Graduated compression stockings for prevention of deep vein thrombosis
Abstract
Background
Hospitalised patients are at increased risk of developing deep vein thrombosis (DVT) in the lower limb and pelvic veins, on a background of prolonged immobilisation associated with their medical or surgical illness. Patients with DVT are at increased risk of developing a pulmonary embolism (PE). The use of graduated compression stockings (GCS) in hospitalised patients has been proposed to decrease the risk of DVT. This is an update of a Cochrane Review first published in 2000, and last updated in 2014.
Objectives
To evaluate the effectiveness and safety of graduated compression stockings in preventing deep vein thrombosis in various groups of hospitalised patients.
Search methods
For this review the Cochrane Vascular Information Specialist searched the Cochrane Vascular Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), and trials registries on 21 March 2017; and the Cochrane Vascular Specialised Register, CENTRAL, MEDLINE Ovid, Embase Ovid, CINAHL Ebsco, AMED Ovid , and trials registries on 12 June 2018.
Selection criteria
Randomised controlled trials (RCTs) involving GCS alone, or GCS used on a background of any other DVT prophylactic method. We combined results from both of these groups of trials.
Data collection and analysis
Two review authors (AS, MD) assessed potentially eligible trials for inclusion. One review author (AS) extracted the data, which a second review author (MD) cross‐checked and authenticated. Two review authors (AS, MD) assessed the methodological quality of trials with the Cochrane 'Risk of bias' tool. Any disagreements were resolved by discussion with the senior review author (TL). For dichotomous outcomes, we calculated the Peto odds ratio and corresponding 95% confidence interval. We pooled data using a fixed‐effect model. We used the GRADE system to evaluate the overall quality of the evidence supporting the outcomes assessed in this review.
Main results
We included 20 RCTs involving a total of 1681 individual participants and 1172 individual legs (2853 analytic units). Of these 20 trials, 10 included patients undergoing general surgery; six included patients undergoing orthopaedic surgery; three individual trials included patients undergoing neurosurgery, cardiac surgery, and gynaecological surgery, respectively; and only one trial included medical patients. Graduated compression stockings were applied on the day before surgery or on the day of surgery and were worn up until discharge or until the participants were fully mobile. In the majority of the included studies DVT was identified by the radioactive I125 uptake test. Duration of follow‐up ranged from seven to 14 days. The included studies were at an overall low risk of bias.
We were able to pool the data from 20 studies reporting the incidence of DVT. In the GCS group, 134 of 1445 units developed DVT (9%) in comparison to the control group (without GCS), in which 290 of 1408 units developed DVT (21%). The Peto odds ratio (OR) was 0.35 (95% confidence interval (CI) 0.28 to 0.43; 20 studies; 2853 units; high‐quality evidence), showing an overall effect favouring treatment with GCS (P < 0.001).
Based on results from eight included studies, the incidence of proximal DVT was 7 of 517 (1%) units in the GCS group and 28 of 518 (5%) units in the control group. The Peto OR was 0.26 (95% CI 0.13 to 0.53; 8 studies; 1035 units; moderate‐quality evidence) with an overall effect favouring treatment with GCS (P < 0.001). Combining results from five studies, all based on surgical patients, the incidence of PE was 5 of 283 (2%) participants in the GCS group and 14 of 286 (5%) in the control group. The Peto OR was 0.38 (95% CI 0.15 to 0.96; 5 studies; 569 participants; low‐quality evidence) with an overall effect favouring treatment with GCS (P = 0.04). We downgraded the quality of the evidence for proximal DVT and PE due to low event rate (imprecision) and lack of routine screening for PE (inconsistency).
We carried out subgroup analysis by speciality (surgical or medical patients). Combining results from 19 trials focusing on surgical patients, 134 of 1365 (9.8%) units developed DVT in the GCS group compared to 282 of 1328 (21.2%) units in the control group. The Peto OR was 0.35 (95% CI 0.28 to 0.44; high‐quality evidence), with an overall effect favouring treatment with GCS (P < 0.001). Based on results from seven included studies, the incidence of proximal DVT was 7 of 437 units (1.6%) in the GCS group and 28 of 438 (6.4%) in the control group. The Peto OR was 0.26 (95% CI 0.13 to 0.53; 875 units; moderate‐quality evidence) with an overall effect favouring treatment with GCS (P < 0.001). We downgraded the evidence for proximal DVT due to low event rate (imprecision).
Based on the results from one trial focusing on medical patients admitted following acute myocardial infarction, 0 of 80 (0%) legs developed DVT in the GCS group and 8 of 80 (10%) legs developed DVT in the control group. The Peto OR was 0.12 (95% CI 0.03 to 0.51; low‐quality evidence) with an overall effect favouring treatment with GCS (P = 0.004). None of the medical patients in either group developed a proximal DVT, and the incidence of PE was not reported.
Limited data were available to accurately assess the incidence of adverse effects and complications with the use of GCS as these were not routinely quantitatively reported in the included studies.
Authors' conclusions
There is high‐quality evidence that GCS are effective in reducing the risk of DVT in hospitalised patients who have undergone general and orthopaedic surgery, with or without other methods of background thromboprophylaxis, where clinically appropriate. There is moderate‐quality evidence that GCS probably reduce the risk of proximal DVT, and low‐quality evidence that GCS may reduce the risk of PE. However, there remains a paucity of evidence to assess the effectiveness of GCS in diminishing the risk of DVT in medical patients.
PICO
Plain language summary
Graduated compression stockings for prevention of deep vein thrombosis during a hospital stay
Background
Deep vein thrombosis (DVT) is a blood clot that forms in a vein deep in the body, usually in the leg or pelvic veins. A number of factors such as reduced mobility, older age, obesity, active cancer, major surgery, major injuries, history of previous DVT, family history of DVT, and recent period of illness may increase the risk of developing a DVT. Hospital patients, who often have one or more of these risk factors, are at particular risk of developing DVT, either immediately after surgery or if they are immobile due to a medical illness.
Symptoms of DVT vary from none to pain and swelling in the legs. A blood clot can move from the leg to the lungs, with the danger of pulmonary embolism (PE) and death. The main treatment for DVT includes the use of blood‐thinning drugs (anticoagulation). Deep vein thrombosis usually resolves, but it can have long‐term effects such as high venous pressure in the leg, leg pain, swelling, darkening of the skin, and inflammation.
Deep vein thrombosis can be prevented with the use of compression or drugs. Drugs can cause bleeding, which is a particular concern in surgical patients. Graduated compression stockings (GCS) help prevent the formation of blood clots in the legs by applying varying amounts of pressure to different parts of the leg.
Study characteristics and key results
We identified 20 randomised controlled trials (studies in which participants are assigned to a treatment group using a random method) (2853 analytic units consisting of 1681 individual patients and 1172 individual legs) in our most recent search on 12 June 2018. Nine trials compared wearing stockings to no stockings, and 11 compared stockings plus another method with that method alone. The other methods used were dextran 70, aspirin, heparin, and mechanical sequential compression. Of the 20 trials, 10 included patients undergoing general surgery; six included patients undergoing orthopaedic surgery; three individual trials included patients undergoing neurosurgery, cardiac surgery, and gynaecological surgery, respectively; and only one trial included medical patients (patients who were admitted to the hospital for reasons other than surgery). The compression stockings were applied on the day before surgery or on the day of surgery and were worn up until discharge or until the patients were fully mobile. Thigh‐length stockings were used in the vast majority of included studies. The included studies were of good quality overall. We found that wearing GCS reduced the overall risk of developing DVT, and probably also DVT in the thighs. We found that GCS may also reduce the risk of PE amongst patients undergoing surgery. As only one trial included medical patients, results for this population are limited. The occurrence of problems associated with wearing GCS was poorly reported in the included studies.
Quality of the evidence
Our review confirmed that GCS are effective in reducing the risk of DVT in hospitalised surgical patients (high‐quality evidence). It also demonstrated that GCS probably reduce the risk of developing DVT in the thighs (proximal DVT, moderate‐quality evidence) and PE (low‐quality evidence). Reasons for downgrading the quality of the evidence included low event rate (i.e. small number of participants who developed DVT) and uncertainty due to only a small number of patients being routinely screened for proximal DVT or PE. Limited evidence was available for hospitalised medical patients, with only one study suggesting that GCS may prevent DVT in such patients.
Authors' conclusions
Summary of findings
| Graduated compression stockings for prevention of deep vein thrombosis | ||||||
| Patient or population: Hospitalised patients1 | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of units/ participants2 | Quality of the evidence | Comments | |
| Risk with no GCS | Risk with GCS | |||||
| Deep vein thrombosis (DVT) Follow‐up: 7 to 14 days, or until discharge | Study population | OR 0.35 | 2853 | ⊕⊕⊕⊕ | Graduated compression stockings reduce the incidence of DVT in hospitalised patients. However, studies did not routinely distinguish between symptomatic and asymptomatic DVTs. | |
| 206 per 1000 | 83 per 1000 | |||||
| Proximal DVT Follow‐up: 7 to 14 days, or until discharge | Study population | OR 0.26 | 1035 | ⊕⊕⊕⊝ | Graduated compression stockings probably reduce the incidence of proximal DVT in hospitalised patients. There was a relatively low event rate overall, and studies did not routinely distinguish between symptomatic and asymptomatic proximal DVTs. | |
| 54 per 1000 | 15 per 1000 | |||||
| Pulmonary embolism Follow‐up: 7 to 30 days | Study population | OR 0.38 | 569 | ⊕⊕⊝⊝ | Graduated compression stockings may slightly reduce the incidence of pulmonary embolism in hospitalised patients. Pulmonary embolism was not routinely assessed in most included studies, and the overall event rate was very low. | |
| 49 per 1000 | 19 per 1000 | |||||
| Adverse effects and complications Follow‐up: until discharge | See comment | ‐ | ‐ | ‐ | Some participants removed stockings due to discomfort or poor fitting, however adverse effects and complications were not routinely reported quantitatively in the included studies. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Included patients admitted for surgical procedures (including abdominal, orthopaedic, neurosurgical, gynaecological surgery) and medical illness (acute myocardial infarction). | ||||||
| Graduated compression stockings for prevention of deep vein thrombosis | ||||||
| Patient or population: Patients hospitalised for surgical procedures1 | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of units/ participants2 | Quality of the evidence | Comments | |
| Risk with no GCS | Risk with GCS | |||||
| Deep vein thrombosis (DVT) Follow‐up: 7 to 14 days, or until discharge | Study population | OR 0.35 (0.28 to 0.44) | 2693 (19 RCTs) | ⊕⊕⊕⊕ | Graduated compression stockings reduce the incidence of DVT in hospitalised surgical patients. However, studies did not routinely distinguish between symptomatic and asymptomatic DVTs. | |
| 212 per 1000 | 86 per 1000 | |||||
| Proximal DVT Follow‐up: 7 to 14 days, or until discharge | Study population | OR 0.26 (0.13 to 0.53) | 875 (7 RCTs) | ⊕⊕⊕⊝ | Graduated compression stockings probably reduce the incidence of proximal DVT in hospitalised surgical patients. However, studies did not routinely distinguish between symptomatic and asymptomatic proximal DVTs. There was a relatively low event rate overall. | |
| 64 per 1000 | 17 per 1000 | |||||
| Pulmonary embolism Follow‐up: 7 to 30 days, or until discharge | Study population | OR 0.38 (0.15 to 0.96) | 569 (5 RCTs) | ⊕⊕⊝⊝ | Graduated compression stockings may slightly reduce the incidence of pulmonary embolism in hospitalised surgical patients. However, pulmonary embolism was not routinely assessed in most included studies, and the overall event rate was very low. | |
| 49 per 1000 | 19 per 1000 | |||||
| Adverse effects and complications Follow‐up: until discharge | See comment | ‐ | ‐ | ‐ | Some participants removed stockings due to discomfort or poor fitting, however adverse effects and complications associated with wearing GCS were not routinely reported quantitatively in the included studies. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Included patients admitted for surgical procedures (including abdominal, orthopaedic, neurosurgical, gynaecological surgery). | ||||||
| Graduated compression stockings for prevention of deep vein thrombosis | ||||||
| Patient or population: Patients hospitalised following acute myocardial infarction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of units | Quality of the evidence | Comments | |
| Risk with no GCS | Risk with GCS | |||||
| Deep vein thrombosis (DVT) Follow‐up: 8 days or discharge or until development of DVT | Study population | OR 0.12 (0.03 to 0.51) | 160 | ⊕⊕⊝⊝ | Graduated compression stockings may reduce incidence of DVT in hospitalised medical patients. However, results are based on a single study on medical patients hospitalised following acute myocardial infarction (Kierkegaard 1993). | |
| 100 per 1000 | 13 per 1000 | |||||
| Proximal DVT Follow‐up: 8 days or discharge or until development of DVT | Study population | Not estimable | 160 (1 RCT) | ‐ | None of the participants in either group of this single RCT with a small sample size developed proximal DVT. | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Pulmonary embolism | See comment | ‐ | ‐ | ‐ | No studies reported on this outcome. There is paucity of evidence to evaluate the effect of GCS on reducing incidence of pulmonary embolism in hospitalised medical patients. | |
| Adverse effects and complications | See comment | ‐ | 160 (1 RCT) | ‐ | There are rare reports of post‐thrombotic changes in participants who developed DVT in the single included RCT. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded the quality of the evidence by two steps as there was only one study and a low event rate in the GCS group (imprecision). | ||||||
Background
Description of the condition
The occurrence of one or more factors of Virchow's triad (stasis of blood, endothelial injury, and hypercoagulability of blood) in the venous system often leads to deep vein thrombosis (DVT) (Virchow 1858). Diagnosis of DVT is difficult as the patient history is not specific, and symptoms vary from no symptoms to pain and swelling in the legs. The sequelae of DVT vary from complete resolution of the clot without any ill effects through to death due to pulmonary embolism (PE).
Risk factors associated with the development of DVT include age over 60 years, active cancer, obesity, major surgery, major trauma, prolonged immobilisation, pregnancy, history of thromboembolic disease, and acute medical illness. Thromboembolic risk factors are particularly common amongst hospitalised patients, with baseline incidence of DVT in 29% of surgical patients and 24% in medical patients, and incidence of symptomatic PE of 3% and 1%, respectively, without the use of thromboprophylaxis (NICE 2010).
Morbidity due to DVT includes post‐thrombotic syndrome (PTS), which encompasses chronic venous hypertension causing limb pain, swelling, hyperpigmentation (darkening of the skin), dermatitis (inflammation of the skin), ulcers, and lipodermatosclerosis (a hardening of the skin that may gain a red or brown pigmentation and is accompanied by wasting of the subcutaneous fat). Data from a prospective multicentre cohort study found that 43% of patients with symptomatic DVT developed features of PTS at two‐year follow‐up (Kahn 2008).
Mortality associated with DVT is greatest in the first 30 days, at 3% to 6%, though the risk of death remains increased even at long‐term follow‐up (Søgaard 2014). Various reports suggest that 28% to 41% of patients with DVT subsequently develop a PE, which is associated with an increase in risk of 30‐day mortality to approximately 12% (White 2003).
Patients who are at risk of developing DVT are categorised into three groups of low, moderate, and high risk according to the International Consensus Statement and Thromboembolic Risk Factor (THRIFT) consensus group guidelines (ICS 2013; THRIFT 1992). However, guidelines on prophylaxis of venous thromboembolism from the National Institute for Health and Care Excellence and the Scottish Intercollegiate Guidelines Network no longer categorise patients into low‐, moderate‐, and high‐risk groups (NICE 2010; SIGN 2010), instead looking at risk factors for developing DVT in hospitalised patients on an individual basis.
Description of the intervention
Both mechanical and pharmacological methods are used in the prevention of DVT. Pharmacological methods alter the blood coagulation profile; the major disadvantage of this is the risk of bleeding, which may be of particular concern in surgical patients. For example, the altered coagulation may lead to joint haematomas following joint replacement surgery and intracranial haemorrhage following head injury or neurosurgery. Mechanical methods include techniques such as intermittent pneumatic compression (IPC) and wearing of graduated compression stockings (GCS).
How the intervention might work
The exact mechanism by which GCS function is unknown. However, there is evidence to suggest that they exert graded circumferential pressure distally to proximally and, when combined with muscular activity in the limb, are thought to displace blood from the superficial to the deep venous system via the perforating veins. It is argued that this effectively increases the velocity and volume of flow in the deep system thereby potentially preventing thrombosis (Benko 2001).
Why it is important to do this review
Despite the theoretical effectiveness and widespread use of compression stockings, their clinical effectiveness needs further appraisal. Improper application of stockings may potentially cause complications such as discomfort, oedema of the legs, DVT, and arterial ischaemia. Stockings may also be contraindicated for medical reasons. The extent to which the leg profile of patients may limit effectiveness has not been addressed. This review did not address the recommendations regarding the ideal length of stockings (knee length versus thigh length), which are assessed by a separate Cochrane Review (Sajid 2012).
The use of GCS amongst surgical patients is estimated to cost the National Health Service (NHS) GBP 63.1 million annually (GAPS). A strong evidence base is therefore needed to justify the routine use of GCS amongst hospitalised patients.
Objectives
To evaluate the effectiveness and safety of graduated compression stockings in preventing deep vein thrombosis in various groups of hospitalised patients.
We tested the following hypotheses:
-
compression stockings are effective in preventing DVT in hospitalised patients (excluding stroke);
-
in all moderate‐risk patients, compression stockings alone are adequate for DVT prophylaxis, except for patients for whom stockings are specifically contraindicated;
-
stockings are unnecessary in low‐risk patients;
-
complications are associated with the use of compression stockings.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised controlled trials (RCTs) that involved the use of compression stockings for DVT prophylaxis.
Types of participants
We included patients of either sex and any age hospitalised for conditions other than stroke.
Types of interventions
We included trials in which the use of graduated compression stockings (GCS) was compared with no prophylaxis, and those studies in which the use of GCS was compared with no stockings on a background of another method of DVT prophylaxis in both the treatment and control group (e.g. aspirin, heparin). We analysed both groups of trials together in this update as they both assessed the same treatment effect (i.e. GCS versus no GCS).
Types of outcome measures
Primary outcomes
-
Diagnosis of DVT, either all DVT or proximal DVT, identified by ultrasound, venogram, or isotope studies
Secondary outcomes
-
Diagnosis of PE, identified by a ventilation perfusion lung scan, pulmonary angiogram, or postmortem examination
-
Complications and adverse effects arising from the use of GCS
Search methods for identification of studies
We placed no restrictions on language or publication status.
Electronic searches
For this update, the Cochrane Vascular Information Specialist (CIS) searched the following databases for relevant trials:
-
the Cochrane Vascular Specialised Register (21 March 2017);
-
the Cochrane Central Register of Controlled Trials (CENTRAL (2017, Issue 2)) via the Cochrane Register of Studies Online (21 March 2017).
See Appendix 1 for details of the search strategy used to search CENTRAL.
The Cochrane Vascular Specialised Register is maintained by the CIS and is constructed from weekly electronic searches of MEDLINE Ovid, Embase Ovid, CINAHL Ebsco (Cumulative Index to Nursing and Allied Health Literature), AMED Ovid (Allied and Complementary Medicine), and through handsearching relevant journals.
The CIS also searched the following trial registries for details of ongoing and unpublished studies (21 March 2017):
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov);
-
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/trialsearch);
-
ISRCTN register (www.isrctn.com/).
See Appendix 2 for details of the search strategies used.
The CIS subsequently performed a top‐up search on 12 June 2018, searching the Cochrane Vascular Specialised Register, CENTRAL, MEDLINE Ovid (2017 and 2018 only), Embase Ovid (2017 and 2018 only), CINAHL Ebsco (2017 and 2018 only), AMED Ovid (2017 and 2018 only), and the trials registries ClinicalTrials.gov and WHO ICTRP. See Appendix 3 for details of the search strategies.
Searching other resources
We searched the reference lists of all potentially eligible studies identified from the electronic searches to find additional trials.
Data collection and analysis
Selection of studies
We have specified the criteria for the selection of trials above. Two review authors (AS, MD) carried out the selection of trials for inclusion in this update, which the senior review author (TL) checked and approved.
One review author (AS) carried out the initial screening of all retrieved studies based on titles and abstracts in order to identify obvious exclusions (i.e. studies not relevant to the review). Where there was uncertainty regarding the relevance of a particular study, a second review author (MD) was consulted. Two review authors (AS, MD) independently assessed the remaining records so as to avoid exclusion of any relevant articles. Next, full papers were extracted for the remaining articles, which two review authors (AS, MD) independently assessed for inclusion (see Criteria for considering studies for this review). In case of disagreement between review authors, consensus was reached by discussion with a third review author (TL). Finally, all eligible, relevant studies based on the abovementioned criteria were included in the review.
Data extraction and management
For this update, one review author (AS) performed data extraction and entered data onto a data extraction form. Another review author (MD) then cross‐checked the data. We extracted the following information.
-
Age
-
Sex
-
DVT risk groups to which participants belonged
-
Duration of application of stockings
-
Types and length of stockings
-
Incidence of DVT
-
PE
-
Adverse effects
-
Investigations used to make the diagnoses
Assessment of risk of bias in included studies
Two review authors (AS, MD) independently assessed the risk of bias for all the included studies based on six domains: random sequence generation, allocation concealment, blinding (participant and assessor), incomplete outcome data, selective reporting, and other biases. We used the 'Risk of bias' assessment tool outlined in the Cochrane Handbook for Systematic Reviews of Interventions to determine whether studies were free of potential bias, assessing studies as at low risk, high risk, or unclear risk of bias if the available information was inadequate (Higgins 2011). Any discrepancies in opinion were discussed and consensus reached. Blinding of participants to their treatment group (i.e. whether they wore GCS or not) was inherently difficult, therefore blinding of assessors to treatment groups was deemed the most appropriate method to minimise risk of bias.
Measures of treatment effect
The effectiveness of treatment (i.e. the use of GCS) was assessed by recording the incidence of DVT in the treatment (stockinged) group compared to that in the control (non‐stockinged) group. Deep vein thrombosis was diagnosed using an objective method of assessment such as ultrasound, venogram, or isotope studies. We did not combine individual patient data from different trials.
We performed analysis of the cumulative data using Peto's odds ratio (OR) with 95% confidence interval (CI) employing a fixed‐effect model. We used fixed‐effect analysis since the vast majority of included studies were based on surgical patients at medium or high risk of developing DVT. Furthermore, we also considered that fixed‐effect analysis would help minimise bias due to the low event rate amongst a number of included studies with a small sample size. We used Review Manager 5, the statistical package provided by Cochrane, for cumulative analysis of the included trials (RevMan 2012).
Unit of analysis issues
Individual patients were the analytic units except in seven trials (Bergqvist 1984; Kierkegaard 1993; Mellbring 1986; Scurr 1977; Scurr 1987; Shirai 1985; Torngren 1980), where one limb was randomised to act as control and the other was treated.
Since a thrombus can embolise from either the control or stockinged leg, trials randomising individual legs could not be used to compare the incidence of PE, therefore where data were available, individual patients were used as the unit of analysis for comparing incidence of PE.
Dealing with missing data
Complete primary outcome data were available for participants excluded postrandomisation in only four trials (Allan 1983; Scurr 1977; Torngren 1980; Wille‐Jorgensen 1991). Participants had been excluded postrandomisation in an additional eight trials (Bergqvist 1984; Fredin 1989; Holford 1976; Hui 1996; Kalodiki 1996; Mellbring 1986; Ohlund 1983; Wille‐Jorgensen 1985), though the confirmation of the presence or absence of DVT amongst these excluded participants was not reported. We were unable to assess Shirai 1985 for missing data, as it was only published in Japanese. There were no reported exclusions postrandomisation in the remaining seven trials (Barnes 1978; Chin 2009; Kierkegaard 1993; Scurr 1987; Tsapogas 1971; Turner 1984; Turpie 1989). Due to the small number of trials reporting outcome data for participants excluded postrandomisation, we performed a per‐protocol analysis.
Similarly, complete outcome data were unavailable for secondary outcomes, therefore we performed a per‐protocol analysis.
Assessment of heterogeneity
We used the I2 statistic to quantify heterogeneity. We considered heterogeneity to be statistically significant for a P < 0.1.
Assessment of reporting biases
We assessed reporting bias by visual inspection of funnel plots.
Data synthesis
In previous versions of this review, we performed data synthesis based on two groups as follows.
-
Group 1: GCS only in the treatment group and no prophylaxis in the control group.
-
Group 2: GCS in the treatment group and another method of DVT prophylaxis in both the treatment and control groups.
Since both of these groups test the same treatment effect (i.e. with stockings versus without stockings), we merged all trials in the 2014 update to increase the power of the review.
We tested comparisons of results using a fixed‐effect model for the meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We subgrouped trials based on the speciality under which the participant had been hospitalised. Most participants underwent either general surgical or orthopaedic surgical procedures. We undertook subgroup analyses in Review Manager 5 using the method described by Deeks 2001.
Sensitivity analysis
We performed sensitivity analyses to assess the effect of the following three potential areas of bias on the robustness of decisions made during the review process.
-
Method of randomisation: trials reporting use of an appropriate method of randomisation versus use of an unclear method of randomisation.
-
Unit of analysis of randomisation: individual legs versus individual participants.
-
Use of a background method of thromboprophylaxis.
-
Method of diagnosis of DVT.
'Summary of findings' table
We presented the main findings of this update in 'Summary of findings' tables. We considered the quality of evidence, magnitude of effect of interventions examined, and the sum of available data for all outcomes of this review (DVT, proximal DVT, PE, adverse events and complications). We presented the findings for all patients (summary of findings Table for the main comparison), surgical patients (summary of findings Table 2), and medical patients (summary of findings Table 3) according to GRADE principles, as described by Higgins 2011 and Atkins 2004. We evaluated evidence on the basis of risk of bias of the included studies, inconsistency, indirectness, imprecision of data, and publication bias. We used GRADEpro GDT software to prepare the 'Summary of findings' tables and the Ryan 2016 publication to prepare GRADE ratings (GRADEpro GDT 2015).
Results
Description of studies
Results of the search
See Figure 1.
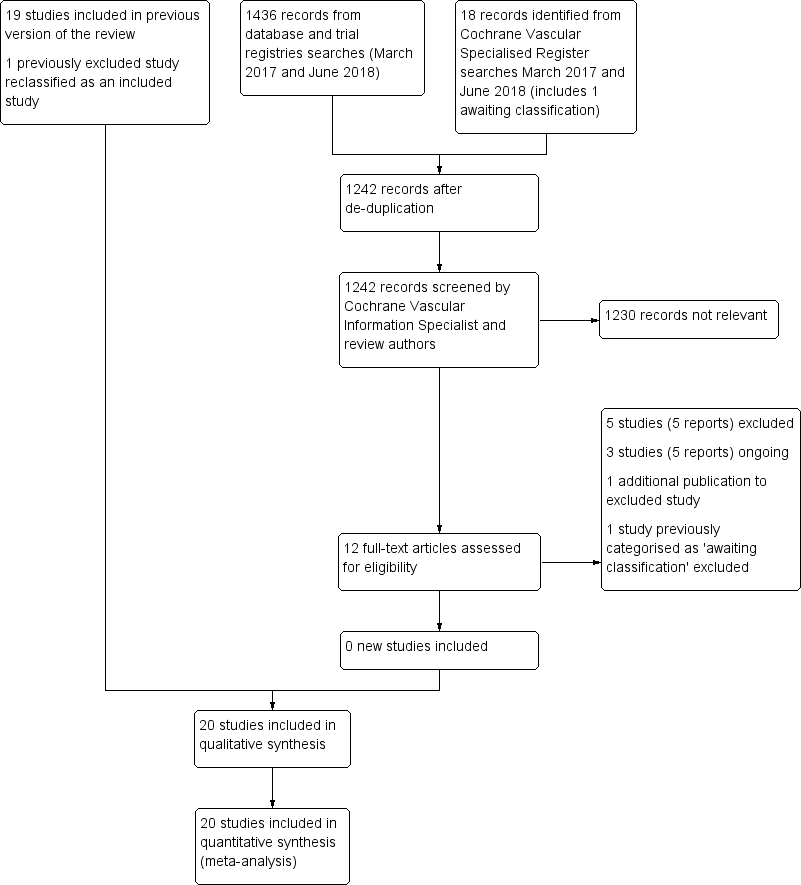
Study flow diagram.
Included studies
We added one additional study in the 2018 update (Mellbring 1986), resulting in a total of 20 RCTs that met the inclusion criteria (Allan 1983; Barnes 1978; Bergqvist 1984; Chin 2009; Fredin 1989; Holford 1976; Hui 1996; Kalodiki 1996; Kierkegaard 1993; Mellbring 1986; Ohlund 1983; Scurr 1977; Scurr 1987; Shirai 1985; Torngren 1980; Tsapogas 1971; Turner 1984; Turpie 1989; Wille‐Jorgensen 1985; Wille‐Jorgensen 1991). See the Characteristics of included studies table. Mellbring 1986 had previously been excluded due to data issues which were overcome for this update.
All trials
The 20 included RCTs provided a total of 2853 analytic units (1681 participants and 1172 legs). Specialties involved:
-
general surgery, 10 trials (Allan 1983; Bergqvist 1984; Holford 1976; Mellbring 1986; Scurr 1977; Scurr 1987; Torngren 1980; Tsapogas 1971; Wille‐Jorgensen 1985; Wille‐Jorgensen 1991);
-
orthopaedics, six trials (Barnes 1978; Chin 2009; Fredin 1989; Hui 1996; Kalodiki 1996; Ohlund 1983);
-
neurosurgery, one trial (Turpie 1989);
-
cardiac surgery, one trial (Shirai 1985);
-
obstetrics and gynaecology, one trial (Turner 1984); and
-
cardiology, one trial (Kierkegaard 1993).
Patients undergoing general surgery formed the largest group (1486 of 2853 analytic units, 52%), followed by patients undergoing orthopaedic surgery (598 of 2853 analytic units, 21%).
All participants in the treatment groups received GCS as the method of DVT prophylaxis, with or without an additional background method of thromboprophylaxis. In nine included trials, participants in the control group received no DVT prophylaxis (Allan 1983; Chin 2009; Holford 1976; Hui 1996; Scurr 1977; Shirai 1985; Tsapogas 1971; Turner 1984; Turpie 1989). In the remaining 11 included trials, where stockings were used over a background method of thromboprophylaxis, participants in the control group received either:
-
dextran 70 (Bergqvist 1984; Fredin 1989; Ohlund 1983);
-
subcutaneous heparin (Torngren 1980; Wille‐Jorgensen 1985; Wille‐Jorgensen 1991);
-
aspirin (Barnes 1978; Kierkegaard 1993);
-
low molecular weight heparin (Kalodiki 1996; Mellbring 1986); or
-
sequential compression (Mellbring 1986; Scurr 1987).
Participants in the treatment group also received GCS.
Methodological differences between trials
In all but one of the RCTs participants were aged 35 years and above. The exception was Turpie 1989, which involved neurosurgical patients aged 16 years and above. One trial involved participants with myocardial infarction who were aged 70 years and over (Kierkegaard 1993), and one trial involved participants undergoing cardiac surgery who were aged 18 to 81 years (Shirai 1985).
All trials used thigh‐length GCS, except Hui 1996, in which one group used thigh‐length stockings and another used knee‐length stockings. We combined these groups for the purposes of this review. One participant in the trial by Turpie 1989 wore knee‐length stockings due to obesity. Five trials did not mention the length of the stockings used (Allan 1983; Chin 2009; Ohlund 1983; Turner 1984; Wille‐Jorgensen 1991).
In all trials GCS were applied either on the day of admission or on the day of operation. This was not critically evaluated on the assumption that all participants were fully mobile prior to surgery. In all but two of the trials the stockings were worn until the day of discharge or until the participants were fully mobile; participants in the remaining two studies wore GCS for 14 days or until discharge (Fredin 1989; Turpie 1989).
Most RCTs (16 of 20 trials) used the radioactive I125 fibrinogen uptake (FUT) assay to screen for DVT postoperatively and phlebography to confirm the diagnosis. One trial used Doppler ultrasonography for screening and phlebography for confirmation of DVT (Barnes 1978); two trials used only phlebography (Hui 1996; Kalodiki 1996); and one trial used only duplex ultrasonography for diagnosis of DVT (Chin 2009).
Excluded studies
We excluded a further six studies in the 2018 update (Barinov 2014; NCT01234064; NCT01935414; Sultan 2014; Wille‐Jorgensen 1986; Zheng 2014), bringing the total number of excluded studies to 56. We excluded studies for the following reasons.
-
Thirty‐five studies did not have appropriate control arms (Ayhan 2013; Barinov 2014; Caprini 1983; Chandhoke 1991; Fasting 1985; Gao 2012; Hansberry 1991; KANT study; Koopmann 1985; Lacut 2005; Lee 1989; Lobastov 2013; Maksimovic 1996; Marston 1995; Maxwell 2000; NCT00333021; NCT01935414; Necioglu 2008; Norgren 1996; Nurmohamed 1996; Orken 2009; Porteous 1989; Rabe 2013; Ryan 2002; Sakon 2012; Serin 2010; Shilpa 2013; Silbersack 2004; Sobieraj‐Teague 2012; Sultan 2014; Vignon 2013; Wille‐Jorgensen 1986; Yang 2009; Zhang 2011; Zheng 2014).
-
Two studies were not designed to assess the effectiveness of stockings in preventing DVT (Ido 1995; Rocca 2012).
-
In six studies DVT was not diagnosed or assessed appropriately (Cohen 2007; Ibegbuna 1997; Manella 1981; Rasmussen 1988; Wilkins 1952; Wilson 1994).
-
Five studies did not use the correct type of GCS (Flanc 1969; Patel 1988; Ramos 1996; Rosengarten 1970; Westrich 1996). Two trials used pneumatic compression (Ramos 1996; Westrich 1996); one trial used Tubigrip (Rosengarten 1970); and one trial used thick elastic compression stockings (Flanc 1969). In one French trial the type of stocking used was not clear (Patel 1988).
-
Three studies did not meet the inclusion criteria of this review based on types of patients included (Belcaro 1993; CLOTS 2009; Muir 2000). One study appeared to evaluate people with recurrent DVT in the community (Belcaro 1993). Two studies evaluated people with acute stroke (CLOTS 2009; Muir 2000); these trials have been included in a separate Cochrane Review focusing on people with acute stroke (Naccarato 2010). The rationale for this is as follows: stockings reduce the cross‐sectional area of the deep veins, making the calf muscle pump more effectively and thereby improving blood flow. The authors of the CLOTS trial have suggested that severe leg weakness in people with acute stroke may therefore account for the ineffectiveness of stockings in this patient group (CLOTS 2009).
-
Five studies were only published as abstracts, making it difficult to accurately assess their methodology and to extract data (Bolton 1978; Brunkwall 1991; NCT01234064; Perkins 1999; Sultan 2011). Further information provided by the authors of the Sultan 2011 trial showed that not all participants in this trial had been hospitalised, therefore it did not meet our inclusion criteria.
See the Characteristics of excluded studies table for further details.
Studies awaiting classification and ongoing studies
We could not adequately assess the study design for Celebi 2001 as the report was not published in English, therefore we have categorised it as awaiting classification. See the Characteristics of studies awaiting classification table for further details.
We identified three ongoing studies (ChiCTR1800014257; GAPS; IRCT2017080935594N1), of which two trials are scheduled to conclude in 2019 (GAPS; IRCT2017080935594N1), and one trial in late 2020 (ChiCTR1800014257). See Characteristics of ongoing studies table for further details.
Risk of bias in included studies
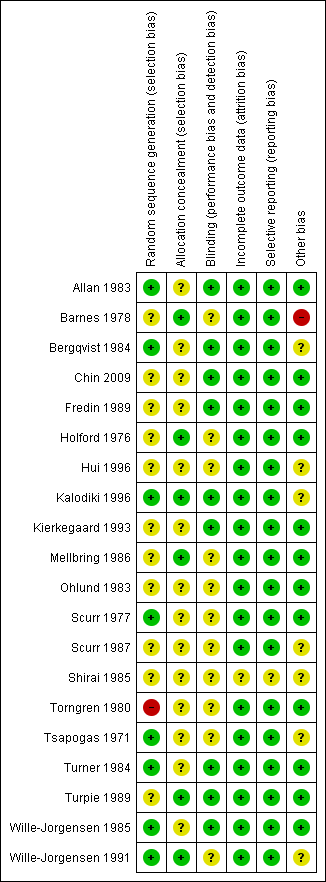
Details regarding risk of bias are provided in the Characteristics of included studies table and are represented in Figure 2 and Figure 3.
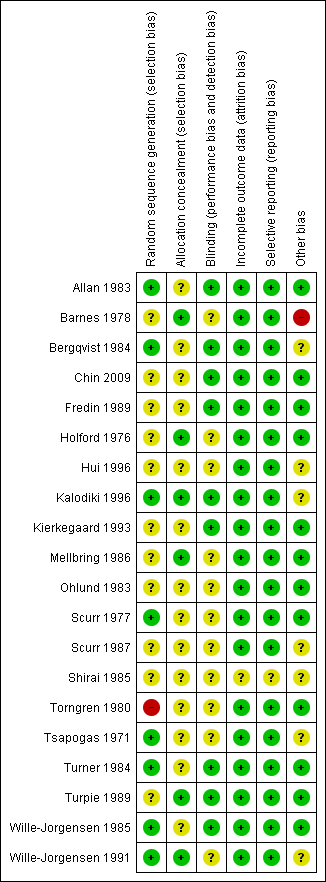
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Allocation
Nine trials described the method of randomisation of participants to treatment and control groups, which was done using:
-
random number tables in six trials (Allan 1983; Bergqvist 1984; Tsapogas 1971; Turner 1984; Wille‐Jorgensen 1985; Wille‐Jorgensen 1991);
-
coin toss in one trial (Scurr 1977);
-
consecutively numbered boxes in one trial (Kalodiki 1996);
-
date of birth in one trial (Torngren 1980), which we deemed to be an inadequate method of randomisation, and so we judged the study to be at high risk of bias.
In Tsapogas 1971, participants were randomised using a random allocation table (low risk of bias). However, there was a discrepancy between the numbers of participants randomised to the treatment and control groups, therefore we deemed this trial to be at unclear risk of bias likely due to unclear allocation concealment.
The method of randomisation was not mentioned in the remaining 11 included trials, so these trials were judged to be at unclear risk of bias (Barnes 1978; Chin 2009; Fredin 1989; Holford 1976; Hui 1996; Kierkegaard 1993; Mellbring 1986; Ohlund 1983; Scurr 1987; Shirai 1985; Turpie 1989). In Hui 1996, participants were randomised in a ratio of 1:1 in the thigh‐length GCS group and 1:4 in the knee‐length GCS group. The control group of the thigh‐length GCS group was also used as the control for the knee‐length GCS group in this trial.
Of note, Chin 2009 was the only included trial published after the publication of the CONSORT statement in 1996 (CONSORT 1996). Despite this, the Chin 2009 trial report did not include a power calculation and did not report the method of randomisation and use of allocation concealment, as advised in the CONSORT statement, suggesting risk of bias.
There was no mention of allocation concealment in 14 of the 20 RCTs judged as at unclear risk of bias. The remaining six studies used sealed envelopes to conceal the allocation of participants to the treatment and control groups and were judged as at low risk of bias (Barnes 1978; Holford 1976; Kalodiki 1996; Mellbring 1986; Turpie 1989; Wille‐Jorgensen 1991).
Blinding
It is inherently difficult to ensure adequate blinding for patients who wear stockings and those who do not. In eight trials, the radiologist reporting the scan results was unaware of whether the participant, or their leg, belonged to the treatment or control group, so these were judged as at low risk of bias (Allan 1983; Bergqvist 1984; Chin 2009; Kalodiki 1996; Turner 1984; Turpie 1989; Wille‐Jorgensen 1985; Wille‐Jorgensen 1991). In two trials the results of the studies were analysed without knowledge of the type of prophylaxis, so these were also judged as at low risk of bias (Fredin 1989; Kierkegaard 1993). We judged the remaining 10 studies as at unclear risk of performance and detection bias (Bergqvist 1984; Holford 1976; Hui 1996; Mellbring 1986; Ohlund 1983; Scurr 1977; Scurr 1987; Shirai 1985; Torngren 1980; Tsapogas 1971).
Incomplete outcome data
In seven trials, results for all included participants were analysed (Barnes 1978; Chin 2009; Kierkegaard 1993; Scurr 1987; Tsapogas 1971; Turner 1984; Turpie 1989). In the remaining trials, participants lost to follow‐up were accounted for, with some participants excluded postrandomisation due to failure to comply with wearing the GCS because they found them uncomfortable. One trial did not report participant attrition during the study period (Shirai 1985), but this report could not be accurately appraised as it was published in Japanese and was therefore found to be at unclear risk of bias. We judged the remainder of the studies as at low risk of bias.
Selective reporting
All included studies reported the incidence of DVTs as stated in their aims. Visual inspection of the funnel plot showed that all included trials came within the expected confidence intervals, though there was a suggestion of minimal publication bias (Figure 4). We found one study to be at unclear risk of bias as it was published in Japanese and we were unable to accurately appraise this report (Shirai 1985). The remainder of the studies were judged as at low risk of bias.

Funnel plot of comparison: Incidence of DVT with stockings and without stockings (all specialties).
Other potential sources of bias
None of the trials stratified participants according to DVT risk level. However, our own analysis of the papers indicated that all participants were in either moderate‐ or high‐risk groups.
Six trials obtained funding or support from pharmaceutical companies or manufacturers of GCS, and we were unclear if this could have influenced the studies. These companies included The Kendall Company (Barnes 1978; Scurr 1987; Wille‐Jorgensen 1991), Beiersdorf AB (Bergqvist 1984), Brevet Hospital Products (Hui 1996), and Rhone‐Poulenc Rorer (Kalodiki 1996).
In addition, Barnes 1978 was terminated early as it was deemed unjustifiable to continue after revealing a major incidence of DVT amongst participants who did not wear stockings; we assessed this study as being at high risk of bias.
In one trial (Tsapogas 1971), participants in the treatment group were given an additional recommendation regarding exercise that was not given to the control group. It is possible this influenced the risk of thrombosis, therefore we judged this study as at unclear risk of bias.
One trial was published in Japanese (Shirai 1985), which made it difficult to accurately appraise the study design.
Effects of interventions
See: Summary of findings for the main comparison Graduated compression stockings for prevention of deep vein thrombosis ‐ all patients; Summary of findings 2 Graduated compression stockings for prevention of deep vein thrombosis in surgical patients; Summary of findings 3 Graduated compression stockings for prevention of deep vein thrombosis in medical patients
The results of the review should be interpreted with caution, paying particular attention to the detailed notes in the Description of studies section, as these may have influenced the analysis due to variations within the included trials, for example the use of the opposite limb as the control, differing background prophylactic methods used, and the age difference in some of the trials. However, in the majority of the included trials a statistically significant difference between the treated participants (those that used GCS) and the control group (those that did not use GCS) was demonstrated in the incidence of DVT (primary outcome).
Incidence of DVT
In the 2014 update, we merged trials assessing the effectiveness of GCS as the sole method of prophylaxis together with trials using a background method of thromboprophylaxis for all participants in addition to the use of GCS in the treatment group. This resulted in a total of 2853 analytic units (1681 individual participants and 1172 individual legs) in the meta‐analysis (Analysis 1.1). In the treatment group (GCS), 134 of the 1445 units developed DVT in comparison to 290 of the 1408 units in the control group (no GCS): Peto's odds ratio (OR) of 0.35 (95% confidence interval (Cl) 0.28 to 0.43; 2853 units; 20 studies; P < 0.001; high‐quality evidence). This amounted to a 9% incidence of DVT in the treatment group in comparison to a 21% incidence in the control group.
The I2 statistic for this analysis suggested 10% heterogeneity, with P = 0.33 (Analysis 1.1). This was supported by the corresponding forest plot (Figure 4), using Peto's ORs, which showed that results for all studies were within or on the 95% CI, suggesting minimal publication bias.
Subgroup analysis by specialty
We performed subgroup analysis based upon the specialty under which the participants were managed (Figure 5). There was no significant difference between specialty subgroups regarding the effectiveness of stockings in reducing the incidence of DVT (P = 0.15).

Number of analytic units from each specialty included in the meta‐analysis.
The majority of participants were general surgical patients, accounting for 1486 of 2853 units (52%). Amongst this cohort, the incidence of DVT was 52 of 741 (7%) in the treatment group and 148 of 745 (20%) in the control group (Peto OR 0.30, 95% CI 0.22 to 0.41; 1486 units; 10 studies; high‐quality evidence) (Allan 1983; Bergqvist 1984; Holford 1976; Mellbring 1986; Scurr 1977; Scurr 1987; Torngren 1980; Tsapogas 1971; Wille‐Jorgensen 1985; Wille‐Jorgensen 1991).
Participants undergoing orthopaedic surgery accounted for 598 of 2853 units (21%) (Barnes 1978; Chin 2009; Fredin 1989; Hui 1996; Kalodiki 1996; Ohlund 1983). Amongst participants undergoing orthopaedic surgery, 70 of 314 units (22%) in the treatment group and 97 of 284 units (34%) in the control group developed DVT (Peto OR 0.47, 95% CI 0.32 to 0.68; 598 units; 6 studies; high‐quality evidence) (Analysis 1.1).
The four remaining trials on participants from other specialties provided small sample sizes for each of the specialities (Kierkegaard 1993; Shirai 1985; Turner 1984; Turpie 1989). Combining the results favoured the use of stockings (Peto OR 0.28, 95% CI 0.16 to 0.48; 769 participants; 4 studies; moderate‐quality evidence). Of note, only one trial considered medical patients (Kierkegaard 1993), making it difficult to confidently judge the effect of stockings in these participants (Analysis 1.1).
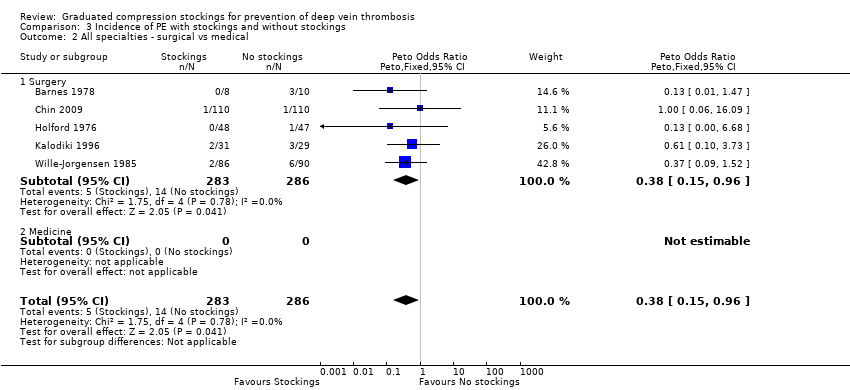
Subgroup analysis to compare all surgery versus medical did not reveal any differences in DVT incidence (P = 0.15). Combining results from 19 trials focusing on surgical patients, 134 of 1365 units (9.8%) developed DVT in the GCS group compared to 282 of 1328 units (21.2%) in the control group. The Peto OR was 0.35 (95% CI 0.28 to 0.44; P < 0.001; high‐quality evidence), with an overall effect favouring treatment with GCS (Analysis 1.2).
Based on the results from one trial focusing on medical patients admitted following acute myocardial infarction (Kierkegaard 1993), 0 of 80 legs (0%) developed DVT in the GCS group compared to 8 of 80 legs (10%) in the control group. The Peto OR was 0.12 (95% CI 0.03 to 0.51; P = 0.004; low‐quality evidence), with an overall effect favouring treatment with GCS (Analysis 1.2).
Incidence of proximal DVT
Proximal DVTs, which occur in the popliteal, femoral, and iliac veins, are considered to be of greatest clinical significance as they are more likely to embolise to the pulmonary veins and can thereby potentially result in fatal PE. We therefore assessed the incidence of proximal DVT in the two experimental arms of the included trials. Eight trials provided data for the incidence of proximal DVT amongst 1035 included units (Barnes 1978; Bergqvist 1984; Chin 2009; Fredin 1989; Kalodiki 1996; Kierkegaard 1993; Scurr 1987; Turpie 1989). The incidence of proximal DVT was 7 of 517 units (1%) in the treatment group and 28 of 518 units (5%) in the control group (Peto OR 0.26, 95% CI 0.13 to 0.53; 1035 units; 8 studies; P < 0.001; moderate‐quality evidence). There was no significant difference between surgical subgroup specialities regarding the effectiveness of stockings in reducing the incidence of proximal DVT (P = 0.79) (Analysis 2.1).
We carried out subgroup analysis investigating differences between surgical and medical patients. Results from seven included studies evaluating surgical patients showed the incidence of proximal DVT as 7 of 437 units (1.6%) in the GCS group and 28 of 438 units (6.4%) in the control group (Barnes 1978; Bergqvist 1984; Chin 2009; Fredin 1989; Kalodiki 1996; Scurr 1987; Turpie 1989). The Peto OR was 0.26 (95% CI 0.13 to 0.53; 875 units; P < 0.001; moderate‐quality evidence), with an overall effect favouring treatment with GCS. We downgraded the evidence for proximal DVT due to low event rate (imprecision). No events of proximal DVT were recorded in the one study involving medical patients (Analysis 2.2) (Kierkegaard 1993).
Incidence of PE
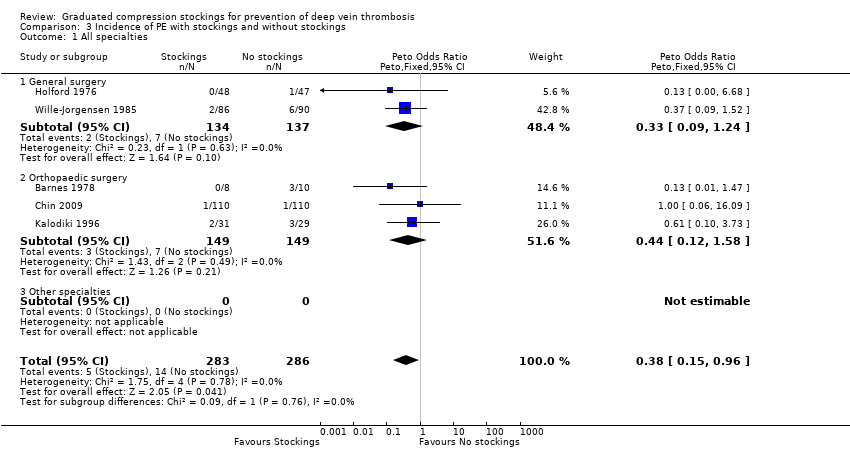
Five trials, all based on surgical patients, provided data for the incidence of PE amongst 569 included participants (Barnes 1978; Chin 2009; Holford 1976; Kalodiki 1996; Wille‐Jorgensen 1985). Routine screening for PE was only conducted in two of these studies using perfusion‐ventilation scintigraphy (Holford 1976; Kalodiki 1996). This method was used to confirm clinically apparent PE in the remaining studies, except in one trial where PE was diagnosed at autopsy (Turpie 1989). The incidence of PE was 5 of 283 participants (2%) in the treatment group and 14 of 286 participants (5%) in the control group (Peto OR 0.38, 95% CI 0.15 to 0.96; 569 participants; 5 studies; P = 0.04; low‐quality evidence). These results should be interpreted with caution in light of the aforementioned limitations in reporting of the incidence of PE in the included trials. (Analysis 3.1).
One further trial reported that one participant was diagnosed with PE at autopsy but did not state to which group this participant belonged (Turpie 1989). However, the cause of death of this participant was found to be cerebral oedema. Two further trials reported a cumulative incidence of three cases of PE but did not specify to which group these participants belonged (Bergqvist 1984; Fredin 1989). Torngren 1980 reported that no participants suffered fatal PE. There was no significant difference between subgroups regarding the effectiveness of stockings in reducing the incidence of PE (P = 0.76). (Analysis 3.1).
Adverse effects and complications from GCS
Seven of 20 trials mentioned the incidence of adverse effects, but none of the trials stated to which groups the participants belonged (Bergqvist 1984; Chin 2009; Fredin 1989; Kalodiki 1996; Kierkegaard 1993; Torngren 1980; Wille‐Jorgensen 1991).
Kierkegaard 1993 reported that some participants experienced postphlebitic changes. Four trials mentioned the incidence of bleeding associated with the background antithrombotic measure used (Bergqvist 1984; Fredin 1989; Kalodiki 1996; Wille‐Jorgensen 1991). Kalodiki 1996 reported no difference in haemorrhagic complications between the treatment and control groups. One trial reported that none of the participants showed any signs of postoperative haemorrhage or side effects (Torngren 1980). Similarly, one further trial reported no adverse events related to the use of GCS (Chin 2009).
Two further trials reported participants' complaints (Hui 1996; Turpie 1989). In one trial, 23% of participants wearing above‐knee stockings and 16% of participants wearing below‐knee stockings found the stockings too uncomfortable and requested their removal (Hui 1996). Ambulant patients in another trial reported disturbance as the stockings fell down easily, which was likely to be due to improper fitting (Turpie 1989).
Discussion
Summary of main results
Meta‐analysis of the 20 included RCTs showed high‐quality evidence that application of GCS decreased the occurrence of DVT in hospitalised patients (Analysis 1.1).
Eight trials reported the incidence of proximal DVT (Barnes 1978; Bergqvist 1984; Chin 2009; Fredin 1989; Kalodiki 1996; Kierkegaard 1993; Scurr 1987; Turpie 1989), and five trials reported the incidence of PE (Barnes 1978; Chin 2009; Holford 1976; Kalodiki 1996; Wille‐Jorgensen 1985). A lower incidence of proximal DVT (moderate‐quality evidence) and PE (low‐quality evidence) was noted amongst participants fitted with GCS (Analysis 2.1; Analysis 3.1), however the low incidence rate coupled with a relatively small sample size limits the power of these meta‐analyses, thereby making it difficult to confidently infer the effectiveness of GCS in preventing these outcomes.
Few adverse events were reported. In one trial some participants removed their stockings earlier than they should have done, presumably due to discomfort (Hui 1996). No other trials reported complications associated with wearing stockings. In one trial some participants developed postphlebitic changes (Kierkegaard 1993). In contrast, four studies mentioned bleeding complications related to the associated use of heparin, dextran, or aspirin, but the numbers were too small and not uniform enough to make any definitive judgement (Bergqvist 1984; Fredin 1989; Kalodiki 1996; Mellbring 1986; Wille‐Jorgensen 1991).
Overall completeness and applicability of evidence
This review included predominantly patients undergoing general surgical and orthopaedic surgical procedures (Figure 5), and thus provides good evidence for the use of GCS amongst these patient groups. However, only one RCT included hospitalised medical patients, and no trial included participants deemed to have low risk of developing DVT (Kierkegaard 1993), hence we cannot comment on the benefits of using GCS in these patient groups.
The available evidence was based predominantly on the use of above‐knee stockings. Only Hui 1996 looked at the difference between thigh‐length GCS versus no stockings and knee‐length GCS versus no stockings. In six trials, the length of the GCS used was not made explicit (Allan 1983; Chin 2009; Kierkegaard 1993; Ohlund 1983; Turner 1984; Wille‐Jorgensen 1991). The numbers were too small to draw any conclusions as to the efficacy of DVT prevention based on the length of the stockings used. This is not the remit of this review but is the subject of another Cochrane Review, which reported insufficient high‐quality evidence to determine whether knee‐length and thigh‐length stockings differ in their effectiveness in reducing the incidence of DVT in hospitalised patients (Sajid 2012).
Duration of routine follow‐up was 7 to 14 days, or until discharge, with a single study routinely screening for symptomatic DVT at 30‐day follow‐up (Chin 2009). This contrasts with epidemiological data, which demonstrates the greatest risk of postoperative DVT at four to six weeks after surgery (Sweetland 2009). The lower duration routine follow‐up in the included studies does not cover this period of increased DVT risk and thus fails to accurately estimate the true magnitude of the effect of GCS in preventing DVT following discharge into the community.
None of the RCTs were uniform in detailing or recommending the duration of time that GCS should be worn postoperatively, that is until discharge, mobilisation, or the next clinic visit. This aspect is important because we know from clinical experience that DVT can still occur at home after discharge, and there have been a number of incidences of death after discharge that were due to DVT and PE. This is further supported by the results from the Million Women Study (Sweetland 2009).
Some of these areas of uncertainty have been addressed in the published protocol of an ongoing study (GAPS), where hospitalised surgical patients will receive thromboprophylaxis (low molecular weight heparin (LMWH) only versus LMWH and GCS), with the primary outcome as incidence of DVT at 90‐day follow‐up. In light of the increased use of chemoprophylaxis for DVT, this trial aims to specifically evaluate the additional benefit of GCS over LMWH as thromboprophylaxis. We will evaluate the final report of this study for inclusion in this review when it is available.
Quality of the evidence
We have included 20 RCTs of good methodological quality providing 2853 analytic units in this meta‐analysis to determine the effectiveness of the use of GCS in hospitalised patients (Analysis 1.1). This sample size provides high‐quality evidence to support the use of GCS to prevent DVT in the clinical setting, especially amongst surgical patients, since most included participants underwent either general surgical or orthopaedic surgical procedures (Figure 5). Moderate‐quality evidence suggests that GCS may prevent proximal DVT; we downgraded the quality of the evidence due to imprecision. We found low‐quality evidence for GCS in preventing PE, downgrading the evidence as routine screening for PE was done in only two of the five RCTs reporting on this outcome, and results were inconsistent. Only one included trial considered hospitalised medical patients, making it difficult to form a judgement regarding this subgroup of patients (Kierkegaard 1993).
Furthermore, small sample sizes limited subgroup analysis based on type of procedure, length of stockings used, or type of background prophylaxis. We were unable to confidently assess the variation in effectiveness of GCS over these parameters.
Potential biases in the review process
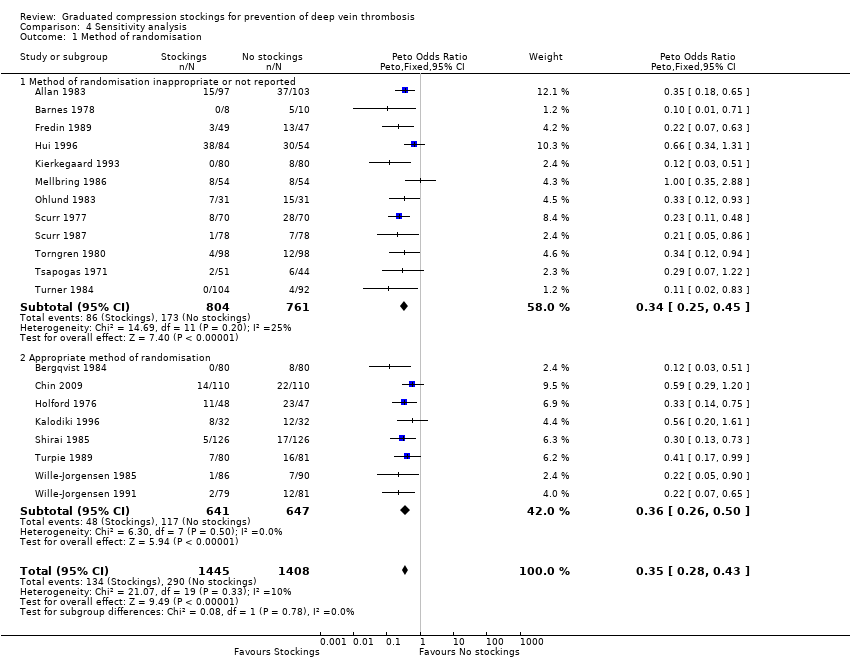
Interestingly, all but one of the 20 included trials were conducted before the CONSORT guidelines for reporting RCTs were published (CONSORT 1996). Most importantly, 11 of 20 trials either did not report sufficiently or used an inappropriate method of randomisation (see the Risk of bias in included studies section). We therefore performed a sensitivity analysis to assess the associated potential risk of bias, which found no significant difference in the effectiveness of stockings in reducing the incidence of DVT amongst trials that reported an appropriate method of randomisation and those that did not (P = 0.78, Analysis 4.1).
Seven of the 20 included RCTs involved the use of the other leg as the control (Bergqvist 1984; Kierkegaard 1993; Mellbring 1986; Scurr 1977; Scurr 1987; Shirai 1985; Torngren 1980). It is possible that GCS applied to one leg could have an effect on the other leg of the same patient (Spiro 1970), although there is no clear evidence for this. If this is true, it may bias the results of these studies. None of the included studies randomising individual legs reported the use of a matched or paired analysis to address statistical bias due to ignoring the pairing. This could result in these studies receiving too little weight in the meta‐analysis due to wider confidence intervals, and thereby possibly disguising clinically important heterogeneity (Higgins 2011). However, since the studies are underweighted rather than overweighted, the analysis is conservative. Despite such concerns, all these trials have demonstrated that GCS reduced the risk of DVT compared to when GCS were not applied. A sensitivity analysis performed to assess this further found stockings to be effective in reducing the incidence of DVT irrespective of whether the unit of randomisation was individual legs or individual participants (Analysis 4.2). In other words, there was no difference in the effectiveness of stockings in reducing the incidence of DVT irrespective of the unit of randomisation (P = 0.24).
As part of a previous update, we merged trials using stockings as the sole method of thromboprophylaxis and those using stockings over a background method of thromboprophylaxis, thereby introducing a potential bias. However, both these groups of trials demonstrated a reduced incidence of DVT, with or without a background method of thromboprophylaxis (Analysis 4.3), with no clear difference in the magnitude of effect (P = 0.25). It must be stressed that results for the use of GCS over a background method of thromboprophylaxis should be interpreted with some caution, as this group of trials was heterogeneous. Background prophylaxis varied between dextran 70, heparin, aspirin, and sequential compression. We could not assess the extent of influence of individual background prophylaxis since further grouping would have reduced the number of participants so much that the data would not be valid.
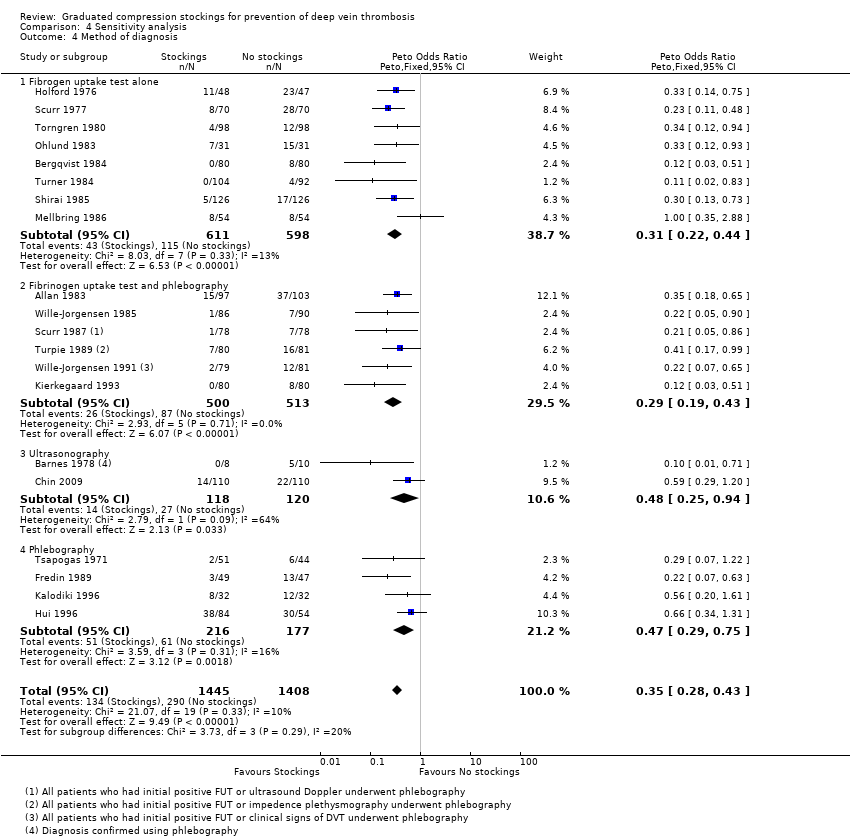
Flordal 1995 and Lensing 1993 have previously highlighted poor efficacy of using radioactive I125 fibrinogen uptake test (FUT) as the only diagnostic modality for DVT. In this review, eight of the 20 included trials relied solely on FUT to diagnose DVT. We therefore performed a sensitivity analysis to assess the associated potential risk of bias by the inclusion of these studies (Analysis 4.4), which found that results of trials using phlebography to confirm the diagnosis of DVT following a positive FUT and those using other modalities for the diagnosis of DVT (ultrasonography, or phlebography alone) also strongly favoured the use of stockings in diminishing the risk of DVT. There was no significant difference in the effectiveness of stockings in reducing the incidence of DVT between subgroups of diagnostic modalities (P = 0.29).
After excluding stroke patients, we identified only one RCT that involved a medical specialty, which was in patients following a myocardial infarction (Kierkegaard 1993). It was thus difficult to confidently judge the effectiveness of GCS in preventing DVT in medical patients, and further trials are required in this cohort of patients.
It is important to note that visual interpretation of the funnel plot demonstrated a borderline risk of publication bias in assessing the effectiveness of stockings in preventing DVT (Figure 4; Analysis 1.1). However, we noted no clear evidence of publication bias in meta‐analyses of the incidence of proximal DVT and PE (Figure 6; Figure 7).

Funnel plot of comparison: Incidence of proximal DVT with stockings and without stockings (all specialties).
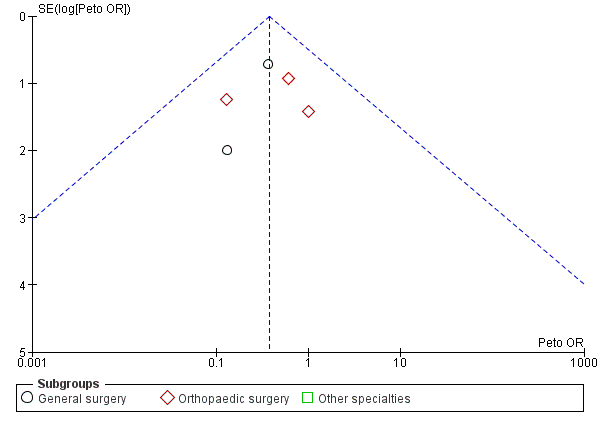
Funnel plot of comparison: Incidence of PE with stockings and without stockings (all specialties).
Agreements and disagreements with other studies or reviews
Results from this review are comparable to those of a previous health technology assessment (Roderick 2005), which found a 66% risk reduction with the application of GCS and a 60% risk reduction when GCS were used on a background of another prophylactic method. The variation in the degree of risk reduction reported by that review, as compared to our results, may be because their analyses were based upon the number of participants originally randomised in the included trials and included participants who were later excluded (i.e. an intention‐to‐treat analysis). Furthermore, a number of trials included in Roderick 2005 did not meet our inclusion criteria.
The National Institute for Health and Care Excellence (NICE) has published guidance for reducing the risk of venous thromboembolism amongst hospitalised patients (NICE 2010). Their recommendations are consistent with our finding that GCS are more effective than no prophylaxis. However, NICE recommends that GCS should not be prescribed to patients admitted for stroke; this was based primarily on the CLOTS 2009 trial in which large proportions of patients were prescribed aspirin, which may have influenced the results. It also raises the issue of lack of evidence concerning the use of mechanical prophylaxis in medical patients.

Study flow diagram.
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
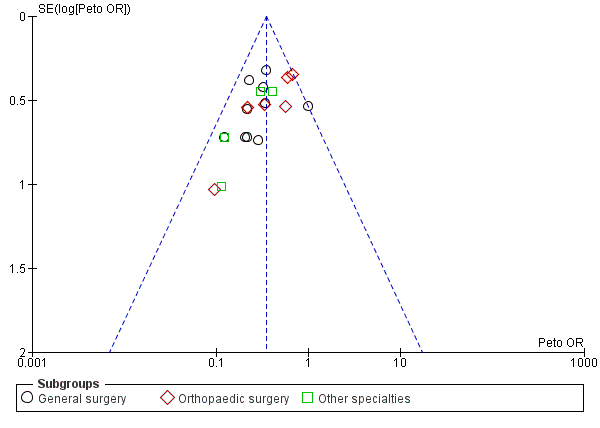
Funnel plot of comparison: Incidence of DVT with stockings and without stockings (all specialties).
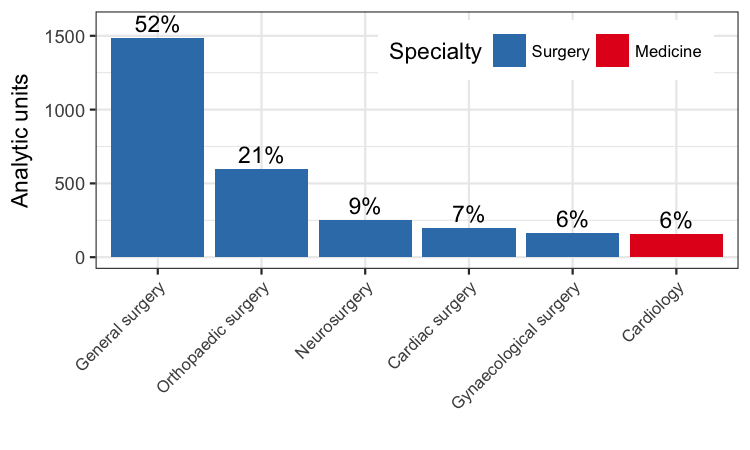
Number of analytic units from each specialty included in the meta‐analysis.
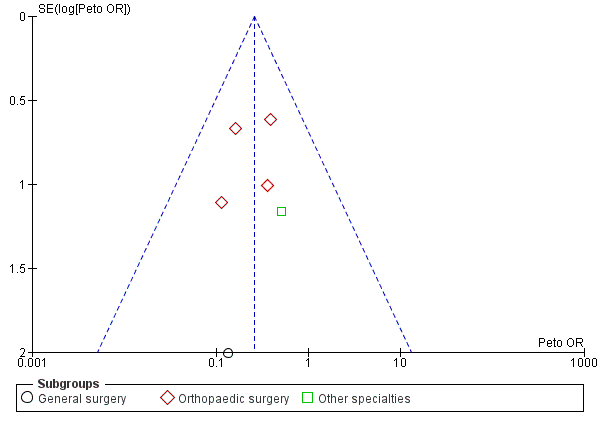
Funnel plot of comparison: Incidence of proximal DVT with stockings and without stockings (all specialties).
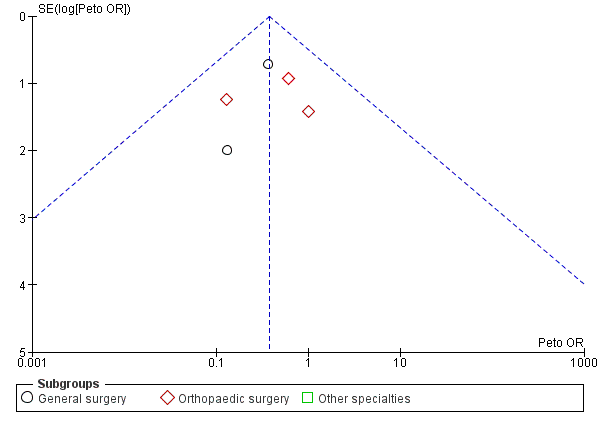
Funnel plot of comparison: Incidence of PE with stockings and without stockings (all specialties).

Comparison 1 Incidence of DVT with stockings and without stockings, Outcome 1 All specialties.

Comparison 1 Incidence of DVT with stockings and without stockings, Outcome 2 All specialties ‐ surgical vs medical.

Comparison 2 Incidence of proximal DVT with stockings and without stockings, Outcome 1 All specialties.

Comparison 2 Incidence of proximal DVT with stockings and without stockings, Outcome 2 All specialties ‐ surgical vs medical.
Comparison 3 Incidence of PE with stockings and without stockings, Outcome 1 All specialties.
Comparison 3 Incidence of PE with stockings and without stockings, Outcome 2 All specialties ‐ surgical vs medical.
Comparison 4 Sensitivity analysis, Outcome 1 Method of randomisation.

Comparison 4 Sensitivity analysis, Outcome 2 Unit of analysis for randomisation.

Comparison 4 Sensitivity analysis, Outcome 3 Use of background method of thromboprophylaxis.
Comparison 4 Sensitivity analysis, Outcome 4 Method of diagnosis.
| Graduated compression stockings for prevention of deep vein thrombosis | ||||||
| Patient or population: Hospitalised patients1 | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of units/ participants2 | Quality of the evidence | Comments | |
| Risk with no GCS | Risk with GCS | |||||
| Deep vein thrombosis (DVT) Follow‐up: 7 to 14 days, or until discharge | Study population | OR 0.35 | 2853 | ⊕⊕⊕⊕ | Graduated compression stockings reduce the incidence of DVT in hospitalised patients. However, studies did not routinely distinguish between symptomatic and asymptomatic DVTs. | |
| 206 per 1000 | 83 per 1000 | |||||
| Proximal DVT Follow‐up: 7 to 14 days, or until discharge | Study population | OR 0.26 | 1035 | ⊕⊕⊕⊝ | Graduated compression stockings probably reduce the incidence of proximal DVT in hospitalised patients. There was a relatively low event rate overall, and studies did not routinely distinguish between symptomatic and asymptomatic proximal DVTs. | |
| 54 per 1000 | 15 per 1000 | |||||
| Pulmonary embolism Follow‐up: 7 to 30 days | Study population | OR 0.38 | 569 | ⊕⊕⊝⊝ | Graduated compression stockings may slightly reduce the incidence of pulmonary embolism in hospitalised patients. Pulmonary embolism was not routinely assessed in most included studies, and the overall event rate was very low. | |
| 49 per 1000 | 19 per 1000 | |||||
| Adverse effects and complications Follow‐up: until discharge | See comment | ‐ | ‐ | ‐ | Some participants removed stockings due to discomfort or poor fitting, however adverse effects and complications were not routinely reported quantitatively in the included studies. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Included patients admitted for surgical procedures (including abdominal, orthopaedic, neurosurgical, gynaecological surgery) and medical illness (acute myocardial infarction). | ||||||
| Graduated compression stockings for prevention of deep vein thrombosis | ||||||
| Patient or population: Patients hospitalised for surgical procedures1 | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of units/ participants2 | Quality of the evidence | Comments | |
| Risk with no GCS | Risk with GCS | |||||
| Deep vein thrombosis (DVT) Follow‐up: 7 to 14 days, or until discharge | Study population | OR 0.35 (0.28 to 0.44) | 2693 (19 RCTs) | ⊕⊕⊕⊕ | Graduated compression stockings reduce the incidence of DVT in hospitalised surgical patients. However, studies did not routinely distinguish between symptomatic and asymptomatic DVTs. | |
| 212 per 1000 | 86 per 1000 | |||||
| Proximal DVT Follow‐up: 7 to 14 days, or until discharge | Study population | OR 0.26 (0.13 to 0.53) | 875 (7 RCTs) | ⊕⊕⊕⊝ | Graduated compression stockings probably reduce the incidence of proximal DVT in hospitalised surgical patients. However, studies did not routinely distinguish between symptomatic and asymptomatic proximal DVTs. There was a relatively low event rate overall. | |
| 64 per 1000 | 17 per 1000 | |||||
| Pulmonary embolism Follow‐up: 7 to 30 days, or until discharge | Study population | OR 0.38 (0.15 to 0.96) | 569 (5 RCTs) | ⊕⊕⊝⊝ | Graduated compression stockings may slightly reduce the incidence of pulmonary embolism in hospitalised surgical patients. However, pulmonary embolism was not routinely assessed in most included studies, and the overall event rate was very low. | |
| 49 per 1000 | 19 per 1000 | |||||
| Adverse effects and complications Follow‐up: until discharge | See comment | ‐ | ‐ | ‐ | Some participants removed stockings due to discomfort or poor fitting, however adverse effects and complications associated with wearing GCS were not routinely reported quantitatively in the included studies. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Included patients admitted for surgical procedures (including abdominal, orthopaedic, neurosurgical, gynaecological surgery). | ||||||
| Graduated compression stockings for prevention of deep vein thrombosis | ||||||
| Patient or population: Patients hospitalised following acute myocardial infarction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of units | Quality of the evidence | Comments | |
| Risk with no GCS | Risk with GCS | |||||
| Deep vein thrombosis (DVT) Follow‐up: 8 days or discharge or until development of DVT | Study population | OR 0.12 (0.03 to 0.51) | 160 | ⊕⊕⊝⊝ | Graduated compression stockings may reduce incidence of DVT in hospitalised medical patients. However, results are based on a single study on medical patients hospitalised following acute myocardial infarction (Kierkegaard 1993). | |
| 100 per 1000 | 13 per 1000 | |||||
| Proximal DVT Follow‐up: 8 days or discharge or until development of DVT | Study population | Not estimable | 160 (1 RCT) | ‐ | None of the participants in either group of this single RCT with a small sample size developed proximal DVT. | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Pulmonary embolism | See comment | ‐ | ‐ | ‐ | No studies reported on this outcome. There is paucity of evidence to evaluate the effect of GCS on reducing incidence of pulmonary embolism in hospitalised medical patients. | |
| Adverse effects and complications | See comment | ‐ | 160 (1 RCT) | ‐ | There are rare reports of post‐thrombotic changes in participants who developed DVT in the single included RCT. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded the quality of the evidence by two steps as there was only one study and a low event rate in the GCS group (imprecision). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All specialties Show forest plot | 20 | 2853 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.28, 0.43] |
| 1.1 General surgery | 10 | 1486 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.30 [0.22, 0.41] |
| 1.2 Orthopaedic surgery | 6 | 598 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.47 [0.32, 0.68] |
| 1.3 Other specialties | 4 | 769 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.16, 0.48] |
| 2 All specialties ‐ surgical vs medical Show forest plot | 20 | 2853 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.28, 0.43] |
| 2.1 Surgery | 19 | 2693 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.28, 0.44] |
| 2.2 Medicine | 1 | 160 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.03, 0.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All specialties Show forest plot | 8 | 1035 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.26 [0.13, 0.53] |
| 1.1 General surgery | 2 | 316 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 6.82] |
| 1.2 Orthopaedic surgery | 4 | 398 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.25 [0.12, 0.53] |
| 1.3 Other specialties | 2 | 321 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.52 [0.05, 5.03] |
| 2 All specialties ‐ surgical vs medical Show forest plot | 8 | 1035 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.26 [0.13, 0.53] |
| 2.1 Surgery | 7 | 875 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.26 [0.13, 0.53] |
| 2.2 Medicine | 1 | 160 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All specialties Show forest plot | 5 | 569 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.15, 0.96] |
| 1.1 General surgery | 2 | 271 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.33 [0.09, 1.24] |
| 1.2 Orthopaedic surgery | 3 | 298 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.44 [0.12, 1.58] |
| 1.3 Other specialties | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 All specialties ‐ surgical vs medical Show forest plot | 5 | 569 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.15, 0.96] |
| 2.1 Surgery | 5 | 569 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.15, 0.96] |
| 2.2 Medicine | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Method of randomisation Show forest plot | 20 | 2853 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.28, 0.43] |
| 1.1 Method of randomisation inappropriate or not reported | 12 | 1565 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.34 [0.25, 0.45] |
| 1.2 Appropriate method of randomisation | 8 | 1288 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.36 [0.26, 0.50] |
| 2 Unit of analysis for randomisation Show forest plot | 20 | 2853 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.28, 0.43] |
| 2.1 Individual participants | 13 | 1681 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.29, 0.49] |
| 2.2 Individual legs | 7 | 1172 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.19, 0.42] |
| 3 Use of background method of thromboprophylaxis Show forest plot | 20 | 2853 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.28, 0.43] |
| 3.1 Trials without background thromboprophylaxis | 9 | 1497 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.29, 0.50] |
| 3.2 Trials with background thromboprophylaxis | 11 | 1356 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.29 [0.20, 0.42] |
| 4 Method of diagnosis Show forest plot | 20 | 2853 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.28, 0.43] |
| 4.1 Fibrogen uptake test alone | 8 | 1209 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.31 [0.22, 0.44] |
| 4.2 Fibrinogen uptake test and phlebography | 6 | 1013 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.29 [0.19, 0.43] |
| 4.3 Ultrasonography | 2 | 238 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.48 [0.25, 0.94] |
| 4.4 Phlebography | 4 | 393 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.47 [0.29, 0.75] |

