.svg)



























