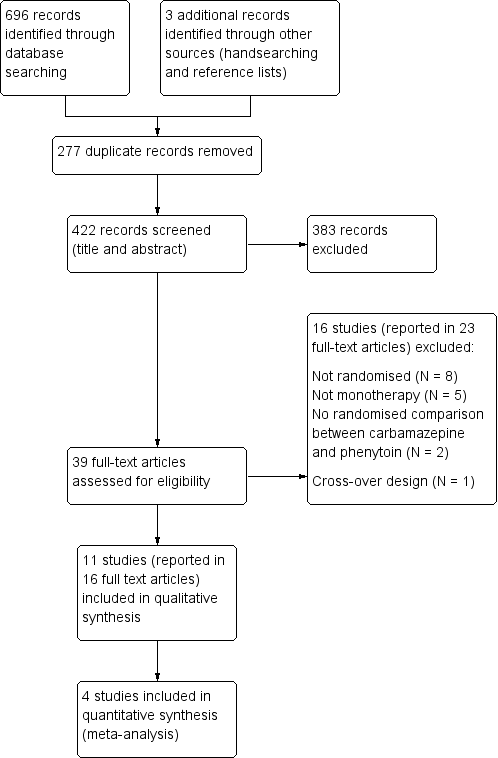
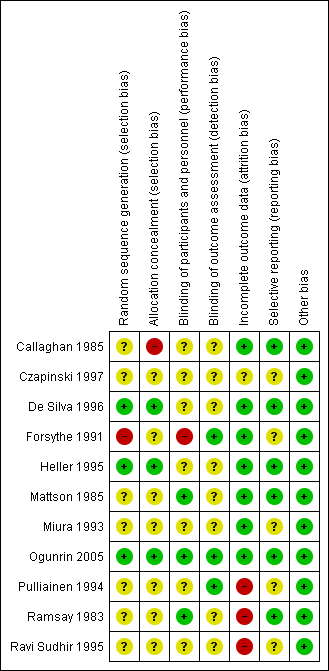
| Outcome | Original analysis | Generalised onset and age at onset > 30 years classified as focal onset | Generalised onset and age at onset > 30 years classified as uncertain seizure type |
| Pooled HR (95% CI) fixed‐effect model | Test of subgroup differences | Pooled HR (95% CI) fixed‐effect model | Test of subgroup differences | Pooled HR (95% CI) fixed‐effect model | Test of subgroup differences |
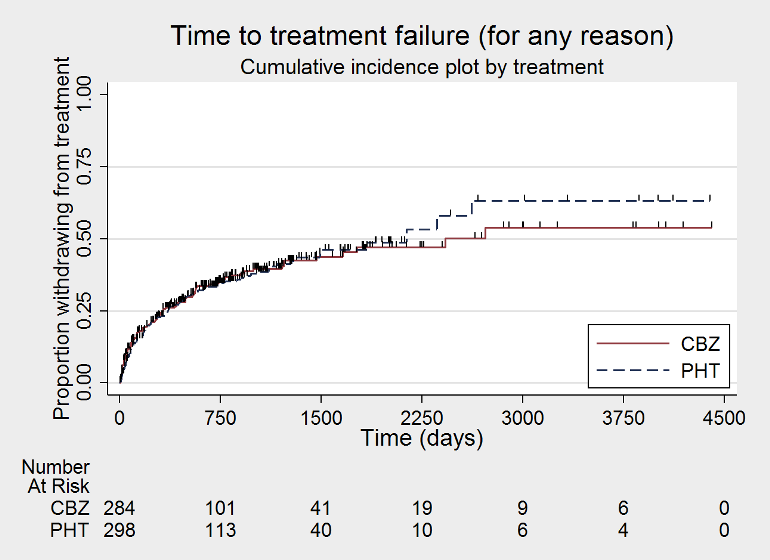
| Time to treatment failure (for any reason related to treatment)a | F: 0.83 (0.61 to 1.13), I2 = 0% G: 2.38 (1.04 to 5.47), I2 = 0% O: 0.94 (0.70 to 1.26), I2 = 35% | Chi2 = 5.45, df = 1, P = 0.02, I2 = 81.7% | F: 0.88 (0.65 to 1.19), I2 = 0% G: 1.96 (0.81 to 4.78), I2 = 0% O: 0.96 (0.72 to 1.27), I2 = 6% | Chi2 = 2.83, df = 1, P = 0.09, I2 = 64.6% | F: 0.83 (0.61 to 1.13), I2 = 0% G: 1.96 (0.81 to 4.78), I2 = 0% U: 5.23 (0.47 to 58.71), I2 = NA O: 0.93 (0.70 to 1.24), I2 = 7% | Chi2 = 5.24, df = 2, P = 0.07, I2 = 61.8% |
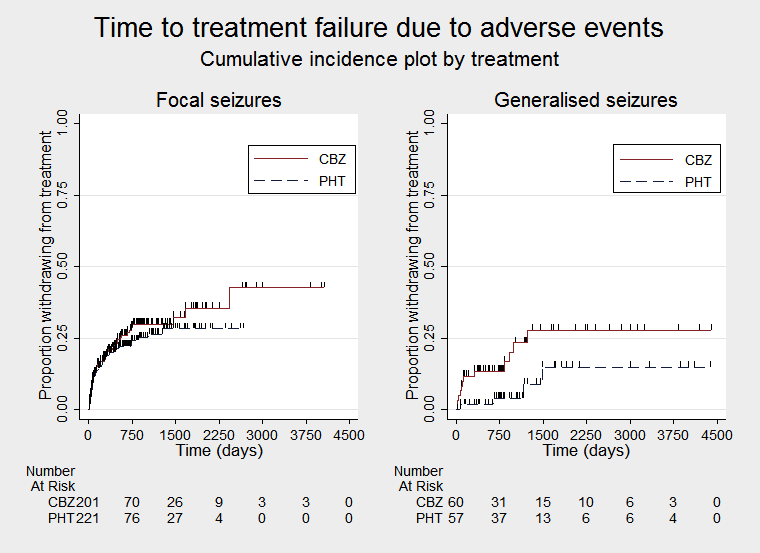
| Time to treatment failure due to adverse eventsa | F: 1.19 (0.80 to 1.78), I2 = 0% G: 2.31 (0.68 to 7.81), I2 = 60% O: 1.27 (0.87 to 1.86), I2 = 3% | Chi2 = 1.02, df = 1, P = 0.31, I2 = 2.2% | F: 1.25 (0.84 to 1.86), I2 = 22% G: 1.72 (0.51 to 5.87), I2 = 0% O: 1.29 (0.88 to 1.88), I2 = 0% | Chi2 = 0.24, df = 1, P = 0.62, I2 = 0% | F: 1.19 (0.80 to 1.78), I2 = 0% G: 1.72 (0.51 to 5.87), I2 = 0% U: Not estimablec O: 1.24 (0.85 to 1.81), I2 = 0% | Chi2 = 0.31, df = 2, P = 0.86, I2 = 0% |
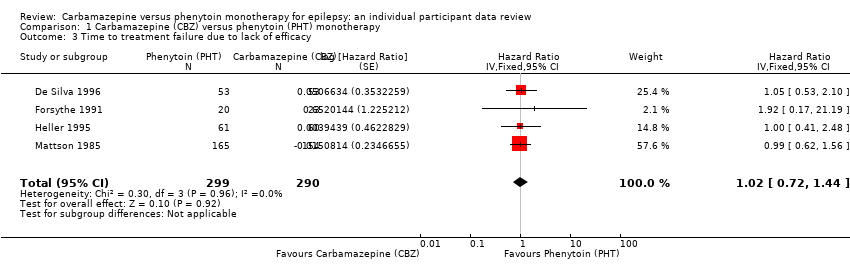
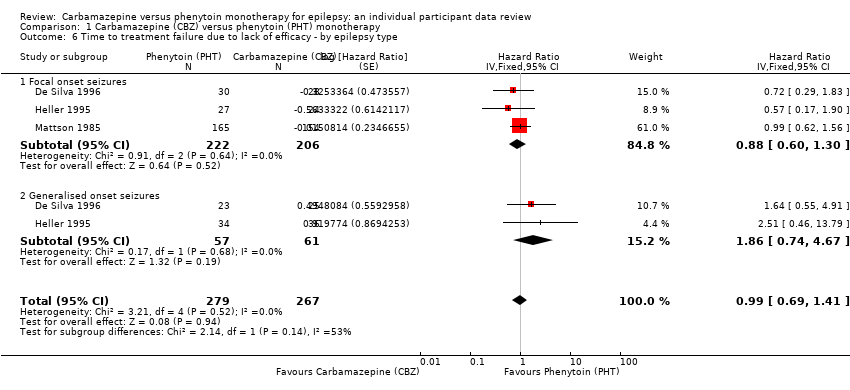
| Time to treatment failure due to lack of efficacya | F: 0.88 (0.60 to 1.30), I2 = 0% G: 1.86 (0.74 to 4.67), I2 = 0% O: 0.99 (0.69 to 1.41), I2 = 0% | Chi2 = 2.14, df = 1, P = 0.14, I2 = 53.2% | F: 0.91 (0.62 to 1.34), I2 = 0% G: 1.81 (0.68 to 4.82), I2 = 0% O: 1.00 (0.70 to 1.43), I2 = 0% | Chi2 = 1.64, df = 1, P = 0.20, I2 = 38.9% | F: 0.88 (0.60 to 1.30), I2 = 0% G: 1.81 (0.68 to 4.82), I2 = 0% U: Not estimablec O: 0.97 (0.68 to 1.40), I2 = 0% | Chi2 = 1.78, df = 2, P = 0.41, I2 = 0% |
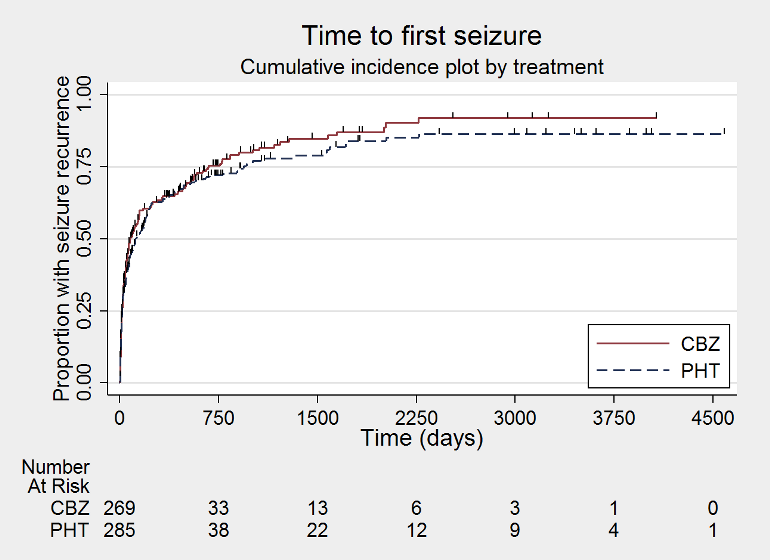
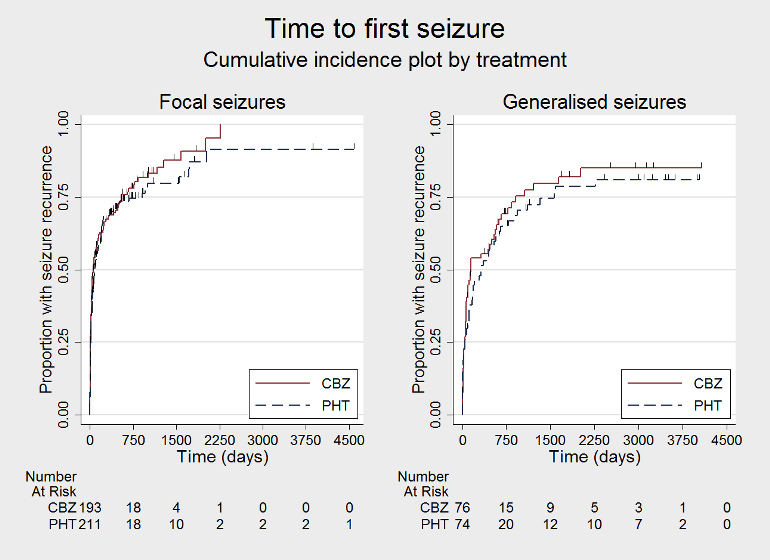
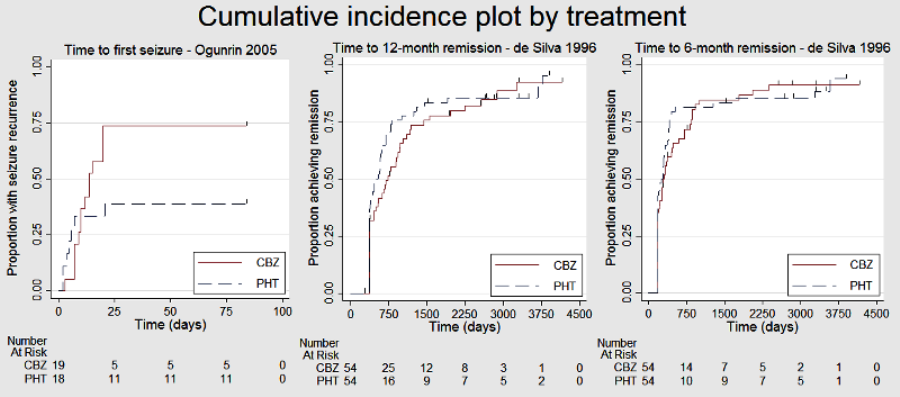
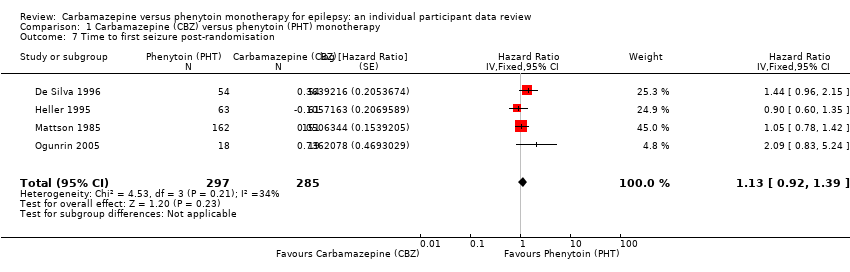
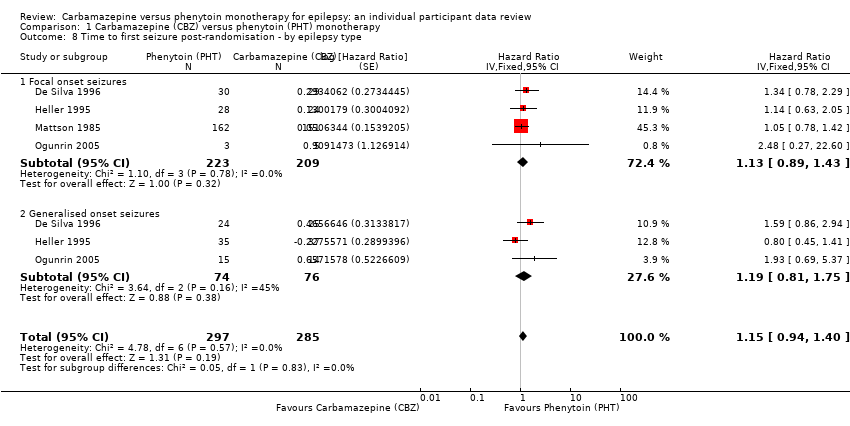
| Time to first seizureb | F: 1.13 (0.89 to 1.43), I2 = 0% G: 1.19 (0.81 to 1.75), I2 = 45% O: 1.15 (0.94 to 1.40), I2 = 0% | Chi2 = 0.05, df = 1, P = 0.83, I2 = 0% | F: 1.15 (0.91 to 1.44), I2 = 0% G: 1.19 (0.77 to 1.84), I2 = 53% O: 1.16 (0.94 to 1.42), I2 = 0% | Chi2 = 0.02, df = 1, P = 0.88, I2 = 0% | F: 1.13 (0.89 to 1.43), I2 = 0% G: 1.19 (0.77 to 1.84), I2 = 53% U: 0.82 (0.29 to 2.34), I2 = NA O: 1.13 (0.92 to 1.39), I2 = 0% | Chi2 = 0.41, df = 2, P = 0.82, I2 = 0% |
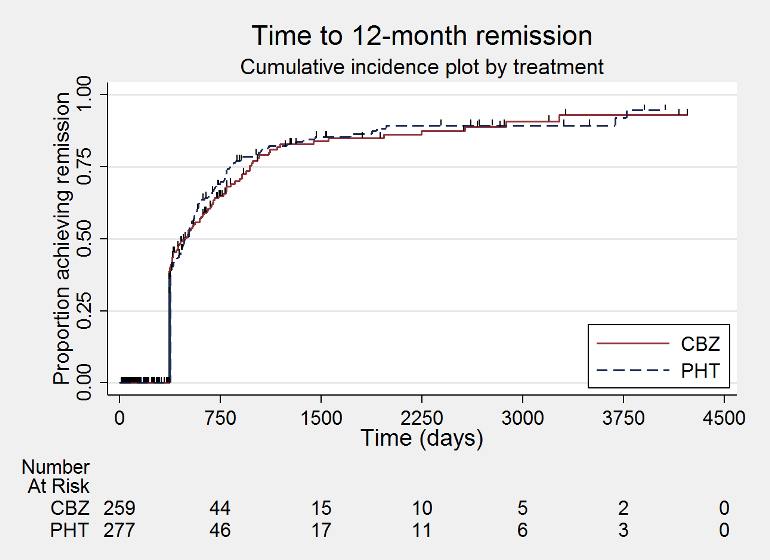
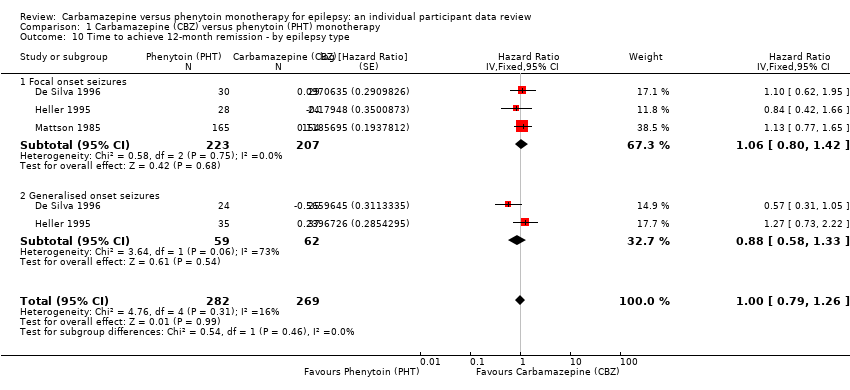
| Time to 12‐month remissiona | F: 1.06 (0.80 to 1.42), I2 = 0% G: 0.88 (0.58 to 1.33), I2 = 73% O: 1.00 (0.79 to 1.26), I2 = 16% | Chi2 = 0.54, df = 1, P = 0.46, I2 = 0% | F: 1.10 (0.84 to 1.45), I2 = 0% G: 0.69 (0.43 to 1.11), I2 = 0% O: 0.98 (0.77 to 1.24), I2 = 0% | Chi2 = 2.79, df = 1, P = 0.09, I2 = 64.2% | F: 1.06 (0.80 to 1.42), I2 = 0% G: 0.69 (0.43 to 1.11), I2 = 0% U: 1.91 (0.74 to 4.90), I2 = NA O: 0.99 (0.78 to 1.25), I2 = 15% | Chi2 = 4.32, df = 2, P = 0.12, I2 = 53.7% |
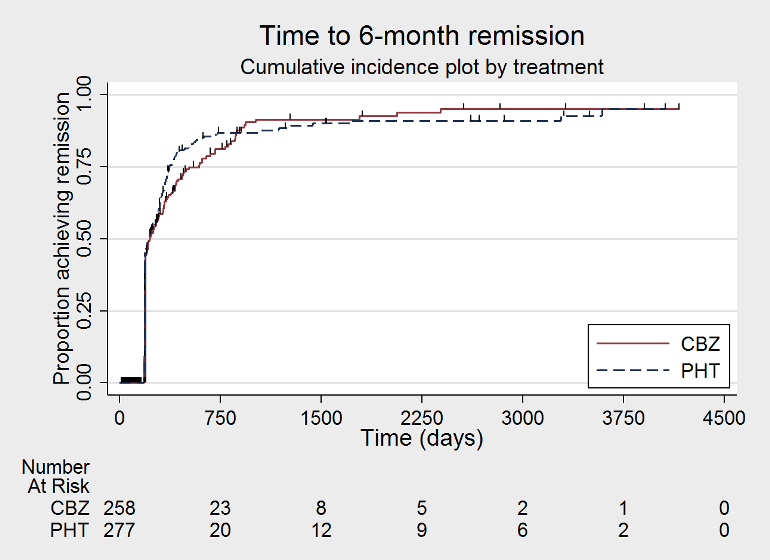
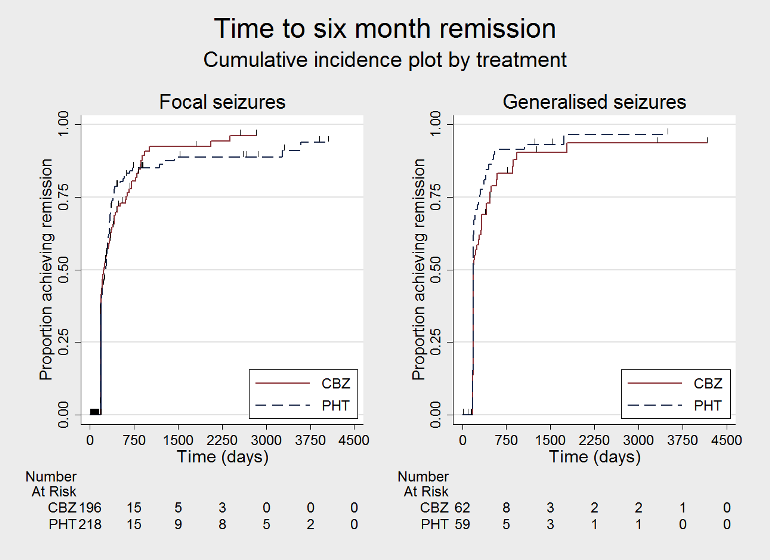
| Time to 6‐month remissiona | F: 0.98 (0.75 to 1.27), I2 = 0% G: 0.77 (0.52 to 1.13), I2 = 39% O: 0.90 (0.73 to 1.12), I2 = 0% | Chi2 = 1.03, df = 1, P = 0.31, I2 = 3.2% | F: 0.98 (0.76 to 1.26), I2 = 0% G: 0.59 (0.37 to 0.93), I2 = 0% O: 0.87 (0.70 to 1.09), I2 = 15% | Chi2 = 3.63, df = 1, P = 0.06, I2 = 72.5% | F: 0.98 (0.75 to 1.27), I2 = 0% G: 0.59 (0.37 to 0.93), I2 = 0% U: 1.20 (0.51 to 2.83), I2 = NA O: 0.88 (0.71 to 1.10), I2 = 2% | Chi2 = 4.01, df = 2, P = 0.13, I2 = 50.1% |