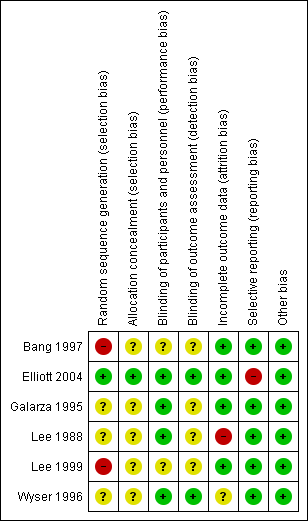
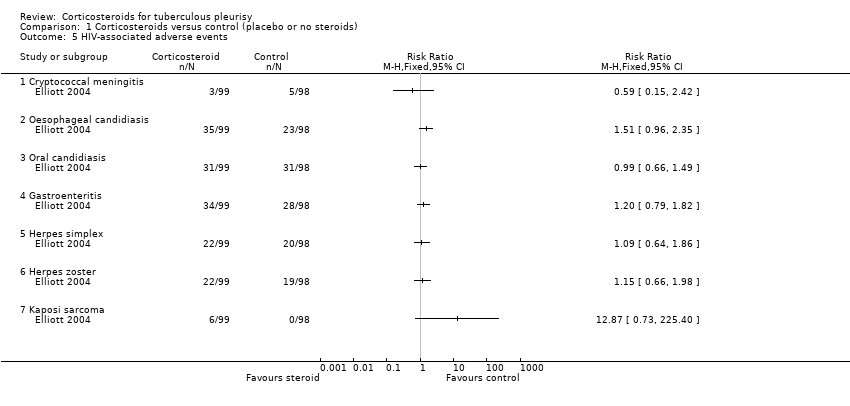
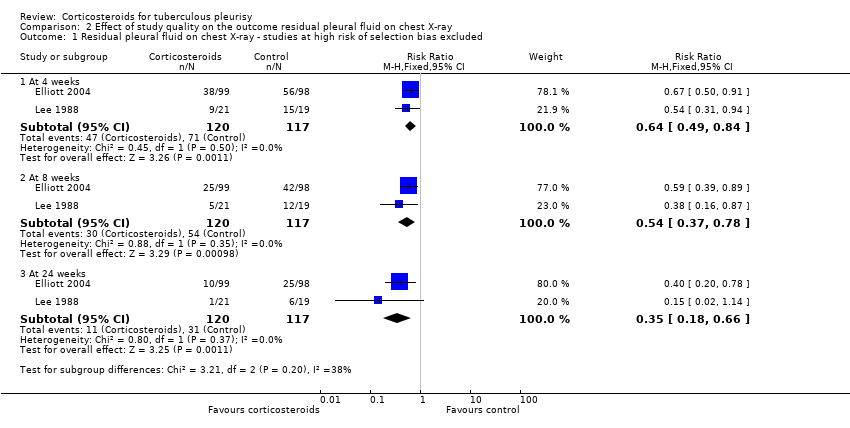
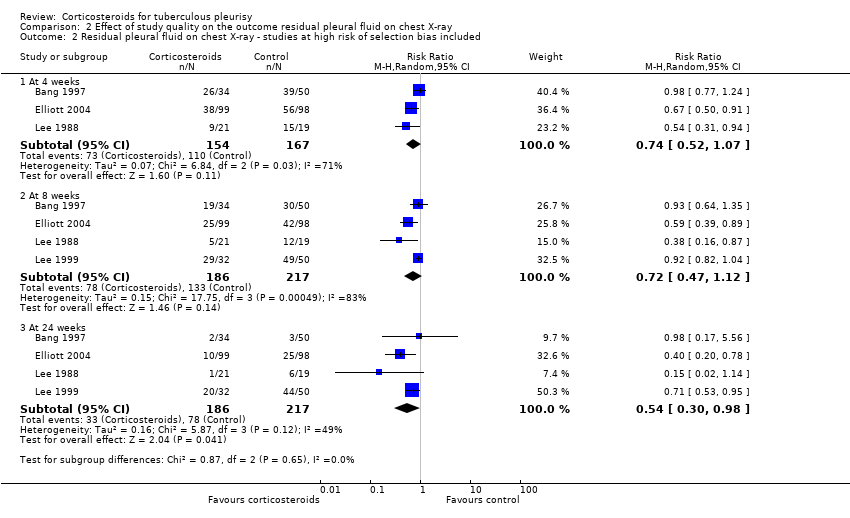
Contenido relacionado
Revisiones y protocolos relacionados
Charles S Wiysonge, Mpiko Ntsekhe, Lehana Thabane, Jimmy Volmink, Dumisani Majombozi, Freedom Gumedze, Shaheen Pandie, Bongani M Mayosi | 13 septiembre 2017
Julia A Critchley, Lois C Orton, Fiona Pearson | 12 noviembre 2014
Moleen Zunza, Diane M Gray, Taryn Young, Mark Cotton, Heather J Zar | 29 agosto 2017
Kameshwar Prasad, Mamta B Singh, Hannah Ryan | 28 abril 2016
Fan Zhang, Christine V Kramer | 30 junio 2014
Bhagteshwar Singh, Derek Cocker, Hannah Ryan, Derek J Sloan | 20 marzo 2019
Geraint R Davies, Stefania Cerri, Luca Richeldi | 17 octubre 2007
Surendra K Sharma, Anju Sharma, Tamilarasu Kadhiravan, Prathap Tharyan | 5 julio 2013
Theresa Avesa, Joshua Tambea, Reed AC Siemieniuk, Lawrence Mbuagbaw | 9 noviembre 2018
Jennifer Onwumeh, Charles I Okwundu, Tamara Kredo | 25 mayo 2017
Respuestas clínicas Cochrane
Sera Tort, Fyezah Jehan | 3 octubre 2017