Monoterapia con carbamazepina versus fenitoína para la epilepsia: una revisión de datos de participantes individuales
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Single‐centre, randomised, parallel‐group trial of people referred for assessment at Cork Regional Hospital, Ireland 3 treatment arms: carbamazepine, phenytoin, sodium valproate Dates conducted: Not stated | |
| Participants | Adults and children with a minimum of 2 untreated generalised or focal seizures in the 6 months preceding the study Number randomised: PHT = 58, CBZ = 59 52 participants (44%) with focal epilepsy. 61 (52%) men Age range: 4 to 75 years. Duration of treatment (range in months): 3 to 47 | |
| Interventions | Monotherapy with PHT or CBZ Mean daily dose achieved: PHT = 5.4 mg/kg, CBZ = 10.9 mg/kg | |
| Outcomes | Seizure control: excellent (complete freedom of seizures) good (> 50% reduction in seizure frequency) poor (< 50% reduction in seizure frequency or no response) Side effects | |
| Notes | Outcomes chosen for this review were not reported. IPD not available Funding: Grants provided by Labaz, Geigy, and Warner‐Lambert. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation based on 2 Latin squares without stratification. The first, second and third preference of drug for the participant appears to have been taken into account in the process. Unclear if assignment was completely random |
| Allocation concealment (selection bias) | High risk | An independent person (department secretary) selected the “drug of first preference” from randomisation list on a sequential basis. Allocation not adequately concealed |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported. Intention‐to‐treat approach taken, all randomised participants analysed |
| Selective reporting (reporting bias) | Low risk | Primary outcomes (seizure control) and secondary outcomes (side effects) reported sufficiently |
| Other bias | Low risk | No other bias detected |
| Methods | 36‐month randomised, comparative study 4 treatment arms: carbamazepine, sodium valproate, phenytoin, phenobarbitone Dates conducted and country: Not stated (assumed conducted in Poland due to author affiliations) | |
| Participants | Adults with newly‐diagnosed epilepsy Number randomised: CBZ = 30, PHT = 30 100% focal epilepsy, Age range: 18 to 40 years Percentage men and range of follow‐up not mentioned (outcome recorded at 3 years) | |
| Interventions | Monotherapy with PHT or CBZ Starting doses CBZ = 400 mg/day, PHT = 200 mg/day. Dose achieved not stated | |
| Outcomes | Proportion achieving 24‐month remission at 3 years and exclusions after randomisation due to adverse effects or no efficacy | |
| Notes | Abstract only. Outcomes chosen for this review were not reported, IPD pledged but not received Funding: Not stated Conflicts of interest: None stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study randomised but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Unclear risk | "Exclusion rates" reported for all treatment groups, no further information provided |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, study available in abstract format only. Outcomes for this review not available |
| Other bias | Low risk | No other bias detected |
| Methods | Randomised, parallel‐group, open‐label paediatric study conducted in 2 centres in the United Kingdom Trial conducted between 1981 and 1987 4 treatment arms: carbamazepine, sodium valproate, phenytoin, phenobarbitone | |
| Participants | Children with newly‐diagnosed epilepsy (2 or more untreated focal or generalised tonic‐clonic seizures in the 12 months preceding the study) Number randomised: CBZ = 54, PHT = 54 64 children (59%) with focal epilepsy. 59 (55%) boys. Mean age (range): 9 (3 to 16) years Range of follow‐up: 3 to 88 (months) | |
| Interventions | Monotherapy with PHT or CBZ. Median daily dose achieved: PHT = 175 mg/day, CBZ = 400 mg/day | |
| Outcomes | Time to first seizure recurrence after start of therapy Time to 12‐month remission from all seizures Adverse effects and withdrawals due to adverse events | |
| Notes | IPD provided for all randomised participants. All outcomes in this review calculated from IPD Funding: support provided by the Medical Research Council, the Health Promotion Trust, Ciba‐Geigy, Parke‐Davis, and Sanofi Conflicts of interest: None stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation list generated using permuted blocks of size 8 or 16 with stratification for centre, seizure type and presence of neurological signs |
| Allocation concealment (selection bias) | Low risk | Allocation concealed by 4 batches of concealed opaque envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unblinded; authors state masking of treatment would not be “practicable or ethical” and would “undermine compliance.” Unclear if lack of masking influenced outcome |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unblinded; authors state masking of treatment would not be “practicable or ethical” and would “undermine compliance.” Unclear if lack of masking influenced outcome |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, all randomised participants analysed from IPD provided1 |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or calculated with IPD provided1 |
| Other bias | Low risk | No other bias detected |
| Methods | Single‐centre, randomised, parallel‐group trial. 3 treatment arms: carbamazepine, phenytoin, sodium valproate Dates conducted and country: Not stated (assumed conducted in United Kingdom due to author affiliations) | |
| Participants | Children with at least 3 newly‐diagnosed generalised or focal seizures within a period of 6 months Number randomised: PHT = 20, CBZ = 23 No information on epilepsy type, sex or range of follow‐up Age range: 5 to 14 years. Study duration: 12 months | |
| Interventions | Monotherapy with PHT or CBZ Mean dose: PHT = 6.1 mg/day, CBZ = 17.9 mg/day | |
| Outcomes | Cognitive assessments Summary of withdrawals from randomised drug | |
| Notes | Outcomes chosen for this review were not reported IPD not available, but could be constructed from the publication for the outcome 'Time to treatment failure' Funding: A grant was obtained from the Yorkshire Regional Health Authority, support for measuring serum levels provided by Ciba‐Geigy PLC and Sanofi PLC. Conflicts of interest: None stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quota allocation by sex, age, seizure type and current treatment is an inadequate randomisation method |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Personnel and participants (and parents) unblinded |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors single‐blinded for cognitive testing |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, results reported and analysed for all participants randomised and all who completed various stages of follow‐up |
| Selective reporting (reporting bias) | Unclear risk | 1 of 4 outcomes for this review reported. Cognitive outcomes described in Methods section well reported in Results section. Adverse effects reported, no seizure outcomes reported and outcomes chosen for this review not reported. No protocol available so unclear if seizure outcomes were planned a priori |
| Other bias | Low risk | No other bias detected |
| Methods | Randomised, parallel‐group, open‐label paediatric study conducted in 2 centres in the United Kingdom Trial conducted between 1981 and 1987 4 treatment arms: carbamazepine, sodium valproate, phenytoin, phenobarbitone | |
| Participants | Adults with newly‐diagnosed epilepsy (2 or more untreated focal or generalised tonic‐clonic seizures in the 12 months preceding the study) Number randomised: CBZ = 61, PHT = 63 52 participants (42%) with focal epilepsy. 64 (52%) men. Mean age (range): 31 (13 to 72) years Range of follow‐up (months): 1 to 91 | |
| Interventions | Monotherapy with PHT or CBZ. Median daily dose achieved: PHT = 300 mg/day, CBZ = 600 mg/day | |
| Outcomes | Time to first seizure recurrence after start of therapy Time to 12‐month remission from all seizures Adverse effects and withdrawals due to adverse events | |
| Notes | IPD provided for all randomised participants. All outcomes in this review calculated from IPD Funding: support provided by the Medical Research Council, the Health Promotion Trust, Ciba‐Geigy, Parke‐Davis, and Sanofi Conflicts of interest: None stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation list generated using permuted blocks of size 8 or 16 with stratification for centre, seizure type and presence of neurological signs |
| Allocation concealment (selection bias) | Low risk | Allocation concealed by 4 batches of concealed opaque envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unblinded; authors state masking of treatment would not be “practical” and would have “introduced bias due to a very large drop‐out rate.” Unclear if outcome was influenced |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unblinded; authors state masking of treatment would not be “practical” and would have “introduced bias due to a very large drop‐out rate.” Unclear if outcome was influenced |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, all randomised participants analysed from IPD provided1 |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or calculated with IPD provided1 |
| Other bias | Low risk | No other bias detected |
| Methods | Multicentre, randomised, parallel‐group, double‐blinded study over 10 centres in the USA with separate randomisation schemes used for each seizure type 4 treatment arms: carbamazepine, phenytoin, phenobarbitone, primidone Dates conducted: Not stated | |
| Participants | Adults with previously untreated or under‐treated simple or complex focal or secondary generalised tonic‐clonic seizures Number randomised: PHT = 165, CBZ = 155 100% focal epilepsy. 278 (87%) men. Mean age (range): 41 (18 to 82) years Range of follow‐up: 0 to 66 months | |
| Interventions | Monotherapy with PHT or CBZ. Median daily dose achieved: PHT = 400 mg/day, CBZ = 800 mg/day | |
| Outcomes | Participant retention/time to drug failure (length of time participant continued to take randomised drug) Composite scores of seizure frequency (seizure rates and total seizure control) and toxicity Incidence of side effects | |
| Notes | IPD provided for all randomised participants. All outcomes in this review calculated from IPD Funding: supported by the Veterans Adminstration Medical Research Service Cooperative Studies Program (CS 118) Conflicts of interest: None stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomised with stratification for seizure type. Method of randomisation not stated and not provided by authors |
| Allocation concealment (selection bias) | Unclear risk | No information provided in the publication or by study authors |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind (participants and personnel) achieved using an additional blank tablet |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear if outcome assessment was blinded, no information provided |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, all randomised participants analysed from IPD provided1 |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or calculated with IPD provided1 |
| Other bias | Low risk | No other bias detected |
| Methods | Prospective randomised study. 3 treatment arms: carbamazepine, phenytoin, sodium valproate Dates conducted and country: Not stated (assumed conducted in Japan due to author affiliation) | |
| Participants | Children aged 1 to 14 with previously untreated focal seizures or generalised tonic‐clonic seizures, or both Number randomised: PHT = 51, CBZ = 66. 84 (72%) with focal seizures. No information on gender Range of follow‐up: 6 to 66 months, mean follow‐up: 37 months in PHT group, 34 in CBZ group | |
| Interventions | Monotherapy with PHT or CBZ. Initial daily dose: PHT = 7.2 ± 1.4 mg/kg/day, CBZ = 13.0 ± 1.6 mg/kg/day | |
| Outcomes | Proportion of all randomised participants with seizure recurrence (by seizure type) Proportion of participants with optimum plasma levels with seizure recurrence (by seizure type) | |
| Notes | Very limited information available.The study is reported in a summary publication of 3 different studies (other 2 studies are not CBZ vs PHT) Outcomes chosen for this review were not reported, and IPD not available Funding: Not stated Conflicts of interest: None stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study is described as "randomised" but no further details are provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided; unclear if the study was blinded or not |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided; unclear if the study was blinded or not |
| Incomplete outcome data (attrition bias) | Low risk | Ranges of follow‐up given for both treatment groups. Results reported "at the end of follow up," no withdrawals or exclusions mentioned, all participants included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Seizure recurrence outcomes described and well reported. No adverse events reported; no protocol available so unclear if adverse events were planned a priori. Outcomes for this review not available |
| Other bias | Low risk | No other bias detected |
| Methods | Double‐blinded, parallel‐group, randomised study conducted in a single centre in Nigeria between October 2000 and October 2002 3 treatment arms: carbamazepine, phenytoin, phenobarbitone | |
| Participants | Consecutive newly‐diagnosed people aged 14 or over presenting at the outpatient neurology clinic of the University Teaching Hopsital, Benin City, Nigeria with recurrent, untreated afebrile seizures Number randomised: PHT = 19, CBZ = 19 8 participants with focal seizures (22%), 23 men (62%). Mean age (range): 29.8 years (14 to 38 years) All participants followed up for 12 weeks | |
| Interventions | Monotherapy with PHT or CBZ. Median daily dose (range): PHT = 200 mg (100 to 300 mg), CBZ = 600 mg (400 to 1200 mg) | |
| Outcomes | Cognitive measures (reaction times, mental speed, memory, attention) | |
| Notes | IPD provided for all randomised participants. Study duration was 12 weeks; all participants completed the study without withdrawing, so outcomes 'Time to treatment failure', 'Time to six‐month remission' and 'Time to 12‐month remission' could not be calculated. 'Time to first seizure' calculated from IPD provided Funding: Not stated Conflicts of interest: None stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study randomised using simple randomisation. Each participant was asked to pick 1 from a table of numbers (1 ‐ 60), numbers corresponded to allocation of 1 of 3 drugs (information provided by author) |
| Allocation concealment (selection bias) | Low risk | Recruitment/randomisation of participants and allocation of treatments took place on different sites (information provided by author) |
| Blinding of participants and personnel (performance bias) | Low risk | Participants single‐blinded. Research assistant recruiting participants and counselling on medication adherence was not blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Investigators performing cognitive assessments were single‐blinded |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants completed the study. All randomised participants analysed from IPD provided1 |
| Selective reporting (reporting bias) | Low risk | 1 outcome for this review calculated from IPD provided1. Other outcomes for this review not available due to short study length. All cognitive outcomes from the study well reported |
| Other bias | Low risk | No other bias detected |
| Methods | Single‐centre, randomised, parallel‐group trial of participants, referrals to the outpatient department of neurology of the Central Hospital of Paijat‐Hame, Finland 2 treatment arms: carbamazepine and phenytoin Dates conducted: Not stated | |
| Participants | Adults (eligible age range 15 to 57) with newly‐diagnosed epilepsy Number randomised: PHT = 20, CBZ = 23* 10 (23%) participants with focal epilepsy. 20 (47%) men Mean age (SD) years: PHT = 31.5 (11.3), CBZ = 26.8 (13.2) | |
| Interventions | Monotherapy with PHT or CBZ. Dose information not reported | |
| Outcomes | Cognitive assessments (visual motor speed, co‐ordination, attention and concentration, verbal and visuospatial learning, visual and recognition memory, reasoning, mood, handedness) Harmful side effects | |
| Notes | *59 participants were randomised but 16 were subsequently excluded. Results were presented only for the 43 participants who completed the entire study Outcomes chosen for this review were not reported. IPD not available Funding: Not stated Conflicts of interest: None stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to treatment groups, method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided; unclear if participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Cognitive outcome assessor was blinded |
| Incomplete outcome data (attrition bias) | High risk | 16/59 (27%) of participants excluded from analysis. Results presented only for 43 participants who completed the study |
| Selective reporting (reporting bias) | Unclear risk | Cognitive outcomes described in Methods section well reported in Results section. Adverse effects reported, no seizure outcomes reported and outcomes chosen for this review not reported. No protocol available so unclear if seizure outcomes were planned a priori |
| Other bias | Low risk | No other bias detected |
| Methods | Randomised, 'two compartment' parallel study, conducted in the USA 2 treatment arms: carbamazepine, phenytoin Dates conducted: Not stated | |
| Participants | Adults, previously untreated, with at least 2 seizures or at least 1 seizure and an EEG with paroxysmal features Number randomised: PHT = 45, CBZ = 42 55 participants (63%) with focal epilepsy. 60 (69%) men. Overall mean age (range) 37.4 (18 to 77) years Study duration: 2 years. Range of follow‐up not reported | |
| Interventions | Monotherapy with PHT or CBZ Mean daily dose achieved (for the 54 participants with no major side effects): PHT = 5.35 mg/kg/day, CBZ = 9.32 mg/kg/day | |
| Outcomes | Laboratory measures Side effects (major and minor) Seizure control/treatment failure | |
| Notes | 7 participants on CBZ and 10 participants on PHT were “dropped for non‐compliance” and excluded from analysis Outcomes chosen for this review were not reported. IPD not available Funding: Supported in part by the Southern Foundation for Brain Research Conflicts of interest: None stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomly assigned to treatment groups; method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind (participants and personnel) achieved with additional blank tablet |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear if outcome assessors were blinded |
| Incomplete outcome data (attrition bias) | High risk | 17/87 (19.5%) of participants excluded from analysis for "non‐compliance". Results presented only for participants who completed the study |
| Selective reporting (reporting bias) | Low risk | All efficacy and tolerability outcomes specified in the Methods sections reported well in the Results section. No protocol available. Outcomes chosen for this review were not reported |
| Other bias | Low risk | No other bias detected |
| Methods | Single‐centre, randomised, parallel‐group study of participants referred to the Neurology Clinic of Nehru Hospital, Chandigarh, India 2 treatment arms: carbamazepine, phenytoin Dates conducted: Not stated | |
| Participants | Newly‐diagnosed and drug naïve adults over the age of 14 attending the Neurology Clinic of Nehru Hospital, Chandigarh, India Number randomised: PHT = 20, CBZ = 20 11 participants with focal epilepsy (27.5%), 28 men (70%) Mean age (range): PHT group 23.4 (14 to 44 years), CBZ 24.4 (14 to 45 years) Study duration 10 to 12 weeks. Range of follow‐up not reported | |
| Interventions | Monotherapy with PHT or CBZ. Initial daily dose: PHT = 5 mg/kg/day, CBZ = 10 mg/kg/day | |
| Outcomes | Cognitive measures before and after treatments (verbal, performance, memory, visuomotor, perceptomotor organisation, visual organisation, dysfunction) | |
| Notes | 6 participants on CBZ and 8 participants on PHT were excluded from final analysis of cognitive assessments who were lost to follow‐up or who had uncontrolled seizures Outcomes chosen for this review were not reported. IPD not available Funding: Not stated Conflicts of interest: None stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The subjects were randomised to one of the two study groups", no further information given on methods of randomisation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided; unclear if study was blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided; unclear if study was blinded |
| Incomplete outcome data (attrition bias) | High risk | 14/40 (35%) of participants excluded from analysis who were lost to follow‐up or experienced uncontrolled seizures. Results presented only for participants who completed the study |
| Selective reporting (reporting bias) | Unclear risk | Cognitive outcomes described in Methods section well reported in Results section. No seizure outcomes or adverse events reported and outcomes chosen for this review not reported. No protocol available, so unclear if seizure outcomes were planned a priori |
| Other bias | Low risk | No other bias detected |
1For studies in which IPD were provided (De Silva 1996; Heller 1995; Mattson 1985; Ogunrin 2005) attrition and reporting bias are reduced as attrition rates and unpublished outcome data are requested.
CBZ: carbamazepine
EEG: electroencephalograph
IPD: individual participant data
PHT: phenytoin
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Unclear whether trial is randomised and unclear whether participants received either CBZ or PHT as monotherapy. Authors could not be contacted to clarify, so trial excluded due to uncertainties. | |
| Comparison between CBZ monotherapy and PHT monotherapy cannot be made. Participants were given phenobarbital initially which was later withdrawn whilst either CBZ or PHT was also introduced | |
| Comparison between CBZ monotherapy and PHT monotherapy cannot be made. No randomised monotherapy comparison between CBZ and PHT. Participants were separated into 2 treatment arms (based on previous drug failure) and randomised to CBZ and clobazam in 1 arm and PHT or clobazam in the other arm | |
| Cross‐over design; cross‐over studies are not an appropriate design for measuring the long‐term outcomes of interest in this review | |
| Comparison between CBZ monotherapy and PHT monotherapy cannot be made. Participants who failed CBZ or PHT monotherapy were randomised to levetiracetam or VPS monotherapy | |
| Participants were randomised to lamotrigine or 'standard therapy' (PHT, CBZ or VPA at the choice of the investigator). No randomised comparison can be made of CBZ and PHT | |
| Comparison between CBZ monotherapy and PHT monotherapy cannot be made. All medication except phenobarbital and primidone were discontinued gradually, whilst dose of randomised drug CBZ or PHT was increased | |
| Study is not randomised; participants were already on CBZ or PHT monotherapy on entry into the study | |
| Unclear if the study was randomised. Comparison between CBZ monotherapy and PHT monotherapy cannot be made. The trial has a cross‐over design with a 2‐week washout period in which both drugs were taken to make a gradual transition | |
| Unclear whether trial is randomised and unclear whether participants received either CBZ or PHT as monotherapy. Authors could not be contacted to clarify therefore trial excluded due to uncertainties. | |
| Not fully randomised: “The treatment was chosen at random unless the individual diagnoses required a specific drug” | |
| Direct comparison between CBZ and PHT not available. The publication reports 2 separate randomised studies, the first compares VPS and PHT and the second compares VPS and CBZ | |
| Study is not randomised | |
| Randomised participants were slowly withdrawn from their previous treatment as part of the trial and therefore a comparison between CBZ and PHT monotherapy cannot be made | |
| All participants received PHT for 2 months prior to entering a randomised cross‐over period. It is unclear whether a comparison between CBZ and PHT monotherapy could be made | |
| The study is not randomised ‐ the investigator made the choice of treatment for each participant |
CBZ: carbamazepine; PHT: phenytoin; VPS: sodium valproate
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to treatment failure (any reason related to the treatment) Show forest plot | 4 | 589 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.76, 1.31] |
| Analysis 1.1  Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 1 Time to treatment failure (any reason related to the treatment). | ||||
| 2 Time to treatment failure due to adverse events Show forest plot | 4 | 589 | Hazard Ratio (Fixed, 95% CI) | 1.35 [0.93, 1.95] |
| Analysis 1.2  Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 2 Time to treatment failure due to adverse events. | ||||
| 3 Time to treatment failure due to lack of efficacy Show forest plot | 4 | 589 | Hazard Ratio (Fixed, 95% CI) | 1.02 [0.72, 1.44] |
| Analysis 1.3  Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 3 Time to treatment failure due to lack of efficacy. | ||||
| 4 Time to treatment failure (any reason related to the treatment) ‐ by epilepsy type Show forest plot | 3 | 546 | Hazard Ratio (Fixed, 95% CI) | 0.94 [0.70, 1.26] |
| Analysis 1.4  Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 4 Time to treatment failure (any reason related to the treatment) ‐ by epilepsy type. | ||||
| 4.1 Focal onset seizures | 3 | 428 | Hazard Ratio (Fixed, 95% CI) | 0.83 [0.61, 1.13] |
| 4.2 Generalised onset seizures | 2 | 118 | Hazard Ratio (Fixed, 95% CI) | 2.38 [1.04, 5.47] |
| 5 Time to treatment failure due to adverse events ‐ by epilepsy type Show forest plot | 3 | 546 | Hazard Ratio (Fixed, 95% CI) | 1.27 [0.87, 1.86] |
| Analysis 1.5  Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 5 Time to treatment failure due to adverse events ‐ by epilepsy type. | ||||
| 5.1 Generalised onset seizures | 2 | 118 | Hazard Ratio (Fixed, 95% CI) | 2.31 [0.68, 7.81] |
| 5.2 Focal onset seizures | 3 | 428 | Hazard Ratio (Fixed, 95% CI) | 1.19 [0.80, 1.78] |
| 6 Time to treatment failure due to lack of efficacy ‐ by epilepsy type Show forest plot | 3 | 546 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.69, 1.41] |
| Analysis 1.6  Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 6 Time to treatment failure due to lack of efficacy ‐ by epilepsy type. | ||||
| 6.1 Focal onset seizures | 3 | 428 | Hazard Ratio (Fixed, 95% CI) | 0.88 [0.60, 1.30] |
| 6.2 Generalised onset seizures | 2 | 118 | Hazard Ratio (Fixed, 95% CI) | 1.86 [0.74, 4.67] |
| 7 Time to first seizure post‐randomisation Show forest plot | 4 | 582 | Hazard Ratio (Fixed, 95% CI) | 1.13 [0.92, 1.39] |
| Analysis 1.7  Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 7 Time to first seizure post‐randomisation. | ||||
| 8 Time to first seizure post‐randomisation ‐ by epilepsy type Show forest plot | 4 | 582 | Hazard Ratio (Fixed, 95% CI) | 1.15 [0.94, 1.40] |
| Analysis 1.8  Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 8 Time to first seizure post‐randomisation ‐ by epilepsy type. | ||||
| 8.1 Focal onset seizures | 4 | 432 | Hazard Ratio (Fixed, 95% CI) | 1.13 [0.89, 1.43] |
| 8.2 Generalised onset seizures | 3 | 150 | Hazard Ratio (Fixed, 95% CI) | 1.19 [0.81, 1.75] |
| 9 Time to achieve 12‐month remission Show forest plot | 3 | 551 | Hazard Ratio (Fixed, 95% CI) | 1.01 [0.80, 1.27] |
| Analysis 1.9  Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 9 Time to achieve 12‐month remission. | ||||
| 10 Time to achieve 12‐month remission ‐ by epilepsy type Show forest plot | 3 | 551 | Hazard Ratio (Fixed, 95% CI) | 1.00 [0.79, 1.26] |
| Analysis 1.10  Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 10 Time to achieve 12‐month remission ‐ by epilepsy type. | ||||
| 10.1 Focal onset seizures | 3 | 430 | Hazard Ratio (Fixed, 95% CI) | 1.06 [0.80, 1.42] |
| 10.2 Generalised onset seizures | 2 | 121 | Hazard Ratio (Fixed, 95% CI) | 0.88 [0.58, 1.33] |
| 11 Time to achieve six‐month remission Show forest plot | 3 | 551 | Hazard Ratio (Fixed, 95% CI) | 0.92 [0.74, 1.14] |
| Analysis 1.11  Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 11 Time to achieve six‐month remission. | ||||
| 12 Time to achieve six‐month remission ‐ by epilepsy type Show forest plot | 3 | 551 | Hazard Ratio (Fixed, 95% CI) | 0.90 [0.73, 1.12] |
| Analysis 1.12  Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 12 Time to achieve six‐month remission ‐ by epilepsy type. | ||||
| 12.1 Focal onset seizures | 3 | 430 | Hazard Ratio (Fixed, 95% CI) | 0.98 [0.75, 1.27] |
| 12.2 Generalised onset seizures | 2 | 121 | Hazard Ratio (Fixed, 95% CI) | 0.77 [0.52, 1.13] |
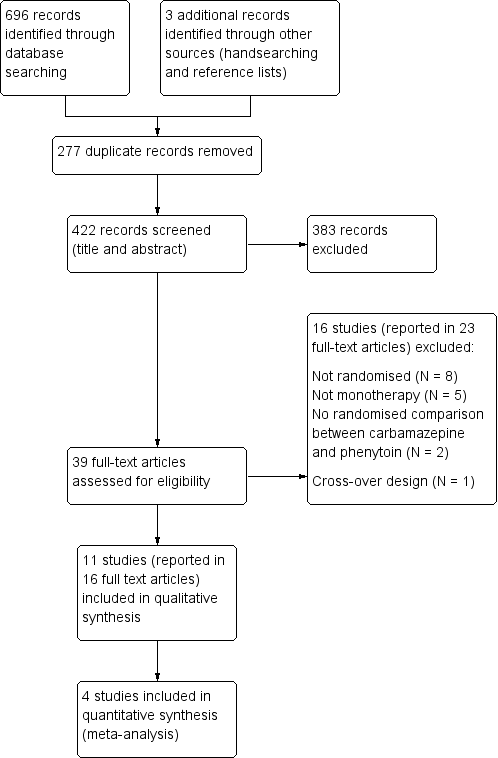
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
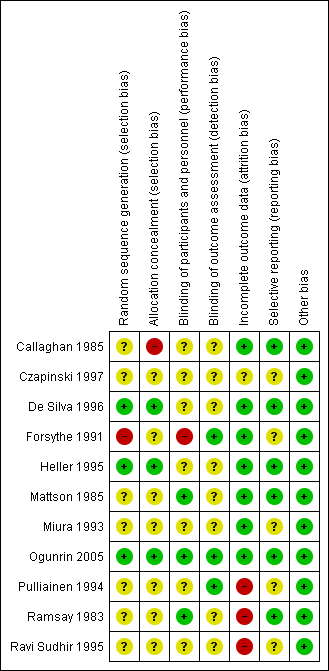
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
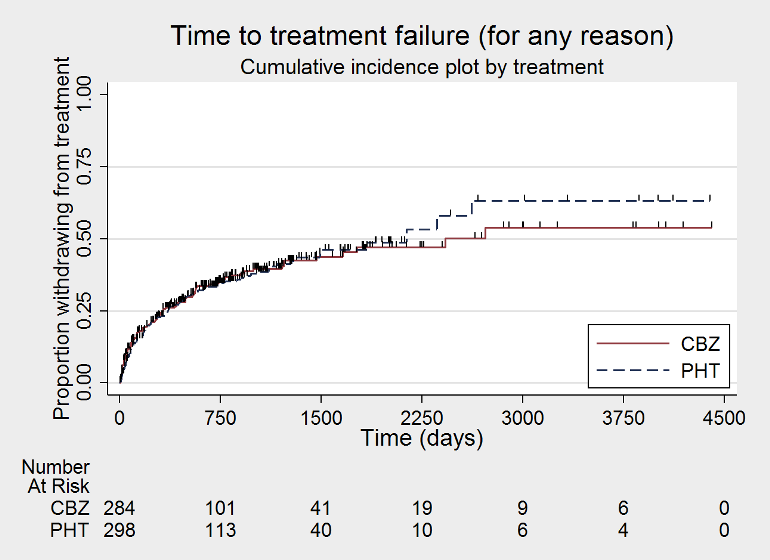
Time to treatment failure ‐ any reason related to the treatment (CBZ: carbamazepine; PHT: phenytoin)

Time to treatment failure ‐ any reason related to the treatment, by epilepsy type (CBZ: carbamazepine; PHT: phenytoin)

Time to treatment failure due to adverse events (CBZ: carbamazepine; PHT: phenytoin)
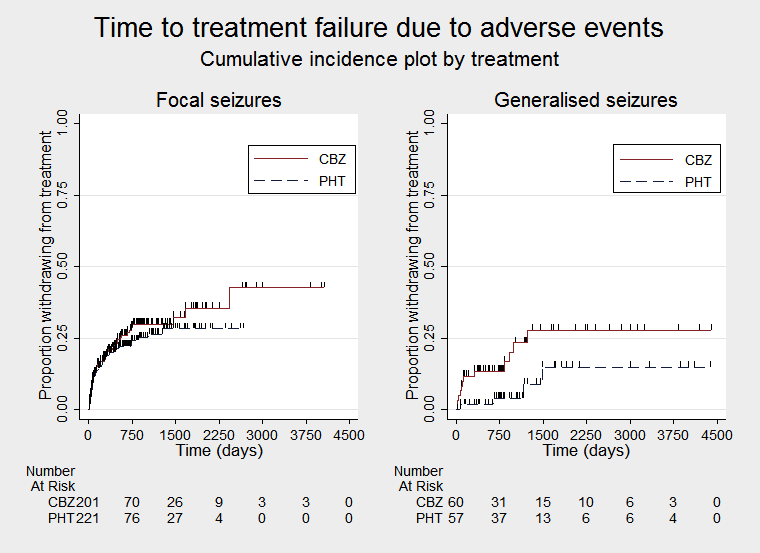
Time to treatment failure due to adverse events, by epilepsy type (CBZ: carbamazepine; PHT: phenytoin)

Time to treatment failure due to lack of efficacy (CBZ: carbamazepine; PHT: phenytoin)

Time to treatment failure due to lack of efficacy, by epilepsy type (CBZ: carbamazepine; PHT: phenytoin)
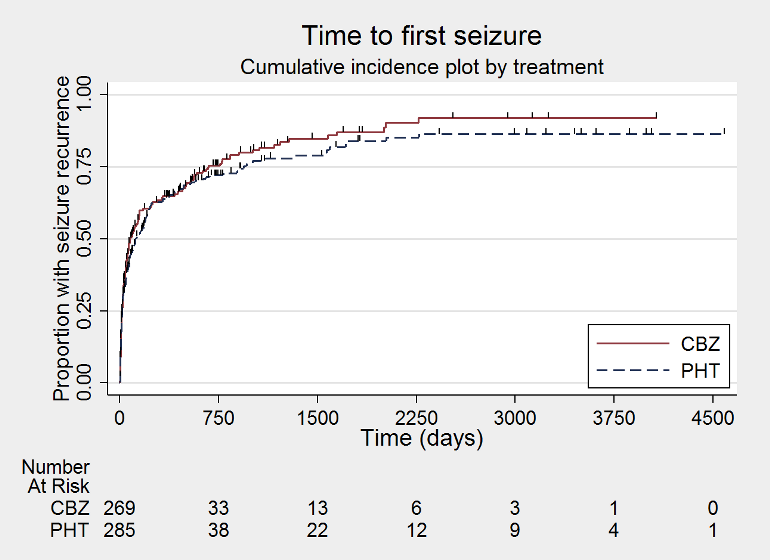
Time to first seizure (CBZ: carbamazepine; PHT: phenytoin)
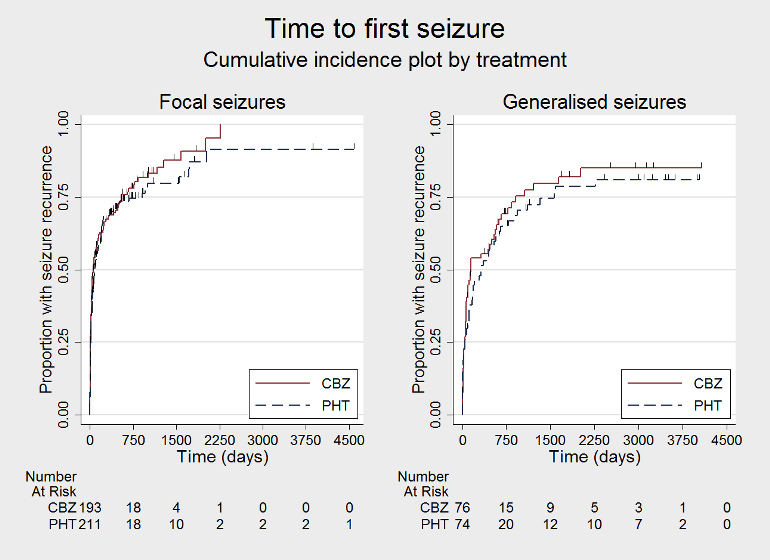
Time to first seizure, by epilepsy type (CBZ: carbamazepine; PHT: phenytoin)
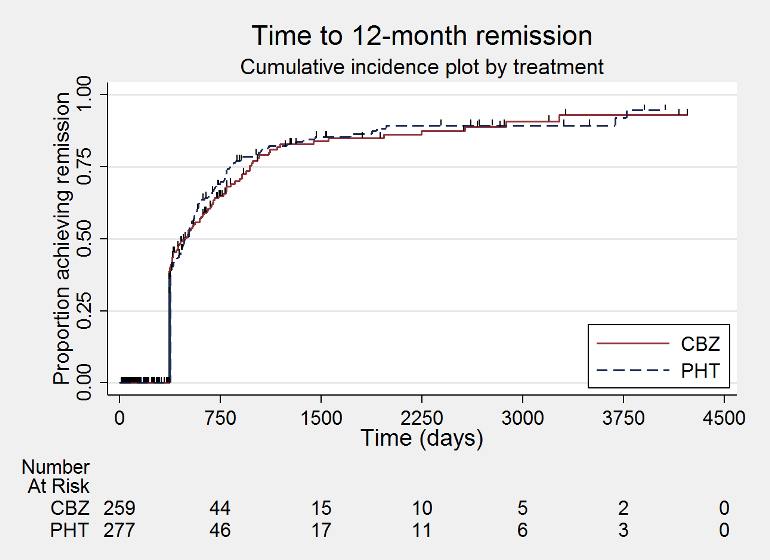
Time to 12 month remission (CBZ: carbamazepine; PHT: phenytoin)

Time to 12 month remission, by epilepsy type (CBZ: carbamazepine; PHT: phenytoin)
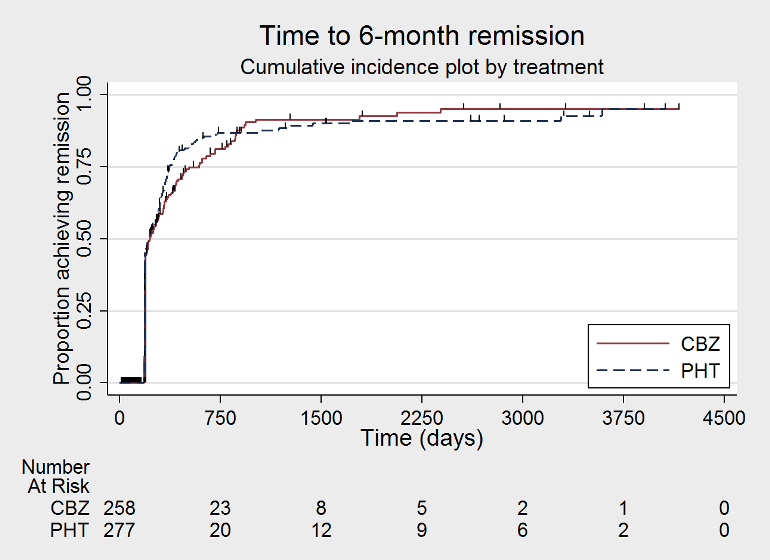
Time to 6 month remission (CBZ: carbamazepine; PHT: phenytoin)
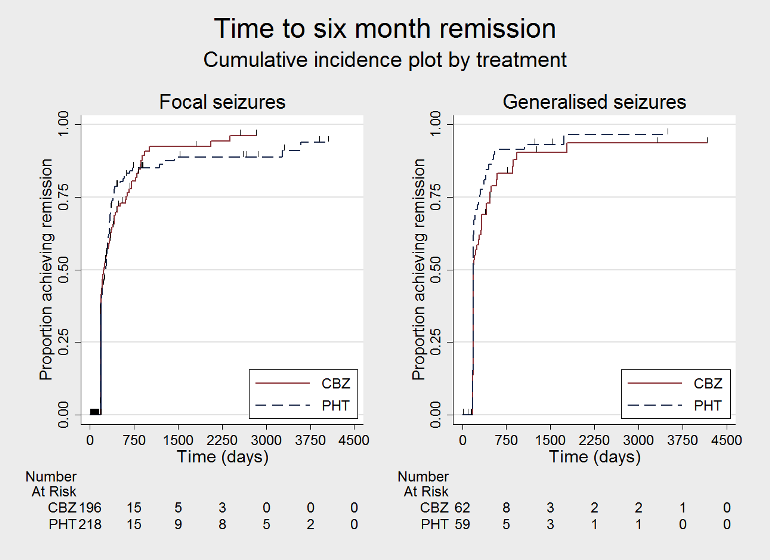
Time to 6 month remission, by epilepsy type (CBZ: carbamazepine; PHT: phenytoin)
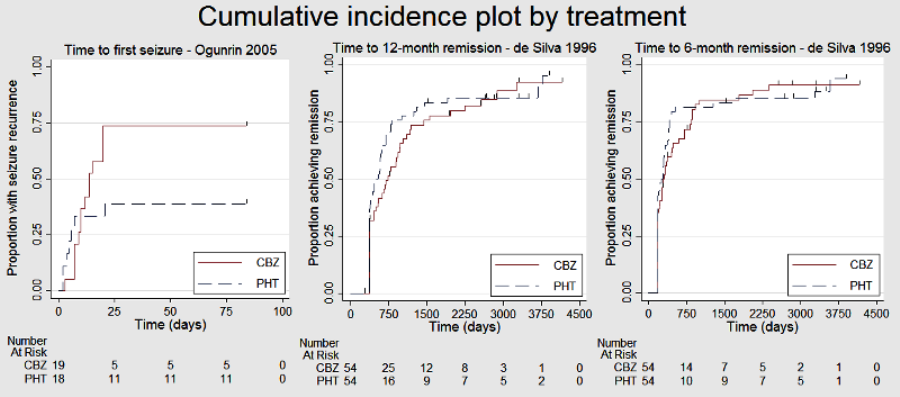
Cumulative incidence plots, proportional hazards assumption checks

Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 1 Time to treatment failure (any reason related to the treatment).

Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 2 Time to treatment failure due to adverse events.
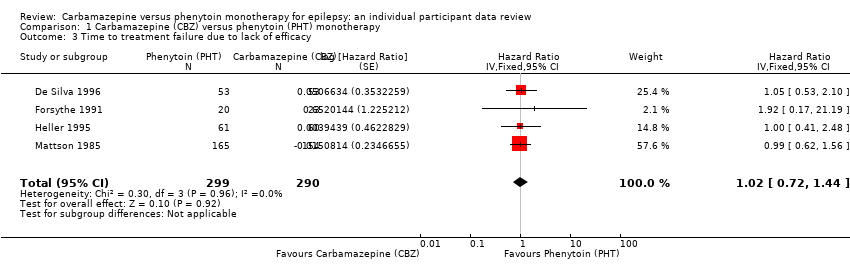
Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 3 Time to treatment failure due to lack of efficacy.

Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 4 Time to treatment failure (any reason related to the treatment) ‐ by epilepsy type.

Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 5 Time to treatment failure due to adverse events ‐ by epilepsy type.
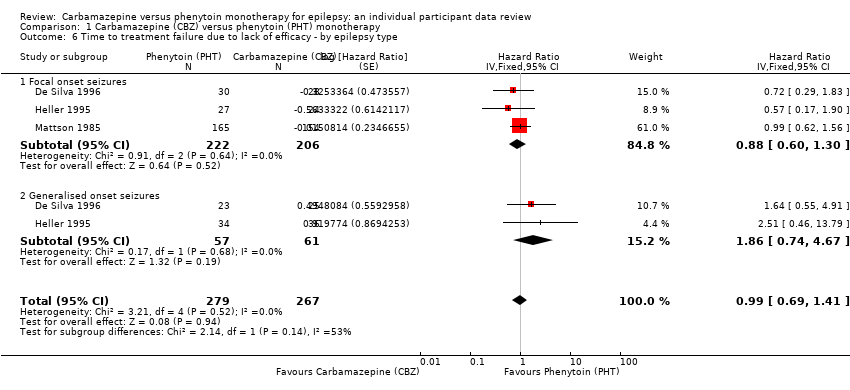
Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 6 Time to treatment failure due to lack of efficacy ‐ by epilepsy type.
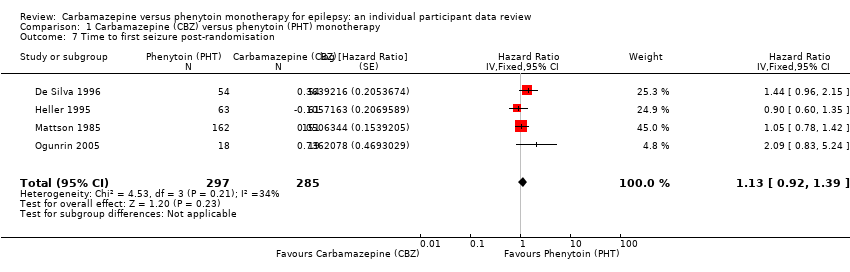
Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 7 Time to first seizure post‐randomisation.
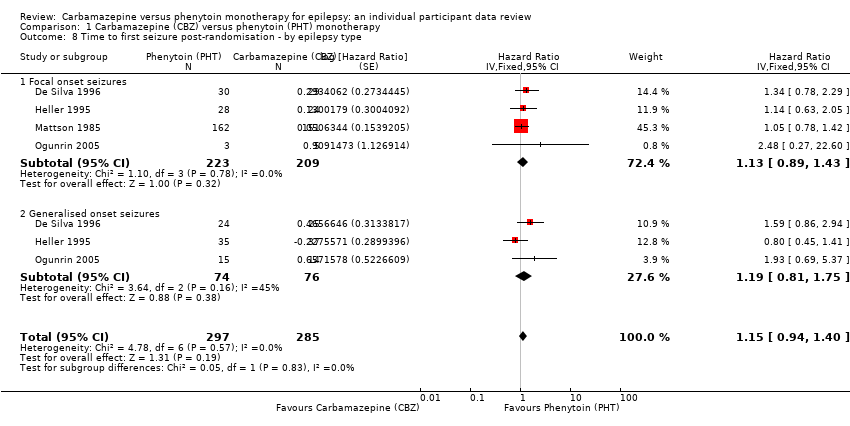
Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 8 Time to first seizure post‐randomisation ‐ by epilepsy type.

Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 9 Time to achieve 12‐month remission.
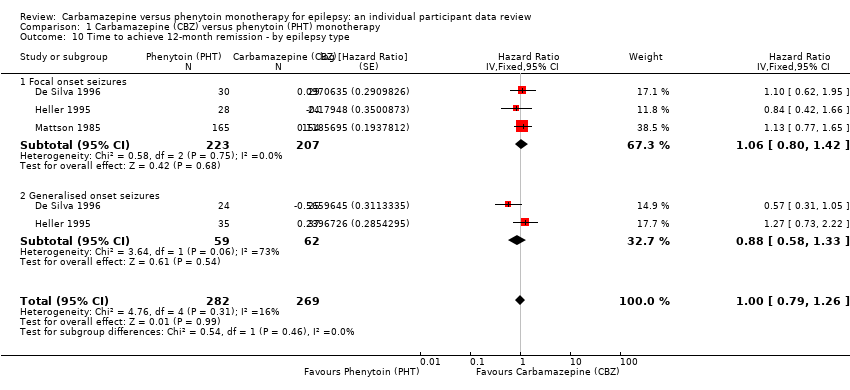
Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 10 Time to achieve 12‐month remission ‐ by epilepsy type.

Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 11 Time to achieve six‐month remission.

Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 12 Time to achieve six‐month remission ‐ by epilepsy type.
| Carbamazepine compared with phenytoin for epilepsy | ||||||
| Patient or population: adults and children with new‐onset focal or generalised epilepsy Settings: outpatients Intervention: carbamazepine Comparison: phenytoin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of Participants | Certainty (quality) of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Phenytoin | Carbamazepine | |||||
| Time to treatment failure (any reason related to treatment) Range of follow‐up:1 day to 4403 days | The median time to treatment failure was 2135 days in the phenytoin group | The median time to treatment failure was 2422 days (307 days longer) in the carbamazepine group | HR 0.94 (0.70 to 1.26)a | 546 (3 studies) | ⊕⊕⊕⊝ moderateb | HR < 1 indicates a clinical advantage for carbamazepine There was also no statistically significant difference between drugs in treatment failure due to adverse events (HR 1.27, 95% CI 0.87 to 1.86; P = 0.21; I2 = 3%), or treatment failure due to lack of efficacy: HR 0.99 (95% CI 0.69 to 1.41; P = 0.94; I2 = 0%) |
| Time to treatment failure (any reason related to treatment) Subgroup: focal onset seizures Range of follow‐up: 1 day to 4064 days | The median time to treatment failure was 1300 days in the phenytoin group | The median time to treatment failure was 2422 days (1122 days longer) in the carbamazepine group | HR 0.83 (0.61 to 1.13) | 428 (3 studies) | ⊕⊕⊕⊝ moderateb | HR < 1 indicates a clinical advantage for carbamazepine There was also no statistically significant difference between drugs in treatment failure due to adverse events (HR 1.19, 95% CI 0.80 to 1.78; P = 0.38, I2 = 0%), or treatment failure due to lack of efficacy: HR 0.88 (95% CI 0.60 to 1.40, P = 0.52, I2 = 0%) |
| Time to treatment failure (any reason related to treatment) Subgroup: generalised Range of follow‐up:1 day to 4403 days | The 10th percentilec | The 10th percentilec | HR 2.38 (1.04 to 5.47) | 118 (2 studies) | ⊕⊕⊝⊝ lowb,d | HR < 1 indicates a clinical advantage for carbamazepine There was no statistically significant difference between drugs in treatment failure due to adverse events (HR 2.31, 95% CI 0.68 to 7.81; P = 0.18; I2 = 60% , or treatment failure due to lack of efficacy: HR 1.86 (95% CI 0.74 to 4.67; P = 0.19; I2 = 0%) but confidence intervals are wide so we cannot rule out an advantage to either drug or no difference between the drugs |
| *Illustrative risks in the carbamazepine and phenytoin groups are calculated at the median time to treatment failure (i.e. the time to 50% of participants failing or withdrawing from allocated treatment) within each group across all trials. The relative effect (pooled hazard ratio) shows the comparison of 'Time to treatment failure' between the treatment groups. CI: 95% confidence interval; HR: hazard ratio | ||||||
| GRADE Working Group grades of evidence High certainty (quality): Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty (quality): Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty (quality): Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very certainty (quality): We are very uncertain about the estimate. | ||||||
| aPooled HR for all participants adjusted for seizure type. All pooled HRs presented calculated with fixed‐effect model. | ||||||
| Carbamazepine compared with phenytoin for epilepsy | ||||||
| Patient or population: adults and children with new‐onset focal or generalised epilepsy Settings: outpatients Intervention: carbamazepine Comparison: phenytoin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of Participants | Certainty (quality) of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Phenytoin | Carbamazepine | |||||
| Time to first seizure after randomisation Range of follow‐up: 0 days to 4589 days | The median time to first seizure was 124 days in the phenytoin group | The median time to first seizure was 79 days (45 days shorter) in the carbamazepine group | HR 1.15 (0.94 to 1.40) | 582 (4 studies) | ⊕⊕⊕⊝ moderateb | HR < 1 indicates a clinical |
| Time to first seizure after randomisation Subgroup: focal onset seizures Range of follow‐up: 0 days to 4589 days | The median time to first seizure was 78 days in the phenytoin group | The median time to first seizure was 62 days (16 days shorter) in the carbamazepine group | HR 1.13 (0.89 to 1.43) | 432 (4 studies) | ⊕⊕⊕⊝ moderateb | HR < 1 indicates a clinical |
| Time to first seizure after randomisation Range of follow‐up: 2 days to 4070 days | The median time to first seizure was 323 days in the phenytoin group | The median time to first seizure was 142 days (181 days shorter) in the carbamazepine group | HR 1.19 (0.81 to 1.75) | 150 (3 studies) | ⊕⊕⊝⊝ lowb,c | HR < 1 indicates a clinical |
| Time to achieve 12‐month remission Range of follow‐up: 0 days to 4222 days | The median time to 12‐month remission was 472 days in the phenytoin group | The median time to 12‐month remission was 481 days (9 days longer) in the carbamazepine group | HR 1.00 (0.79 to 1.26) | 551 (3 studies) | ⊕⊕⊕⊝ moderateb | HR < 1 indicates a clinical |
| Time to achieve 12‐month remission Range of follow‐up: 0 days to 4222 days | The median time to 12‐month remission was 531 days in the phenytoin group | The median time to 12‐month remission was 515 days (16 days shorter) in the carbamazepine group | HR 1.06 (0.80 to 1.42) | 430 (3 studies) | ⊕⊕⊕⊝ moderateb | HR < 1 indicates a clinical |
| Time to achieve 12‐month remission Range of follow‐up: 7 days to 4163 days | The median time to 12‐month remission was 366 days in the phenytoin group | The median time to 12‐month remission was 375 days (9 days longer) in the carbamazepine group | HR 0.88 (0.58 to 1.33) | 121 (2 studies) | ⊕⊕⊝⊝ lowb,d | HR < 1 indicates a clinical |
| *Illustrative risks in the carbamazepine and phenytoin groups are calculated at the median time to first seizure or time to 12‐month remission (i.e. the time to 50% of participants experiencing a first seizure or 12‐months of remission) within each group across all trials. The relative effect (pooled hazard ratio) shows the comparison of 'Time to first seizure' or 'Time to 12‐month remission' between the treatment groups. CI: 95% confidence interval; HR: hazard ratio | ||||||
| GRADE Working Group grades of evidence High certainty (quality): Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty (quality): Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty (quality): Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very certainty (quality): We are very uncertain about the estimate. | ||||||
| aPooled HR for all participants adjusted for seizure type. All pooled HRs presented calculated with a fixed‐effect model. | ||||||
| Trial | Outcomes reported | Summary of results |
| 1. Seizure control: excellent (seizure‐free) | 1. PHT (n = 58); CBZ (n = 59) PHT: 39 (67%); CBZ: 22 (37%) | |
| 1. Proportion achieving 24‐month remission at 3 years 2. Proportion excluded after randomisation due to adverse effects or no efficacy | 1. PHT: 59%; CBZ: 62% 2. PHT: 23%; CBZ: 30% | |
| 1. Cognitive assessments 2. Withdrawals from randomised drug | 1. No significant differences between the two treatment groups on any cognitive tests | |
| 1. Proportion of all randomised participants with seizure recurrence (by seizure type) 2. Proportion of participants with optimum plasma levels with seizure recurrence (by seizure type) | PHT (n = 51); CBZ (n = 66) 1. PHT (focal): 10/31 (32%); PHT (generalised): 7/20 (35%); 2. PHT (focal): 4/17 (24%); PHT (generalised): 1/8 (13%); | |
| 1. Cognitive assessments (visual motor speed, co‐ordination, attention and concentration, verbal and visual‐spatial learning, visual and recognition memory, reasoning, mood, handedness) 2. Harmful side effects | 1. Compared to CBZ, participants on PHT became slower (motor speed of the hand) and their visual memory decreased. There was an equal decrease in negative mood (helplessness, irritability, depression) on PHT and CBZ
| |
| 1. Side effects (major and minor) 3. Laboratory results | 1. Incidence of:
2. Treatment failures among analysed participants: Seizure control (among analysed participants with no major side effects): PHT: 23/27 participants (86%); CBZ: 22/27 participants (82%) 3. Significantly lower mean LDH level at 24 weeks in CBZ participants than PHT participants (P < 0.01). Other laboratory results similar across treatment groups | |
| 1. Cognitive measures (verbal, performance, memory, visual‐motor, perceptomotor organisation, visual organisation, dysfunction) | 1. No significant differences between any tests of cognitive function taken before treatment and after 10 ‐ 12 weeks for both treatment groups | |
| CBZ = carbamazepine; LDH = lactate dehydrogenase; PHT= phenytoin | ||
| Trial | Focal seizures: n (%) | Male participants: | Age at entry (years): Mean (SD), rangeb | Epilepsy duration | Number of seizures in prior 6 months, median (range)d | |||||
| CBZ | PHT | CBZ | PHT | CBZ | PHT | CBZ | PHT | CBZ | PHT | |
| 29 (54%) | 30 (56%) | 30 (56%) | 34 (63%) | 9.2 (3.8) 2 to 15 | 9.5 (3.4) (3 to 16) | 1.7 (2.6), 0 to 12 | 1.0 (2.1) (0 to 14) | 3 (1 to 500) | 3 (1 to 404) | |
| 24 (39%) | 28 (44%) | 30 (49%) | 34 (54%) | 29.3 (14.1) 13 to 69 | 33.5 (14.3) (14 to 72) | 4.4 (7.4), 0.1 to 40 | 3.8 (5.4) (0 to 24) | 2 (1 to 354) | 2 (1 to 575) | |
| 155 (100%) | 165 (100%) | 133 (87%) | 145 (88%) | 42.1 (15.9) 18 to 82 | 40.8 (15.3) (18 to 81) | 5.9 (9.1), 0.5 to 55 | 6.6 (9.1) (0.5 to 59) | 1 (1 to 100) | 1 (1 to 26) | |
| 5 (26%) | 3 (17%) | 12 (63%) | 11 (61%) | 28.2 (5.8) 14 to 38 | 18.8 (2.6) (15 to 26) | NA | NA | 18 (6 to 36) | 12 (6 to 18) | |
| n: number of participants; CBZ: carbamazepine; NA: not available; PHT:phenytoin SD: standard deviation aSex was missing for two participants on CBZ from Mattson 1985. eRandomised drug missing for six participants in De Silva 1996. | ||||||||||
| Trial | Number randomised | Time to treatment failure (any reason, adverse events, lack of efficacy) | Time to 12‐month remission | Time to 6‐month remission | Time to first seizure | ||||||||||
| PHT | CBZ | Total | PHT | CBZ | Total | PHT | CBZ | Total | PHT | CBZ | Total | PHT | CBZ | Total | |
| 54 | 54 | 108 | 53 | 53 | 106 | 54 | 54 | 108 | 54 | 54 | 108 | 54 | 54 | 108 | |
| 63 | 61 | 124 | 61 | 60 | 121 | 63 | 61 | 124 | 63 | 61 | 124 | 63 | 61 | 124 | |
| 165 | 155 | 320 | 165 | 154 | 319 | 165 | 154 | 319 | 165 | 154 | 319 | 162 | 151 | 313 | |
| 20 | 23 | 43 | 20 | 23 | 43 | Information not available | Information not available | Information not available | |||||||
| 18 | 19 | 37 | Information not available | Information not available | Information not available | 18 | 19 | 37 | |||||||
| Total | 320 | 312 | 632 | 299 | 290 | 589 | 282 | 269 | 551 | 282 | 269 | 551 | 297 | 285 | 582 |
| CBZ = carbamazepine, PHT= phenytoin aIndividual participant data (IPD) supplied for 114 participants recruited in De Silva 1996; randomised drug not recorded in six participants. Reasons for treatment failure not available for two participants (one randomised to CBZ and one to PHT); these participants are not included in analysis of time to treatment failure. | |||||||||||||||
| Reason for early termination | Heller 1995a,b | Totalc | |||||||||
| CBZ | PHT | CBZ | PHT | CBZ | PHT | CBZ | PHT | CBZ | PHT | Total | |
| Adverse events (Event) | 3 | 2 | 4 | 1 | 8 | 1 | 11 | 8 | 26 | 12 | 38 |
| Seizure recurrence (Event) | 12 | 10 | 2 | 1 | 5 | 8 | 3 | 6 | 22 | 25 | 47 |
| Both seizure recurrence and adverse events (Event) | 6 | 5 | 0 | 0 | 4 | 2 | 31 | 33 | 31 | 40 | 81 |
| Non‐compliance/participant choice (Event) | 0 | 0 | 3 | 4 | 0 | 0 | 11 | 26 | 14 | 30 | 44 |
| Participant went into remission (Censored) | 18 | 24 | 0 | 0 | 6 | 14 | 0 | 0 | 24 | 38 | 62 |
| Lost to follow‐up (Censored) | 0 | 0 | 0 | 0 | 0 | 0 | 26 | 19 | 26 | 19 | 45 |
| Death (Censored)d | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 5 | 4 | 5 | 9 |
| Other (Censored)e | 0 | 0 | 0 | 0 | 0 | 0 | 16 | 11 | 16 | 11 | 27 |
| Completed the study (did not withdraw) (Censored) | 14 | 12 | 14 | 14 | 37 | 38 | 53 | 57 | 118 | 121 | 239 |
| Total | 53 | 53 | 23 | 20 | 60 | 63 | 155 | 165 | 281 | 301 | 592 |
| n = number of individuals contributing to the outcome 'Time to treatment failure’ aOne participant for Heller 1995 (CBZ) and two for De Silva 1996 (one PHT and one CBZ) have missing reasons for treatment failure. | |||||||||||
| Outcome | Original analysis | Generalised onset and age at onset > 30 years classified as focal onset | Generalised onset and age at onset > 30 years classified as uncertain seizure type | |||
| Pooled HR (95% CI) fixed‐effect model | Test of subgroup differences | Pooled HR (95% CI) fixed‐effect model | Test of subgroup differences | Pooled HR (95% CI) fixed‐effect model | Test of subgroup differences | |
| Time to treatment failure (for any reason related to treatment)a | F: 0.83 (0.61 to 1.13), I2 = 0% G: 2.38 (1.04 to 5.47), I2 = 0% O: 0.94 (0.70 to 1.26), I2 = 35% | Chi2 = 5.45, df = 1, P = 0.02, I2 = 81.7% | F: 0.88 (0.65 to 1.19), I2 = 0% G: 1.96 (0.81 to 4.78), I2 = 0% O: 0.96 (0.72 to 1.27), I2 = 6% | Chi2 = 2.83, df = 1, P = 0.09, I2 = 64.6% | F: 0.83 (0.61 to 1.13), I2 = 0% G: 1.96 (0.81 to 4.78), I2 = 0% U: 5.23 (0.47 to 58.71), I2 = NA O: 0.93 (0.70 to 1.24), I2 = 7% | Chi2 = 5.24, df = 2, P = 0.07, I2 = 61.8% |
| Time to treatment failure due to adverse eventsa | F: 1.19 (0.80 to 1.78), I2 = 0% G: 2.31 (0.68 to 7.81), I2 = 60% O: 1.27 (0.87 to 1.86), I2 = 3% | Chi2 = 1.02, df = 1, P = 0.31, I2 = 2.2% | F: 1.25 (0.84 to 1.86), I2 = 22% G: 1.72 (0.51 to 5.87), I2 = 0% O: 1.29 (0.88 to 1.88), I2 = 0% | Chi2 = 0.24, df = 1, P = 0.62, I2 = 0% | F: 1.19 (0.80 to 1.78), I2 = 0% G: 1.72 (0.51 to 5.87), I2 = 0% U: Not estimablec O: 1.24 (0.85 to 1.81), I2 = 0% | Chi2 = 0.31, df = 2, P = 0.86, I2 = 0% |
| Time to treatment failure due to lack of efficacya | F: 0.88 (0.60 to 1.30), I2 = 0% G: 1.86 (0.74 to 4.67), I2 = 0% O: 0.99 (0.69 to 1.41), I2 = 0% | Chi2 = 2.14, df = 1, P = 0.14, I2 = 53.2% | F: 0.91 (0.62 to 1.34), I2 = 0% G: 1.81 (0.68 to 4.82), I2 = 0% O: 1.00 (0.70 to 1.43), I2 = 0% | Chi2 = 1.64, df = 1, P = 0.20, I2 = 38.9% | F: 0.88 (0.60 to 1.30), I2 = 0% G: 1.81 (0.68 to 4.82), I2 = 0% U: Not estimablec O: 0.97 (0.68 to 1.40), I2 = 0% | Chi2 = 1.78, df = 2, P = 0.41, I2 = 0% |
| Time to first seizureb | F: 1.13 (0.89 to 1.43), I2 = 0% G: 1.19 (0.81 to 1.75), I2 = 45% O: 1.15 (0.94 to 1.40), I2 = 0% | Chi2 = 0.05, df = 1, P = 0.83, I2 = 0% | F: 1.15 (0.91 to 1.44), I2 = 0% G: 1.19 (0.77 to 1.84), I2 = 53% O: 1.16 (0.94 to 1.42), I2 = 0% | Chi2 = 0.02, df = 1, P = 0.88, I2 = 0% | F: 1.13 (0.89 to 1.43), I2 = 0% G: 1.19 (0.77 to 1.84), I2 = 53% U: 0.82 (0.29 to 2.34), I2 = NA O: 1.13 (0.92 to 1.39), I2 = 0% | Chi2 = 0.41, df = 2, P = 0.82, I2 = 0% |
| Time to 12‐month remissiona | F: 1.06 (0.80 to 1.42), I2 = 0% G: 0.88 (0.58 to 1.33), I2 = 73% O: 1.00 (0.79 to 1.26), I2 = 16% | Chi2 = 0.54, df = 1, P = 0.46, I2 = 0% | F: 1.10 (0.84 to 1.45), I2 = 0% G: 0.69 (0.43 to 1.11), I2 = 0% O: 0.98 (0.77 to 1.24), I2 = 0% | Chi2 = 2.79, df = 1, P = 0.09, I2 = 64.2% | F: 1.06 (0.80 to 1.42), I2 = 0% G: 0.69 (0.43 to 1.11), I2 = 0% U: 1.91 (0.74 to 4.90), I2 = NA O: 0.99 (0.78 to 1.25), I2 = 15% | Chi2 = 4.32, df = 2, P = 0.12, I2 = 53.7% |
| Time to 6‐month remissiona | F: 0.98 (0.75 to 1.27), I2 = 0% G: 0.77 (0.52 to 1.13), I2 = 39% O: 0.90 (0.73 to 1.12), I2 = 0% | Chi2 = 1.03, df = 1, P = 0.31, I2 = 3.2% | F: 0.98 (0.76 to 1.26), I2 = 0% G: 0.59 (0.37 to 0.93), I2 = 0% O: 0.87 (0.70 to 1.09), I2 = 15% | Chi2 = 3.63, df = 1, P = 0.06, I2 = 72.5% | F: 0.98 (0.75 to 1.27), I2 = 0% G: 0.59 (0.37 to 0.93), I2 = 0% U: 1.20 (0.51 to 2.83), I2 = NA O: 0.88 (0.71 to 1.10), I2 = 2% | Chi2 = 4.01, df = 2, P = 0.13, I2 = 50.1% |
| Chi2: Chi2 statistic; CI: confidence interval; df: degrees of freedom of Chi2 distribution; F: focal epilepsy; G: generalised epilepsy; HR: Hazard Ratio; O: overall (all participants); U: uncertain epilepsy; P: P value (< 0.05 are classified as statistically significant) a29 participants reclassified to focal epilepsy or uncertain epilepsy type from Heller 1995. | ||||||
| Trial | Adverse event dataa | Summary of reported results | |
| Carbamazepine (CBZ) | Phenytoin (PHT) | ||
| All adverse events according to drug (note: no participants withdrew due to adverse events) | CBZ (n = 59): drowsiness (n = 2), rash (n = 3) | PHT (n = 58): gum hypertrophy (n = 2), rash (n = 2), ataxia (n = 2) | |
| “Exclusions” due to adverse events or no efficacy” | Proportion “excluded”: CBZ: 30% (out of 30 randomised to CBZ) | Proportion “excluded”: PHT: 23.3% (out of 30 randomised to PHT) | |
| “Unacceptable” adverse events leading to drug withdrawald | CBZ (n = 54): drowsiness (n = 1), blood dyscrasia (n = 1) | PHT (n = 54): drowsiness (n = 2), skin rash (n = 1), blood dyscrasia (n = 1), hirsutism (n = 1) | |
| Withdrawal due to adverse events (no other adverse event data reported) | 4 participants out of 23 randomised to CBZ withdrew for the following reasons (some withdrew for more than adverse event): slowing of mental function, headache, anorexia, nausea, abdominal pain, fatigue and drowsiness2 | 1 participant out of 20 randomised to PHT withdrew from the study due to depression and anorexia | |
| “Unacceptable” adverse events leading to drug withdrawald | CBZ (n = 61): drowsiness (n = 3), rash (n = 2), headache (n = 1), depression (n = 1) | PHT (n = 63): myalgia (n = 1), irritability (n = 1) | |
| Narrative report of ‘Adverse effects’ and ‘Serious side effects’ | CBZ (n = 155): motor disturbance (ataxia, incoordination, nystagmus, tremor: 33%); dysmorphic and idiosyncratic side effects (gum hypertrophy, hirsutism, acne and rash: 14%); gastrointestinal problems (27%); decreased libido or impotence (13%); No serious side effects | PHT (n = 165); motor disturbance (ataxia, inco‐ordination, nystagmus, tremor: 28%); dysmorphic and idiosyncratic side effects (gum hypertrophy, hirsutism, acne and rash: 22 %); gastrointestinal problems (24%); decreased libido or impotence (11%) 1 serious side effect – 1 participant has confirmed lymphoma, rash improved rapidly following discontinuation of PHT | |
| No adverse events reported | N/A | N/A | |
| Participant reported symptomatic complaints (provided as IPD) | CBZ (n = 19): memory impairment (n = 9) psychomotor retardation (n = 1) inattention (n = 1) transient rash (n = 1) CBZ‐induced cough (n = 1) | PHT (n = 18): memory impairment (n = 7) psychomotor retardation (n = 1) inattention (n = 2) transient rash (n = 1) | |
| Participant‐reported adverse events | 1 participant on CBZ complained of facial skin problems; 1 participant on CBZ complained of tiredness and memory problems | 3 participants on PHT complained of tiredness | |
| Major and minor side effects | CBZ (n = 35): Major side effects: rash (n = 1), pruritus (n = 1), impotence (n = 2), dizziness (n = 1), headaches (n = 1), impaired cognition (n = 1), elevated liver enzymes (n = 1) Mild side effects: nausea (33%), headaches (24%), cognitive impairment (33%), nystagmus (52%), sedation (33%), fine tremor (20%) | PHT (n = 35): Major side effects: rash (n = 4), exfoliative dermatitis (n = 1), impotence (n = 1), dizziness (n = 1), nausea/vomiting (n = 1) Mild side effects: nausea (38%), gingival hyperplasia (12%), headaches (32%), cognitive impairment (15%), nystagmus (40%), sedation (15%), fine tremor (28%) | |
| No adverse events reported | N/A | N/A | |
| CBZ = carbamazepine, N/A = not available, PHT= phenytoin aAdverse event data are recorded as reported narratively in the publications, so exact definition of a symptom may vary. Adverse event data supplied as IPD for Ogunrin 2005. Adverse event data were not requested in original IPD requests (De Silva 1996; Heller 1995; Mattson 1985) but will be for all future IPD requests. For numbers of treatment withdrawals due to adverse events in studies for which IPD were provided (De Silva 1996; Heller 1995; Mattson 1985) see Table 4. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to treatment failure (any reason related to the treatment) Show forest plot | 4 | 589 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.76, 1.31] |
| 2 Time to treatment failure due to adverse events Show forest plot | 4 | 589 | Hazard Ratio (Fixed, 95% CI) | 1.35 [0.93, 1.95] |
| 3 Time to treatment failure due to lack of efficacy Show forest plot | 4 | 589 | Hazard Ratio (Fixed, 95% CI) | 1.02 [0.72, 1.44] |
| 4 Time to treatment failure (any reason related to the treatment) ‐ by epilepsy type Show forest plot | 3 | 546 | Hazard Ratio (Fixed, 95% CI) | 0.94 [0.70, 1.26] |
| 4.1 Focal onset seizures | 3 | 428 | Hazard Ratio (Fixed, 95% CI) | 0.83 [0.61, 1.13] |
| 4.2 Generalised onset seizures | 2 | 118 | Hazard Ratio (Fixed, 95% CI) | 2.38 [1.04, 5.47] |
| 5 Time to treatment failure due to adverse events ‐ by epilepsy type Show forest plot | 3 | 546 | Hazard Ratio (Fixed, 95% CI) | 1.27 [0.87, 1.86] |
| 5.1 Generalised onset seizures | 2 | 118 | Hazard Ratio (Fixed, 95% CI) | 2.31 [0.68, 7.81] |
| 5.2 Focal onset seizures | 3 | 428 | Hazard Ratio (Fixed, 95% CI) | 1.19 [0.80, 1.78] |
| 6 Time to treatment failure due to lack of efficacy ‐ by epilepsy type Show forest plot | 3 | 546 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.69, 1.41] |
| 6.1 Focal onset seizures | 3 | 428 | Hazard Ratio (Fixed, 95% CI) | 0.88 [0.60, 1.30] |
| 6.2 Generalised onset seizures | 2 | 118 | Hazard Ratio (Fixed, 95% CI) | 1.86 [0.74, 4.67] |
| 7 Time to first seizure post‐randomisation Show forest plot | 4 | 582 | Hazard Ratio (Fixed, 95% CI) | 1.13 [0.92, 1.39] |
| 8 Time to first seizure post‐randomisation ‐ by epilepsy type Show forest plot | 4 | 582 | Hazard Ratio (Fixed, 95% CI) | 1.15 [0.94, 1.40] |
| 8.1 Focal onset seizures | 4 | 432 | Hazard Ratio (Fixed, 95% CI) | 1.13 [0.89, 1.43] |
| 8.2 Generalised onset seizures | 3 | 150 | Hazard Ratio (Fixed, 95% CI) | 1.19 [0.81, 1.75] |
| 9 Time to achieve 12‐month remission Show forest plot | 3 | 551 | Hazard Ratio (Fixed, 95% CI) | 1.01 [0.80, 1.27] |
| 10 Time to achieve 12‐month remission ‐ by epilepsy type Show forest plot | 3 | 551 | Hazard Ratio (Fixed, 95% CI) | 1.00 [0.79, 1.26] |
| 10.1 Focal onset seizures | 3 | 430 | Hazard Ratio (Fixed, 95% CI) | 1.06 [0.80, 1.42] |
| 10.2 Generalised onset seizures | 2 | 121 | Hazard Ratio (Fixed, 95% CI) | 0.88 [0.58, 1.33] |
| 11 Time to achieve six‐month remission Show forest plot | 3 | 551 | Hazard Ratio (Fixed, 95% CI) | 0.92 [0.74, 1.14] |
| 12 Time to achieve six‐month remission ‐ by epilepsy type Show forest plot | 3 | 551 | Hazard Ratio (Fixed, 95% CI) | 0.90 [0.73, 1.12] |
| 12.1 Focal onset seizures | 3 | 430 | Hazard Ratio (Fixed, 95% CI) | 0.98 [0.75, 1.27] |
| 12.2 Generalised onset seizures | 2 | 121 | Hazard Ratio (Fixed, 95% CI) | 0.77 [0.52, 1.13] |

