Corticosteroides para la pleuresía tuberculosa
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Setting: Inha University Hospital, South Korea Date: recruitment from June 1991 to September 1994 Trial design: prospective randomized study Follow‐up: duration not clearly reported, 8 to 9 months. Chest X‐rays were performed weekly while participants were hospitalized, then monthly after discharge. Participants were asked to complete a questionnaire detailing their symptoms every day until resolution of all symptoms. | |
| Participants | Number of participants: 84 adults (83 included in analysis, 1 participant was excluded because they experienced increased epigastric pain after commencing steroids and so study drug was stopped); 49 male (59%), 34 female (41%) Age: mean 34 years, range 18 to 50 years Inclusion criteria: patients who were admitted to the hospital and diagnosed with tuberculous pleurisy. All participants had pleural biopsy and diagnostic pleurocentesis performed on the 1st or 2nd day of admission. Diagnosis of tuberculosis (TB) pleurisy was based upon the following: histological findings corresponding to TB on pleural biopsy, acid‐fast bacilli (AFB) positive on smear microscopy or culture positive from sputum, pleural fluid, or pleural biopsy. Exclusion criteria
HIV status: all participants were HIV‐negative. The trial took place during a period when HIV infection was very uncommon in South Korea. | |
| Interventions | Intervention: ATT plus prednisolone 1 mg/kg twice daily, tapered by 10 mg each week until complete cessation Control: ATT alone ATT: isoniazid (400 mg/day), rifampicin (600 mg/day; 450 mg if weight 50 kg or less), pyrazinamide (1500 mg/day), ethambutol (800 mg/day) for 2 months followed by same regimen minus pyrazinamide for 7 months | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The trial authors did not state the method of randomization, and the number of participants in each group appears imbalanced (steroid group N = 34, control group N = 50). “Patients deemed eligible for this study were randomized to the steroid group and the non‐steroid group.” |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not report this information. |
| Blinding of participants and personnel (performance bias) | Unclear risk | The trial authors did not report this information. |
| Blinding of outcome assessment (detection bias) | Unclear risk | The trial authors did not report this information. |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up were reported. |
| Selective reporting (reporting bias) | Low risk | The trial protocol was unavailable, but the trial authors reported all outcomes specified in the introduction in the results. |
| Other bias | Low risk | We did not identify any other sources of bias. |
| Methods | Setting: National TB Treatment centre, Mulago Hospital, Kampala, Uganda Date: recruitment from November 1998 to January 2002 Trial design: randomized, doubled‐blind, placebo‐controlled trial Follow‐up: all participants were followed up until July 2002; median follow‐up time was 1.48 years in the steroid group, and 1.65 years in the placebo group. Participants were managed in hospital or as daily ward attenders for the first week of treatment, and after that were discharged home and visited regularly to monitor treatment for 8 weeks. Participants then attended clinic monthly until the end of ATT, and then 3‐monthly after ATT completion. | |
| Participants | Number of participants (% female): 197, 83 (42%) female. 98 received placebo and 99 received prednisolone. Three participants were lost to follow‐up and excluded from the analysis (1 from placebo group, 2 from prednisolone group) Age: mean 34 years Inclusion criteria: participants were eligible for screening if they were > 18 years old with clinical features consistent with pleural TB and a pleural effusion occupying at least 1/3 of 1 hemithorax on chest X‐ray. Screening procedures consisted of medical examination; blood samples including glucose, HIV, and cryptococcal antigen tests; urine sample for dipstick; diagnostic pleural aspiration, and pleural biopsy if possible. Pleural TB was considered to be confirmed if a patient had a positive culture for M. tuberculosis from pleural biopsy, pleural fluid, or sputum or if histopathological analysis of pleural tissue was consistent with tuberculous pleurisy. Exclusion criteria: people recently treated with glucocorticoids, pregnant, or breast‐feeding women, and people not resident in Kampala were excluded from screening. The trial excluded people after screening if they failed to completed the screening procedures, pleural fluid could not be obtained, they had empyema, they had a second major HIV‐related disease, they had risk factors for serious steroid‐related adverse events (history of diabetes or finding of glycosuria, history or finding of hypertension, history of peptic ulcer disease, or mental illness), they could not receive standard doses of antituberculous treatment (ATT) (for example, concurrent liver disease), they were HIV‐negative. HIV status: the trial excluded HIV‐negative people | |
| Interventions | Intervention: ATT plus prednisolone 50 mg daily for 2 weeks, then 40 mg daily for 2 weeks, then 25 mg daily for 2 weeks, then 15 mg daily for 2 weeks, then stopped. Control: ATT plus placebo. ATT: daily rifampicin, isoniazid, pyrazinamide, and ethambutol for 2 months, followed by daily rifampicin and isoniazid for 4 months; doses adjusted for weight using standard criteria. | |
| Outcomes |
| |
| Notes | The trial authors did not mention antiretroviral therapy in this trial. We contacted the trial authors to check whether any of the participants were given antiretroviral therapy, and they reported that to the best of their knowledge none of the participants were taking antiretroviral therapy at any time during the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The randomization sequence was generated by a statistician who was not involved in the care of the patients, by use of STATA (version 5; Stata Corporation). Randomization was done in blocks of 20.” |
| Allocation concealment (selection bias) | Low risk | “Prednisolone and matching placebo tablets were packaged in identical plastic bags, which were labeled with randomization code numbers by two people who were not involved in the study. Medical staff gave participants the next number in the sequence in the order in which they were enrolled.” |
| Blinding of participants and personnel (performance bias) | Low risk | “All participants and medical, laboratory, and statistical staff remained blinded to the treatment allocation until all data collection had been completed.” |
| Blinding of outcome assessment (detection bias) | Low risk | “All participants and medical, laboratory, and statistical staff remained blinded to the treatment allocation until all data collection had been completed.” |
| Incomplete outcome data (attrition bias) | Low risk | Nine participants were lost to follow‐up, 6/98 in the steroid group and 3/99 in the placebo group, representing 5% of the total participants. |
| Selective reporting (reporting bias) | High risk | The study protocol was not available. The primary outcome was all‐cause mortality and this is clearly stated, but the other outcomes are not specified in the introduction or methods section. "The use of prednisolone was associated with more‐rapid improvement in all of the principal symptoms and signs of pleural tuberculosis. This effect was statistically significant, particularly during the first few weeks of treatment, for anorexia, weight loss, and cough (figure 3A–C)." Only data related to the statistically significant outcomes for symptomatic improvement are reported. |
| Other bias | Low risk | We did not identify any other potential sources of bias. |
| Methods | Location: Hospital Universitari de Bellvitge, Barcelona, Spain Date: January 1985 and December 1992 Trial design: prospective, randomized, double blind, placebo controlled study Follow‐up: 6 months | |
| Participants | Number of participants (% female): 117, 58 (48%) female. 60 received placebo, 57 received prednisone. No losses to follow‐up reported. Age: 11 to 53 years Inclusion criteria: diagnosis of tuberculous pleurisy was made if patients met at least one of the following criteria.
Diagnostic algorithm: thoracocentesis with analysis of pleural biopsy and pleural fluid Exclusion criteria: diagnostic investigations not consistent with the inclusion criteria, HIV seropositive HIV status: the trial excluded HIV‐positive people | |
| Interventions | Intervention: prednisone plus standard regimen Prednisone: single oral dose of 1 mg/kg/day for 15 days tapering off over the next 15 days Standard regimen: isoniazid (5 mg/kg/day; max 300 mg/day); rifampicin (10 mg/kg/day; max 600 mg/day); once daily as a combination tablet for 6 months | |
| Outcomes |
| |
| Notes | A definite microbiological or pathological diagnosis was confirmed in 63% of participants. Before discharge, pleural fluid was drained until 1/3 of hemithorax was occupied on standard chest X‐ray in all participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not describe the method of randomization. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | “Patients were randomly assigned to receive, in a double blind fashion, either prednisolone or placebo”. |
| Blinding of outcome assessment (detection bias) | Unclear risk | The trial authors did not mention the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) | Low risk | The trial authors did not report any losses to follow‐up; there were no unexplained defaulters. |
| Selective reporting (reporting bias) | Low risk | No protocol available, but the trial authors reported on the 2 main outcomes specified in the introduction. |
| Other bias | Low risk | We did not identify any other source of bias. |
| Methods | Location: Chang Gung Memorial Hospital, Taipei, Taiwan Date: October 1983 with recruitment until June 1987 Trial design: double‐blind, placebo‐controlled, randomized study Follow‐up: up to 24 months | |
| Participants | Number of participants (% female): 45 recruited to study, 16/40 (40%) female. 40 participants included in analysis, 21 in steroid group, 19 in placebo group. Five participants were excluded from the analysis, 1 due to a diagnosis of renal cell carcinoma and 4 were lost to follow‐up. The trial authors did not report which group the excluded participants were randomized to. Age: mean age 28.7 years, range 18 to 45 years Inclusion criteria: under 45 years, new pleural effusion not previously treated, with pleural biopsy reported at TB or chronic granulomatous inflammation Exclusion criteria: history of pulmonary TB, diagnosis of alternative cause of pleural effusion such as heart failure, malignancy, pneumonia, history of other pulmonary disease or condition that contraindicated the use of steroids such as diabetes, peptic ulcer, hypertension. HIV status: not reported | |
| Interventions | Intervention: prednisolone plus standard regimen Prednisolone initially given as a single oral dose (0.75 mg/kg/day), tapered gradually over 2 to 3 months once radiological improvement was seen by 5 mg per week until discontinued. Standard regimen: isoniazid (300 mg/day); rifampicin (450 mg/day) for 9 to 12 months; and ethambutol (20 mg/kg/day) for 3 months | |
| Outcomes |
| |
| Notes | Diagnostic thoracocentesis (< 50 mL) performed on the first day for all participants; no participants reported as having therapeutic thoracocentesis. Criteria for tapering prednisolone dose as follows
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Those who were eligible for the study were randomly assigned to treatment with either prednisolone plus antituberculosis drugs (steroid group) or placebo with antituberculosis drugs (placebo group." |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | The trial authors described this as “double blind”. |
| Blinding of outcome assessment (detection bias) | Unclear risk | The trial authors did not mention whether blinding of outcome assessors took place or not. |
| Incomplete outcome data (attrition bias) | High risk | The trial excluded 5 participants excluded from the final analysis; 1 due to diagnosis of renal cell carcinoma and 4 due to loss to follow‐up with no further information given. Therefore the trial authors included 89% of recruited participants in the final analysis. The trial authors did not report which groups the five excluded participants were randomized to. |
| Selective reporting (reporting bias) | Low risk | There was no protocol available, but the trial authors reported all outcomes that they clearly stated in the methods. |
| Other bias | Low risk | We did not identify any other sources of bias. |
| Methods | Setting: Chung‐Ang University Hospital, South Korea Date: February 1990 to February 1997 Trial design: prospective randomized study Follow‐up: participants were followed up at 2 months and 6 months, and in a final visit to the out‐patient department after treatment. Median follow‐up was 9 months in the steroid group and 12 months in the control group. | |
| Participants | Number of participants: 82, 29 (35%) female; 32 participants in the steroid group and 50 in the control group. No losses to follow‐up reported. Age: mean 32 years, range 17 to 51 years Inclusion criteria: people admitted to hospital with a diagnosis of TB pleurisy based on TB on pleural biopsy, or pleural effusion with AFB stain positive on microscopy of sputum, pleural fluid, or pleural biopsy, or M. tuberculosis culture positive on sputum, pleural fluid, or pleural biopsy. Pleural biopsy and diagnostic pleurocentesis were performed on all patients on the 1st or 2nd day of admission. Exclusion criteria: people with pleural effusion due to other causes (not specified by the trial authors). People with diabetes, hypertension, or peptic ulcer disease who could not receive corticosteroids. People who were "not cooperative". HIV status: not mentioned. | |
| Interventions | Intervention: ATT plus prednisolone 30 mg once daily for 1 month and then tapered over the following month. | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The trial did not state the method of randomization, and the number of participants in each group appears imbalanced (steroid group N = 50, control group N = 32). “… patients were randomized to the steroid group and the non‐steroid group". |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not report on the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | The trial authors did not report on blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | Unclear risk | The trial did not report on blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Low risk | There were no losses to follow‐up. |
| Selective reporting (reporting bias) | Low risk | The trial protocol was unavailable, but the trial authors reported all outcomes specified in the introduction in the results. |
| Other bias | Low risk | We did not identify any other sources of bias. |
| Methods | Setting: Tygerberg Hospital, Cape Town, South Africa Date: April 1994 with recruitment until January 1995 Trial design: double‐blind, placebo‐controlled, randomized study Follow‐up: 6 months | |
| Participants | Number of participants (% female): 74 participants randomized, 70 included in analysis, 36 in placebo group, 34 in prednisone group, 27/70 (36.5%) female. The trial authors excluded 4 participants from analysis, 3 due to non‐compliance with treatment, 1 due to diagnosis of oesophageal cancer at follow‐up. The trial authors did not report to which group the excluded participants were randomized to. Age: mean age 33 years Inclusion criteria: pleural biopsy specimen proving TB pleurisy, based on presence of caseating granulomata with or without AFB on histological examination, or a positive culture for M. tuberculosis. Diagnostic algorithm: thoracoscopy followed by bronchoscopy under general anaesthesia, with biopsies of the parietal pleura taken for histological examination and culture. Exclusion criteria: people with other causes of exudative effusion such as pneumonia or cancer. People with contraindications to corticosteroids such as diabetes mellitus, uncontrolled hypertension, peptic ulcer disease, and empyema. People with HIV. HIV status: the trial authors excluded HIV‐positive people. | |
| Interventions |
Prednisone: oral dose of 0.75 mg/kg/day for 2 to 4 weeks; dose tapered by 5 mg/day over 2 weeks after clinical and radiological improvement Standard regimen: isoniazid (8 mg/kg/day), rifampicin (10 mg/kg/day), and pyrazinamide (25 mg/kg/day) as a fixed combination tablet (Rifater); and pyridoxine (25 mg/kg/day) for 6 months | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “All eligible patients were randomly assigned in a double‐blind fashion to treatment with either prednisone plus standard anti‐TB therapy (prednisone group) or placebo plus standard anti‐TB therapy (placebo group).” |
| Allocation concealment (selection bias) | Unclear risk | The trial described the placebo tablets as “identical”. The trial authors did not clearly describe the method of concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | The trial authors described the trial as “double blind”. |
| Blinding of outcome assessment (detection bias) | Low risk | The outcome assessors were “blinded to the clinical history”. |
| Incomplete outcome data (attrition bias) | Unclear risk | The trial authors excluded four participants from the analysis; 3 due to “non‐compliance with the treatment”. |
| Selective reporting (reporting bias) | Low risk | The protocol was unavailable, but the trial authors reported the outcomes described in the introduction. |
| Other bias | Low risk | We did not identify any other source of bias. |
Abbreviations: AFB: acid‐fast bacilli; ATT: antituberculous treatment; FVC: forced vital capacity; HIV: human immunodeficiency virus; TB: tuberculosis.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| No randomization | |
| Participants did not have pleurisy ‐ cases of pulmonary tuberculosis (TB) | |
| Case series | |
| Review | |
| Numbers of participants in each trial arm not clearly stated | |
| No randomization | |
| Diagnosis of TB not confirmed | |
| No randomization | |
| Participants did not have pleurisy ‐ cases of pulmonary TB | |
| Letter referring to included trial (Galarza 1995) | |
| No randomization | |
| No randomization | |
| No randomization | |
| Participants did not have pleurisy ‐ cases of pulmonary TB | |
| No randomization | |
| Compared prednisolone to another steroid (cortivazol) | |
| No randomization | |
| Participants did not have pleurisy ‐ cases of pulmonary TB | |
| No randomization | |
| No randomization | |
| No randomization | |
| No randomization |
Abbreviations: TB: tuberculosis.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A multi‐center, randomized, double‐blind, parallel placebo trial to evaluate the clinical efficacy of glucocorticosteroid for tuberculous pleurisy |
| Methods | Interventional randomized parallel control trial |
| Participants | Inclusion criteria
Exclusion criteria
Target sample size: Group A: 500; Group B: 500; Total: 1000 |
| Interventions | Group A: prednisone orally taken 30 mg once daily for 2 weeks, then reduce to 20 mg once daily for 3rd week; finally reduce to 10 mg for 4th week; Group B: placebo orally taken 30 mg once daily for 2 weeks, then reduce to 20 mg once daily for 3rd week; finally reduce to 10 mg for 4th week |
| Outcomes | Primary outcome
Secondary outcome
|
| Starting date | Date of first enrolment: 1 January 2010 Last refreshed: 29 June 2014 |
| Contact information | Huangzhong Shi, Department of Respiratory of Wuhan Union Hospital, No. 1277 Liberation Avenue, Wuhan 430022. Tel: +86 027 85726010. Email: [email protected] JIianbo Xin, Department of Respiratory of Wuhan Union Hospital, No. 1277 Liberation Avenue, Wuhan 430022. Tel: +86 027 85726757. Email: [email protected] |
| Notes | Sponsors: Wuhan Union Hospital; National Science Fund for Distinguished Young Scholars, National Natural Science Foundation of China Trial is identical to ChiCTR‐TRC‐09000747 (www.chictr.org.cn/showproj.aspx?proj=8789) We contacted the trial authors for further information but did not receive a response to date |
| Trial name or title | A Multicenter, Placebo‐Controlled, Double‐Blind, Randomized Clinical Trial to Evaluate the Efficacy and Safety of Corticosteroids for Treatment of Patients With Tuberculous Pleurisy |
| Methods | Interventional double‐blinded randomized parallel safety/efficacy study |
| Participants | Inclusion criteria
Exclusion criteria
Target sample size: 1500 |
| Interventions | Prednisolone versus placebo |
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | Date of registration: 19 June 2006 Date of first enrollment: July 2006 |
| Contact information | Xin Zhou, MD, Department of Respiratory Diseases, First Affiliated Hospital, Shanghai Jiaotong University, Shanghai, China Zhan‐Cheng Gao, MD, PhD, Department of Respiratory Diseases, People's Hospital, Peking University, Beijing, China Huan‐Zhong Shi, MD, PhD, Institute of Respiratory Diseases, First Affiliated Hospital, Guangxi Medical University, Nanning 530021, Guangxi, China |
| Notes | Sponsors: Guangxi Medical University; Bureau of Science and Technology of Guangxi Province, China; Ministry of Education, China; National Natural Science Foundation of China Recruitment completed. We contacted the trial authors but have not received a reply to date. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Residual pleural effusion on chest X‐ray Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1 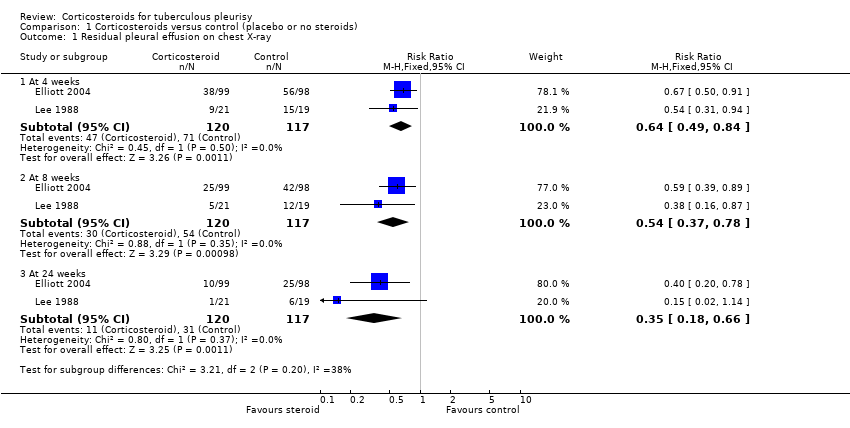 Comparison 1 Corticosteroids versus control (placebo or no steroids), Outcome 1 Residual pleural effusion on chest X‐ray. | ||||
| 1.1 At 4 weeks | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.49, 0.84] |
| 1.2 At 8 weeks | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.37, 0.78] |
| 1.3 At 24 weeks | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.18, 0.66] |
| 2 Pleural changes at the end of treatment (pleural thickening and pleural adhesions) Show forest plot | 5 | 393 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.57, 0.92] |
| Analysis 1.2 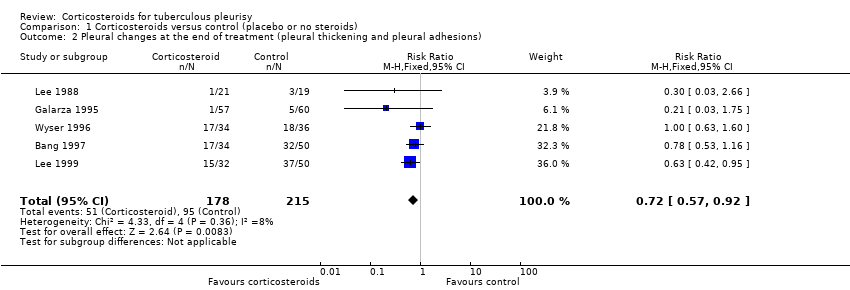 Comparison 1 Corticosteroids versus control (placebo or no steroids), Outcome 2 Pleural changes at the end of treatment (pleural thickening and pleural adhesions). | ||||
| 3 Death from any cause Show forest plot | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.64, 1.31] |
| Analysis 1.3 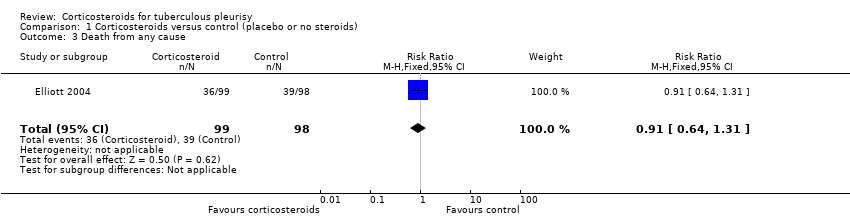 Comparison 1 Corticosteroids versus control (placebo or no steroids), Outcome 3 Death from any cause. | ||||
| 4 Adverse events leading to study drug discontinuation Show forest plot | 6 | 590 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.78 [1.11, 6.94] |
| Analysis 1.4  Comparison 1 Corticosteroids versus control (placebo or no steroids), Outcome 4 Adverse events leading to study drug discontinuation. | ||||
| 5 HIV‐associated adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.5  Comparison 1 Corticosteroids versus control (placebo or no steroids), Outcome 5 HIV‐associated adverse events. | ||||
| 5.1 Cryptococcal meningitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Oesophageal candidiasis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Oral candidiasis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Gastroenteritis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 Herpes simplex | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.6 Herpes zoster | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.7 Kaposi sarcoma | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Residual pleural fluid on chest X‐ray ‐ studies at high risk of selection bias excluded Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Effect of study quality on the outcome residual pleural fluid on chest X‐ray, Outcome 1 Residual pleural fluid on chest X‐ray ‐ studies at high risk of selection bias excluded. | ||||
| 1.1 At 4 weeks | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.49, 0.84] |
| 1.2 At 8 weeks | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.37, 0.78] |
| 1.3 At 24 weeks | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.18, 0.66] |
| 2 Residual pleural fluid on chest X‐ray ‐ studies at high risk of selection bias included Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 Effect of study quality on the outcome residual pleural fluid on chest X‐ray, Outcome 2 Residual pleural fluid on chest X‐ray ‐ studies at high risk of selection bias included. | ||||
| 2.1 At 4 weeks | 3 | 321 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.52, 1.07] |
| 2.2 At 8 weeks | 4 | 403 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.47, 1.12] |
| 2.3 At 24 weeks | 4 | 403 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.30, 0.98] |

Study flow diagram.

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included trials.
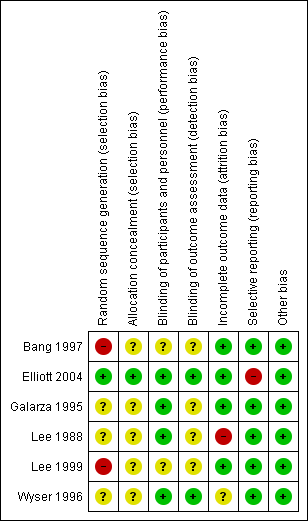
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included trial.

Comparison 1 Corticosteroids versus control (placebo or no steroids), Outcome 1 Residual pleural effusion on chest X‐ray.

Comparison 1 Corticosteroids versus control (placebo or no steroids), Outcome 2 Pleural changes at the end of treatment (pleural thickening and pleural adhesions).

Comparison 1 Corticosteroids versus control (placebo or no steroids), Outcome 3 Death from any cause.

Comparison 1 Corticosteroids versus control (placebo or no steroids), Outcome 4 Adverse events leading to study drug discontinuation.
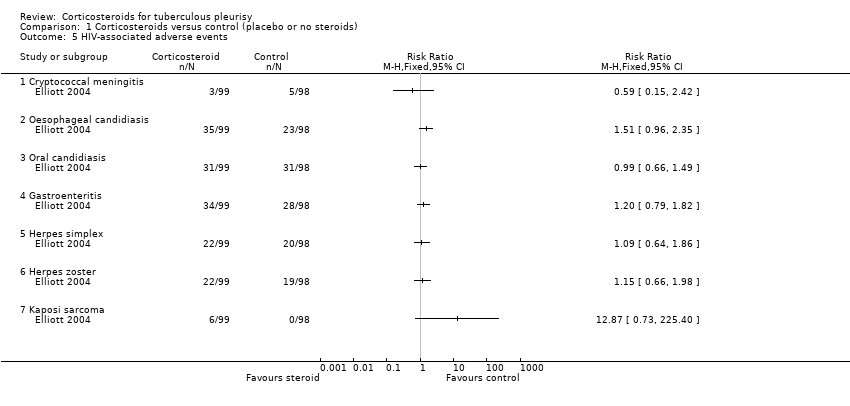
Comparison 1 Corticosteroids versus control (placebo or no steroids), Outcome 5 HIV‐associated adverse events.
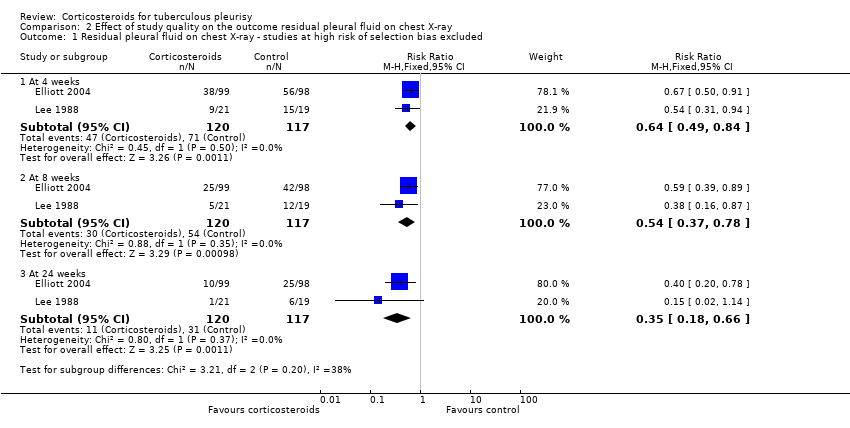
Comparison 2 Effect of study quality on the outcome residual pleural fluid on chest X‐ray, Outcome 1 Residual pleural fluid on chest X‐ray ‐ studies at high risk of selection bias excluded.
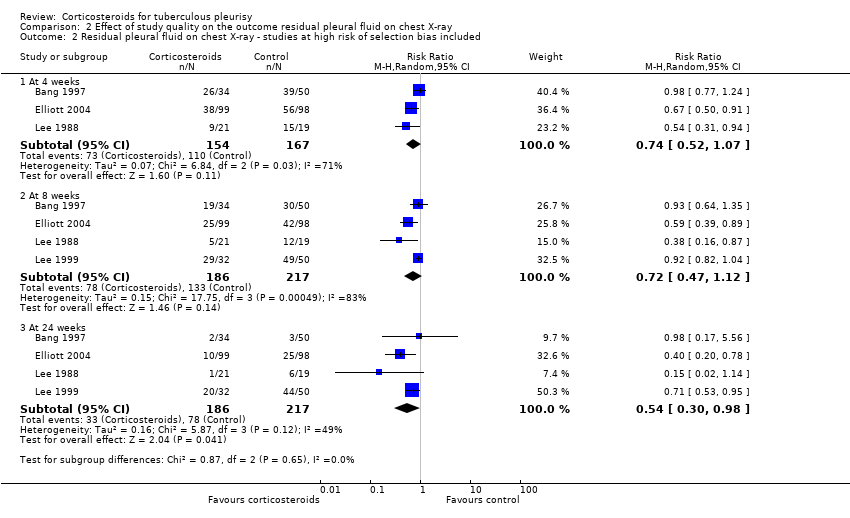
Comparison 2 Effect of study quality on the outcome residual pleural fluid on chest X‐ray, Outcome 2 Residual pleural fluid on chest X‐ray ‐ studies at high risk of selection bias included.
| Steroids compared with placebo for pleural TB | |||||
| Patient or population: adults and adolescents with pleural TB Settings: hospital care and community follow‐up Intervention: corticosteroids Comparison: placebo | |||||
| Outcomes | Illustrative comparative risks¹ (95% CI) | Relative effect | Number of participants | Certainty of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Control | Corticosteroids | ||||
| Residual pleural fluid on chest X‐ray at 8 weeks | 62 per 100 | 33 per 100 (23 to 48) | RR 0.54 (0.37 to 0.78) | 237 (2 trials) | ⊕⊕⊝⊝1,2,3,4 |
| Residual pleural fluid on chest X‐ray at 24 weeks | 29 per 100 | 10 per 100 (5 to 19) | RR 0.35 (0.18 to 0.66) | 237 (2 trials) | ⊕⊕⊝⊝1,2,3,4 low |
| Pleural changes at the end of follow‐up (pleural adhesions or pleural thickening on chest X‐ray; follow‐up 6 to 24 months) | 50 per 100 | 36 per 100 (29 to 46) | RR 0.72 (0.57 to 0.92) | 393 (5 trials) | ⊕⊕⊝⊝5,6,7 |
| Long‐term functional respiratory impairment (> 6 months) | — | — | Average percentage predicted FVC similar in corticosteroid and control groups. | 187 (2 trials) | ⊕⊝⊝⊝8 |
| Adverse events leading to treatment discontinuation (follow‐up 6 to 24 months) | 1 per 100 | 3 per 100 | RR 2.78 (1.11 to 6.94) | 590 | ⊕⊕⊝⊝9,10 |
| HIV‐related infections (cryptococcal meningitis) | 5 per 100 | 3 per 100 (1 to 12) | RR 0.59 (0.15 to 2.42) | 103 (1 trial) | ⊕⊝⊝⊝11,12 |
| HIV‐related cancer (Kaposi's sarcoma) | 14 per 100013 | 180 per 1000 (1 to 316) | RR 12.87 (0.73 to 225.40) | 103 (1 trial) | ⊕⊝⊝⊝14,15 |
| ¹The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by one for risk of bias: of the four trials that reported this outcome, we excluded two trials from the final analysis due to high risk of selection bias, after a subgroup analysis suggested the pooled estimate including these studies could be misleading (Bang 1997; Lee 1999). We judged this to be our best estimate of effect. However because we excluded trials from this analysis this generates uncertainty, so we have downgraded the quality of the evidence. | |||||
| Clinical Feature | Isolated pleural TB | Pleuro‐pulmonary TB |
| Sputum microscopy/culture | Negative | Some positive |
| Pleural fluid | Usually demonstrates exudative effusion Usually negative for M. tuberculosis on smear and culture | Usually demonstrates exudative effusion Usually negative for M. tuberculosis on smear and culture |
| Chest X‐ray | Discrete pleural effusion, or pleural thickening, or both | Pleural effusion with other changes such as consolidation, cavities, atelectasis, or hilar enlargement |
| Chest computed tomography (CT) | May demonstrate underlying lung infection | Demonstrates underlying lung infection |
| Pathogenesis | Predominantly driven by delayed type hypersensitivity reaction | Predominantly driven by TB infection of the lung |
| Prognosis | Most people will improve with no antituberculous treatment (ATT), but may experience a relapse of TB infection | People may deteriorate and die without ATT |
| Trial | Country | Year | Participants | Adults or children | HIV status | ATT regimen | Therapeutic thoracocentesis performed | |
| Steroid group | Control group | |||||||
| South Korea | 1991 to 1994 | 34 | 50 | Adults | Not reported | 2RHZE/7RHE | No | |
| Uganda | 1998 to 2002 | 99 | 98 | Adults | Positive | 2RHZE/4RH | No | |
| Spain | 1985 to 1992 | 57 | 60 | Both | Negative | 6RH | Yes | |
| Taiwan | 1983 to 1987 | 21 | 19 | Adults | Not reported | 3RHE/6‐9RH | No | |
| South Korea | 1990 to 1997 | 50 | 32 | Adults | Not reported | 6RHZE or 2RHZS/4RHZ | No | |
| South Africa | 1994 to 1995 | 34 | 36 | Adults | Negative | 6RHZ | Yes | |
| Abbreviations: ATT: antituberculous treatment; E: ethambutol; H: isoniazid; R: rifampicin; S: streptomycin; Z: pyrazinamide. | ||||||||
| Trial | Diagnostic criteria for pleural TB | Other diagnostic tests |
| Microscopy positive for AFB or culture positive from sputum, pleural fluid, or pleural biopsy. |
| |
| Positive culture from pleural biopsy, pleural fluid, or sputum, or histopathologic analysis of pleural biopsy consistent with tuberculous pleurisy |
| |
| At least one of the following
|
| |
| Pleural biopsy reported as pleural TB or chronic granulomatous inflammation |
| |
| TB on pleural biopsy, or pleural effusion plus AFB stain positive or culture positive from sputum, pleural fluid, or pleural biopsy |
| |
| Pleural biopsy with caseating granulomata with or without AFB on histological examination, or positive culture. |
| |
| Abbreviations: ADA: adenosine deaminase activity; AFB: acid‐fast bacilli; CT: computed tomography; ELISA: enzyme‐linked immunosorbent assay; FEV1: forced expiratory volume at one second; FVC: forced vital capacity; HIV: human immunodeficiency virus; PPD: purified protein derivative; PPD‐S: purified protein derivative‐standard; TB: tuberculosis | ||
| Trial | Steroid | Regimen |
| Prednisolone | 1 mg/kg twice daily, tapered by 10 mg each week until cessation | |
| Prednisolone | 50 mg daily for 2 weeks, 40 mg daily for 2 weeks, then 25 mg daily for 2 weeks, then 15 mg daily for 2 weeks, then stopped | |
| Prednisone | 1 mg/kg/day for 15 days, tapering over the next 15 days | |
| Prednisolone | 0.75 mg/kg/day, tapered by 5 mg per week until discontinued once radiological improvement was seen | |
| Prednisolone | 30 mg four times daily for 1 month and tapered over the following month | |
| Prednisone | 0.75 mg/kg/day for 2 to 4 weeks; dose tapered by 5 mg/day over 2 weeks after clinical and radiological improvement | |
| Abbreviations: mg: milligrams | ||
| Trial | Indicator | Units | Corticosteroids | Control | |
| Mean values | “Fever, pleuritic pain, malaise and breathlessness” | Mean days to resolution | 3.8 (N = 34) | 7.41 (N = 50) | |
| “Fever duration” | Mean days | 3.32 (N = 57) | 4.15 (N = 60) | ||
| “Fever, pleuritic pain, malaise and breathlessness” | Mean days to resolution | 2.4 (N = 21) | 5.6 (N = 19) | ||
| Cut‐offs (categorical) | “Anorexia” | Number of participants with anorexia at 4 weeks | 3/99 (3%) | 18/98 (18.4%) | |
| “Cough” | Number of participants with cough at 4 weeks | 35/99 (35.4%) | 57/98 (58.2%) | ||
| “Weight” | Mean weight in kg at 4 weeks | 57 | 52.5 | ||
| Symptoms resolved in all patients (VAS score) | Weeks | 12 | 16 | ||
| Abbreviations: kg: kilograms; VAS: visual analogue scale | |||||
| Trial | Units | Corticosteroids | Control | |
| Mean values | Mean days to resolution | 88 (N=34) | 100 (N=50) | |
| Mean days to resolution | 54.5 (N=21) | 123.2 (N=19) | ||
| Reabsorption index1 at 4 weeks | 93% | 89%2 | ||
| Categorical values | Number of participants with residual effusion at 4 weeks | 26/34 (76.5%) | 39/50 (78%) | |
| Number of participants with residual effusion at 8 weeks | 19/34 (55.9%) | 30/50 (60%) | ||
| Number of participants with residual effusion at 24 weeks | 2/34 (5.9%) | 3/50 (6%) | ||
| Number of participants with residual effusion at 4 weeks | 38/99 (38.4%) | 56/98 (57.1%) | ||
| Number of participants with residual effusion at 8 weeks | 25/99 (30.3%) | 42/98 (56.1%) | ||
| Number of participants with residual effusion at 24 weeks | 10/99 (10.1%) | 25/98 (25.5%)3 | ||
| Number of participants with residual effusion at 4 weeks | 9/21 (42.9%) | 15/19 (78.9%) | ||
| Number of participants with residual effusion at 8 weeks | 5/21 (23.8%) | 12/19 (63.2%) | ||
| Number of participants with residual effusion at 24 weeks | 1/21 (4.8%) | 6/19 (31.6%) | ||
| Number of participants with residual effusion at 8 weeks | 29/32 (90.6%) | 49/50 (98%) | ||
| Number of participants with residual effusion at 24 weeks | 20/32 (62.5%) | 44/50 (88%) | ||
| Abbreviations: N: number of participants | ||||
| Trial | Indicator | Units | Corticosteroids | Control |
| Percentage predicted FVC | Mean percentage predicted FVC | 95% (N = 57) | 95% (N = 60)1 | |
| Percentage predicted FVC | Mean percentage predicted FVC | 85% (N = 34) | 80% (N = 36)2 | |
| Lung function impairment | Number of participants with restrictive PFT results | 11/34 (33.3%) | 14/36 (39.4%)3 | |
| Abbreviations: FVC: forced vital capacity; N: number of participants; PFT: pulmonary function tests | ||||
| Trial | Corticosteroid | Control |
| 1/34 (2.9%)1 | 0/50 | |
| 9/99 (9.1%)2 | 2/98 (2.0%) | |
| 0/57 | NR | |
| 1/21 (4.8%)3 | NR | |
| NR | NR | |
| 4/34 (11.8%) | 3/36 (8.3%)4 | |
| Abbreviations: NR: not reported 1Aggravation of epigastric pain in one patient, steroids stopped, and patient withdrawn from the trial. | ||
| Trial | Indicator | Control (N/98) | Corticosteroid (N/99) |
| Kaposi’s sarcoma | 0 | 6 (6.1%) | |
| Cryptococcal meningitis | 5 (5.1%) | 3 (3.0%) | |
| Oesophageal candidiasis | 23 (23.5%) | 35 (35.4%) | |
| Oral candidiasis | 31 (32.6%) | 31 (31.3%) | |
| Herpes zoster | 19 (19.4%) | 22 (22.2%) | |
| Oral or genital herpes simplex | 20 (20.4%) | 22 (22.2%) | |
| Gastroenteritis | 28 (28.6%) | 34 (34.3%) | |
| Abbreviations: N: number of participants | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Residual pleural effusion on chest X‐ray Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 At 4 weeks | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.49, 0.84] |
| 1.2 At 8 weeks | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.37, 0.78] |
| 1.3 At 24 weeks | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.18, 0.66] |
| 2 Pleural changes at the end of treatment (pleural thickening and pleural adhesions) Show forest plot | 5 | 393 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.57, 0.92] |
| 3 Death from any cause Show forest plot | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.64, 1.31] |
| 4 Adverse events leading to study drug discontinuation Show forest plot | 6 | 590 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.78 [1.11, 6.94] |
| 5 HIV‐associated adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Cryptococcal meningitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Oesophageal candidiasis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Oral candidiasis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Gastroenteritis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 Herpes simplex | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.6 Herpes zoster | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.7 Kaposi sarcoma | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Residual pleural fluid on chest X‐ray ‐ studies at high risk of selection bias excluded Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 At 4 weeks | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.49, 0.84] |
| 1.2 At 8 weeks | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.37, 0.78] |
| 1.3 At 24 weeks | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.18, 0.66] |
| 2 Residual pleural fluid on chest X‐ray ‐ studies at high risk of selection bias included Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 At 4 weeks | 3 | 321 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.52, 1.07] |
| 2.2 At 8 weeks | 4 | 403 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.47, 1.12] |
| 2.3 At 24 weeks | 4 | 403 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.30, 0.98] |

