Monoterapia con carbamazepina versus fenitoína para la epilepsia: una revisión de datos de participantes individuales
Información
- DOI:
- https://doi.org/10.1002/14651858.CD001911.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 18 julio 2019see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Epilepsia
- Copyright:
-
- Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
SJN assessed trials for inclusion in the review update, obtained individual participant data from trial investigators for the review update, assessed risks of bias in all included trials, performed analyses in Stata version 14, added survival plots and a 'Summary of findings' table, and updated the text of the review.
CTS was the lead investigator on the original review, assessed eligibility and methodological quality of original individual trials, organised and cleaned the IPD sets, performed data validation checks and statistical analyses, and co‐wrote the original review.
AGM obtained IPD from trial investigators, provided guidance with the clinical interpretation of results, assessed eligibility and methodological quality of individual trials, and co‐wrote the original review.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
Medical Research Council, UK.
-
National Institute for Health Research (NIHR), UK.
Declarations of interest
SJN: none known
AGM: a consortium of pharmaceutical companies (GSK, EISAI, UCB Pharma) funded the National Audit of Seizure Management in Hospitals (NASH) through grants paid to the University of Liverpool. Professor Tony Marson is part funded by National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care North West Coast (NIHR CLAHRC NWC).
CTS: none known
Acknowledgements
This review update was supported by the National Institute for Health Research, via Cochrane Infrastructure Grant funding to the Epilepsy Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
We are greatly indebted to all of the original trialists that have provided individual participant data (IPD) and input into this review.
We are grateful to the Cochrane Epilepsy Group Information Specialist, Graham Chan, for performing all electronic searches.
We acknowledge Jennifer Weston, Paula Williamson and Helen Clough for contributions to the original review and previous versions of the review.
Version history
| Published | Title | Stage | Authors | Version |
| 2019 Jul 18 | Carbamazepine versus phenytoin monotherapy for epilepsy: an individual participant data review | Review | Sarah J Nevitt, Anthony G Marson, Catrin Tudur Smith | |
| 2017 Feb 27 | Carbamazepine versus phenytoin monotherapy for epilepsy: an individual participant data review | Review | Sarah J Nevitt, Anthony G Marson, Jennifer Weston, Catrin Tudur Smith | |
| 2015 Aug 14 | Carbamazepine versus phenytoin monotherapy for epilepsy: an individual participant data review | Review | Sarah J Nolan, Anthony G Marson, Jennifer Weston, Catrin Tudur Smith | |
| 2002 Apr 22 | Carbamazepine versus phenytoin monotherapy for epilepsy | Review | Catrin Tudur Smith, Anthony G Marson, Helen E Clough, Paula R Williamson | |
Differences between protocol and review
December 2014: the title was changed to specify that the review uses individual participant data (IPD).
Update 2015: we added sensitivity analyses following identification of potential misclassification of seizure type. The existence of misclassification in the individual studies could not have been known at the time of writing the original protocol.
Update 2015: we added the outcomes 'Time to six‐month remission' and 'Adverse events' for consistency with the other reviews in the series of Cochrane IPD reviews investigating pair‐wise monotherapy comparisons.
Update 2015: we added 'Summary of findings' tables to the update in 2015 and added text in the Methods section for 'Summary of findings' tables in August 2016.
Update 2018: 'Time to withdrawal of allocated treatment' was re‐defined as 'Time to treatment failure', due to feedback received from the Cochrane Editorial Unit regarding potential confusion regarding 'withdrawal' as a positive or negative outcome of anti‐epileptic monotherapy.
Additional analyses of 'Time to treatment failure' (due to lack of efficacy and due to adverse events), following feedback on published anti‐epileptic drug monotherapy reviews that these sub‐outcomes would be useful for clinical practice.
The term 'partial' has been replaced by 'focal', in accordance with the most recent classification of epilepsies of the International League Against Epilepsy (Scheffer 2017).
We exclude cross‐over designs, as this design is not appropriate for measuring the long‐term outcomes of the review; previously included cross‐over studies are now excluded from the review.
Notes
Sarah J Nolan (lead author of 2015 update) is now Sarah J Nevitt
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Anticonvulsants [adverse effects, *therapeutic use];
- Carbamazepine [adverse effects, *therapeutic use];
- Epilepsy [*drug therapy];
- Phenytoin [adverse effects, *therapeutic use];
- Randomized Controlled Trials as Topic;
- Remission Induction;
- Seizures [prevention & control];
- Treatment Failure;
- Treatment Outcome;
Medical Subject Headings Check Words
Child; Humans;
PICO
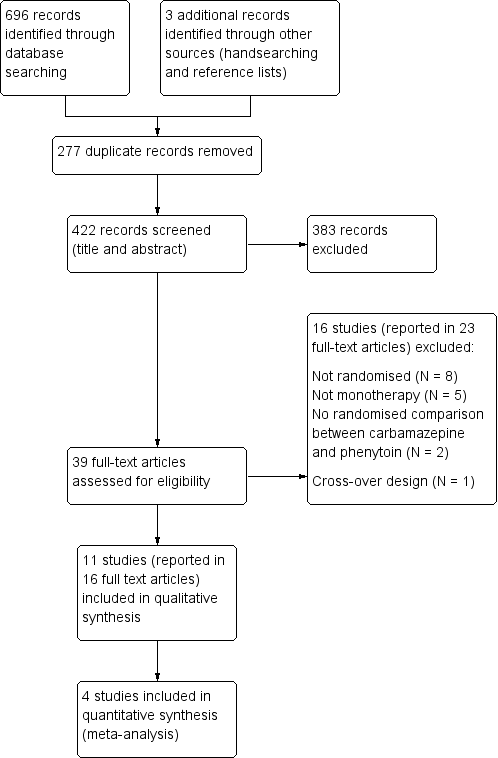
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
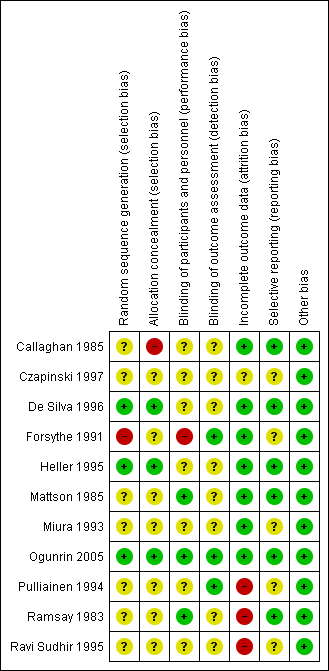
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
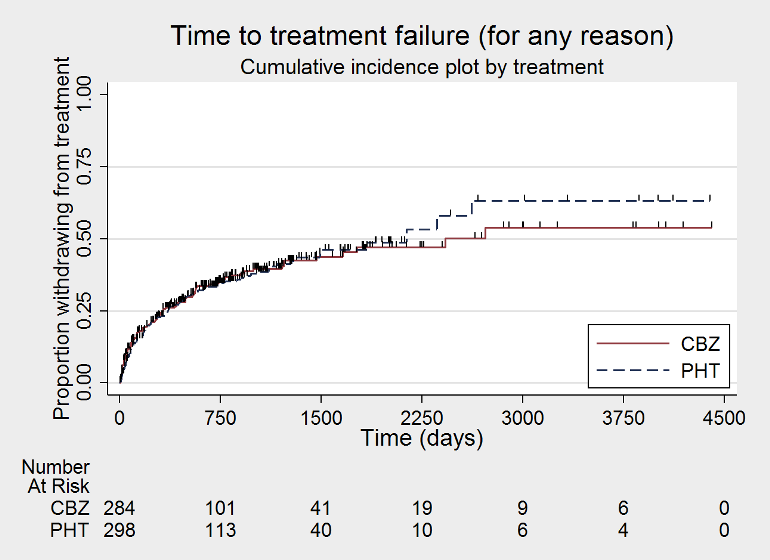
Time to treatment failure ‐ any reason related to the treatment (CBZ: carbamazepine; PHT: phenytoin)

Time to treatment failure ‐ any reason related to the treatment, by epilepsy type (CBZ: carbamazepine; PHT: phenytoin)

Time to treatment failure due to adverse events (CBZ: carbamazepine; PHT: phenytoin)
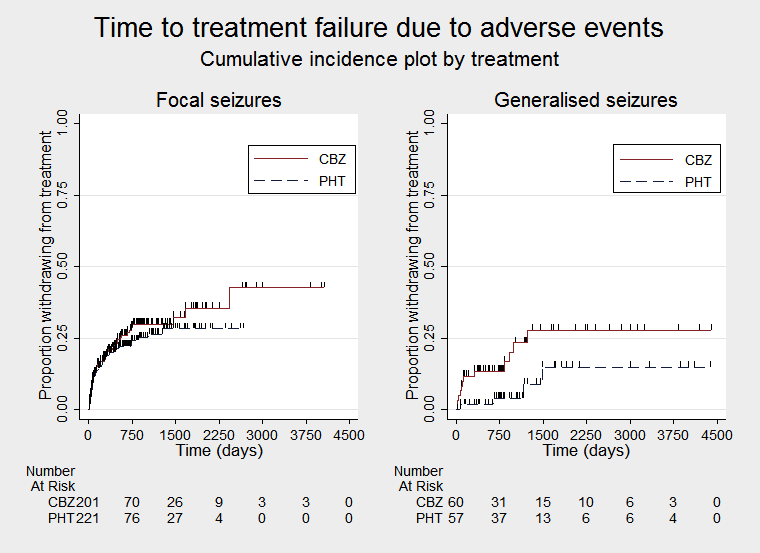
Time to treatment failure due to adverse events, by epilepsy type (CBZ: carbamazepine; PHT: phenytoin)

Time to treatment failure due to lack of efficacy (CBZ: carbamazepine; PHT: phenytoin)

Time to treatment failure due to lack of efficacy, by epilepsy type (CBZ: carbamazepine; PHT: phenytoin)
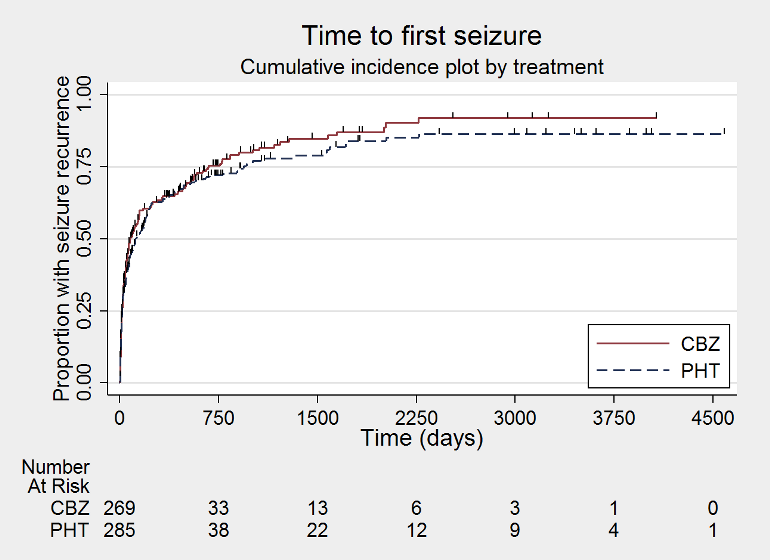
Time to first seizure (CBZ: carbamazepine; PHT: phenytoin)
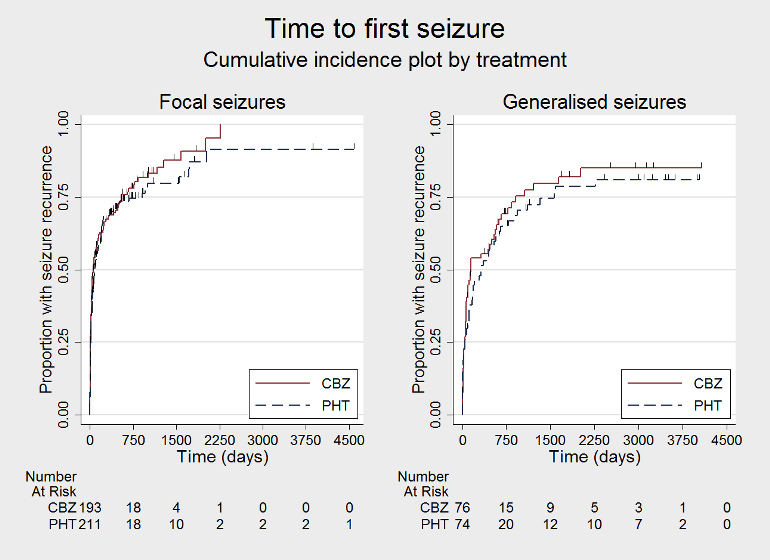
Time to first seizure, by epilepsy type (CBZ: carbamazepine; PHT: phenytoin)
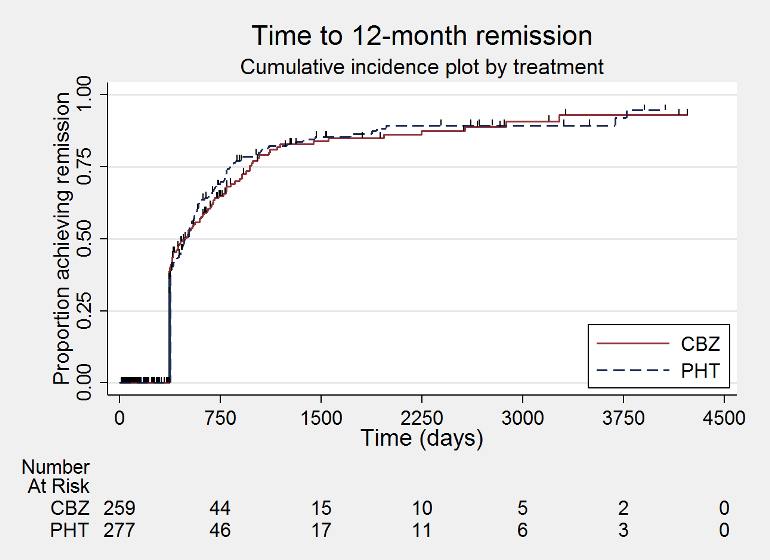
Time to 12 month remission (CBZ: carbamazepine; PHT: phenytoin)

Time to 12 month remission, by epilepsy type (CBZ: carbamazepine; PHT: phenytoin)
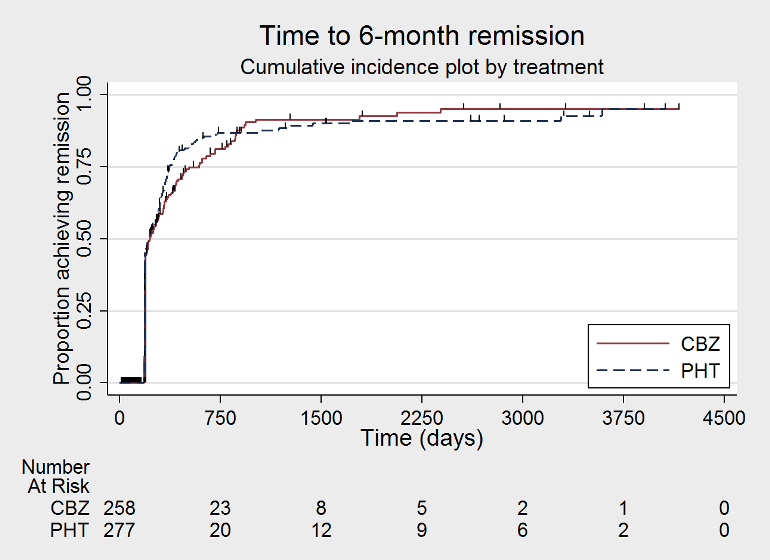
Time to 6 month remission (CBZ: carbamazepine; PHT: phenytoin)
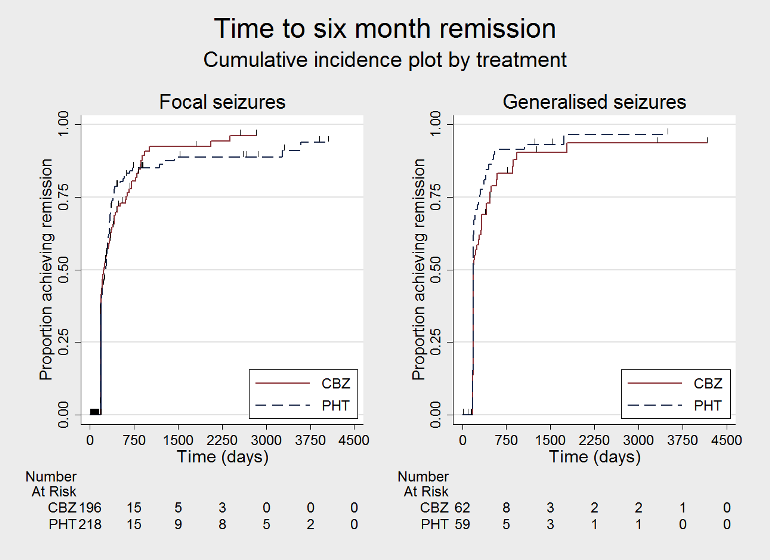
Time to 6 month remission, by epilepsy type (CBZ: carbamazepine; PHT: phenytoin)
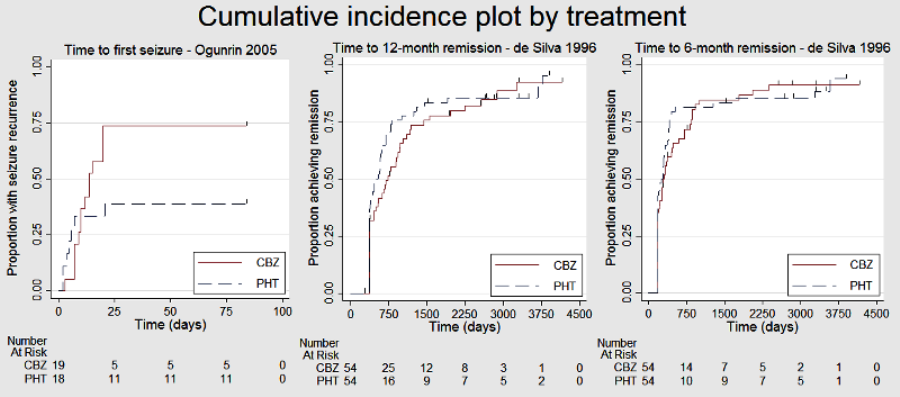
Cumulative incidence plots, proportional hazards assumption checks

Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 1 Time to treatment failure (any reason related to the treatment).

Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 2 Time to treatment failure due to adverse events.
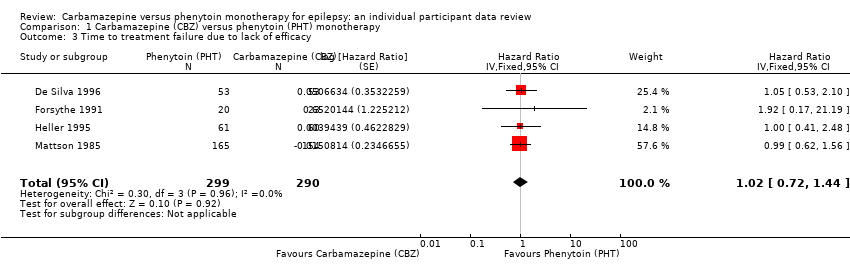
Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 3 Time to treatment failure due to lack of efficacy.

Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 4 Time to treatment failure (any reason related to the treatment) ‐ by epilepsy type.

Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 5 Time to treatment failure due to adverse events ‐ by epilepsy type.
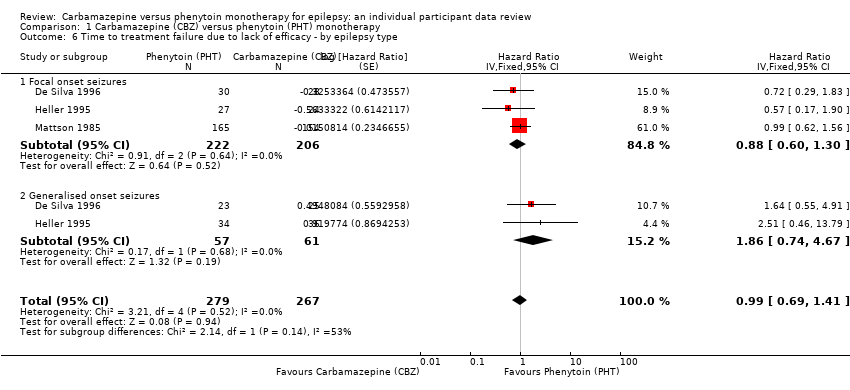
Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 6 Time to treatment failure due to lack of efficacy ‐ by epilepsy type.
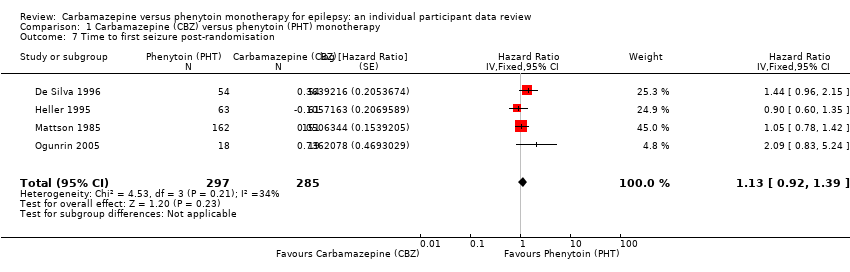
Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 7 Time to first seizure post‐randomisation.
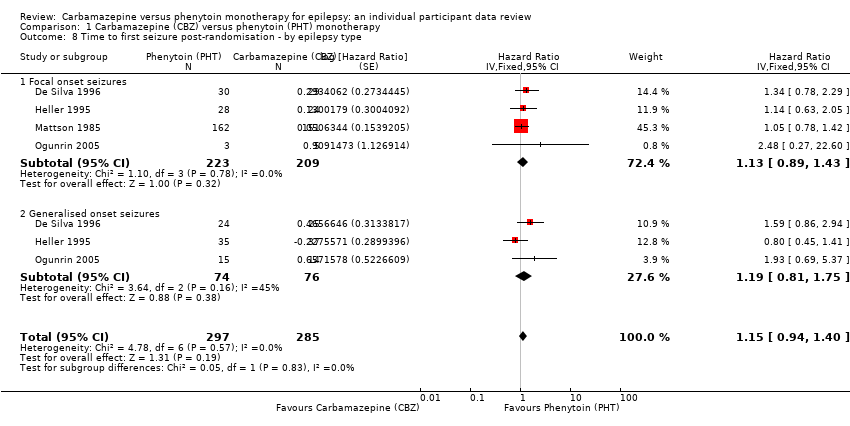
Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 8 Time to first seizure post‐randomisation ‐ by epilepsy type.

Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 9 Time to achieve 12‐month remission.
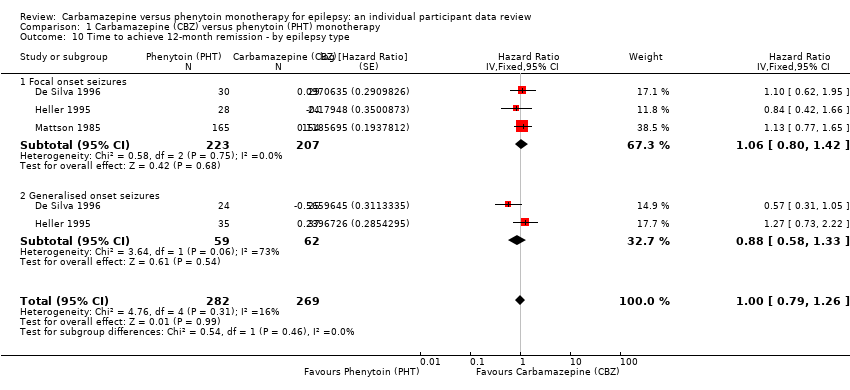
Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 10 Time to achieve 12‐month remission ‐ by epilepsy type.

Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 11 Time to achieve six‐month remission.

Comparison 1 Carbamazepine (CBZ) versus phenytoin (PHT) monotherapy, Outcome 12 Time to achieve six‐month remission ‐ by epilepsy type.
| Carbamazepine compared with phenytoin for epilepsy | ||||||
| Patient or population: adults and children with new‐onset focal or generalised epilepsy Settings: outpatients Intervention: carbamazepine Comparison: phenytoin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of Participants | Certainty (quality) of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Phenytoin | Carbamazepine | |||||
| Time to treatment failure (any reason related to treatment) Range of follow‐up:1 day to 4403 days | The median time to treatment failure was 2135 days in the phenytoin group | The median time to treatment failure was 2422 days (307 days longer) in the carbamazepine group | HR 0.94 (0.70 to 1.26)a | 546 (3 studies) | ⊕⊕⊕⊝ moderateb | HR < 1 indicates a clinical advantage for carbamazepine There was also no statistically significant difference between drugs in treatment failure due to adverse events (HR 1.27, 95% CI 0.87 to 1.86; P = 0.21; I2 = 3%), or treatment failure due to lack of efficacy: HR 0.99 (95% CI 0.69 to 1.41; P = 0.94; I2 = 0%) |
| Time to treatment failure (any reason related to treatment) Subgroup: focal onset seizures Range of follow‐up: 1 day to 4064 days | The median time to treatment failure was 1300 days in the phenytoin group | The median time to treatment failure was 2422 days (1122 days longer) in the carbamazepine group | HR 0.83 (0.61 to 1.13) | 428 (3 studies) | ⊕⊕⊕⊝ moderateb | HR < 1 indicates a clinical advantage for carbamazepine There was also no statistically significant difference between drugs in treatment failure due to adverse events (HR 1.19, 95% CI 0.80 to 1.78; P = 0.38, I2 = 0%), or treatment failure due to lack of efficacy: HR 0.88 (95% CI 0.60 to 1.40, P = 0.52, I2 = 0%) |
| Time to treatment failure (any reason related to treatment) Subgroup: generalised Range of follow‐up:1 day to 4403 days | The 10th percentilec | The 10th percentilec | HR 2.38 (1.04 to 5.47) | 118 (2 studies) | ⊕⊕⊝⊝ lowb,d | HR < 1 indicates a clinical advantage for carbamazepine There was no statistically significant difference between drugs in treatment failure due to adverse events (HR 2.31, 95% CI 0.68 to 7.81; P = 0.18; I2 = 60% , or treatment failure due to lack of efficacy: HR 1.86 (95% CI 0.74 to 4.67; P = 0.19; I2 = 0%) but confidence intervals are wide so we cannot rule out an advantage to either drug or no difference between the drugs |
| *Illustrative risks in the carbamazepine and phenytoin groups are calculated at the median time to treatment failure (i.e. the time to 50% of participants failing or withdrawing from allocated treatment) within each group across all trials. The relative effect (pooled hazard ratio) shows the comparison of 'Time to treatment failure' between the treatment groups. CI: 95% confidence interval; HR: hazard ratio | ||||||
| GRADE Working Group grades of evidence High certainty (quality): Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty (quality): Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty (quality): Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very certainty (quality): We are very uncertain about the estimate. | ||||||
| aPooled HR for all participants adjusted for seizure type. All pooled HRs presented calculated with fixed‐effect model. | ||||||
| Carbamazepine compared with phenytoin for epilepsy | ||||||
| Patient or population: adults and children with new‐onset focal or generalised epilepsy Settings: outpatients Intervention: carbamazepine Comparison: phenytoin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of Participants | Certainty (quality) of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Phenytoin | Carbamazepine | |||||
| Time to first seizure after randomisation Range of follow‐up: 0 days to 4589 days | The median time to first seizure was 124 days in the phenytoin group | The median time to first seizure was 79 days (45 days shorter) in the carbamazepine group | HR 1.15 (0.94 to 1.40) | 582 (4 studies) | ⊕⊕⊕⊝ moderateb | HR < 1 indicates a clinical |
| Time to first seizure after randomisation Subgroup: focal onset seizures Range of follow‐up: 0 days to 4589 days | The median time to first seizure was 78 days in the phenytoin group | The median time to first seizure was 62 days (16 days shorter) in the carbamazepine group | HR 1.13 (0.89 to 1.43) | 432 (4 studies) | ⊕⊕⊕⊝ moderateb | HR < 1 indicates a clinical |
| Time to first seizure after randomisation Range of follow‐up: 2 days to 4070 days | The median time to first seizure was 323 days in the phenytoin group | The median time to first seizure was 142 days (181 days shorter) in the carbamazepine group | HR 1.19 (0.81 to 1.75) | 150 (3 studies) | ⊕⊕⊝⊝ lowb,c | HR < 1 indicates a clinical |
| Time to achieve 12‐month remission Range of follow‐up: 0 days to 4222 days | The median time to 12‐month remission was 472 days in the phenytoin group | The median time to 12‐month remission was 481 days (9 days longer) in the carbamazepine group | HR 1.00 (0.79 to 1.26) | 551 (3 studies) | ⊕⊕⊕⊝ moderateb | HR < 1 indicates a clinical |
| Time to achieve 12‐month remission Range of follow‐up: 0 days to 4222 days | The median time to 12‐month remission was 531 days in the phenytoin group | The median time to 12‐month remission was 515 days (16 days shorter) in the carbamazepine group | HR 1.06 (0.80 to 1.42) | 430 (3 studies) | ⊕⊕⊕⊝ moderateb | HR < 1 indicates a clinical |
| Time to achieve 12‐month remission Range of follow‐up: 7 days to 4163 days | The median time to 12‐month remission was 366 days in the phenytoin group | The median time to 12‐month remission was 375 days (9 days longer) in the carbamazepine group | HR 0.88 (0.58 to 1.33) | 121 (2 studies) | ⊕⊕⊝⊝ lowb,d | HR < 1 indicates a clinical |
| *Illustrative risks in the carbamazepine and phenytoin groups are calculated at the median time to first seizure or time to 12‐month remission (i.e. the time to 50% of participants experiencing a first seizure or 12‐months of remission) within each group across all trials. The relative effect (pooled hazard ratio) shows the comparison of 'Time to first seizure' or 'Time to 12‐month remission' between the treatment groups. CI: 95% confidence interval; HR: hazard ratio | ||||||
| GRADE Working Group grades of evidence High certainty (quality): Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty (quality): Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty (quality): Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very certainty (quality): We are very uncertain about the estimate. | ||||||
| aPooled HR for all participants adjusted for seizure type. All pooled HRs presented calculated with a fixed‐effect model. | ||||||
| Trial | Outcomes reported | Summary of results |
| 1. Seizure control: excellent (seizure‐free) | 1. PHT (n = 58); CBZ (n = 59) PHT: 39 (67%); CBZ: 22 (37%) | |
| 1. Proportion achieving 24‐month remission at 3 years 2. Proportion excluded after randomisation due to adverse effects or no efficacy | 1. PHT: 59%; CBZ: 62% 2. PHT: 23%; CBZ: 30% | |
| 1. Cognitive assessments 2. Withdrawals from randomised drug | 1. No significant differences between the two treatment groups on any cognitive tests | |
| 1. Proportion of all randomised participants with seizure recurrence (by seizure type) 2. Proportion of participants with optimum plasma levels with seizure recurrence (by seizure type) | PHT (n = 51); CBZ (n = 66) 1. PHT (focal): 10/31 (32%); PHT (generalised): 7/20 (35%); 2. PHT (focal): 4/17 (24%); PHT (generalised): 1/8 (13%); | |
| 1. Cognitive assessments (visual motor speed, co‐ordination, attention and concentration, verbal and visual‐spatial learning, visual and recognition memory, reasoning, mood, handedness) 2. Harmful side effects | 1. Compared to CBZ, participants on PHT became slower (motor speed of the hand) and their visual memory decreased. There was an equal decrease in negative mood (helplessness, irritability, depression) on PHT and CBZ
| |
| 1. Side effects (major and minor) 3. Laboratory results | 1. Incidence of:
2. Treatment failures among analysed participants: Seizure control (among analysed participants with no major side effects): PHT: 23/27 participants (86%); CBZ: 22/27 participants (82%) 3. Significantly lower mean LDH level at 24 weeks in CBZ participants than PHT participants (P < 0.01). Other laboratory results similar across treatment groups | |
| 1. Cognitive measures (verbal, performance, memory, visual‐motor, perceptomotor organisation, visual organisation, dysfunction) | 1. No significant differences between any tests of cognitive function taken before treatment and after 10 ‐ 12 weeks for both treatment groups | |
| CBZ = carbamazepine; LDH = lactate dehydrogenase; PHT= phenytoin | ||
| Trial | Focal seizures: n (%) | Male participants: | Age at entry (years): Mean (SD), rangeb | Epilepsy duration | Number of seizures in prior 6 months, median (range)d | |||||
| CBZ | PHT | CBZ | PHT | CBZ | PHT | CBZ | PHT | CBZ | PHT | |
| 29 (54%) | 30 (56%) | 30 (56%) | 34 (63%) | 9.2 (3.8) 2 to 15 | 9.5 (3.4) (3 to 16) | 1.7 (2.6), 0 to 12 | 1.0 (2.1) (0 to 14) | 3 (1 to 500) | 3 (1 to 404) | |
| 24 (39%) | 28 (44%) | 30 (49%) | 34 (54%) | 29.3 (14.1) 13 to 69 | 33.5 (14.3) (14 to 72) | 4.4 (7.4), 0.1 to 40 | 3.8 (5.4) (0 to 24) | 2 (1 to 354) | 2 (1 to 575) | |
| 155 (100%) | 165 (100%) | 133 (87%) | 145 (88%) | 42.1 (15.9) 18 to 82 | 40.8 (15.3) (18 to 81) | 5.9 (9.1), 0.5 to 55 | 6.6 (9.1) (0.5 to 59) | 1 (1 to 100) | 1 (1 to 26) | |
| 5 (26%) | 3 (17%) | 12 (63%) | 11 (61%) | 28.2 (5.8) 14 to 38 | 18.8 (2.6) (15 to 26) | NA | NA | 18 (6 to 36) | 12 (6 to 18) | |
| n: number of participants; CBZ: carbamazepine; NA: not available; PHT:phenytoin SD: standard deviation aSex was missing for two participants on CBZ from Mattson 1985. eRandomised drug missing for six participants in De Silva 1996. | ||||||||||
| Trial | Number randomised | Time to treatment failure (any reason, adverse events, lack of efficacy) | Time to 12‐month remission | Time to 6‐month remission | Time to first seizure | ||||||||||
| PHT | CBZ | Total | PHT | CBZ | Total | PHT | CBZ | Total | PHT | CBZ | Total | PHT | CBZ | Total | |
| 54 | 54 | 108 | 53 | 53 | 106 | 54 | 54 | 108 | 54 | 54 | 108 | 54 | 54 | 108 | |
| 63 | 61 | 124 | 61 | 60 | 121 | 63 | 61 | 124 | 63 | 61 | 124 | 63 | 61 | 124 | |
| 165 | 155 | 320 | 165 | 154 | 319 | 165 | 154 | 319 | 165 | 154 | 319 | 162 | 151 | 313 | |
| 20 | 23 | 43 | 20 | 23 | 43 | Information not available | Information not available | Information not available | |||||||
| 18 | 19 | 37 | Information not available | Information not available | Information not available | 18 | 19 | 37 | |||||||
| Total | 320 | 312 | 632 | 299 | 290 | 589 | 282 | 269 | 551 | 282 | 269 | 551 | 297 | 285 | 582 |
| CBZ = carbamazepine, PHT= phenytoin aIndividual participant data (IPD) supplied for 114 participants recruited in De Silva 1996; randomised drug not recorded in six participants. Reasons for treatment failure not available for two participants (one randomised to CBZ and one to PHT); these participants are not included in analysis of time to treatment failure. | |||||||||||||||
| Reason for early termination | Heller 1995a,b | Totalc | |||||||||
| CBZ | PHT | CBZ | PHT | CBZ | PHT | CBZ | PHT | CBZ | PHT | Total | |
| Adverse events (Event) | 3 | 2 | 4 | 1 | 8 | 1 | 11 | 8 | 26 | 12 | 38 |
| Seizure recurrence (Event) | 12 | 10 | 2 | 1 | 5 | 8 | 3 | 6 | 22 | 25 | 47 |
| Both seizure recurrence and adverse events (Event) | 6 | 5 | 0 | 0 | 4 | 2 | 31 | 33 | 31 | 40 | 81 |
| Non‐compliance/participant choice (Event) | 0 | 0 | 3 | 4 | 0 | 0 | 11 | 26 | 14 | 30 | 44 |
| Participant went into remission (Censored) | 18 | 24 | 0 | 0 | 6 | 14 | 0 | 0 | 24 | 38 | 62 |
| Lost to follow‐up (Censored) | 0 | 0 | 0 | 0 | 0 | 0 | 26 | 19 | 26 | 19 | 45 |
| Death (Censored)d | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 5 | 4 | 5 | 9 |
| Other (Censored)e | 0 | 0 | 0 | 0 | 0 | 0 | 16 | 11 | 16 | 11 | 27 |
| Completed the study (did not withdraw) (Censored) | 14 | 12 | 14 | 14 | 37 | 38 | 53 | 57 | 118 | 121 | 239 |
| Total | 53 | 53 | 23 | 20 | 60 | 63 | 155 | 165 | 281 | 301 | 592 |
| n = number of individuals contributing to the outcome 'Time to treatment failure’ aOne participant for Heller 1995 (CBZ) and two for De Silva 1996 (one PHT and one CBZ) have missing reasons for treatment failure. | |||||||||||
| Outcome | Original analysis | Generalised onset and age at onset > 30 years classified as focal onset | Generalised onset and age at onset > 30 years classified as uncertain seizure type | |||
| Pooled HR (95% CI) fixed‐effect model | Test of subgroup differences | Pooled HR (95% CI) fixed‐effect model | Test of subgroup differences | Pooled HR (95% CI) fixed‐effect model | Test of subgroup differences | |
| Time to treatment failure (for any reason related to treatment)a | F: 0.83 (0.61 to 1.13), I2 = 0% G: 2.38 (1.04 to 5.47), I2 = 0% O: 0.94 (0.70 to 1.26), I2 = 35% | Chi2 = 5.45, df = 1, P = 0.02, I2 = 81.7% | F: 0.88 (0.65 to 1.19), I2 = 0% G: 1.96 (0.81 to 4.78), I2 = 0% O: 0.96 (0.72 to 1.27), I2 = 6% | Chi2 = 2.83, df = 1, P = 0.09, I2 = 64.6% | F: 0.83 (0.61 to 1.13), I2 = 0% G: 1.96 (0.81 to 4.78), I2 = 0% U: 5.23 (0.47 to 58.71), I2 = NA O: 0.93 (0.70 to 1.24), I2 = 7% | Chi2 = 5.24, df = 2, P = 0.07, I2 = 61.8% |
| Time to treatment failure due to adverse eventsa | F: 1.19 (0.80 to 1.78), I2 = 0% G: 2.31 (0.68 to 7.81), I2 = 60% O: 1.27 (0.87 to 1.86), I2 = 3% | Chi2 = 1.02, df = 1, P = 0.31, I2 = 2.2% | F: 1.25 (0.84 to 1.86), I2 = 22% G: 1.72 (0.51 to 5.87), I2 = 0% O: 1.29 (0.88 to 1.88), I2 = 0% | Chi2 = 0.24, df = 1, P = 0.62, I2 = 0% | F: 1.19 (0.80 to 1.78), I2 = 0% G: 1.72 (0.51 to 5.87), I2 = 0% U: Not estimablec O: 1.24 (0.85 to 1.81), I2 = 0% | Chi2 = 0.31, df = 2, P = 0.86, I2 = 0% |
| Time to treatment failure due to lack of efficacya | F: 0.88 (0.60 to 1.30), I2 = 0% G: 1.86 (0.74 to 4.67), I2 = 0% O: 0.99 (0.69 to 1.41), I2 = 0% | Chi2 = 2.14, df = 1, P = 0.14, I2 = 53.2% | F: 0.91 (0.62 to 1.34), I2 = 0% G: 1.81 (0.68 to 4.82), I2 = 0% O: 1.00 (0.70 to 1.43), I2 = 0% | Chi2 = 1.64, df = 1, P = 0.20, I2 = 38.9% | F: 0.88 (0.60 to 1.30), I2 = 0% G: 1.81 (0.68 to 4.82), I2 = 0% U: Not estimablec O: 0.97 (0.68 to 1.40), I2 = 0% | Chi2 = 1.78, df = 2, P = 0.41, I2 = 0% |
| Time to first seizureb | F: 1.13 (0.89 to 1.43), I2 = 0% G: 1.19 (0.81 to 1.75), I2 = 45% O: 1.15 (0.94 to 1.40), I2 = 0% | Chi2 = 0.05, df = 1, P = 0.83, I2 = 0% | F: 1.15 (0.91 to 1.44), I2 = 0% G: 1.19 (0.77 to 1.84), I2 = 53% O: 1.16 (0.94 to 1.42), I2 = 0% | Chi2 = 0.02, df = 1, P = 0.88, I2 = 0% | F: 1.13 (0.89 to 1.43), I2 = 0% G: 1.19 (0.77 to 1.84), I2 = 53% U: 0.82 (0.29 to 2.34), I2 = NA O: 1.13 (0.92 to 1.39), I2 = 0% | Chi2 = 0.41, df = 2, P = 0.82, I2 = 0% |
| Time to 12‐month remissiona | F: 1.06 (0.80 to 1.42), I2 = 0% G: 0.88 (0.58 to 1.33), I2 = 73% O: 1.00 (0.79 to 1.26), I2 = 16% | Chi2 = 0.54, df = 1, P = 0.46, I2 = 0% | F: 1.10 (0.84 to 1.45), I2 = 0% G: 0.69 (0.43 to 1.11), I2 = 0% O: 0.98 (0.77 to 1.24), I2 = 0% | Chi2 = 2.79, df = 1, P = 0.09, I2 = 64.2% | F: 1.06 (0.80 to 1.42), I2 = 0% G: 0.69 (0.43 to 1.11), I2 = 0% U: 1.91 (0.74 to 4.90), I2 = NA O: 0.99 (0.78 to 1.25), I2 = 15% | Chi2 = 4.32, df = 2, P = 0.12, I2 = 53.7% |
| Time to 6‐month remissiona | F: 0.98 (0.75 to 1.27), I2 = 0% G: 0.77 (0.52 to 1.13), I2 = 39% O: 0.90 (0.73 to 1.12), I2 = 0% | Chi2 = 1.03, df = 1, P = 0.31, I2 = 3.2% | F: 0.98 (0.76 to 1.26), I2 = 0% G: 0.59 (0.37 to 0.93), I2 = 0% O: 0.87 (0.70 to 1.09), I2 = 15% | Chi2 = 3.63, df = 1, P = 0.06, I2 = 72.5% | F: 0.98 (0.75 to 1.27), I2 = 0% G: 0.59 (0.37 to 0.93), I2 = 0% U: 1.20 (0.51 to 2.83), I2 = NA O: 0.88 (0.71 to 1.10), I2 = 2% | Chi2 = 4.01, df = 2, P = 0.13, I2 = 50.1% |
| Chi2: Chi2 statistic; CI: confidence interval; df: degrees of freedom of Chi2 distribution; F: focal epilepsy; G: generalised epilepsy; HR: Hazard Ratio; O: overall (all participants); U: uncertain epilepsy; P: P value (< 0.05 are classified as statistically significant) a29 participants reclassified to focal epilepsy or uncertain epilepsy type from Heller 1995. | ||||||
| Trial | Adverse event dataa | Summary of reported results | |
| Carbamazepine (CBZ) | Phenytoin (PHT) | ||
| All adverse events according to drug (note: no participants withdrew due to adverse events) | CBZ (n = 59): drowsiness (n = 2), rash (n = 3) | PHT (n = 58): gum hypertrophy (n = 2), rash (n = 2), ataxia (n = 2) | |
| “Exclusions” due to adverse events or no efficacy” | Proportion “excluded”: CBZ: 30% (out of 30 randomised to CBZ) | Proportion “excluded”: PHT: 23.3% (out of 30 randomised to PHT) | |
| “Unacceptable” adverse events leading to drug withdrawald | CBZ (n = 54): drowsiness (n = 1), blood dyscrasia (n = 1) | PHT (n = 54): drowsiness (n = 2), skin rash (n = 1), blood dyscrasia (n = 1), hirsutism (n = 1) | |
| Withdrawal due to adverse events (no other adverse event data reported) | 4 participants out of 23 randomised to CBZ withdrew for the following reasons (some withdrew for more than adverse event): slowing of mental function, headache, anorexia, nausea, abdominal pain, fatigue and drowsiness2 | 1 participant out of 20 randomised to PHT withdrew from the study due to depression and anorexia | |
| “Unacceptable” adverse events leading to drug withdrawald | CBZ (n = 61): drowsiness (n = 3), rash (n = 2), headache (n = 1), depression (n = 1) | PHT (n = 63): myalgia (n = 1), irritability (n = 1) | |
| Narrative report of ‘Adverse effects’ and ‘Serious side effects’ | CBZ (n = 155): motor disturbance (ataxia, incoordination, nystagmus, tremor: 33%); dysmorphic and idiosyncratic side effects (gum hypertrophy, hirsutism, acne and rash: 14%); gastrointestinal problems (27%); decreased libido or impotence (13%); No serious side effects | PHT (n = 165); motor disturbance (ataxia, inco‐ordination, nystagmus, tremor: 28%); dysmorphic and idiosyncratic side effects (gum hypertrophy, hirsutism, acne and rash: 22 %); gastrointestinal problems (24%); decreased libido or impotence (11%) 1 serious side effect – 1 participant has confirmed lymphoma, rash improved rapidly following discontinuation of PHT | |
| No adverse events reported | N/A | N/A | |
| Participant reported symptomatic complaints (provided as IPD) | CBZ (n = 19): memory impairment (n = 9) psychomotor retardation (n = 1) inattention (n = 1) transient rash (n = 1) CBZ‐induced cough (n = 1) | PHT (n = 18): memory impairment (n = 7) psychomotor retardation (n = 1) inattention (n = 2) transient rash (n = 1) | |
| Participant‐reported adverse events | 1 participant on CBZ complained of facial skin problems; 1 participant on CBZ complained of tiredness and memory problems | 3 participants on PHT complained of tiredness | |
| Major and minor side effects | CBZ (n = 35): Major side effects: rash (n = 1), pruritus (n = 1), impotence (n = 2), dizziness (n = 1), headaches (n = 1), impaired cognition (n = 1), elevated liver enzymes (n = 1) Mild side effects: nausea (33%), headaches (24%), cognitive impairment (33%), nystagmus (52%), sedation (33%), fine tremor (20%) | PHT (n = 35): Major side effects: rash (n = 4), exfoliative dermatitis (n = 1), impotence (n = 1), dizziness (n = 1), nausea/vomiting (n = 1) Mild side effects: nausea (38%), gingival hyperplasia (12%), headaches (32%), cognitive impairment (15%), nystagmus (40%), sedation (15%), fine tremor (28%) | |
| No adverse events reported | N/A | N/A | |
| CBZ = carbamazepine, N/A = not available, PHT= phenytoin aAdverse event data are recorded as reported narratively in the publications, so exact definition of a symptom may vary. Adverse event data supplied as IPD for Ogunrin 2005. Adverse event data were not requested in original IPD requests (De Silva 1996; Heller 1995; Mattson 1985) but will be for all future IPD requests. For numbers of treatment withdrawals due to adverse events in studies for which IPD were provided (De Silva 1996; Heller 1995; Mattson 1985) see Table 4. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to treatment failure (any reason related to the treatment) Show forest plot | 4 | 589 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.76, 1.31] |
| 2 Time to treatment failure due to adverse events Show forest plot | 4 | 589 | Hazard Ratio (Fixed, 95% CI) | 1.35 [0.93, 1.95] |
| 3 Time to treatment failure due to lack of efficacy Show forest plot | 4 | 589 | Hazard Ratio (Fixed, 95% CI) | 1.02 [0.72, 1.44] |
| 4 Time to treatment failure (any reason related to the treatment) ‐ by epilepsy type Show forest plot | 3 | 546 | Hazard Ratio (Fixed, 95% CI) | 0.94 [0.70, 1.26] |
| 4.1 Focal onset seizures | 3 | 428 | Hazard Ratio (Fixed, 95% CI) | 0.83 [0.61, 1.13] |
| 4.2 Generalised onset seizures | 2 | 118 | Hazard Ratio (Fixed, 95% CI) | 2.38 [1.04, 5.47] |
| 5 Time to treatment failure due to adverse events ‐ by epilepsy type Show forest plot | 3 | 546 | Hazard Ratio (Fixed, 95% CI) | 1.27 [0.87, 1.86] |
| 5.1 Generalised onset seizures | 2 | 118 | Hazard Ratio (Fixed, 95% CI) | 2.31 [0.68, 7.81] |
| 5.2 Focal onset seizures | 3 | 428 | Hazard Ratio (Fixed, 95% CI) | 1.19 [0.80, 1.78] |
| 6 Time to treatment failure due to lack of efficacy ‐ by epilepsy type Show forest plot | 3 | 546 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.69, 1.41] |
| 6.1 Focal onset seizures | 3 | 428 | Hazard Ratio (Fixed, 95% CI) | 0.88 [0.60, 1.30] |
| 6.2 Generalised onset seizures | 2 | 118 | Hazard Ratio (Fixed, 95% CI) | 1.86 [0.74, 4.67] |
| 7 Time to first seizure post‐randomisation Show forest plot | 4 | 582 | Hazard Ratio (Fixed, 95% CI) | 1.13 [0.92, 1.39] |
| 8 Time to first seizure post‐randomisation ‐ by epilepsy type Show forest plot | 4 | 582 | Hazard Ratio (Fixed, 95% CI) | 1.15 [0.94, 1.40] |
| 8.1 Focal onset seizures | 4 | 432 | Hazard Ratio (Fixed, 95% CI) | 1.13 [0.89, 1.43] |
| 8.2 Generalised onset seizures | 3 | 150 | Hazard Ratio (Fixed, 95% CI) | 1.19 [0.81, 1.75] |
| 9 Time to achieve 12‐month remission Show forest plot | 3 | 551 | Hazard Ratio (Fixed, 95% CI) | 1.01 [0.80, 1.27] |
| 10 Time to achieve 12‐month remission ‐ by epilepsy type Show forest plot | 3 | 551 | Hazard Ratio (Fixed, 95% CI) | 1.00 [0.79, 1.26] |
| 10.1 Focal onset seizures | 3 | 430 | Hazard Ratio (Fixed, 95% CI) | 1.06 [0.80, 1.42] |
| 10.2 Generalised onset seizures | 2 | 121 | Hazard Ratio (Fixed, 95% CI) | 0.88 [0.58, 1.33] |
| 11 Time to achieve six‐month remission Show forest plot | 3 | 551 | Hazard Ratio (Fixed, 95% CI) | 0.92 [0.74, 1.14] |
| 12 Time to achieve six‐month remission ‐ by epilepsy type Show forest plot | 3 | 551 | Hazard Ratio (Fixed, 95% CI) | 0.90 [0.73, 1.12] |
| 12.1 Focal onset seizures | 3 | 430 | Hazard Ratio (Fixed, 95% CI) | 0.98 [0.75, 1.27] |
| 12.2 Generalised onset seizures | 2 | 121 | Hazard Ratio (Fixed, 95% CI) | 0.77 [0.52, 1.13] |

