Oral non‐steroidal anti‐inflammatory drug therapy for lung disease in cystic fibrosis
Información
- DOI:
- https://doi.org/10.1002/14651858.CD001505.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 07 abril 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Fibrosis quística y enfermedades genéticas
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Anna Crighton had the original idea for the review, drafted the protocol, extracted data and co‐authored the review. Carol Dezateux drafted the protocol and data extraction forms, extracted and analyzed data and was responsible for writing the review.
Anna Crighton and Carol Dezateux stepped down from the review as from February 2006.
Larry Lands took over as lead reviewer in May 2004. He led on the update of the review and acts as guarantor of the review.
Sanja Stanojevic joined the review in June 2006. She extracted and analyzed data for the subsequent updates and co‐authored the review.
Sources of support
Internal sources
-
Institute of Child Health, Ninewells Hospital and Medical School, Dundee DD1 9SY, UK.
External sources
-
Tenovus, Scotland, UK.
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
Dr Larry Lands was the lead investigator in the Trans‐Canadian Trial (Lands 2007).
Acknowledgements
We are grateful to Professor Rosalind Smyth, Jill Motley, Nikki Jahnke and the Cochrane Cystic Fibrosis and Genetic Disorders Editorial Group for their invaluable support; to Professor Carol Dezateux and Anna Crighton for their input into previous versions of this review; to Dr Michael Konstan for his helpful comments on an earlier draft of the original review; and to Dr Somnath Mukhopadhyay for his encouragement.
Version history
| Published | Title | Stage | Authors | Version |
| 2019 Sep 09 | Oral non‐steroidal anti‐inflammatory drug therapy for lung disease in cystic fibrosis | Review | Larry C Lands, Sanja Stanojevic | |
| 2016 Apr 07 | Oral non‐steroidal anti‐inflammatory drug therapy for lung disease in cystic fibrosis | Review | Larry C Lands, Sanja Stanojevic | |
| 2013 Jun 13 | Oral non‐steroidal anti‐inflammatory drug therapy for lung disease in cystic fibrosis | Review | Larry C Lands, Sanja Stanojevic | |
| 2007 Oct 17 | Oral non‐steroidal anti‐inflammatory drug therapy for lung disease in cystic fibrosis | Review | Larry C Lands, Sanja Stanojevic | |
| 1999 Apr 26 | Oral non‐steroidal anti‐inflammatory drug therapy for cystic fibrosis | Review | Larry C Lands, Carol Dezateux, A Crighton, Sanja Stanojevic | |
Differences between protocol and review
There was a post‐hoc subgroup analysis of the lung function data split by age (two groups: 'under 13 years at randomisation' and '13 years and over at randomisation').
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adolescent; Adult; Child; Child, Preschool; Humans;
PICO

Comparison 1 Oral nonsteroidal anti‐inflammatory drug versus placebo, Outcome 1 Annual rate of change in % predicted FEV1.
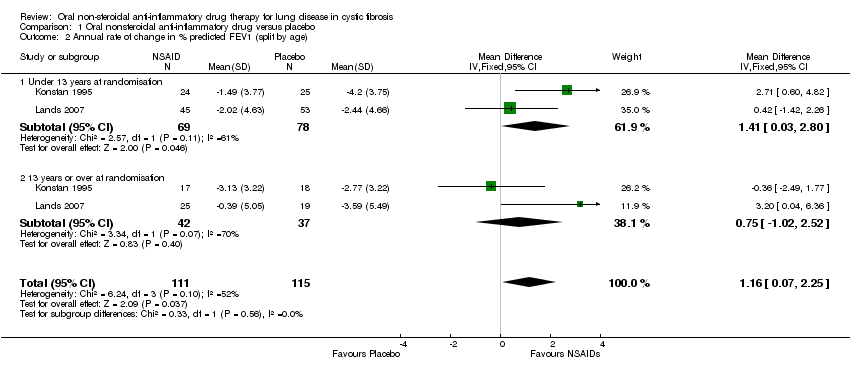
Comparison 1 Oral nonsteroidal anti‐inflammatory drug versus placebo, Outcome 2 Annual rate of change in % predicted FEV1 (split by age).

Comparison 1 Oral nonsteroidal anti‐inflammatory drug versus placebo, Outcome 3 Annual rate of change in % predicted FVC.
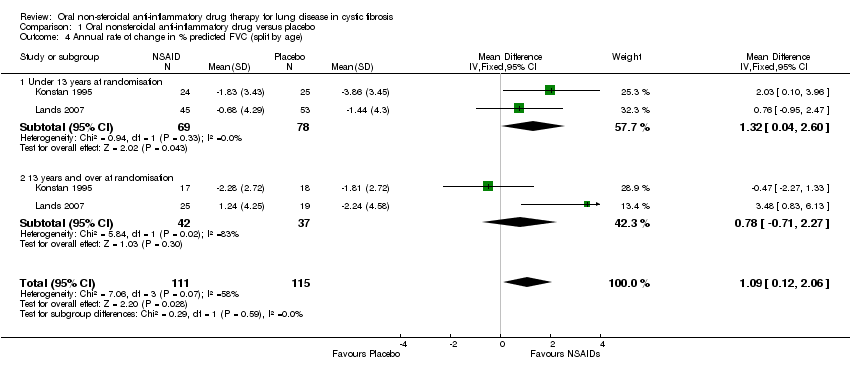
Comparison 1 Oral nonsteroidal anti‐inflammatory drug versus placebo, Outcome 4 Annual rate of change in % predicted FVC (split by age).
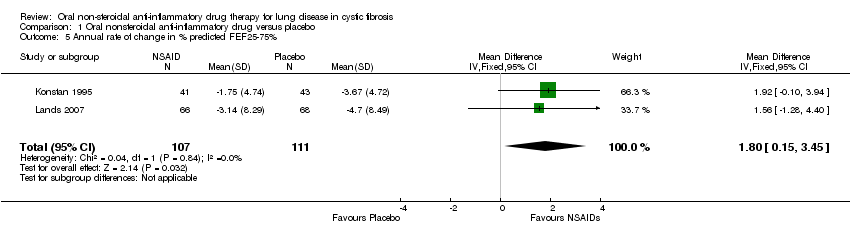
Comparison 1 Oral nonsteroidal anti‐inflammatory drug versus placebo, Outcome 5 Annual rate of change in % predicted FEF25‐75%.
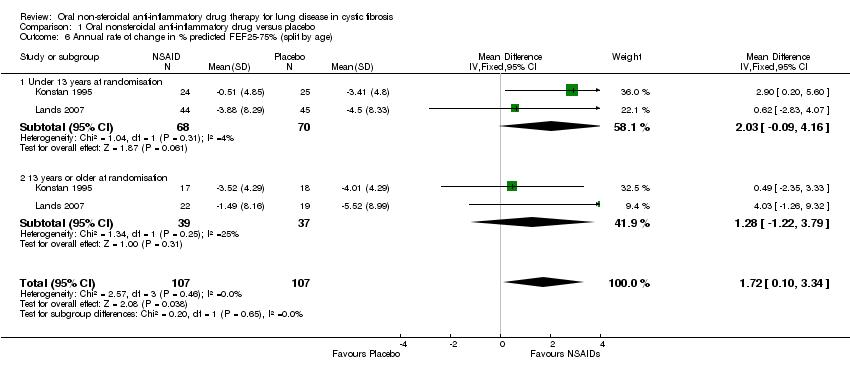
Comparison 1 Oral nonsteroidal anti‐inflammatory drug versus placebo, Outcome 6 Annual rate of change in % predicted FEF25‐75% (split by age).

Comparison 1 Oral nonsteroidal anti‐inflammatory drug versus placebo, Outcome 7 Proportion with at least one respiratory hospitalisation.
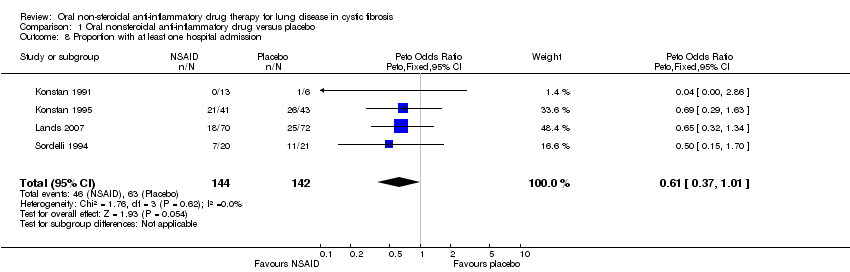
Comparison 1 Oral nonsteroidal anti‐inflammatory drug versus placebo, Outcome 8 Proportion with at least one hospital admission.
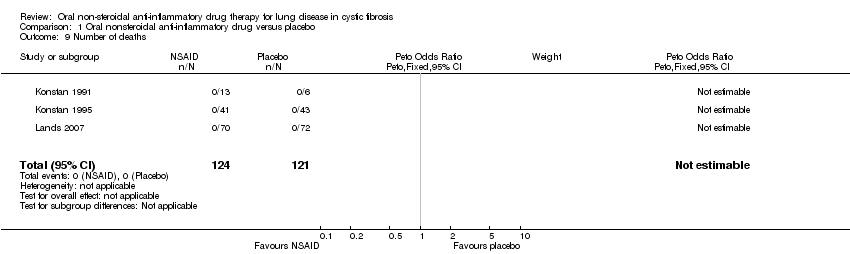
Comparison 1 Oral nonsteroidal anti‐inflammatory drug versus placebo, Outcome 9 Number of deaths.

Comparison 1 Oral nonsteroidal anti‐inflammatory drug versus placebo, Outcome 10 Annual rate of change in % ideal body weight.

Comparison 1 Oral nonsteroidal anti‐inflammatory drug versus placebo, Outcome 11 Annual rate of change in % ideal body weight (split by age).

Comparison 1 Oral nonsteroidal anti‐inflammatory drug versus placebo, Outcome 12 Chest X‐ray score.
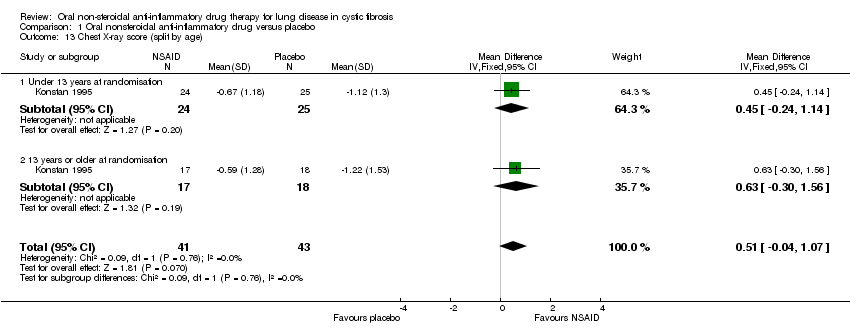
Comparison 1 Oral nonsteroidal anti‐inflammatory drug versus placebo, Outcome 13 Chest X‐ray score (split by age).
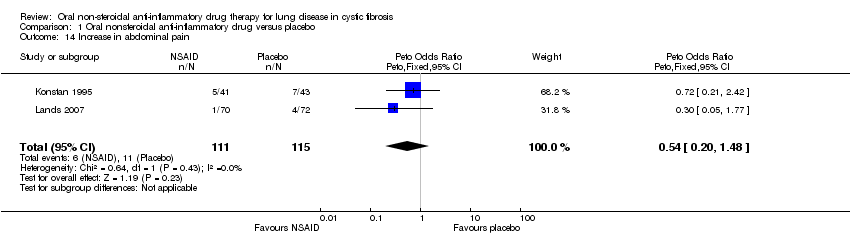
Comparison 1 Oral nonsteroidal anti‐inflammatory drug versus placebo, Outcome 14 Increase in abdominal pain.

Comparison 1 Oral nonsteroidal anti‐inflammatory drug versus placebo, Outcome 15 Decrease in abdominal pain.
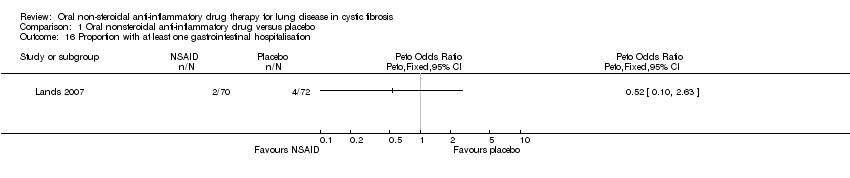
Comparison 1 Oral nonsteroidal anti‐inflammatory drug versus placebo, Outcome 16 Proportion with at least one gastrointestinal hospitalisation.
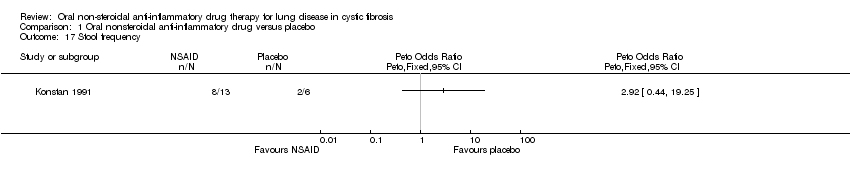
Comparison 1 Oral nonsteroidal anti‐inflammatory drug versus placebo, Outcome 17 Stool frequency.

Comparison 1 Oral nonsteroidal anti‐inflammatory drug versus placebo, Outcome 18 Occult blood.

Comparison 1 Oral nonsteroidal anti‐inflammatory drug versus placebo, Outcome 19 Increase in epistaxis.
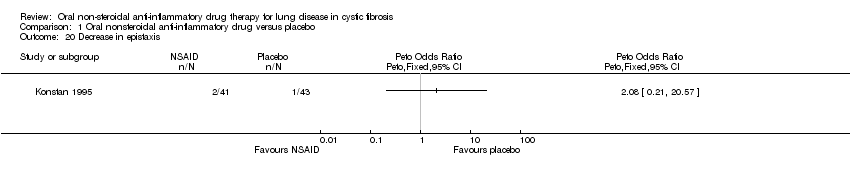
Comparison 1 Oral nonsteroidal anti‐inflammatory drug versus placebo, Outcome 20 Decrease in epistaxis.

Comparison 1 Oral nonsteroidal anti‐inflammatory drug versus placebo, Outcome 21 Increase in conjunctivitis.

Comparison 1 Oral nonsteroidal anti‐inflammatory drug versus placebo, Outcome 22 Decrease in conjunctivitis.

Comparison 1 Oral nonsteroidal anti‐inflammatory drug versus placebo, Outcome 23 Increase in nausea.

Comparison 1 Oral nonsteroidal anti‐inflammatory drug versus placebo, Outcome 24 Increase in diarrhoea.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Annual rate of change in % predicted FEV1 Show forest plot | 2 | 226 | Mean Difference (IV, Fixed, 95% CI) | 1.32 [0.21, 2.42] |
| 2 Annual rate of change in % predicted FEV1 (split by age) Show forest plot | 2 | 226 | Mean Difference (IV, Fixed, 95% CI) | 1.16 [0.07, 2.25] |
| 2.1 Under 13 years at randomisation | 2 | 147 | Mean Difference (IV, Fixed, 95% CI) | 1.41 [0.03, 2.80] |
| 2.2 13 years or over at randomisation | 2 | 79 | Mean Difference (IV, Fixed, 95% CI) | 0.75 [‐1.02, 2.52] |
| 3 Annual rate of change in % predicted FVC Show forest plot | 2 | 226 | Mean Difference (IV, Fixed, 95% CI) | 1.27 [0.26, 2.28] |
| 4 Annual rate of change in % predicted FVC (split by age) Show forest plot | 2 | 226 | Mean Difference (IV, Fixed, 95% CI) | 1.09 [0.12, 2.06] |
| 4.1 Under 13 years at randomisation | 2 | 147 | Mean Difference (IV, Fixed, 95% CI) | 1.32 [0.04, 2.60] |
| 4.2 13 years and over at randomisation | 2 | 79 | Mean Difference (IV, Fixed, 95% CI) | 0.78 [‐0.71, 2.27] |
| 5 Annual rate of change in % predicted FEF25‐75% Show forest plot | 2 | 218 | Mean Difference (IV, Fixed, 95% CI) | 1.80 [0.15, 3.45] |
| 6 Annual rate of change in % predicted FEF25‐75% (split by age) Show forest plot | 2 | 214 | Mean Difference (IV, Fixed, 95% CI) | 1.72 [0.10, 3.34] |
| 6.1 Under 13 years at randomisation | 2 | 138 | Mean Difference (IV, Fixed, 95% CI) | 2.03 [‐0.09, 4.16] |
| 6.2 13 years or older at randomisation | 2 | 76 | Mean Difference (IV, Fixed, 95% CI) | 1.28 [‐1.22, 3.79] |
| 7 Proportion with at least one respiratory hospitalisation Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 8 Proportion with at least one hospital admission Show forest plot | 4 | 286 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.61 [0.37, 1.01] |
| 9 Number of deaths Show forest plot | 3 | 245 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Annual rate of change in % ideal body weight Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11 Annual rate of change in % ideal body weight (split by age) Show forest plot | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | 0.81 [0.08, 1.53] |
| 11.1 Under 13 years at randomisation | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | 1.45 [0.33, 2.57] |
| 11.2 13 years or older at randomisation | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 0.34 [‐0.61, 1.29] |
| 12 Chest X‐ray score Show forest plot | 2 | 226 | Mean Difference (IV, Fixed, 95% CI) | 0.37 [‐0.08, 0.81] |
| 13 Chest X‐ray score (split by age) Show forest plot | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | 0.51 [‐0.04, 1.07] |
| 13.1 Under 13 years at randomisation | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | 0.45 [‐0.24, 1.14] |
| 13.2 13 years or older at randomisation | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 0.63 [‐0.30, 1.56] |
| 14 Increase in abdominal pain Show forest plot | 2 | 226 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.54 [0.20, 1.48] |
| 15 Decrease in abdominal pain Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 16 Proportion with at least one gastrointestinal hospitalisation Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 17 Stool frequency Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 18 Occult blood Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 19 Increase in epistaxis Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 20 Decrease in epistaxis Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 21 Increase in conjunctivitis Show forest plot | 2 | 226 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.72 [0.22, 2.40] |
| 22 Decrease in conjunctivitis Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 23 Increase in nausea Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 24 Increase in diarrhoea Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |

