Dosis única de paracetamol oral (acetaminofeno), con y sin codeína, para el dolor postoperatorio en adultos
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | RCT, single oral dose, 4 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at baseline the hourly to 4 hours | |
| Participants | Impacted third molar extraction Mean age mid 20's N = 128 | |
| Interventions | Paracetamol+codeine 1000/60 mg, n = 41 Paracetamol 1000 mg, n = 41 Codeine 60 mg, n = 21 Placebo, n = 17 | |
| Outcomes | PI: non std 10 point scale PR: std 5 point scale Time to use of rescue medication Number of patients using rescue medication Number of patients reporting any adverse event | |
| Notes | Oxford Quality Score: R1, DB1, W1 | |
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate (more than 40 mm on 100 mm VAS) to severe (greater than 60 mm on a std 100 mm VAS) intensity Pain assessed at 0, 30, 60 minutes then hourly to 6 hours | |
| Participants | Caesarean section Age: 27 ‐ 37 years N = 125 | |
| Interventions | Paracetamol+codeine 800/60 mg, n = 50 Paracetamol 1000 mg, n = 50 Placebo, n = 25 | |
| Outcomes | PI: std 4 point scale and std 100 mm VAS ("no pain" to "unbearable pain") PR: std 5 point scale Number of patients reporting any adverse event and serious adverse events | |
| Notes | Oxford Quality Score: R1, DB2, W1 Patients asked to refrain from rescue medication for 1 hour | |
| Methods | RCT, DB single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 30, 60 minutes the hourly to 4 hours. Multiple dose phase continued after initial 4 hours | |
| Participants | Orthopaedic surgery Mean age 46 years N = 153 M = 83, F = 70 | |
| Interventions | Paracetamol+codeine 300/30 mg, n = 55 Paracetamol+tramadol 325/37.5 mg, n = 49 Placebo, n = 49 | |
| Outcomes | PI: std 4 point scale PR: non std 6 point scale Number of patients using rescue medication Number of patients withdrawing due to adverse event | |
| Notes | Oxford Quality Score: R2, DB2, W1 | |
| Methods | RCT, DB single oral dose, dummy, 5 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at baseline the every 30 minutes to 8 hours | |
| Participants | Impacted third molar extraction Mean age 25 years N = 120 M = 44, F = 76 | |
| Interventions | Paracetamol+codeine 1000/60 mg, n = 24 Paracetamol 1000 mg, n = 22 Diclofenac 100 mg, n = 22 Paracetamol+diclofenac 1000/100 mg, n = 24 Paracetamol+diclofenac+codeine 1000/100/60 mg, n = 24 | |
| Outcomes | PI: std 100 mm VAS PR: std 5 point scale PGE: non std 4 point scale Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event | |
| Notes | Oxford Quality Score: R2, DB2, W1 | |
| Methods | RCT, DB single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 30, 60 minutes then hourly to 6 hours | |
| Participants | Impacted third molar extraction Mean age 21 years N = 292 M = 122, F = 371 | |
| Interventions | Paracetamol+codeine 600/60 mg, n = 180 Rofecoxib 50mg, n = 182 Placebo, n = 31 | |
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Time to use of rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event | |
| Notes | Oxford Quality Score: R1, DB2, W1 | |
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at: 0, 0.5, 1, 2, 3, 4, 5, 6, 24 hours | |
| Participants | Impacted third molar extraction Mean age 19 years | |
| Interventions | Paracetamol+codeine 600/60 mg, n = 180 Rofecoxib 50 mg, n = 180 Placebo, n = 30 | |
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 pt scale (patients reporting "very good" or "excellent") Time to use of rescue medication Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event | |
| Notes | Oxford Quality Score: R1, DB2, W1 Patients asked to refrain from rescue medication for 1.5 hours | |
| Methods | RCT, DB, single oral dose, 5 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at baseline then hourly to 4 hours | |
| Participants | Impacted third molar extraction Mean age early 20s N = 248 | |
| Interventions | Paracetamol 650 mg, n = 37 Paracetamol+codeine 650/60 mg, n = 42 Paracetamol+d‐propoxyphene 650/100 mg, n = 42 Ibuprofen 200 mg, n = 42 Placebo, n = 37 | |
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 pt scale (patients reporting "very good" or "excellent") Time to use of rescue medication Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event | |
| Notes | Oxford Quality Score R1, DB2, W1 Patients asked to refrain from rescue medication for 1 hour | |
| Methods | RCT, DB, single oral dose, 5 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at baseline then hourly to 4 hours | |
| Participants | Impacted third molar extraction Age 18‐57 years N =165 | |
| Interventions | Paracetamol 600 mg, n = 36 Paracetamol+codeine 600/60 mg, n = 31 Placebo, n = 40 | |
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Number of patients using rescue medication Number of patients withdrawing due to adverse event | |
| Notes | Oxford Quality Score R1, DB2, W1 Patients asked to refrain from rescue medication for 1 hour | |
| Methods | RCT, DB, single oral dose, 6 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 30, 60 minutes then hourly to 6 hours | |
| Participants | Impacted tooth removal Age "young adults' n=247 | |
| Interventions | Paracetamol 650 mg, n = 37 Paracetamol+codeine 650/60 mg, n = 39 Zomepirac 100 mg, n = 23 Flurbiprofen 50 mg, n = 42 Flurbiprofen 100 mg, n = 41 Placebo, n = 44 | |
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Time to use of rescue medication Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event | |
| Notes | Oxford Quality Score R1, DB1, W1 Patients asked to refrain from rescue medication for 1 hour | |
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 30, 60 minutes, then hourly to 6 hours | |
| Participants | Oral Surgery Age 18+ years N = 137 | |
| Interventions | Paracetamol+codeine 300/30 mg, n = 39 Aspirin+butalbital+caffeine+codeine 325/50/40/30 mg, n = 43 Placebo, n = 41 | |
| Outcomes | PI: std 4 point scale PR: std 5 point scale Time to use of rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event | |
| Notes | Oxford Quality Score: R1, DB2, W1 Patients asked to refrain from rescue medication for 1 hour | |
| Methods | RCT, DB, single oral dose, 5 parallel groups Medication administered when baseline pain reached moderate to severe intensity Pain assessed at baseline then hourly to 6 hours | |
| Participants | Impacted third molar removal N = 135 | |
| Interventions | Paracetamol 500 mg, n = 72 Piroxicam 20 mg, n = 76 Piroxicam cyclodextrin =20 mg, n = 74 Placebo, n = 76 | |
| Outcomes | PI: std 4 point scale PR: std 5 point scale Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event | |
| Notes | Oxford Quality Score R1, DB1, W1 | |
| Methods | RCT, DB, single oral dose, 5 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at baseline then hourly to 12 hours | |
| Participants | Impacted third molar removal Age 15+ years N = 177 | |
| Interventions | Paracetamol 600 mg, n = 34 Paracetamol+codeine 600/60 mg, n = 31 Diflusinal 500 mg, n = 32 Diflusinal 1000 mg, n = 32 Placebo, n = 30 | |
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Time to use of rescue medication Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event | |
| Notes | Oxford Quality Score R1, DB2, W1 Patients asked to refrain from rescue medication for 2 hours | |
| Methods | RCT, DB, single oral dose, 5 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 30, 60 ,90, 120 mins then hourly to 12 hours | |
| Participants | General, gynaecological or orthopaedic surgery Age 19+ years N = 132 | |
| Interventions | Paracetamol+codeine 600/60 mg, n = 26 Paracetamol 600 mg, n = 26 Diflusinal 500 mg, n = 26 Diflusinal 1000 mg, n = 28 Placebo, n = 26 | |
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Time to use of rescue medication Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event | |
| Notes | Oxford Quality Score R2, DB2, W1 Patients asked to refrain from rescue medication for 1 hour | |
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at baseline the hourly to 6 hours | |
| Participants | Removal of impacted 3rd molar Age 15+ years N = 146 | |
| Interventions | Paracetamol+codeine 300/30 mg, n = 43 Aspirin+butalbital+caffeine+codeine 325/50/40/15 mg, n = 41 Placebo, n = 38 | |
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Time to use of rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event | |
| Notes | Oxford Quality Score: R2, DB2, W1 Patients asked to refrain from rescue medication for 1 hour | |
| Methods | RCT, DB, single oral dose, 4 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at baseline then hourly to 12 hours | |
| Participants | Impacted third molar removal Age 15+ years N = 107 | |
| Interventions | Paracetamol 600 mg, n = 22 Paracetamol+codeine 600/60 mg, n = 17 Flurbiprofen 100 mg, n = 26 Placebo, n = 23 | |
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Time to use of rescue medication Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event | |
| Notes | Oxford Quality Score R2, DB2, W1 Patients asked to refrain from rescue medication for 2 hours | |
| Methods | RCT, DB, single oral dose, 6 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at baseline then hourly to 6 hours | |
| Participants | Removal of impacted 3rd molar (1 or more) Age 15+ years N = 162 | |
| Interventions | Paracetamol+codeine 600/60 mg, n = 27 Aspirin 650 mg, n = 32 Ketorolac 10 mg, n = 37 Placebo, n = 32 | |
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Time to use of rescue medication Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event | |
| Notes | Oxford Quality Score R2, DB2, W1 Patients asked to refrain from rescue medication for 2 hours | |
| Methods | RCT, DB, single oral dose, 6 parallel groups. Followed by multiple dose phase Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at baseline then hourly to 6 hours | |
| Participants | Removal of impacted 3rd molar (1 or more) Age 15+ years N = 206 | |
| Interventions | Paracetamol+codeine 600/60 mg, n = 38 Paracetamol 600 mg, n = 36 Ketorolac 10 mg, n = 31 Ketorolac 20 mg, n = 35 ibuprofen 400 mg, n = 32 Placebo, n = 34 | |
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Time to use of rescue medication Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event | |
| Notes | Oxford Quality Score: R2, DB2, W1 Patients asked to refrain from rescue medication for 2 hours | |
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 30, 60 minutes the hourly to 6 hours | |
| Participants | Removal of impacted 3rd molar Age 15+ years N = 324 | |
| Interventions | Paracetamol+codeine 300/30 mg, n = 93 Paracetamol+hydrocodone bitartrate 500/7.5 mg, n = 94 Placebo, n = 45 | |
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Time to use of rescue medication Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event | |
| Notes | Oxford Quality Score: R2, DB2, W1 Patients asked to refrain from rescue medication for 2 hours | |
| Methods | RCT, DB, single oral dose, 2 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 30, 60 minutes then hourly to 5 hours | |
| Participants | Elective orthopaedic or general surgery Age 16 ‐ 65 years N = 116 | |
| Interventions | Paracetamol+codeine 1000/60 mg, n = 45 Paracetamol 1000 mg, n = 45 | |
| Outcomes | PI: std 4 point scale and std 100mm VAS PR: std 5 point scale PGE: non std 4 point scale Time to use of rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event | |
| Notes | Oxford Quality Score: R1, DB1, W1 Patients asked to refrain from rescue medication for 1 hour | |
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 30, 60 minutes then hourly to 6 hours | |
| Participants | Orthopaedic surgery Age 18‐65 years N = 120 | |
| Interventions | Paracetamol+codeine 300/30 mg, n = 40 Ibuprofen 400 mg, n = 40 Placebo, n = 40 | |
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Time to use of rescue medication Number of patients using rescue medication Number of patients reporting any adverse event | |
| Notes | Oxford Quality Score: R1, DB1, W0 | |
| Methods | RCT, DB, single oral dose, 4 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 30, 60 mins then hourly to 6 hours | |
| Participants | Elective abdominal, orthopaedic, rectal, thoracic and vascular surgery Age 19‐87 years N = 116 | |
| Interventions | Paracetamol 600 mg, n = 28 Paracetamol+codeine 600/60 mg, n = 28 Codeine 60 mg, n = 28 Placebo, n = 25 | |
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Number of patients using rescue medication Number of serious adverse events | |
| Notes | Oxford Quality Score R1, DB2, W0 | |
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 30, 60, 90, 120 minutes, then hourly to 8 hours, then at 10, 12, 20 and 24 hours | |
| Participants | Impacted third molar removal Mean age 23 years N = 201 M = 97, F = 104 | |
| Interventions | Paracetamol+codeine 600/60 mg, n = 50 Naproxen sodium 550mg, n = 50 Etoricoxib 120mg, n = 50 Placebo, n = 50 | |
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Time to use of rescue medication Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event | |
| Notes | Oxford Quality Score:R2, DB2, W1 | |
| Methods | RCT, DB, single oral dose, 4 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 30, 60 minutes, the hourly to 6 hours | |
| Participants | Removal of impacted third molar N = 100 | |
| Interventions | Paracetamol+codeine 600/60 mg, n = 23 Placebo, n = 26 | |
| Outcomes | PI: std 4 point scale PR: std 5 point scale | |
| Notes | Oxford Quality Score: R1, DB2, W1 Patients asked to refrain from rescue medication for 1 hour | |
| Methods | RCT, DB single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 30, 60 minutes then hourly to 4 hours | |
| Participants | Orthopaedic surgery Mean age 47 years N = 305 M = 215, F = 90 | |
| Interventions | Paracetamol+codeine 300/30 mg, n = 109 Paracetamol+tramadol 325/37.5 mg, n = 98 Placebo, n = 98 | |
| Outcomes | PI: std 4 point scale PR: non std 6 point scale Number of patients using rescue medication | |
| Notes | Oxford Quality Score: R2, DB2, W1 | |
| Methods | RCT, DB, single oral dose, 4 parallel groups Baseline pain was >60 mm on VAS scale (severe) Pain assessed at 0, 30, 60 minutes then hourly to 6 hours | |
| Participants | Orthopaedic surgery Age: Adults N = 144 | |
| Interventions | Paracetamol+codeine 1000/60 mg, n = 36 Tramadol 50 mg, n = 33 Tramadol 100 mg, n = 35 Placebo, n = 33 | |
| Outcomes | PI: std 100 mm VAS Time to use of rescue medication Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events | |
| Notes | Oxford Quality score: R1, DB2, W1 | |
| Methods | RCT, DB, single oral dose, 6 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 30, 60 minutes, then hourly to 6 hours | |
| Participants | Removal of impacted third molar Age 16+ years N =182 | |
| Interventions | Paracetamol 650 mg, n = 30 Paracetamol+codeine 650/60 mg, n = 31 Flurbiprofen 50 mg, n = 31 Flurbiprofen 100 mg, n = 29 Zomepirac 100 mg, n = 31 Placebo, n = 30 | |
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event 'Overall improvement': non std 7 point scale | |
| Notes | Oxford Quality Score R2, DB2, W1 Patients asked to refrain from rescue medication for 1 hour | |
| Methods | RCT, DB, single oral dose, 4 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 30, 60 minutes then hourly to 6 hours | |
| Participants | Elective surgery ‐ mainly orthopaedic, abdominal, gynaecological and urological Age 18+ years N = 161 | |
| Interventions | Paracetamol+codeine 650/60 mg, n = 39 Ketoprofen 50 mg, n = 41 Ketoprofen 150 mg, n = 39 Placebo, n = 41 | |
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Time to use of rescue medication Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event | |
| Notes | Oxford Quality Score: R1, DB1, W1 Patients asked to refrain from rescue medication for 1 hour | |
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 30, 60, 90 and 120 minutes then hourly to 8 hours | |
| Participants | Impacted third molar extraction Mean age 25 years N = 125 M = 50, F = 75 | |
| Interventions | Paracetamol+codeine 600/60 mg, n = 49 Ibuprofen+hydrocodone 400/150 mg, n = 49 Placebo, n = 27 | |
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: non std 5 point scale Time to use of rescue medication Number of patients reporting any adverse event and serious adverse events | |
| Notes | Oxford Quality Score: R2, DB2, W0 | |
DB: double blind; F: female; M: male; N: total number of participants in study; n: number of participants in treatment arm; R: randomisation RCT: randomised controlled trial; PI: pain intensity; PR: pain relief; PGR: patient global response; std: standard; W: withdrawals
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Pain only assessed for 2 hours after administration of the interventions | |
| Interventions were given when pain was "of sufficient intensity that analgesia would normally be given" and data was only presented for pain relief over the first hour | |
| No appropriate control (Paracetamol with codeine vs codeine CR) | |
| Inadequate description of method. Excluded as did not state whether allocation was randomised or if the studies (summary of five trials) were double blind | |
| No appropriate control (Lysine Clonixinate (LC) vs Paracetamol with codeine) | |
| Inadequate description of method. Excluded as did not state whether allocation was randomised | |
| No appropriate control (Diflunisal v paracetamol plus codeine) | |
| No appropriate control (paracetamol plus codeine v paracetamol plus dextropropoxyphene) | |
| PI scale was 5 point and therefore not validated for the data extraction method. No results for pain relief which would allow the calculation of TOTPAR were presented. Global evaluation was in the opinion of the investigator and not the patient | |
| No appropriate control (Paracetamol with codeine vs. ibuprofen) | |
| Insufficient baseline pain (not of moderate or severe intensity, less than 30 mm on 100 mm VAS). No placebo group | |
| Intervention administered immediately after surgery before anaesthetic wore off. Therefore inadequate baseline pain | |
| No appropriate control (paracetamol plus codeine v flurbiprofen) | |
| No appropriate control (paracetamol plus codeine v naproxen) | |
| Fewer than 10 patients in the treatment arm | |
| Fewer than 10 patients in the treatment arm | |
| No appropriate control (ibuprofen vs. paracetamol with codeine) | |
| Single blind study | |
| Patients instructed to take tablets "when pain relief was needed". Mean baseline pain minus 2 standard deviations was less than 30 mm for all interventions (>30 mm equates to at least moderate pain), therefore it is probable that patients with mild pain were included | |
| Patients instructed to take tablets "when pain relief was needed". Mean baseline pain minus 2 standard deviations was less than 30 mm for all interventions (>30 mm equates to at least moderate pain), therefore it is probable that patients with mild pain were included | |
| No appropriate control (paracetamol v codeine) | |
| No appropriate control (ibuprofen vs paracetamol with codeine) | |
| No appropriate control (paracetamol plus codeine v APC v naproxen) | |
| No appropriate control (paracetamol v aspirin) | |
| Outline of 5 studies. Study 3 and 4 compare paracetamol plus codeine to placebo. Study 4 is a duplicate of an included RCT. Study 3 cannot be included as the report fails to state whether the allocation to each intervention was randomised | |
| Intervention administered immediately after surgery before anaesthetic wore off. Therefore inadequate baseline pain | |
| Intervention administered immediately after surgery before anaesthetic wore off. Therefore inadequate baseline pain | |
| No appropriate control (paracetamol plus codeine v ketorolac) | |
| No appropriate control (paracetamol plus codeine v ibuprofen) |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with at least 50% pain relief over 4 to 6 hours Show forest plot | 3 | 192 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.34 [2.93, 13.73] |
| Analysis 1.1  Comparison 1 Paracetamol 800 to 1000 mg plus codeine 60 mg versus placebo, Outcome 1 Participants with at least 50% pain relief over 4 to 6 hours. | ||||
| 2 Participants with any adverse event Show forest plot | 3 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.58, 1.39] |
| Analysis 1.2  Comparison 1 Paracetamol 800 to 1000 mg plus codeine 60 mg versus placebo, Outcome 2 Participants with any adverse event. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with at least 50% pain relief over 4 to 6 hours Show forest plot | 4 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.06, 1.62] |
| Analysis 2.1  Comparison 2 Paracetamol 800 to 1000 mg plus codeine 60 mg versus paracetamol 1000 mg, Outcome 1 Participants with at least 50% pain relief over 4 to 6 hours. | ||||
| 2 Participants using rescue medication over 4 to 6 hours Show forest plot | 2 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.41, 0.89] |
| Analysis 2.2  Comparison 2 Paracetamol 800 to 1000 mg plus codeine 60 mg versus paracetamol 1000 mg, Outcome 2 Participants using rescue medication over 4 to 6 hours. | ||||
| 3 Participants with any adverse event Show forest plot | 4 | 324 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.70, 1.81] |
| Analysis 2.3  Comparison 2 Paracetamol 800 to 1000 mg plus codeine 60 mg versus paracetamol 1000 mg, Outcome 3 Participants with any adverse event. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with at least 50% pain relief over 4 to 6 hours Show forest plot | 17 | 1413 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.64 [2.17, 3.21] |
| Analysis 3.1  Comparison 3 Paracetamol 600 to 650 mg plus codeine 60 mg versus placebo, Outcome 1 Participants with at least 50% pain relief over 4 to 6 hours. | ||||
| 2 Participants with at least 50% pain relief over 4 to 6 hours, dental Show forest plot | 14 | 1221 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.70 [2.18, 3.35] |
| Analysis 3.2  Comparison 3 Paracetamol 600 to 650 mg plus codeine 60 mg versus placebo, Outcome 2 Participants with at least 50% pain relief over 4 to 6 hours, dental. | ||||
| 3 Participants with at least 50% pain relief over 4 to 6 hours, other surgery Show forest plot | 3 | 192 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.36 [1.49, 3.73] |
| Analysis 3.3  Comparison 3 Paracetamol 600 to 650 mg plus codeine 60 mg versus placebo, Outcome 3 Participants with at least 50% pain relief over 4 to 6 hours, other surgery. | ||||
| 4 Participants using rescue medication over 4 to 6 hours Show forest plot | 10 | 657 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.68, 0.82] |
| Analysis 3.4  Comparison 3 Paracetamol 600 to 650 mg plus codeine 60 mg versus placebo, Outcome 4 Participants using rescue medication over 4 to 6 hours. | ||||
| 5 Participants with any adverse event Show forest plot | 14 | 1258 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.27, 1.93] |
| Analysis 3.5  Comparison 3 Paracetamol 600 to 650 mg plus codeine 60 mg versus placebo, Outcome 5 Participants with any adverse event. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with at least 50% pain relief over 4 to 6 hours Show forest plot | 10 | 622 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.11, 1.52] |
| Analysis 4.1  Comparison 4 Paracetamol 600 to 650 mg plus codeine 60 mg versus paracetamol 600‐650 mg, Outcome 1 Participants with at least 50% pain relief over 4 to 6 hours. | ||||
| 2 Participants with at least 50% pain relief over 4 to 6 hours, dental Show forest plot | 8 | 512 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.11, 1.57] |
| Analysis 4.2  Comparison 4 Paracetamol 600 to 650 mg plus codeine 60 mg versus paracetamol 600‐650 mg, Outcome 2 Participants with at least 50% pain relief over 4 to 6 hours, dental. | ||||
| 3 Participants with at least 50% pain relief over 4 to 6 hours, other surgery Show forest plot | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.83, 1.77] |
| Analysis 4.3  Comparison 4 Paracetamol 600 to 650 mg plus codeine 60 mg versus paracetamol 600‐650 mg, Outcome 3 Participants with at least 50% pain relief over 4 to 6 hours, other surgery. | ||||
| 4 Participants using rescue medication over 4 to 6 hours Show forest plot | 7 | 436 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.64, 0.88] |
| Analysis 4.4  Comparison 4 Paracetamol 600 to 650 mg plus codeine 60 mg versus paracetamol 600‐650 mg, Outcome 4 Participants using rescue medication over 4 to 6 hours. | ||||
| 5 Participants with any adverse event Show forest plot | 7 | 443 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.79, 1.57] |
| Analysis 4.5  Comparison 4 Paracetamol 600 to 650 mg plus codeine 60 mg versus paracetamol 600‐650 mg, Outcome 5 Participants with any adverse event. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with at least 50% pain relief over 4 to 6 hours Show forest plot | 6 | 690 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.42, 2.47] |
| Analysis 5.1  Comparison 5 Paracetamol 300 mg plus codeine 30 mg versus placebo, Outcome 1 Participants with at least 50% pain relief over 4 to 6 hours. | ||||
| 2 Participants with at least 50% pain relief over 4 to 6 hours, dental Show forest plot | 3 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.31 [1.76, 6.23] |
| Analysis 5.2  Comparison 5 Paracetamol 300 mg plus codeine 30 mg versus placebo, Outcome 2 Participants with at least 50% pain relief over 4 to 6 hours, dental. | ||||
| 3 Participants with at least 50% pain relief over 4 to 6 hours, other surgery Show forest plot | 3 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.11, 2.05] |
| Analysis 5.3  Comparison 5 Paracetamol 300 mg plus codeine 30 mg versus placebo, Outcome 3 Participants with at least 50% pain relief over 4 to 6 hours, other surgery. | ||||
| 4 Participants using rescue medication over 4 to 6 hours Show forest plot | 4 | 529 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.68, 0.91] |
| Analysis 5.4  Comparison 5 Paracetamol 300 mg plus codeine 30 mg versus placebo, Outcome 4 Participants using rescue medication over 4 to 6 hours. | ||||
| 5 Participants with any adverse event Show forest plot | 3 | 344 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.50, 1.45] |
| Analysis 5.5  Comparison 5 Paracetamol 300 mg plus codeine 30 mg versus placebo, Outcome 5 Participants with any adverse event. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fewer than 40 patients in active and placebo groups, participants with at least 50% pain relief over 4 to 6 hours Show forest plot | 10 | 572 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.01 [2.31, 3.92] |
| Analysis 6.1  Comparison 6 Sensitivity analysis (paracetamol with codeine versus placebo), Outcome 1 Fewer than 40 patients in active and placebo groups, participants with at least 50% pain relief over 4 to 6 hours. | ||||
| 2 More than 40 patients in active and placebo groups, participants with at least 50% pain relief over 4 to 6 hours Show forest plot | 5 | 629 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.21, 2.03] |
| Analysis 6.2  Comparison 6 Sensitivity analysis (paracetamol with codeine versus placebo), Outcome 2 More than 40 patients in active and placebo groups, participants with at least 50% pain relief over 4 to 6 hours. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fewer than 40 patients in active and control groups, Participants with at least 50% pain relief over 4 to 6 hours Show forest plot | 10 | 588 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [1.15, 1.60] |
| Analysis 7.1  Comparison 7 Sensitivity analysis (paracetamol with codeine versus paracetamol), Outcome 1 Fewer than 40 patients in active and control groups, Participants with at least 50% pain relief over 4 to 6 hours. | ||||
| 2 More than 40 patients in active and control groups, Participants with at least 50% pain relief over 4 to 6 hours Show forest plot | 3 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.02, 1.65] |
| Analysis 7.2  Comparison 7 Sensitivity analysis (paracetamol with codeine versus paracetamol), Outcome 2 More than 40 patients in active and control groups, Participants with at least 50% pain relief over 4 to 6 hours. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with any adverse event Show forest plot | 20 | 1811 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.15, 1.63] |
| Analysis 8.1  Comparison 8 Paracetamol plus codeine (all doses) versus placebo, Outcome 1 Participants with any adverse event. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with any adverse event Show forest plot | 11 | 767 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.87, 1.40] |
| Analysis 9.1  Comparison 9 Paracetamol plus codeine (all doses) versus paracetamol alone, Outcome 1 Participants with any adverse event. | ||||
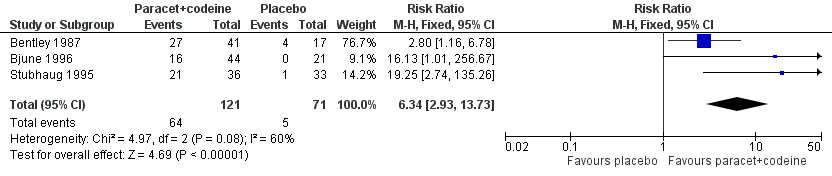
Forest plot of comparison: 3 Paracetamol 800 to 1000 mg plus codeine 60 mg versus placebo, outcome: 1.1 Participants with at least 50% pain relief over 4 to 6 hours.
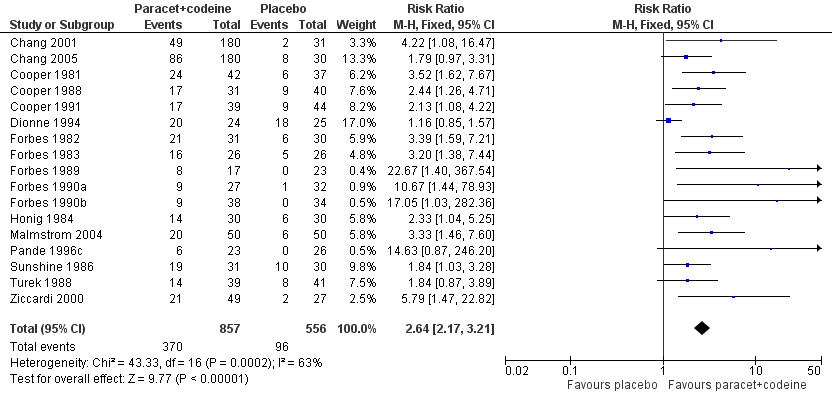
Forest plot of comparison: 5 Paracetamol 600 to 650 mg plus codeine 60 mg versus placebo, outcome: 3.1 Participants with at least 50% pain relief over 4 to 6 hours.
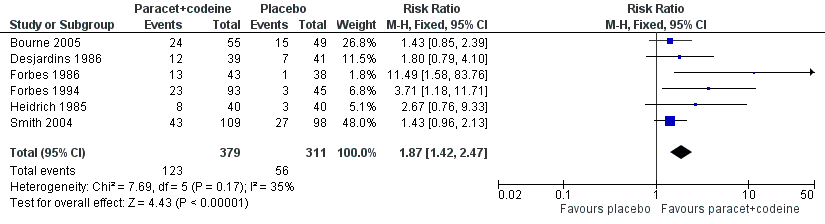
Forest plot of comparison: 7 Paracetamol 300 mg plus codeine 30 mg versus placebo, outcome: 5.1 Participants with at least 50% pain relief over 4 to 6 hours.

Forest plot of comparison: 4 Paracetamol 800 to 1000 mg plus codeine 60 mg versus paracetamol 1000 mg, outcome: 2.1 Participants with at least 50% pain relief over 4 to 6 hours.
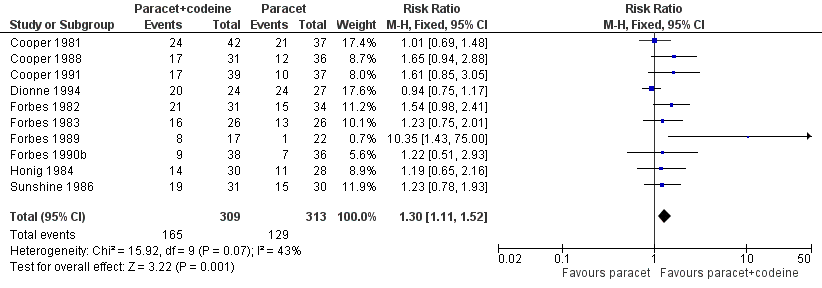
Forest plot of comparison: 6 Paracetamol 600 to 650 mg plus codeine 60 mg versus paracetamol 600 to 650 mg, outcome: 4.1 Participants with at least 50% pain relief over 4 to 6 hours.
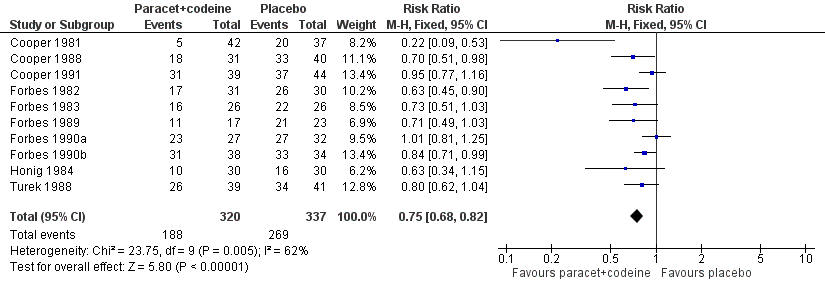
Forest plot of comparison: 5 Paracetamol 600 to 650 mg plus codeine 60 mg versus placebo, outcome: 3.4 Participants using rescue medication over 4 to 6 hours.

Forest plot of comparison: 7 Paracetamol 300 mg plus codeine 30 mg versus placebo, outcome: 5.4 Participants using rescue medication over 4 to 6 hours.

Forest plot of comparison: 6 Paracetamol 600 to 650 mg plus codeine 60 mg versus paracetamol 600 to 650 mg, outcome: 4.4 Participants using rescue medication over 4 to 6 hours.
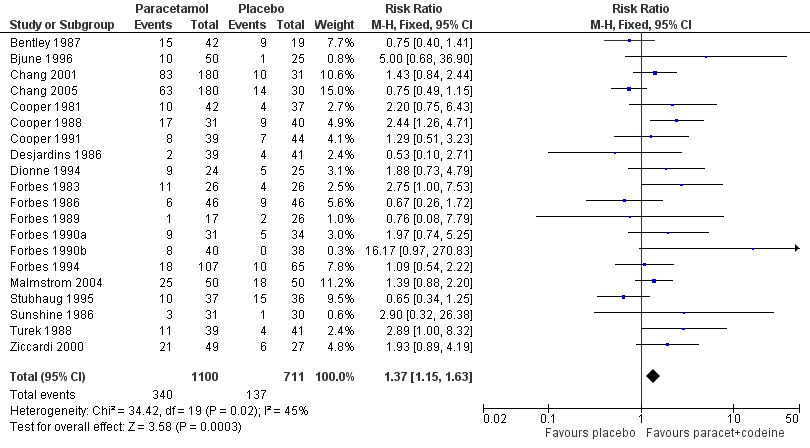
Forest plot of comparison: 8 Paracetamol plus codeine (all doses) versus placebo, outcome: 8.1 Participants with at least one adverse event.
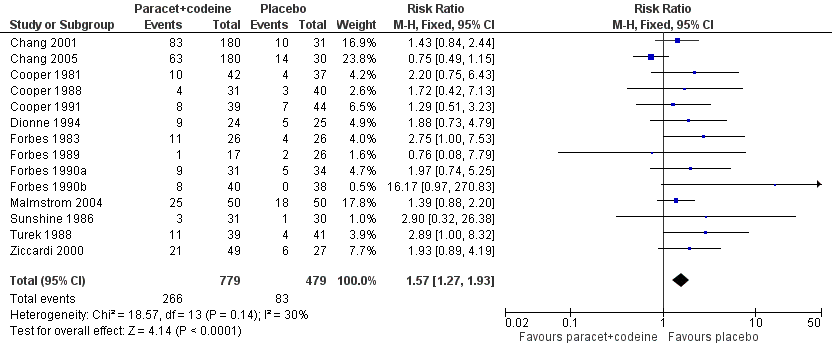
Forest plot of comparison: 5 Paracetamol 600 to 650 mg plus codeine 60 mg versus placebo, outcome: 3.5 Participants with any adverse event.

Forest plot of comparison: 9 Paracetamol plus codeine (all doses) versus paracetamol alone, outcome: 9.1 Participants with any adverse event.

Comparison 1 Paracetamol 800 to 1000 mg plus codeine 60 mg versus placebo, Outcome 1 Participants with at least 50% pain relief over 4 to 6 hours.
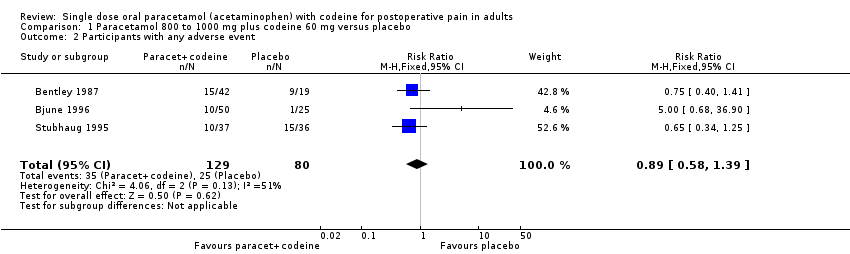
Comparison 1 Paracetamol 800 to 1000 mg plus codeine 60 mg versus placebo, Outcome 2 Participants with any adverse event.
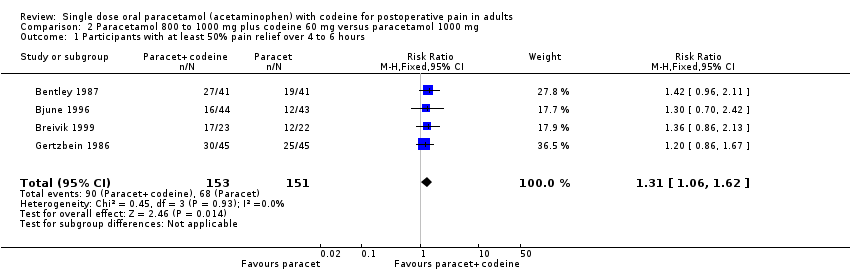
Comparison 2 Paracetamol 800 to 1000 mg plus codeine 60 mg versus paracetamol 1000 mg, Outcome 1 Participants with at least 50% pain relief over 4 to 6 hours.

Comparison 2 Paracetamol 800 to 1000 mg plus codeine 60 mg versus paracetamol 1000 mg, Outcome 2 Participants using rescue medication over 4 to 6 hours.

Comparison 2 Paracetamol 800 to 1000 mg plus codeine 60 mg versus paracetamol 1000 mg, Outcome 3 Participants with any adverse event.

Comparison 3 Paracetamol 600 to 650 mg plus codeine 60 mg versus placebo, Outcome 1 Participants with at least 50% pain relief over 4 to 6 hours.

Comparison 3 Paracetamol 600 to 650 mg plus codeine 60 mg versus placebo, Outcome 2 Participants with at least 50% pain relief over 4 to 6 hours, dental.
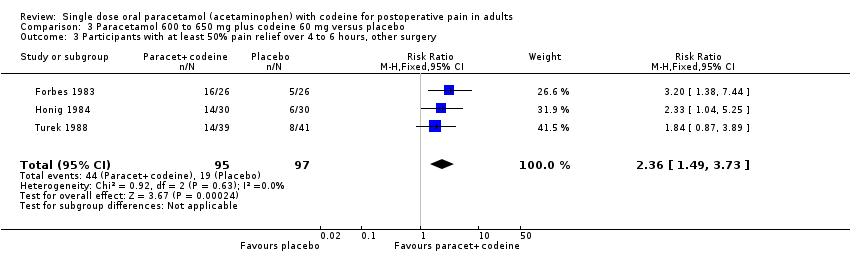
Comparison 3 Paracetamol 600 to 650 mg plus codeine 60 mg versus placebo, Outcome 3 Participants with at least 50% pain relief over 4 to 6 hours, other surgery.

Comparison 3 Paracetamol 600 to 650 mg plus codeine 60 mg versus placebo, Outcome 4 Participants using rescue medication over 4 to 6 hours.

Comparison 3 Paracetamol 600 to 650 mg plus codeine 60 mg versus placebo, Outcome 5 Participants with any adverse event.

Comparison 4 Paracetamol 600 to 650 mg plus codeine 60 mg versus paracetamol 600‐650 mg, Outcome 1 Participants with at least 50% pain relief over 4 to 6 hours.
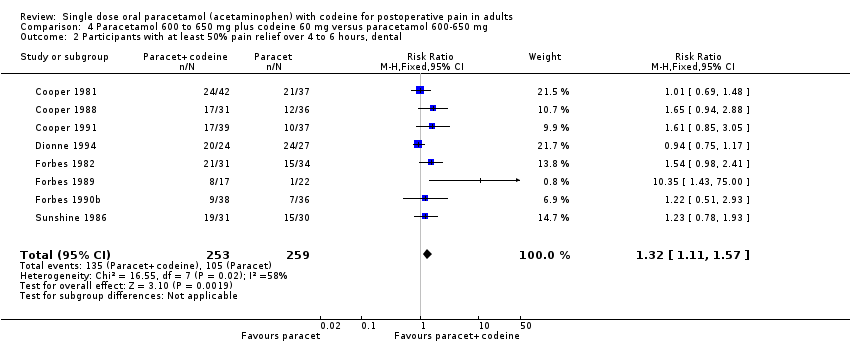
Comparison 4 Paracetamol 600 to 650 mg plus codeine 60 mg versus paracetamol 600‐650 mg, Outcome 2 Participants with at least 50% pain relief over 4 to 6 hours, dental.

Comparison 4 Paracetamol 600 to 650 mg plus codeine 60 mg versus paracetamol 600‐650 mg, Outcome 3 Participants with at least 50% pain relief over 4 to 6 hours, other surgery.

Comparison 4 Paracetamol 600 to 650 mg plus codeine 60 mg versus paracetamol 600‐650 mg, Outcome 4 Participants using rescue medication over 4 to 6 hours.

Comparison 4 Paracetamol 600 to 650 mg plus codeine 60 mg versus paracetamol 600‐650 mg, Outcome 5 Participants with any adverse event.

Comparison 5 Paracetamol 300 mg plus codeine 30 mg versus placebo, Outcome 1 Participants with at least 50% pain relief over 4 to 6 hours.

Comparison 5 Paracetamol 300 mg plus codeine 30 mg versus placebo, Outcome 2 Participants with at least 50% pain relief over 4 to 6 hours, dental.
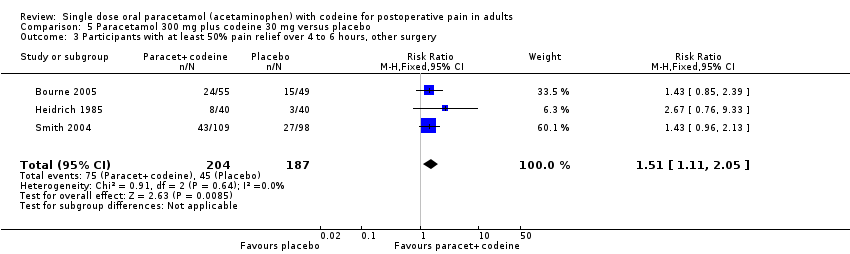
Comparison 5 Paracetamol 300 mg plus codeine 30 mg versus placebo, Outcome 3 Participants with at least 50% pain relief over 4 to 6 hours, other surgery.
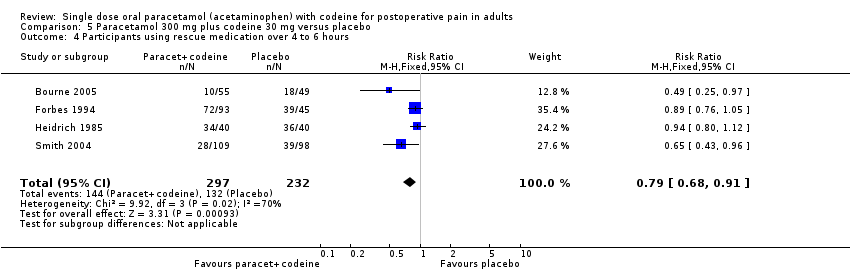
Comparison 5 Paracetamol 300 mg plus codeine 30 mg versus placebo, Outcome 4 Participants using rescue medication over 4 to 6 hours.
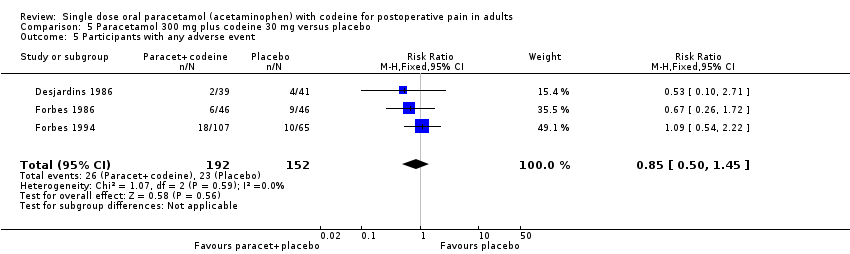
Comparison 5 Paracetamol 300 mg plus codeine 30 mg versus placebo, Outcome 5 Participants with any adverse event.

Comparison 6 Sensitivity analysis (paracetamol with codeine versus placebo), Outcome 1 Fewer than 40 patients in active and placebo groups, participants with at least 50% pain relief over 4 to 6 hours.

Comparison 6 Sensitivity analysis (paracetamol with codeine versus placebo), Outcome 2 More than 40 patients in active and placebo groups, participants with at least 50% pain relief over 4 to 6 hours.

Comparison 7 Sensitivity analysis (paracetamol with codeine versus paracetamol), Outcome 1 Fewer than 40 patients in active and control groups, Participants with at least 50% pain relief over 4 to 6 hours.
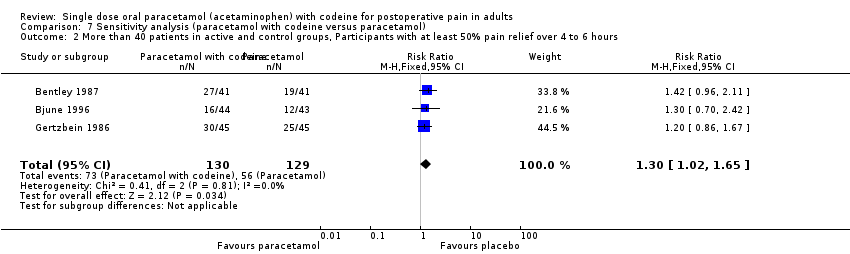
Comparison 7 Sensitivity analysis (paracetamol with codeine versus paracetamol), Outcome 2 More than 40 patients in active and control groups, Participants with at least 50% pain relief over 4 to 6 hours.
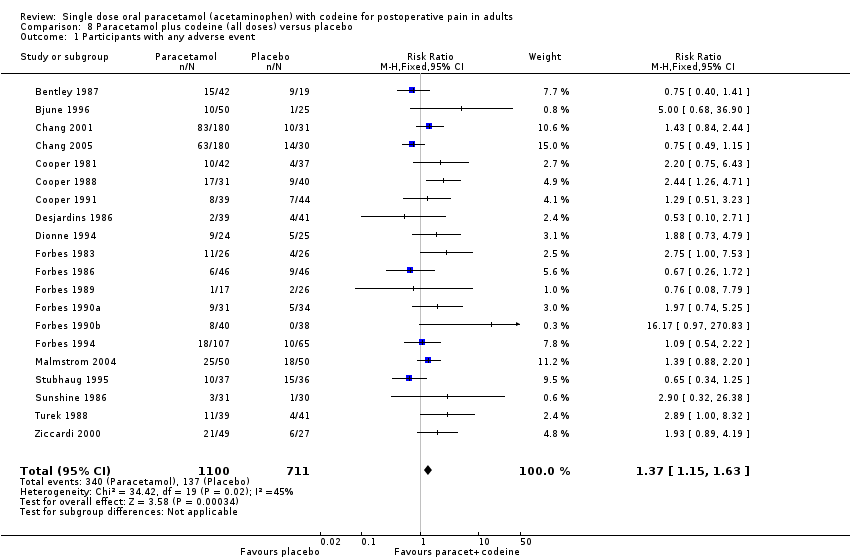
Comparison 8 Paracetamol plus codeine (all doses) versus placebo, Outcome 1 Participants with any adverse event.
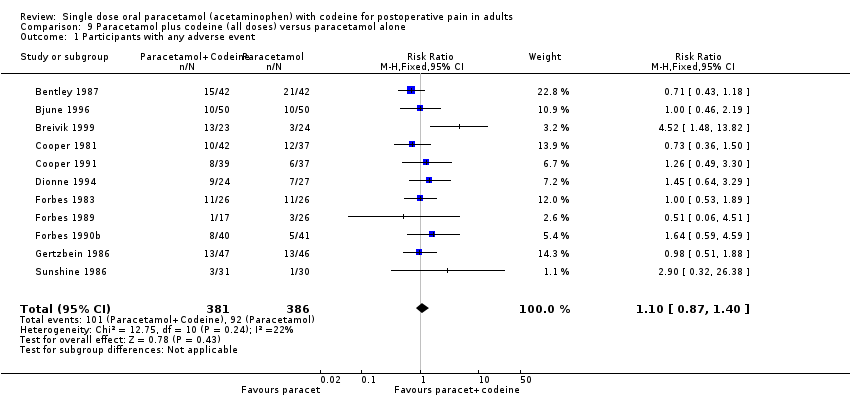
Comparison 9 Paracetamol plus codeine (all doses) versus paracetamol alone, Outcome 1 Participants with any adverse event.
| Analgesia | Rescue medication | |||||
| Study ID | Treatment | PI or PR | Number with 50% PR | PGE: v good or excellent | Median time to use (hr) | Number using |
| (1) Paracetamol+codeine 1000/60 mg, n=41 (2) Paracetamol 1000 mg, n=41 (3) Codeine 60 mg, n=21 (4) Placebo, n=17 | TOTPAR 5: (1) 11.5 (2) 8.7 (4) 4.9 | (1) 27/41 (2) 19/41 (4) 4/17 | No data | (1) 4.1 (2) 3.3 (4) 1.4 | at 4 hr: (1) 44 (2) 68 (4) 81 | |
| (1) Paracetamol+codeine 800/60 mg, n=50 (2) Paracetamol 1000 mg, n=50 (3) Placebo, n=25 | TOTPAR 6: severe pain (1) 10.5 (2) 6.4 (3) 0 moderate pain (1) 6.5 (2) 8.0 (3) 1.5 | (1) 16/44 (2) 12/43 (3) 0/21 | No usable data | No data | No data | |
| (1) Paracetamol+codeine 300/30 mg, n=55 (2) Paracetamol+tramadol 325/37.5 mg, n=49 (3) Placebo, n=49 | SPID 4: (1) 2.8 (3) 2.1 Baseline PI: (1) 2.1 (2) 2.2 | (1) 24/55 (3) 15/49 | No usable data | No data | at 4 hr: (1) 18 (3) 37 | |
| (1) Paracetamol+codeine 1000/60 mg n=24 (2) Paracetamol 1000 mg, n=22 (3) Diclofenac 100 mg, n=22 (4) Paracetamol+diclofenac 1000/100 mg, n=24 (5) Paracetamol+diclofenac+codeine 1000/100/60 mg, n=24 | TOTPAR 6: (1) 15.5 (2) 12.1 | (1) 17/23 (2) 12/22 | No data | No data | at 6 hr: (1) 17 (2) 38 | |
| (1) Paracetamol+codeine 600/60 mg n=180 (2) Rofecoxib 50mg n=182 (3) Placebo n=31 | TOTPAR 6: (1) 7.0 (3) 3.4 | (1) 49/180 (3) 2/31 | (1) 27/180 (3) 0/31 | (1) 2.3 (3) 1.6 | No data | |
| (1) Paracetamol+codeine 600/60 mg n=180 (2) Rofecoxib 50 mg n=180 (3) Placebo n=30 | TOTPAR 6: (1) 10.7 (3) 6.7 | (1) 86/180 (3) 8/30 | (1) 123/180 (3) 3/30 | (1) 6.5 (3) 3.2 | at 24 hr: (1) 86 (3) 73 | |
| (1) Paracetamol+codeine 650/60 mg, n=42 (2) Paracetamol 650 mg, n=37 (3) Paracetamol+d‐propoxyphene 650/100 mg, n=42 (4) Ibuprofen 200 mg, n=42 (5) Placebo, n=37 | TOTPAR 4: (1) 8.4 (2) 8.2 (5) 3.4 | (1) 24/42 (2) 21/37 (5) 6/37 | No usable data | Mean: (1) 3.1 (2) 3.5 (5) 2.9 | at 4 hr: (1) 12 (2) 5 (5) 54 | |
| (1) Paracetamol+codeine 600/60 mg, n=31 (2) Paracetamol 600 mg, n=36 (3) Meclofenamate Na 100 mg, n=36 (2) Placebo, n=40 | TOTPAR 6: (1) 12.0 (2) 8.0 (4) 6.3 | (1) 17/31 (2) 12/36 (4) 9/40 | (1) 15/31 (2) 12/36 (4) 8/40 | No data | at 6 hr: (1) 58 (2) 78 (4) 82 | |
| (1) Paracetamol+codeine 650/60 mg, n=39 (2) Paracetamol 650 mg, n=37 (3) Zomepirac 100 mg, n=23 (4) Flurbiprofen 50 mg, n=42 (5) Flurbiprofen 100 mg, n=41 (6) Placebo, n=44 | TOTPAR 6: (1) 10.1 (2) 6.8 (6) 5.7 | (1) 17/39 (2) 10/37 (6) 9/44 | (1) 14/39 (2) 3/37 (6) 2/44 | Mean: (1) 3.5 (2) 3.2 (6) 3.1 | at 6 hr: (1) 80 (2) 95 (6) 84 | |
| (1) Paracetamol+codeine 300/30 mg, n=39 (2) Aspirin+butalbital+caffeine+codeine 325/50/40/30 mg, n=43 (3) Placebo, n=41 | TOTPAR 6: (1) 7.8 (3) 5.1 | (1) 12/39 (3) 7/41 | No usable data | Mean: (1) 3.4 (3) 3.0 | No data | |
| (1) Paracetamol+codeine 650/60 mg, n=24 (2) Paracetamol 650 mg, n=27 (3) Flurbiprofen 50 mg, n=25 (4) Flurbiprofen 100 mg, n=22 (5) Placebo, n=25 | TOTPAR 6: (1) 16.9 (2) 18.4 (5) 14.9 | (1) 20/24 (2) 24/27 (5) 18/25 | No usable data | No data | No data | |
| (1) Paracetamol+codeine 600/60 mg, n=31 (2) Paracetamol 600 mg, n=34 (3) Diflusinal 500 mg, n=32 (4) Diflusinal 1000 mg, n=32 (5) Placebo, n=30 | TOTPAR 4: (1) 9.7 (2) 8.9 (5) 3.8 | (1) 21/31 (2) 15/34 (5) 6/30 | No usable data | (1) 5.3 (2) 3.5 (5) 2.4 | at 6 hr: (1) 56 (2) 70 (5) 82 | |
| (1) Paracetamol+codeine 600/60 mg, n=26 (2) Paracetamol 600 mg, n=26 (3) Diflusinal 500 mg, n=26 (4) Diflusinal 1000 mg, n=28 (5) Placebo, n=26 | TOTPAR 6: (1) 13.5 (2) 11.2 (5) 5.4 | (1) 16/26 (2) 13/26 (5) 5/26 | No usable data | (1) 5.3 (2) 4.0 (5) 2.4 | at 6 hr: (1) 63 (2) 73 (5) 86 | |
| (1) Paracetamol+codeine 300/30 mg, n=43 (2) Aspirin+butalbital+caffeine+codeine 325/50/40/15 mg, n=41 (3) Placebo, n=38 | TOTPAR 6: (1) 7.4 (3) 2.4 | (1) 13/43 (2) 1/38 | No usable data | Mean: (1) 4.5 (2) 3.0 | No data | |
| (1) Paracetamol+codeine 600/60 mg, n=17 (2) Paracetamol 600 mg, n=22 (3) Flurbiprofen 100 mg, n=26 (4) Placebo, n=23 | TOTPAR 6: (1) 10.5 (2) 4.5 (3) 2.0 | (1) 8/17 (2) 1/22 (4) 0/23 | No usable data | (1) 5.1 (2) 2.8 (4) 1.7 | at 6 hr: (1) 64 (2) 82 (4) 91 | |
| (1) Paracetamol+codeine 600/60 mg, n=27 (2) Aspirin 650 mg, n=32 (3) Ketorolac 10 mg, n=37 (4) Placebo, n=32 | TOTPAR 6: (1) 8.6 (4) 2.9 | (1) 9/27 (4) 1/32 | No usable data | Mean: (1) 4.3 (4) 3.1 | at 6 hr: (1) 85 (4) 84 | |
| (1) Paracetamol+codeine 600/60 mg, n=38 (2) Paracetamol 600 mg, n=36 (3) Ketorolac 10 mg, n=31 (4) Ketorolac 20 mg, n=35 (5) ibuprofen 400 mg, n=32 (6) Placebo, n=34 | TOTPAR 6: (1) 6.2 (2) 5.8 (6) 1.9 | (1) 9/38 (2) 7/36 (6) 0/34 | No usable data | (1) 2.6 (2) 3.0 (6) 1.8 | at 6 hr: (1) 82 (2) 81 (6) 97 | |
| (1) Paracetamol+codeine 300/30 mg, n=93 (2) Paracetamol+hydrocodone bitartrate 500/7.5 mg, n=94 (3) Placebo, n=45 | TOTPAR 6: (1) 6.6 (3) 3.4 | (1) 23/93 (3) 3/45 | No usable data | Mean: (1) 3.9 (3) 3.4 | at 6 hr: (1) 77 (3) 87 | |
| (1) Paracetamol+codeine 1000/60 mg, n=45 (2) Paracetamol 1000 mg, n=45 | TOTPAR 5: (1) 11.5 (2) 10.2 | (1) 30/45 (2) 25/45 | No usable data | Mean: (1) 3.8 (2) 3.6 | No data | |
| (1) Paracetamol+codeine 300/30 mg, n=40 (2) Ibuprofen 400 mg, n=40 (3) Placebo, n=40 | TOTPAR 6: (1) 5.5 (3) 3.3 | (1) 8/40 (3) 3/40 | No data | Mean: (1) 4.7 (3) 3.5 | (1) 85 (3) 90 | |
| (1) Paracetamol+codeine 600/60 mg, n=30 (2) Paracet 600 mg, n=28 (3) Codeine 60 mg, n=28 (4) Placebo, n=30 | TOTPAR 6: (1) 10.6 (2) 8.9 (4) 5.9 | (1) 14/30 (2) 11/28 (4) 6/30 | (1) 12/30 (2) 8/28 (4) 4/30 | No data | at 6 hr: (1) 33 (2) 43 (4) 53 | |
| (1) Paracetamol+codeine 600/60 mg, n=50 (2) Etoricoxib 120 mg, n=50 (3) Naproxen sodium 550 mg, n=50 (4) Placebo, n=50 | TOTPAR 6: (1) 9.2 (4) 4.2 | (1) 20/50 (4) 6/50 | at 8 hr: (1) 24/50 (4) 7/50 | (1) 3.6 (4) 1.6 | at 24 hr: (1) 76 (4) 99 | |
| (1) Paracetamol+codeine 600/60 mg, n=23 (2) Placebo, n=26 | SPID 6: (1) 3.1 (2) 0 Baseline PI: 2.5 | (1) 6/23 (2) 0/26 | No usable data | No data | No data | |
| 1)Paracetamol+codeine 300/30 mg, n=109 2) Paracetamol+tramadol 325/37.5 mg, n=98 3) Placebo n=98 | SPID 4: (1) 2.7 (3) 2.0 | (1) 43/109 (3) 27/98 | No usable data | No data | at 4 hr: (1) 26 (3) 40 | |
| (1) Paracetamol+codeine 1000/60 mg, n=36 (2) Tramadol 50 mg, n=33 (3) Tramadol 100 mg, n=35 (4) Placebo, n=33 | VAS SPID 6: (1) 204 (4) 17 Baseline PI: (1) 67 (4) 66 | (1) 21/36 (4) 1/33 | No usable data | (1) >6 (4) 2.8 | at 6 hr: (1) 36 (4) 82 | |
| (1) Paracetamol+codeine 650/60 mg, n=31 (2) Paracetamol 650 mg, n=30 (3) Flurbiprofen 50 mg, n=31 (4) Flurbiprofen 100 mg, n=29 (5) Zomepirac 100 mg, n=31 (6) Placebo, n=30 | TOTPAR 6: (1) 13.4 (2) 11.1 (6) 8.3 | (1) 19/31 (2) 15/30 (6) 10/30 | No usable data | No data | at 6 hr: (1) 30 (2) 47 (6) 43 | |
| (1) Paracetamol+codeine 650/60 mg, n=39 (2) Ketoprofen 50 mg, n=41 (3) Ketoprofen 150 mg, n=39 (4) Placebo, n=41 | TOTPAR 6: (1) 8.1 (4) 4.6 | (1) 14/39 (4) 8/41 | No usable data | Mean: (1) 3.5 (4) 2.2 | at 6 hr: (1) 67 (4) 83 | |
| (1) Paracetamol+codeine 300/30 mg, n=49 (2) Ibuprofen+hydrocodone 200/75 mg, n=49 (3) Placebo, n=27 | TOTPAR 6: (1) 9.8 (3) 3.5 | (1) 21/49 (3) 2/27 | No usable data | (1) 3.0 (3) 1.0 | No data | |
| Adverse events | Withdrawals | ||||
| Study ID | Treatment | Any | Serious | Adverse event | Other |
| (1) Paracetamol+codeine 1000/60 mg, n=41 (2) Paracetamol 1000 mg, n=41 (3) Codeine 60 mg, n=21 (4) Placebo, n=17 | (1) 15/42 (2) 21/42 (4) 9/19 | No data | None reported | 1 pt lost to follow up, 4 had invalid data | |
| (1) Paracetamol+codeine 800/60 mg, n=50 (2) Paracetamol 1000 mg, n=50 (3) Placebo, n=25 | (1) 10/50 (2) 10/50 (3) 1/25 | None | None reported | 6 paracetamol+codeine pts, 7 paracetamol pts, 4 placebo pts had invalid data | |
| (1) Paracetamol+codeine 300/30 mg, n = 55 (2) Paracetamol+tramadol 325/37.5 mg, n = 49 (3) Placebo, n = 49 | No single dose data | No single dose data | No single dose data | None | |
| (1) Paracetamol+codeine 1000/60 mg n=24 (2) Paracetamol 1000mg, n=22 (3) Diclofenac 100 mg, n=22 (4) Paracetamol+diclofenac 1000/100 mg, n=24 (5) Paracetamol+diclofenac+codeine 1000/100/60 mg, n=24 | (1) 13/23 (2) 3/24 | None | None | 1 pt in paracetamol+codeine group lost to follow up, 4 pts (2 paracetamol, 2 diclofenac) had invalid data | |
| (1) Paracetamol+codeine 600/60 mg n=180 (2) Rofecoxib 50 mg n=182 (3) Placebo n=31 | At 24 hr: (1) 83/180 (2) 10/31 | None | None | 6 pts lost to follow up, 1 para/codeine group vomited medication | |
| (1) Paracetamol+codeine 600/60 mg n=180 (2) Rofecoxib 50 mg n=180 (3) Placebo n=30 | At 24 hr: (1) 63/180 (3) 14/30 | None | None | 1 rofecoxib pt lost to follow up | |
| (1) Paracet+codeine 650/60 mg, n=42 (2) Paracetamol 650 mg, n=37 (3) Paracetamol+d‐propoxyphene 650/100 mg, n=42 (4) Ibuprofen 200 mg, n=42 (5) Placebo, n=37 | (1) 10/42 (2) 12/37 (5) 4/37 | None | None | 31 pts had invalid data | |
| (1) Paracetamol+codeine 600/60 mg, n=31 (2) Paracetamol 600 mg, n=36 (3) Meclofenamate Na 100 mg, n=36 (4) Placebo, n=40 | 15 pts in total reported adverse events | None | None | 11 pts lost to follow up, 3 had invalid data | |
| (1) Paracetamol+codeine 650/60 mg, n=39 (2) Paracetamol 650 mg, n=37 (3) Zomepirac 100 mg, n=23 (4) Flurbiprofen 50 mg, n=42 (5) Flurbiprofen 100 mg, n=41 (6) Placebo, n=44 | (1) 8/39 (2) 6/37 (6) 7/44 | None | None reported | 3 lost to follow up, 5 had invalid data | |
| (1) Paracetamol+codeine 300/30 mg, n=39 (2) Aspirin+butalbital+caffeine+codeine 325/50/40/30 mg, n=43 (3) Placebo, n=41 | (1) 2/39 (3) 4/41 | None | None | 14 pts did not medicate, lost to follow up, invalid data | |
| (1) Paracetamol+codeine 650/60 mg, n=24 (2) Paracetamol 650 mg, n=27 (3) Flurbiprofen 50 mg, n=25 (4) Flurbiprofen 100 mg, n=22 (5) Placebo, n=25 | (1) 9/24 (2) 7/27 (5) 5/25 | None | None reported | 11 pts had invalid data | |
| (1) Paracetamol+codeine 600/60 mg, n=31 (2) Paracetamol 600 mg, n=34 (3) Diflusinal 500 mg, n=32 (4) Diflusinal 1000 mg, n=32 (5) Placebo, n=30 | 15% in total reported adverse events | None | None | 4 pts lost to follow up, 11 had invalid data | |
| (1) Paracetamol+codeine 600/60 mg, n=26 (2) Paracetamol 600 mg, n=26 (3) Diflusinal 500 mg, n=26 (4) Diflusinal 1000 mg, n=28 (5) Placebo, n=26 | (1) 11/26 (2) 11/26 (5) 4/26 | None reported | None | None | |
| (1) Paracetamol+codeine 300/30 mg, n=43 (2) Aspirin+butalbital+caffeine+codeine 325/50/40/15 mg, n=41 (3) Placebo, n=38 | (1) 6/46 (2) 9/46 | None | None | 1 pt lost to follow up, 17 had invalid data | |
| (1) Paracetamol+codeine 600/60 mg, n=17 (2) Paracetamol 600 mg, n=22 (3) Flurbiprofen 100 mg, n=26 (4) Placebo, n=23 | (1) 1/17 (2) 3/26 (4) 2/26 | None | None | 10 pts had invalid data | |
| (1) Paracetamol+codeine 600/60 mg, n=27 (2) Aspirin 650 mg, n=32 (3) Ketorolac 10 mg, n=37 (4) Placebo, n=32 | (1) 9/31 (4) 5/34 | None | None | 1 lost to follow up, 14 had invalid data | |
| (1) Paracetamol+codeine 600/60 mg, n=38 (2) Paracetamol 600 mg, n=36 (3) Ketorolac 10 mg, n=31 (4) Ketorolac 20 mg, n=35 (5) Ibuprofen 400 mg, n=32 (6) Placebo, n=34 | (1) 8/40 (2) 5/41 (6) 0/38 | None | None | 3 pts lost to follow up, 27 had invalid data | |
| (1) Paracetamol+codeine 300/30 mg, n=93 (2) Paracetamol+hydrocodone bitartrate 500/7.5 mg, n=94 (3) Placebo, n=45 | (1) 18/107 (3) 10/65 | None | None | 1 pt lost to follow up, 59 had invalid data | |
| (1) Paracetamol+codeine 1000/60 mg, n=45 (2) Paracetamol 1000 mg, n=45 | (1) 13/47 (2) 13/46 | None | None | 1 pt withdrew consent, 2 pts had invalid data (2 paracetamol+codeine group, 1 paracetamol group) | |
| (1) Paracetamol+codeine 300/30 mg, n=40 (2) Ibuprofen 400 mg, n=40 (3) Placebo, n=40 | No sig diff between groups | No data | None | No data | |
| (1) Paracetamol+codeine 600/60 mg, n=30 (2) Paracetamol 600 mg, n=28 (3) Codeine 60 mg, n=28 (4) Placebo, n = 30 | No data | None reported | None reported | None reported | |
| (1) Paracetamol+codeine 600/60 mg, n=50 (2) Etoricoxib 120 mg, n=50 (3) Naproxen sodium 550 mg, n=50 (4) Placebo, n=50 | Within 10 days: (1) 25/50 (4) 18/50 | None | None | 4 pts lost to follow up (2 paracetamol+codeine group, 1 placebo group) | |
| (1) Paracetamol+codeine 600/60 mg, n=23 (2) Placebo, n=26 | no data | None | None reported | None reported | |
| (1)Paracetamol+codeine 300/30 mg, n = 109 (2) Paracetamol+tramadol 325/37.5 mg, n = 98 (3) Placebo n = 98 | No single dose data | No single dose data | No single dose data | 1 pt had invalid data | |
| (1) Paracetamol+codeine 1000/60 mg, n=36 (2) Tramadol 50 mg, n=33 (3) Tramadol 100 mg, n=35 (4) Placebo, n=33 | (1) 10/37 (2) 15/36 | None | None reported | 7 pts had invalid data | |
| (1) Paracetamol+codeine 650/60 mg, n=31 (2) Paracetamol 650 mg, n=30 (3) Flurbiprofen 50 mg, n=31 (4) Flurbiprofen 100 mg, n=29 (5) Zomepirac 100 mg, n=31 (6) Placebo, n=30 | (1) 3/31 (2) 1/30 (6) 1/30 | None | None reported | None | |
| (1) Paracetamol+codeine 650/60 mg, n=39 (2) Ketoprofen 50 mg, n=41 (3) Ketoprofen 150 mg, n=39 (4) Placebo, n=41 | (1) 11/39 (4) 4/41 | None | None | 1 placebo pt had invalid data | |
| (1) Paracetamol+codeine 300/30 mg, n=49 (2) Ibuprofen+hydrocodone 200/75 mg, n=49 (3) Placebo, n= 27 | (1) 21/49 (3) 6/27 | None | No data | None reported | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with at least 50% pain relief over 4 to 6 hours Show forest plot | 3 | 192 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.34 [2.93, 13.73] |
| 2 Participants with any adverse event Show forest plot | 3 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.58, 1.39] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with at least 50% pain relief over 4 to 6 hours Show forest plot | 4 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.06, 1.62] |
| 2 Participants using rescue medication over 4 to 6 hours Show forest plot | 2 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.41, 0.89] |
| 3 Participants with any adverse event Show forest plot | 4 | 324 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.70, 1.81] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with at least 50% pain relief over 4 to 6 hours Show forest plot | 17 | 1413 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.64 [2.17, 3.21] |
| 2 Participants with at least 50% pain relief over 4 to 6 hours, dental Show forest plot | 14 | 1221 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.70 [2.18, 3.35] |
| 3 Participants with at least 50% pain relief over 4 to 6 hours, other surgery Show forest plot | 3 | 192 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.36 [1.49, 3.73] |
| 4 Participants using rescue medication over 4 to 6 hours Show forest plot | 10 | 657 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.68, 0.82] |
| 5 Participants with any adverse event Show forest plot | 14 | 1258 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.27, 1.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with at least 50% pain relief over 4 to 6 hours Show forest plot | 10 | 622 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.11, 1.52] |
| 2 Participants with at least 50% pain relief over 4 to 6 hours, dental Show forest plot | 8 | 512 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.11, 1.57] |
| 3 Participants with at least 50% pain relief over 4 to 6 hours, other surgery Show forest plot | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.83, 1.77] |
| 4 Participants using rescue medication over 4 to 6 hours Show forest plot | 7 | 436 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.64, 0.88] |
| 5 Participants with any adverse event Show forest plot | 7 | 443 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.79, 1.57] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with at least 50% pain relief over 4 to 6 hours Show forest plot | 6 | 690 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.42, 2.47] |
| 2 Participants with at least 50% pain relief over 4 to 6 hours, dental Show forest plot | 3 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.31 [1.76, 6.23] |
| 3 Participants with at least 50% pain relief over 4 to 6 hours, other surgery Show forest plot | 3 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.11, 2.05] |
| 4 Participants using rescue medication over 4 to 6 hours Show forest plot | 4 | 529 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.68, 0.91] |
| 5 Participants with any adverse event Show forest plot | 3 | 344 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.50, 1.45] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fewer than 40 patients in active and placebo groups, participants with at least 50% pain relief over 4 to 6 hours Show forest plot | 10 | 572 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.01 [2.31, 3.92] |
| 2 More than 40 patients in active and placebo groups, participants with at least 50% pain relief over 4 to 6 hours Show forest plot | 5 | 629 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.21, 2.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fewer than 40 patients in active and control groups, Participants with at least 50% pain relief over 4 to 6 hours Show forest plot | 10 | 588 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [1.15, 1.60] |
| 2 More than 40 patients in active and control groups, Participants with at least 50% pain relief over 4 to 6 hours Show forest plot | 3 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.02, 1.65] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with any adverse event Show forest plot | 20 | 1811 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.15, 1.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with any adverse event Show forest plot | 11 | 767 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.87, 1.40] |

