نقش جورابهای فشارنده مدرج برای پیشگیری از ترومبوز ورید عمقی
Appendices
Appendix 1. CENTRAL search strategy March 2017
| #1 | MESH DESCRIPTOR Venous Thrombosis EXPLODE ALL TREES | 2041 |
| #2 | MESH DESCRIPTOR Thromboembolism EXPLODE ALL TREES | 1433 |
| #3 | MESH DESCRIPTOR Thrombosis | 1267 |
| #4 | thrombo*:TI,AB,KY | 31059 |
| #5 | thrombu*:TI,AB,KY | 1336 |
| #6 | embol*:TI,AB,KY | 5962 |
| #7 | (dvt* or PE or VTE):TI,AB,KY | 4996 |
| #8 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 | 37392 |
| #9 | MESH DESCRIPTOR Stockings, Compression | 165 |
| #10 | MESH DESCRIPTOR Compression Bandages | 99 |
| #11 | (stocking* or hosiery* or tights* or sock* or bandage* or compres* ):TI,AB,KY | 8388 |
| #12 | (VenoTrain* or Ulcertec or SurePress* or ComfortPro or Comfort‐Pro or "Ulcer Kit" or Sigvaris):TI,AB,KY | 23 |
| #13 | #9 OR #10 OR #11 OR #12 | 8388 |
| #14 | #8 AND #13 | 1023 |
| #15 | * NOT SR‐PVD:CC AND 31/03/2014 TO 31/03/2017:DL | 272119 |
| #16 | #14 AND #15 | 238 |
Appendix 2. Trials registry searches March 2017
ClinicalTrials.gov
147 studies found for: compression AND (thrombosis OR embolism OR DVT OR PE)
World Health Organization International Clinical Trials Registry Platform
30 records for 30 trials; compression or stockings in Title and (thrombosis OR embolism OR DVT OR PE) in Condition
27 records for 27 trials; (thrombosis OR embolism OR DVT OR PE) in Condition AND (compression or stockings) Intervention
ISRCTN Register
24 results compression stockings
Appendix 3. Database searches June 2018
| Source | Search strategy | Hits retrieved |
| 1. VASCULAR REGISTER IN CRSW | Stocking AND DVT AND 2017 OR 2018 | 0 |
| 2. CENTRAL | #1 MESH DESCRIPTOR Venous Thrombosis EXPLODE ALL TREES 2377 #2 MESH DESCRIPTOR Thromboembolism EXPLODE ALL TREES 1748 #3 MESH DESCRIPTOR Thrombosis 1618 #4 thrombo*:TI,AB,KY 37896 #5 thrombu*:TI,AB,KY 1701 #6 embol*:TI,AB,KY 7568 #7 (dvt* or PE or VTE):TI,AB,KY 6320 #8 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 45955 #9 MESH DESCRIPTOR Stockings, Compression 188 #10 MESH DESCRIPTOR Compression Bandages 123 #11 (stocking* or hosiery* or tights* or sock* or bandage* or compres* ):TI,AB,KY 10338 #12 (VenoTrain* or Ulcertec or SurePress* or ComfortPro or Comfort‐Pro or "Ulcer Kit" or Sigvaris):TI,AB,KY 25 #13 #9 OR #10 OR #11 OR #12 10338 #14 #8 AND #13 1202 #15 01/01/2017 TO 11/06/2018:CD 285491 #16 #14 AND #15 235 | 235 |
| 3. ClinicalTrials.gov | compression | thrombosis OR embolism OR DVT OR PE0 | Start date on or after 01/01/2017 | Last update posted on or before 06/12/2018 | 22 |
| 4. ICTRP Search Portal | compression or stockings in Title and (thrombosis OR embolism OR DVT OR PE) (thrombosis OR embolism OR DVT OR PE) in Condition AND (compression or stockings) Intervention | 41 50 |
| 5. MEDLINE (2017 AND 2018 ONLY) | 1 exp Venous Thrombosis/ 51417 2 exp THROMBOEMBOLISM/ 51479 3 THROMBOSIS/ 65664 4 thrombo*.ti,ab. 328580 5 thrombu*.ti,ab. 34506 6 embol*.ti,ab. 116862 7 (dvt* or PE or VTE).ti,ab. 47049 8 or/1‐7 503827 9 Stockings, Compression/ 1337 10 Compression Bandages/ 779 11 (stocking* or hosiery* or tights* or sock* or bandage* or compres*).ti,ab. 153878 12 (VenoTrain* or Ulcertec or SurePress* or ComfortPro or Comfort‐Pro or "Ulcer Kit" or Sigvaris).ti,ab. 30 13 or/9‐12 154511 14 8 and 13 8944 15 randomized controlled trial.pt. 463544 16 controlled clinical trial.pt. 92478 17 randomized.ab. 414978 18 placebo.ab. 189985 19 drug therapy.fs. 2026770 20 randomly.ab. 292442 21 trial.ab. 431981 22 groups.ab. 1807008 23 or/15‐22 4228218 24 exp animals/ not humans.sh. 4470062 25 23 not 24 3655192 26 14 and 25 1938 27 (2017* or 2018*).ed. 1410268 28 26 and 27 120 | 120 |
| 6. Embase (2017 AND 2018 ONLY) | 1 exp vein thrombosis/ 117897 2 exp thromboembolism/ 447229 3 thrombosis/ 124510 4 thrombo*.ti,ab. 468161 5 thrombu*.ti,ab. 51801 6 embol*.ti,ab. 166543 7 (dvt* or PE or VTE).ti,ab. 75124 8 or/1‐7 801765 9 compression stocking/ 2205 10 compression bandage/ 2132 11 (stocking* or hosiery* or tights* or sock* or bandage* or compres*).ti,ab. 188533 12 (VenoTrain* or Ulcertec or SurePress* or ComfortPro or Comfort‐Pro or "Ulcer Kit" or Sigvaris).ti,ab. 62 13 or/9‐12 190524 14 8 and 13 14787 15 randomized controlled trial/ 505723 16 controlled clinical trial/ 460951 17 random$.ti,ab. 1309942 18 randomization/ 78407 19 intermethod comparison/ 235704 20 placebo.ti,ab. 273502 21 (compare or compared or comparison).ti. 470023 22 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 1753437 23 (open adj label).ti,ab. 64566 24 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 209146 25 double blind procedure/ 150639 26 parallel group$1.ti,ab. 21829 27 (crossover or cross over).ti,ab. 93000 28 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 283079 29 (assigned or allocated).ti,ab. 332145 30 (controlled adj7 (study or design or trial)).ti,ab. 295234 31 (volunteer or volunteers).ti,ab. 224581 32 trial.ti. 251314 33 or/15‐32 4039780 34 14 and 33 2822 35 (2017* or 2018*).em. 3562214 36 34 and 35 483 37 from 36 keep 1‐483 483 | 483 |
| 7. CINAHL (2017 AND 2018 ONLY) | S31 S29 AND S30 18 S30 EM 2017 OR EM 2018 361,822 S29 S14 AND S28 425 S28 S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 341,275 S27 MH "Random Assignment" 38,519 S26 MH "Triple‐Blind Studies" 85 S25 MH "Double‐Blind Studies" 24,789 S24 MH "Single‐Blind Studies" 7,975 S23 MH "Factorial Design" 919 S22 MH "Placebos" 8,349 S21 MH "Clinical Trials" 93,065 S20 TX "multi‐centre study" OR "multi‐center study" OR "multicentre study" OR "multicenter study" OR "multi‐site study" 4,466 S19 TX crossover OR "cross‐over" 14,523 S18 AB placebo* 28,225 S17 TX random* 218,477 S16 TX trial* 249,805 S15 TX "latin square" 142 S14 S8 AND S13 1,660 S13 S9 OR S10 OR S11 OR S12 25,775 S12 TX VenoTrain* or Ulcertec or SurePress* or ComfortPro or Comfort‐Pro or "Ulcer Kit" or Sigvaris 13 S11 TX stocking* or hosiery* or tights* or sock* or bandage* or compres* 25,772 S10 (MH "Elastic Bandages") 112 S9 (MH "Compression Garments") 1,652 S8 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 56,491 S7 TX dvt* or PE or VTE 11,032 S6 TX embol* 15,079 S5 TX thrombu* 2,453 S4 TX thrombo* 39,098 S3 (MH "Thrombosis") 4,634 S2 (MH "Thromboembolism+") 7,488 S1 (MH "Venous Thrombosis+") 6,387 | 18 |
| 8. AMED (2017 AND 2018 ONLY) | 1 exp THROMBOEMBOLISM/ 72 2 THROMBOSIS/ 199 3 thrombo*.ti,ab. 740 4 thrombu*.ti,ab. 39 5 embol*.ti,ab. 226 6 (dvt* or PE or VTE).ti,ab. 246 7 or/1‐6 1064 8 Bandages/ 427 9 (stocking* or hosiery* or tights* or sock* or bandage* or compres*).ti,ab. 2606 10 (VenoTrain* or Ulcertec or SurePress* or ComfortPro or Comfort‐Pro or "Ulcer Kit" or Sigvaris).ti,ab. 0 11 or/8‐10 2914 12 7 and 11 52 13 ("2017" or "2018").yr. 2075 14 12 and 13 1 15 from 14 keep 1 1 | 1 |

Study flow diagram.
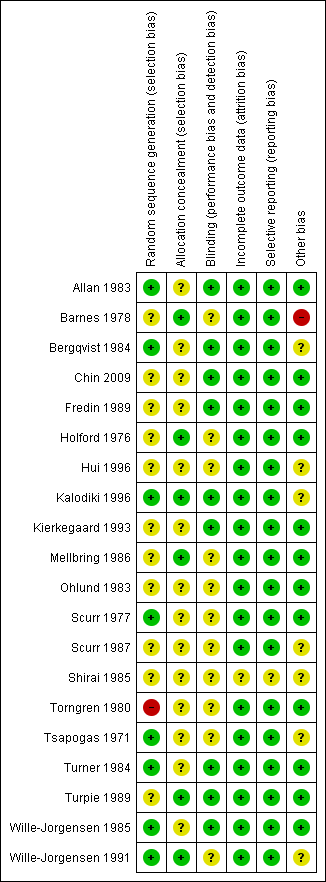
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
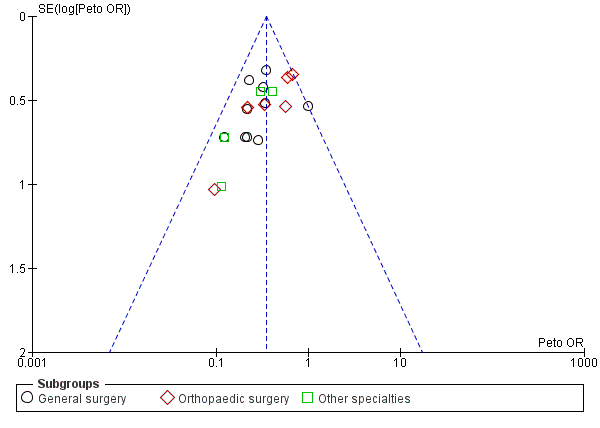
Funnel plot of comparison: Incidence of DVT with stockings and without stockings (all specialties).
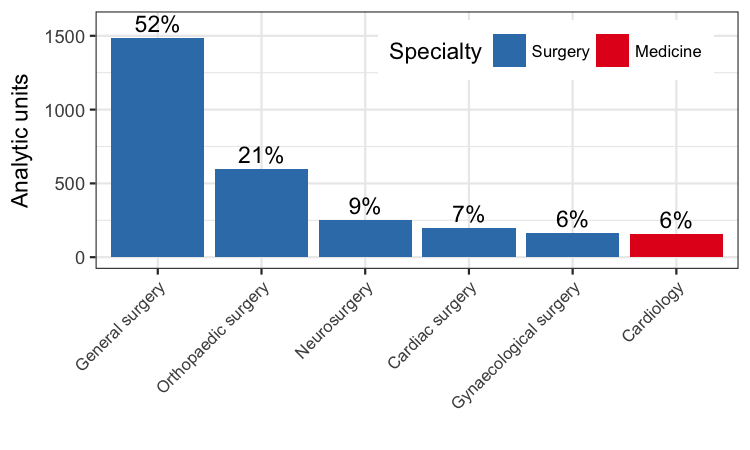
Number of analytic units from each specialty included in the meta‐analysis.
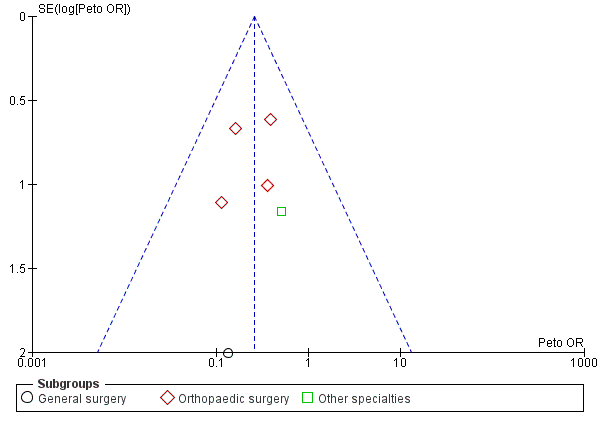
Funnel plot of comparison: Incidence of proximal DVT with stockings and without stockings (all specialties).
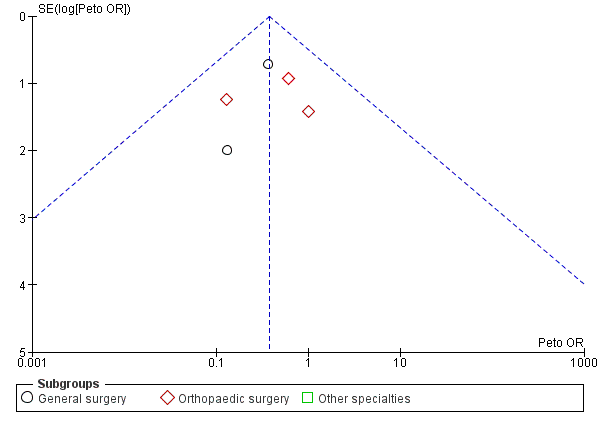
Funnel plot of comparison: Incidence of PE with stockings and without stockings (all specialties).

Comparison 1 Incidence of DVT with stockings and without stockings, Outcome 1 All specialties.

Comparison 1 Incidence of DVT with stockings and without stockings, Outcome 2 All specialties ‐ surgical vs medical.

Comparison 2 Incidence of proximal DVT with stockings and without stockings, Outcome 1 All specialties.

Comparison 2 Incidence of proximal DVT with stockings and without stockings, Outcome 2 All specialties ‐ surgical vs medical.
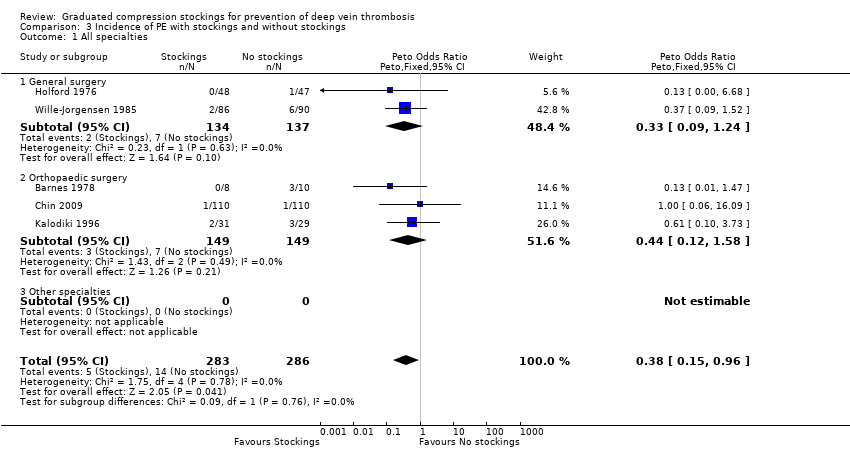
Comparison 3 Incidence of PE with stockings and without stockings, Outcome 1 All specialties.
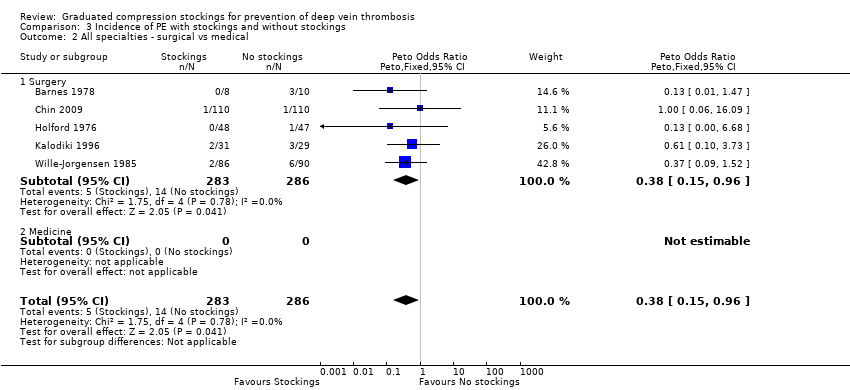
Comparison 3 Incidence of PE with stockings and without stockings, Outcome 2 All specialties ‐ surgical vs medical.
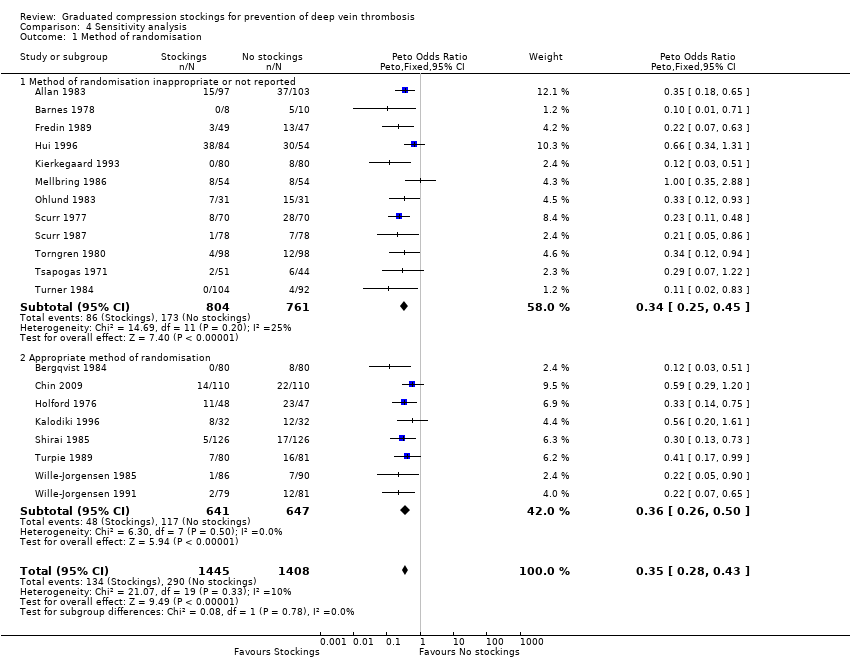
Comparison 4 Sensitivity analysis, Outcome 1 Method of randomisation.

Comparison 4 Sensitivity analysis, Outcome 2 Unit of analysis for randomisation.

Comparison 4 Sensitivity analysis, Outcome 3 Use of background method of thromboprophylaxis.
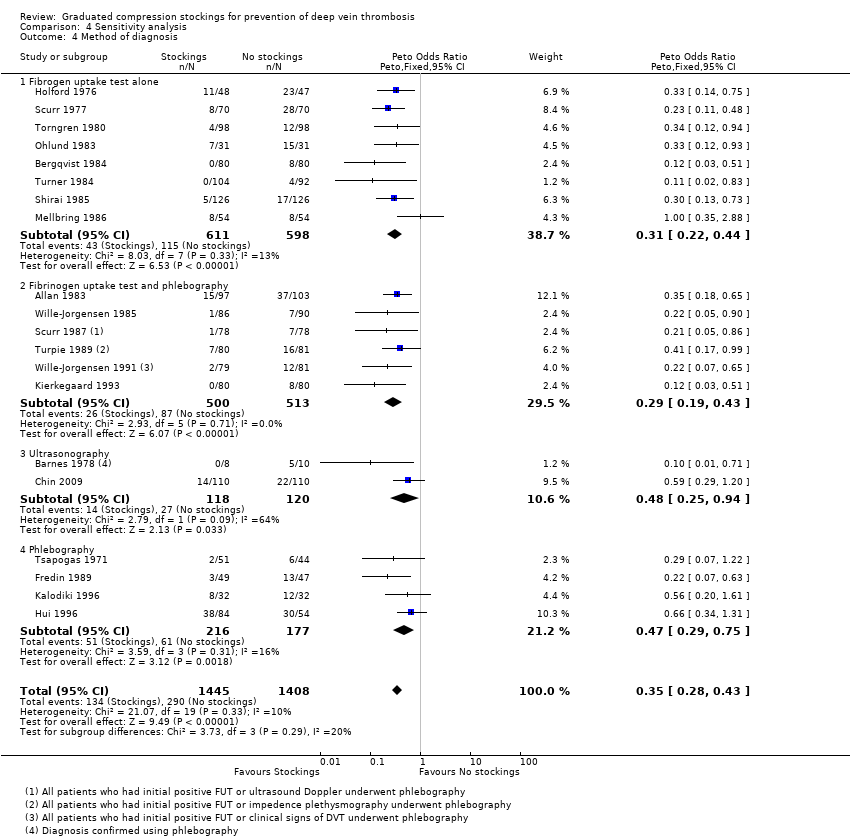
Comparison 4 Sensitivity analysis, Outcome 4 Method of diagnosis.
| Graduated compression stockings for prevention of deep vein thrombosis | ||||||
| Patient or population: Hospitalised patients1 | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of units/ participants2 | Quality of the evidence | Comments | |
| Risk with no GCS | Risk with GCS | |||||
| Deep vein thrombosis (DVT) Follow‐up: 7 to 14 days, or until discharge | Study population | OR 0.35 | 2853 | ⊕⊕⊕⊕ | Graduated compression stockings reduce the incidence of DVT in hospitalised patients. However, studies did not routinely distinguish between symptomatic and asymptomatic DVTs. | |
| 206 per 1000 | 83 per 1000 | |||||
| Proximal DVT Follow‐up: 7 to 14 days, or until discharge | Study population | OR 0.26 | 1035 | ⊕⊕⊕⊝ | Graduated compression stockings probably reduce the incidence of proximal DVT in hospitalised patients. There was a relatively low event rate overall, and studies did not routinely distinguish between symptomatic and asymptomatic proximal DVTs. | |
| 54 per 1000 | 15 per 1000 | |||||
| Pulmonary embolism Follow‐up: 7 to 30 days | Study population | OR 0.38 | 569 | ⊕⊕⊝⊝ | Graduated compression stockings may slightly reduce the incidence of pulmonary embolism in hospitalised patients. Pulmonary embolism was not routinely assessed in most included studies, and the overall event rate was very low. | |
| 49 per 1000 | 19 per 1000 | |||||
| Adverse effects and complications Follow‐up: until discharge | See comment | ‐ | ‐ | ‐ | Some participants removed stockings due to discomfort or poor fitting, however adverse effects and complications were not routinely reported quantitatively in the included studies. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Included patients admitted for surgical procedures (including abdominal, orthopaedic, neurosurgical, gynaecological surgery) and medical illness (acute myocardial infarction). | ||||||
| Graduated compression stockings for prevention of deep vein thrombosis | ||||||
| Patient or population: Patients hospitalised for surgical procedures1 | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of units/ participants2 | Quality of the evidence | Comments | |
| Risk with no GCS | Risk with GCS | |||||
| Deep vein thrombosis (DVT) Follow‐up: 7 to 14 days, or until discharge | Study population | OR 0.35 (0.28 to 0.44) | 2693 (19 RCTs) | ⊕⊕⊕⊕ | Graduated compression stockings reduce the incidence of DVT in hospitalised surgical patients. However, studies did not routinely distinguish between symptomatic and asymptomatic DVTs. | |
| 212 per 1000 | 86 per 1000 | |||||
| Proximal DVT Follow‐up: 7 to 14 days, or until discharge | Study population | OR 0.26 (0.13 to 0.53) | 875 (7 RCTs) | ⊕⊕⊕⊝ | Graduated compression stockings probably reduce the incidence of proximal DVT in hospitalised surgical patients. However, studies did not routinely distinguish between symptomatic and asymptomatic proximal DVTs. There was a relatively low event rate overall. | |
| 64 per 1000 | 17 per 1000 | |||||
| Pulmonary embolism Follow‐up: 7 to 30 days, or until discharge | Study population | OR 0.38 (0.15 to 0.96) | 569 (5 RCTs) | ⊕⊕⊝⊝ | Graduated compression stockings may slightly reduce the incidence of pulmonary embolism in hospitalised surgical patients. However, pulmonary embolism was not routinely assessed in most included studies, and the overall event rate was very low. | |
| 49 per 1000 | 19 per 1000 | |||||
| Adverse effects and complications Follow‐up: until discharge | See comment | ‐ | ‐ | ‐ | Some participants removed stockings due to discomfort or poor fitting, however adverse effects and complications associated with wearing GCS were not routinely reported quantitatively in the included studies. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Included patients admitted for surgical procedures (including abdominal, orthopaedic, neurosurgical, gynaecological surgery). | ||||||
| Graduated compression stockings for prevention of deep vein thrombosis | ||||||
| Patient or population: Patients hospitalised following acute myocardial infarction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of units | Quality of the evidence | Comments | |
| Risk with no GCS | Risk with GCS | |||||
| Deep vein thrombosis (DVT) Follow‐up: 8 days or discharge or until development of DVT | Study population | OR 0.12 (0.03 to 0.51) | 160 | ⊕⊕⊝⊝ | Graduated compression stockings may reduce incidence of DVT in hospitalised medical patients. However, results are based on a single study on medical patients hospitalised following acute myocardial infarction (Kierkegaard 1993). | |
| 100 per 1000 | 13 per 1000 | |||||
| Proximal DVT Follow‐up: 8 days or discharge or until development of DVT | Study population | Not estimable | 160 (1 RCT) | ‐ | None of the participants in either group of this single RCT with a small sample size developed proximal DVT. | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Pulmonary embolism | See comment | ‐ | ‐ | ‐ | No studies reported on this outcome. There is paucity of evidence to evaluate the effect of GCS on reducing incidence of pulmonary embolism in hospitalised medical patients. | |
| Adverse effects and complications | See comment | ‐ | 160 (1 RCT) | ‐ | There are rare reports of post‐thrombotic changes in participants who developed DVT in the single included RCT. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded the quality of the evidence by two steps as there was only one study and a low event rate in the GCS group (imprecision). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All specialties Show forest plot | 20 | 2853 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.28, 0.43] |
| 1.1 General surgery | 10 | 1486 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.30 [0.22, 0.41] |
| 1.2 Orthopaedic surgery | 6 | 598 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.47 [0.32, 0.68] |
| 1.3 Other specialties | 4 | 769 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.16, 0.48] |
| 2 All specialties ‐ surgical vs medical Show forest plot | 20 | 2853 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.28, 0.43] |
| 2.1 Surgery | 19 | 2693 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.28, 0.44] |
| 2.2 Medicine | 1 | 160 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.03, 0.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All specialties Show forest plot | 8 | 1035 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.26 [0.13, 0.53] |
| 1.1 General surgery | 2 | 316 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 6.82] |
| 1.2 Orthopaedic surgery | 4 | 398 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.25 [0.12, 0.53] |
| 1.3 Other specialties | 2 | 321 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.52 [0.05, 5.03] |
| 2 All specialties ‐ surgical vs medical Show forest plot | 8 | 1035 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.26 [0.13, 0.53] |
| 2.1 Surgery | 7 | 875 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.26 [0.13, 0.53] |
| 2.2 Medicine | 1 | 160 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All specialties Show forest plot | 5 | 569 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.15, 0.96] |
| 1.1 General surgery | 2 | 271 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.33 [0.09, 1.24] |
| 1.2 Orthopaedic surgery | 3 | 298 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.44 [0.12, 1.58] |
| 1.3 Other specialties | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 All specialties ‐ surgical vs medical Show forest plot | 5 | 569 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.15, 0.96] |
| 2.1 Surgery | 5 | 569 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.15, 0.96] |
| 2.2 Medicine | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Method of randomisation Show forest plot | 20 | 2853 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.28, 0.43] |
| 1.1 Method of randomisation inappropriate or not reported | 12 | 1565 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.34 [0.25, 0.45] |
| 1.2 Appropriate method of randomisation | 8 | 1288 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.36 [0.26, 0.50] |
| 2 Unit of analysis for randomisation Show forest plot | 20 | 2853 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.28, 0.43] |
| 2.1 Individual participants | 13 | 1681 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.29, 0.49] |
| 2.2 Individual legs | 7 | 1172 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.19, 0.42] |
| 3 Use of background method of thromboprophylaxis Show forest plot | 20 | 2853 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.28, 0.43] |
| 3.1 Trials without background thromboprophylaxis | 9 | 1497 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.29, 0.50] |
| 3.2 Trials with background thromboprophylaxis | 11 | 1356 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.29 [0.20, 0.42] |
| 4 Method of diagnosis Show forest plot | 20 | 2853 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.28, 0.43] |
| 4.1 Fibrogen uptake test alone | 8 | 1209 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.31 [0.22, 0.44] |
| 4.2 Fibrinogen uptake test and phlebography | 6 | 1013 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.29 [0.19, 0.43] |
| 4.3 Ultrasonography | 2 | 238 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.48 [0.25, 0.94] |
| 4.4 Phlebography | 4 | 393 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.47 [0.29, 0.75] |

