Antimicrobial prophylaxis for colorectal surgery
Abstract
Background
Research shows that administration of prophylactic antibiotics before colorectal surgery prevents postoperative surgical wound infection. The best antibiotic choice, timing of administration and route of administration remain undetermined.
Objectives
To establish the effectiveness of antimicrobial prophylaxis for the prevention of surgical wound infection in patients undergoing colorectal surgery. Specifically to determine:
1. whether antimicrobial prophylaxis reduces the risk of surgical wound infection;
2. the target spectrum of bacteria (aerobic or anaerobic bacteria, or both);
3. the best timing and duration of antibiotic administration;
4. the most effective route of antibiotic administration (intravenous, oral or both);
5. whether any antibiotic is clearly more effective than the currently recommended gold standard specified in published guidelines;
6. whether antibiotics should be given before or after surgery.
Search methods
For the original review published in 2009 we searched the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE (Ovid) and EMBASE (Ovid). For the update of this review we rewrote the search strategies and extended the search to cover from 1954 for MEDLINE and 1974 for EMBASE up to 7 January 2013. We searched CENTRAL on the same date (Issue 12, 2012).
Selection criteria
Randomised controlled trials of prophylactic antibiotic use in elective and emergency colorectal surgery, with surgical wound infection as an outcome.
Data collection and analysis
Data were abstracted and reviewed by one review author and checked by another only for the single, dichotomous outcome of surgical wound infection. Quality of evidence was assessed using GRADE methods.
Main results
This updated review includes 260 trials and 68 different antibiotics, including 24 cephalosporins and 43,451 participants. Many studies had multiple variables that separated the two study groups; these could not be compared to other studies that tested one antibiotic and had a single variable separating the two groups. We did not consider the risk of bias arising from attrition and lack of blinding of outcome assessors to affect the results for surgical wound infection.
Meta‐analyses demonstrated a statistically significant difference in postoperative surgical wound infection when prophylactic antibiotics were compared to placebo/no treatment (risk ratio (RR) 0.34, 95% confidence interval (CI) 0.28 to 0.41, high quality evidence). This translates to a reduction in risk from 39% to 13% with prophylactic antibiotics. The slightly higher risk of wound infection with short‐term compared with long‐term duration antibiotic did not reach statistical significance (RR 1.10, 95% CI 0.93 to 1.30). Similarly risk of would infection was slightly higher with single‐dose antibiotics when compared with multiple dose antibiotics, but the results are compatible with benefit and harm (RR 1.30, 95% CI 0.81 to 2.10). Additional aerobic coverage and additional anaerobic coverage both showed statistically significant improvements in surgical wound infection rates (RR 0.44, 95% CI 0.29 to 0.68 and RR 0.47, 95% CI 0.31 to 0.71, respectively), as did combined oral and intravenous antibiotic prophylaxis when compared to intravenous alone (RR 0.56, 95% CI 0.43 to 0.74), or oral alone (RR 0.56, 95% CI 0.40 to 0.76). Comparison of an antibiotic with anaerobic specificity to one with aerobic specificity showed no significant advantage for either one (RR 0.84, 95% CI 0.30 to 2.36). Two small studies compared giving antibiotics before or after surgery and no significant difference in this timing was found (RR 0.67, 95% CI 0.21 to 2.15). Established gold‐standard regimens recommended in major guidelines were no less effective than any other antibiotic choice.
Authors' conclusions
This review has found high quality evidence that antibiotics covering aerobic and anaerobic bacteria delivered orally or intravenously (or both) prior to elective colorectal surgery reduce the risk of surgical wound infection. Our review shows that antibiotics delivered within this framework can reduce the risk of postoperative surgical wound infection by as much as 75%. It is not known whether oral antibiotics would still have these effects when the colon is not empty. This aspect of antibiotic dosing has not been tested. Further research is required to establish the optimal timing and duration of dosing, and the frequency of longer‐term adverse effects such as Clostridium difficile pseudomembranous colitis.
PICO
Plain language summary
Antibiotics administered to patients prior to colorectal surgery
When people undergo surgical operations of their abdomen they are at risk of infection which will often be cured by an antibiotic. However, it might be better to give this before the operation to prevent the infection (prophylaxis or prophylactic use), rather than wait until an infection occurs before giving it. This review looks at the evidence for giving an antibiotic before surgery takes place.
The review found 260 studies which had recruited over 43 thousand people undergoing abdominal surgery. The studies had some limitations in relation to the number of people who remained in the studies and the possibility that the results were affected because some of the researchers in the studies knew which people had received antibiotics before surgery. However, when the results were analysed effect of prophylactic antibiotics was consistently beneficial meaning that these limitations were unlikely to have had a major impact on the nature of the overall results. Abdominal surgical wound infection in patients having operations on the large intestine occurs in about 40% of patients if antibiotics are not given. This risk can be greatly diminished by the administration of antibiotics prophylactically before surgery. The antibiotic(s) given usuallly needs to cover different types of bacteria some of which need oxygen (aerobic bacteria) and others which do not need oxygen (anaerobic bacteria).. They are usually given via a canula injected into a vein, though there is evidence that a combination of oral and intravenous antibiotics may provide more protection. This last finding raises a problem in that current clinical practice is to avoid mechanical cleansing of the colon because it is not thought to be necessary before surgery (and not popular with patients). Studies that found a benefit to oral antibiotics were done at a time when mechanical cleansing of the colon was routinely done. In the light of current practice regarding mechanical cleansing before surgery of the colon, the benefit of oral antibiotics is uncertain.
Authors' conclusions
Summary of findings
| Antibiotic versus no antibiotic/placebo for colorectal surgery | ||||||
| Patient or population: patients undergoing colorectal surgery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antibiotic versus no antibiotic/placebo | |||||
| Surgical wound infection (SWI) | Study population | RR 0.34 | 2455 | ⊕⊕⊕⊕ | ||
| 368 per 1000 | 125 per 1000 | |||||
| Moderate | ||||||
| 391 per 1000 | 133 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We consider our results not to be affected by either post‐randomisation attrition or clinical heterogeneity. | ||||||
| Combined oral and intravenous compared to oral or intravenous alone for colorectal surgery | ||||||
| Patient or population: colorectal surgery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| oral or intravenous alone | Combined oral and intravenous | |||||
| Surgical wound infection: oral + iv versus iv alone | Study population | RR 0.55 | 2929 | high | ||
| 128 per 1000 | 70 per 1000 | |||||
| Moderate | ||||||
| 230 per 1000 | 126 per 1000 | |||||
| Surgical wound infection: combined oral and iv versus oral alone | Study population | RR 0.52 | 1880 | high | ||
| 79 per 1000 | 41 per 1000 | |||||
| Moderate | ||||||
| 146 per 1000 | 76 per 1000 | |||||
| Study population | not estimable | ( studies) | ||||
| 0 per 1000 | 0 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
Background
Abdominal surgical wound infections in patients having operations on the large intestine occur in about 40% of those who do not receive antibiotic prophylaxis (Baum 1981). When an infection does occur, it often involves more than simple drainage of subcutaneous pus and dressing changes at home. Indeed, the risk of death is doubled , intensive care unit admission is more likely and average hospital stay is lengthened by five days (Kirkland 1999). In patients with surgical wound infection, length of stay is one week longer and hospital cost averages USD 13,746 more than in those without infection (USD 2,009 Mahmoud 2009) and an average of USD 6,200 in home care costs are incurred after discharge (USD 2,004, Smith 2004). The risk of hospital readmission is also greatly increased (Kirkland 1999). Contrary to traditional beliefs about pus being a good sign, it seems that survival rates in patients who have colon cancer removed are reduced when wound infection has occurred (Nespoli 2004), though the reasons for this are unknown. Reducing the risk of surgical wound infection is clearly a priority in terms of patient safety and cost containment of medical care.
In 1981, an early systematic review that compared wound infection risk in elective colorectal surgery patients receiving antibiotic prophylaxis to those randomised to placebo or no treatment found that infection risk was so diminished with antibiotics that the review concluded that future studies in this field that included no treatment controls would no longer be ethical (Baum 1981). It was also stated that a gold‐standard antibiotic should be established, so that in all future studies one arm of the study would include the gold standard as the acceptable benchmark from which to judge the new antibiotic. Since then, guidelines have been published that suggest an optimal choice of antibiotic and also dosage regimens (Medical Letter 2012). However, a survey of American hospitals found that these guidelines are followed approximately half of the time (Bratzler 2005).
Published summaries of evidence are found in a number of systematic reviews. The first, cited above, dealt only with any antibiotic versus no treatment controls (Baum 1981). Another looked only at one aspect of route of administration: oral plus intravenous antibiotics versus intravenous antibiotics alone (Lewis 2002), as did Bellows 2011. A fourth was a global review of studies published from 1984 to 1997, but focused more on individual comparisons rather than the global issues mentioned above (Song 1998). This is just a sample of many other such reviews. This systematic review was undertaken in order to determine whether evidence exists to reconfirm the need for antibiotic prophylaxis, to determine what spectrum of bacteria needs to be addressed by the choice of antibiotic (e.g. Gram‐negative versus Gram‐positive bacteria), to determine the best timing and route of antibiotic administration and, finally, to see whether any antibiotic performs better than the gold standard currently recommended in published guidelines.
Objectives
This systematic review has been undertaken to assess the relevant literature in order to establish the effectiveness of antimicrobial prophylaxis in patients undergoing colorectal surgery for the prevention of surgical wound infection.
The review addresses the following questions:
-
Does antibiotic prophylaxis diminish the risk of surgical wound infection in colorectal surgery?
-
What spectrum of bacteria (aerobes or anaerobes) needs to be addressed by antibiotic choice?
-
What is the best timing of antibiotic administration and, specifically, should antibiotics be continued into the postoperative period?
-
What route of antibiotic administration (intravenous, oral or both) is most effective in preventing surgical wound infection?
-
Does any antibiotic choice exceed the efficacy of the currently recommended gold‐standard prophylactic antibiotic therapy? (For definitions see 'Methods of the review').
-
Should antibiotics be given before or after surgery?
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials that assess the effectiveness of antimicrobial prophylaxis in the prevention of postoperative surgical wound infections in patients undergoing colorectal surgery.
Types of participants
Patients (adults and children) undergoing either elective or emergency colorectal surgery, in which sepsis was not suspected preoperatively. This might include bowel obstruction without perforation . We found no trial that included children.
Types of interventions
We considered all antimicrobial prophylaxis regimens delivered orally, intravenously or by intramuscular injection used to prevent postoperative infection. Antibiotics had to be administered before the onset of infection and studies in which antibiotics were given before surgery for suspected appendicitis or diverticulitis were excluded because the antibiotics are treating an established infection for which surgery is required. Administration of antibiotics by other means, notably topically, were excluded as they will be the topic of a separate systematic review.
Types of outcome measures
The abdominal wound has the greatest risk of infection; most commonly from contamination by endogenous colorectal bacteria (contained within the patient's bowel) during surgical procedures (Pollock 1987). In this review, therefore, the rate of surgical wound infection was used as the primary and sole outcome measure to assess the relative effectiveness of antimicrobial prophylaxis in colorectal surgery. The definition and diagnosis of surgical wound infections often varies between published studies, but has the common feature of pus expressed from the surgical incision. We included only abdominal wound infections, because they are more reliably identified than, for example, perineal infections.
Search methods for identification of studies
For the original review published in 2009 (Nelson 2009) we searched the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE (Ovid) and EMBASE (Ovid).
For the update of this review we rewrote the search strategies and extended the searches to cover from 1954 for MEDLINE and 1974 for EMBASE to 7 January 2013. We searched CENTRAL on the same date (Issue 12, 2012) (see Appendix 1). We broadened the search for this update to go backwards to 1954/1974 as well as to extend to 7 January 2013 because of the concern that earlier searches missed studies(always a possibility, but significantly so).
We also searched the references of the identified trials to identify further relevant trials.
Data collection and analysis
Quality assessment
Due to the large number of studies published in this area, once the decision had been made to include a study, the methodological quality (validity) of the study was assessed by one review author (AMG or FS for the original 1998 review and RN for this update) and checked by another (AMG or FS for the original review and AMG for initial publication of this review in 2009, by RN for this update), using the following check‐points:
-
Was the assignment to the treatment groups really random (versus quasi‐randomised by birth dates or hospital numbers etc.)? All studies are specified as randomised trials. If the randomisation method was specified and appropriate, we classified it as low risk of bias. If birth date or hospital number was used, then we classified it as high risk of bias. If it was unspecified, which was by far the greatest likelihood, we classified it as unclear risk of bias.
-
Were those assessing outcomes blind to the treatment allocation? We classified this as low risk of bias if blinding was specified; more commonly we classified this as unclear risk of bias as it was rarely specified.
-
Were the control and treatment groups comparable at entry, i.e. were there significant differences in clinical parameters such as age/gender/diagnosis?
-
Were the groups treated identically, other than for the named interventions?
-
Were the operative procedures defined and described?
-
Was a written definition of wound infection and other outcome measures included?
-
Was relatively complete follow‐up achieved (i.e. greater than 90%)?
-
Were the outcomes of people who withdrew described and included in the analysis?
-
Was the outcome assessor blind as to treatment group. When this was not specified we assumed that the surgical team did the outcome assessment and that there was a high risk of bias.
We resolved any disagreements arising at this stage by discussion.
Data abstraction
Data were extracted from included trials by one review author and checked by another using a data abstraction form (Appendix 2).
Data synthesis
We grouped studies according to the following comparisons:
-
Antimicrobial prophylaxis versus no treatment control or placebo.
-
Short‐ versus long‐term use of an antimicrobial (either alone or in combination). A subgroup analysis included patients who received only a single preoperative dose versus those who received at least a second intraoperative dose of antibiotic or postoperative dosing (or both).
-
Any antimicrobial prophylaxis regimen with added aerobic bacterial coverage versus same regimen with no additional aerobic coverage.
-
Any antimicrobial prophylaxis regimen with added anaerobic bacterial coverage versus same regimen with no additional anaerobic coverage.
In most cases of the third and fourth comparisons above, the primary (non‐test) antibiotic did not have a bacterial coverage spectrum that might overlap with the test antibiotic.
-
Aerobic bacterial coverage only versus anaerobic bacterial coverage only.
-
Antimicrobial prophylaxis administered orally only versus intravenously only.
-
Combined oral and intravenous antibiotic prophylaxis versus either oral or intravenous antibiotic prophylaxis alone.
-
Antibiotic given before surgery versus after surgery.
-
Comparison of any antibiotic therapy to an established gold‐standard antibiotic prophylaxis regimen. This is in accordance with the recommendation of Baum 1981, that once the efficacy of any antibiotic prophylaxis had been established ‐ as it had been in 1980, when compared to placebo ‐ then all future antibiotic trials should consist of comparisons of trial antibiotics to those gold standards (i.e. those antibiotics in common clinical use, as recommended by widely accepted published clinical guidelines), to see whether any new antibiotic regimen clearly exceeded the efficacy of widely recommended choices and might, therefore, be worthy of further study. Alternatively, where the efficacy of any regimen was much worse than the established choices, such that it should not be recommended for use in future.
The gold standards used in these comparisons included:
-
oral neomycin/erythromycin base (Medical Letter 2012);
-
intravenous cefoxitin or cefotetan (Medical Letter 2012);
-
intravenous and oral doxycycline (Andaker 1992).
We examined heterogeneity firstly by examining the characteristics in the Characteristics of included studies table. We assessed statistical heterogeneity using the Chi2 test (P value less than 0.1) and by assessing the I2 statistic (significant if > 30%). This describes the percentage of the variability in effect estimates that is due to heterogeneity rather than chance). Where heterogeneity existed, we investigated by subgroup analysis.
We used the random‐effects method throughout this review because even in the absence of statistical heterogeneity, there was such broad clinical heterogeneity in the included studies (such as 19 different antibiotics used in the 30 placebo based trials) (Analysis 1.1).
Dichotomous outcomes (e.g. surgical wound infection present or not) are presented as risk ratios (RR) with 95% confidence intervals (CI).
Results
Description of studies
The prior published review included a total of 182 trials, published between January 1980 and December 2007, and included 30,880 patients. Studies added in this update bring the total to 260 included studies with 43,451 participating patients. We reclassified nine studies from the original 2009 review as excluded, by far the most common reason being that they involved topical antimicrobial therapy, which will be the subject of a separate review. Thus 87 studies were added in this update.
A total of 68 different antibiotics have been assessed in the included studies; 24 were cephalosporins, the antibiotics thought to have most risk of causing Clostridium difficile colitis (Nelson 2011). Ninety‐six studies were excluded from the review for reasons outlined in the Characteristics of excluded studies table. Seventeen of the included trials were published in German, nine in French, two each in Danish and Italian and one each in Chinese, Finnish, Japanese, Korean, Portuguese and Spanish. All other trials were published in English. Full details of the participants, interventions and outcomes assessed are presented in the Characteristics of included studies table.
Risk of bias in included studies
The biggest risk of bias in this review is attrition. Many patients were excluded from analysis after randomisation. This was not due to loss to follow‐up, since these patients were in hospital recovering from a major operation. However, if a patient had their surgery cancelled, was found to have an intra‐abdominal infection such as ruptured diverticulitis, or if study drugs were delivered off schedule or in error, the follow‐up of that patient ceased rather than continued to the outcome endpoint. At the time that these studies were performed, there was clearly an essential misunderstanding of what was meant by randomisation. Rates of attrition varied from 0% to nearly 50% (Hagen 1980). In most cases where there were post‐randomisation exclusions, it was not specified to which group the exclusions belonged. Therefore all of these studies are analysed as treated. We established a threshold of 10% attrition and in the 'Risk of bias' figures (Figure 1; Figure 2) in the third column (attrition bias) green indicates less than 10% attrition and red any percentage over 10%.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
If a valid method of randomisation was specified the Figure 1 allocation sequence column is shown as green. If it was known that an invalid method of randomisation was used, such as birth date or hospital number, then it is shown as red. However, by far the most common finding was that although the text stated that patients were randomised, the method was not specified and we therefore classified the study as unclear; this appears in Figure 1 as an amber question mark. Allocation concealment was similarly rarely specified. In such situations we also classified it as unclear (so amber). On the other hand, if blinding of the outcome assessor was not specified then clinical experience suggests that the surgical team did the outcome assessment. Therefore, if blinding was specified it shows in the figures as green and in all other situations as red.
Antimicrobial prophylaxis versus no treatment control/placebo
Thirty trials (2435 patients), published between 1971 and 2009, were identified that compared some form of antimicrobial prophylaxis and either no treatment or placebo. In only nine trials was the method of randomisation specified. Blind outcome assessment was undertaken in 13/30 trials and attrition of less than 10% was seen in 20/30 trials (Analysis 1.1; Figure 1).
Short‐ versus long‐term use of an antimicrobial
Thirty‐three trials (4988 patients) examined variation in the duration of the antimicrobial regimen. The trials were published between 1978 and 2011. Analysis 2.1 contains all trials in which two different durations are specified, one short and the other longer. Of these 33 trials, 10 specified a valid allocation sequence, 17 trials had attrition of less than 10% and eight specified blinding of outcome assessment (Figure 1)
Antimicrobial prophylaxis regimen with additional aerobic coverage versus same regimen with no additional aerobic coverage
Fifteen trials (1869 patients) examined the effectiveness of additional aerobic cover. The trials were published between 1980 and 1986. Six of these trials specified the allocation sequence generation, eight had attrition of less than 10% and nine specified blinding of outcome assessment (; Figure 1). In all of these trials the non‐variable antibiotic was one with a purely anaerobic spectrum of bacterial sensitivity and of course the test antibiotic had an aerobic spectrum. This is therefore a pure test of the need for aerobic coverage, in contrast to Analysis 4.1 in which the non‐variable antibiotic(s) might have had both aerobic and anaerobic spectra and in those situations the test antibiotic only augmented the anaerobic coverage.
Antimicrobial prophylaxis regimen with additional anaerobic coverage versus same regimen with no additional anaerobic coverage
Eighteen trials (2625 patients), published between 1977 and 1993, examined the effectiveness of additional anaerobic cover. Seven of these studies specified the allocation generation sequence. Seven had attrition of less than 10% and 10 specified blinding of outcome assessment (; Figure 1).
Antimicrobial prophylaxis regimen with only aerobic coverage versus a regimen with only anaerobic coverage
This is a new comparison treating patients with antibiotics active only against aerobic bacteria versus an antibiotic active only against anaerobic bacteria. There are four studies in this analysis studying 546 patients. Only one study described an adequate randomisation sequence and the same study also had blinding of the outcome assessment (Figure 1). The other three studies all had attrition of less than 10%.
Antimicrobial prophylaxis administered orally versus intravenous administration
Three trials (237 patients) compared the effectiveness of the same antibiotics given by different routes of administration . Only one trial had an adequate randomisation method and another had blinding of outcome assessment. All three had attrition of less than 10% (Figure 1).
Antimicrobial prophylaxis administered orally and intravenously versus intravenously alone
Fourteen reports (2445 patients) assessed combined oral and intravenous prophylaxis versus intravenous alone. In no case was the same antibiotic used for both routes of administration as it had been in the previous section. Seven studies used adequate randomisation and four used adequate concealment of outcome assessment. Five studies had drop‐out rates of 10% or greater (Figure 1). The reported drop‐out (attrition) rate was as high as 34% (Khubchandani 1989).
Antibiotic prophylaxis delivered orally and intravenously versus orally alone
Ten studies (2364 patients) compared antibiotic prophylaxis in this manner (Analysis 7.2) published between 1979 and 2007. Randomisation method was specified in four trials and outcome blinding was specified in four trials as well. Attrition was less than 10% in six trials (Figure 1).
Antibiotic prophylaxis delivered before or after surgery
Only two studies gave the same antibiotic either before or after surgery (127 patients). Neither study adequately described randomisation or blinding of outcome assessment. Only one had attrition of less than 10% ( Figure 1).
Antibiotic prophylaxis of any antibiotic compared to an established gold‐standard prophylaxis regimen
There are several antibiotic regimens that are so broadly used as to be regarded as gold standards to which other antibiotic choices should be compared, recommended in guidelines cited in the Background. These include oral neomycin/erythromycin base, intravenous cefoxitin or cefotetan and, formerly, intravenous doxycycline. Forty‐three trials (6492 patients) included such comparisons. Twelve of these trials had adequate randomisation, only eight specified blinding of outcome assessment and 19 had attrition of less than 10%. However, these studies are meant only to be viewed individually, to see if an outlier might be better than any of the gold standards (see below).
Effects of interventions
See: Summary of findings for the main comparison Antibiotic versus no antibiotic/placebo for colorectal surgery; Summary of findings 2 Combined oral and intravenous compared to oral or intravenous alone for colorectal surgery
Many studies had results that could not provide useful clinical direction because two or more of the study groups contained multiple variables (i.e. variations in the type of antibiotic, dosing schedule and route of administration), which made it impossible to determine which of these three factors might have been responsible for any difference in results. Other studies employed antibiotic combinations that were neither compared to established techniques of prophylaxis, nor offered a rational approach to prophylaxis based upon what is known about colonic bacterial flora and the nature of surgical wound infection in colorectal surgery. Thus in the initial review, 76 studies, though eligible by our inclusion criteria, ultimately were not used in any of the meta‐analyses. The remaining 106 studies that included a total of 15,859 participants were included in the analysis. In this first update this was less of a problem, with 79 of the 260 studies included in the meta‐analyses. The total number of participants now included in the meta‐analyses is 25,314.
Antimicrobial prophylaxis versus no treatment control/placebo (Comparison 01)
Despite the recommendations of Baum 1981 that no further trials of antimicrobials versus placebo be performed, an additional 20 placebo‐controlled trials have been published since 1981, the last in 2009 (Sato 2009). The combined analysis of 30 randomised controlled trials (RCTs) involving 2435 participants showed a statistically significant benefit in favour of antibiotic prophylaxis (risk ratio (RR) 0.34, 95% confidence interval (CI) 0.28 to 0.41; P value less than 0.00001), reducing the overall surgical wound infection rate from 39% to 10%, with no statistical heterogeneity (P value 0.24; I2 = 15%; Analysis 1.1; Summary of findings table 1). Since Baum 1981, it is worth noting that no study effect falls to the right of 1.0 in the forest plot and each individual study either shows a statistically significant benefit of prophylaxis or a benefit that is nearly significant. This shows that the question of whether antimicrobial prophylaxis is better than no treatment, a control or placebo for colorectal surgery does not need to be asked again. Despite the absence of statistical heterogeneity, these studies are clinically extremely heterogeneous. Nineteen different antibiotics were assessed in these trials.
Short‐ versus long‐term use of an antimicrobial (Comparison 02)
Duration of antibiotic dosing was studied in a number of formats and the combined analysis showed no advantage with longer dosing (RR 1.10, 95% CI 0.93 to 1.30; P value 0.26) (Analysis 2.1), with no heterogeneity (P value 0.65; I2 = 0%). In a subgroup analysis, nine studies that specifically compared a single preoperative dose of antibiotic to either a second intraoperative dose, or early postoperative dose, or both, also showed no advantage with extended dosing (RR 1.30, 95% CI 0.81 to 2.10; P value 0.58) (Analysis 2.2). This addresses a specific recommendation that an antibiotic with a short serum half‐life should be given with a second dose in longer operations (Medical Letter 2012). The heterogeneity found in this subgroup analysis again was not seen in the broader analysis, which is curious. These eleven trials come from ten publications, since two of the trials come from a single publication, Kow 1995 and Kow 1995a. This multiple trials in a single publication occurs in several other studies: Anders 1984; Anders 1984a; Anders 1984b, Corman 1993; Corman 1993 A, McArdle 1995; McArdle 1995 A, and Menzel 1993; Menzel 1993 A.
Of the 11 trials which contain 2005 patients, five specified valid allocation sequence generation, six had attrition of less than 10% and three had blinded outcome assessment. The statistical heterogeneity in this second comparison (P = 0.11) which disappears if the study Fujita 2007 is eliminated (P = 0.53) and the risk ratio changes from 1.21 (95% CI 0.82 to 1.80) to 1.06 (95% CI 0.77 to 1.45). Fujita 2007 (Fujita 2007) is a large study and reasonably well conducted. The antibiotic used was cefmetazole, a second‐generation cephalosporin used principally against Staphylococcus, an aerobic organism not generally found in the bowel, nor therefore the usual infecting agent after bowel surgery, which are bacteria found in the lumen of the bowel (Medical Letter 2012). In other words clinically this study is very much an outlier due to an inappropriate antibiotic choice.
Antimicrobial prophylaxis regimen with additional aerobic coverage versus same regimen with no additional aerobic coverage (Comparison 03)
In patients receiving an antibiotic that principally covered anaerobic bacteria, the addition of an antibiotic that covered aerobic bacteria significantly reduced the incidence of surgical wound infection (RR 0.44, 95% CI 0.29 to 0.68; P value 0.0002), with only modest statistical heterogeneity (P value 0.11; I2 = 32%) (Analysis 3.1). The study with the greatest effect on this heterogeneity is Kläy 1983, though review of its conduct reveals no specific cause other than an overall low wound infection rate.
Antimicrobial prophylaxis regimen with additional anaerobic coverage versus same regimen with no additional anaerobic coverage (Comparison 04)
The addition of anaerobic coverage to aerobic coverage also resulted in a statistically significant reduction in surgical wound infection rates (RR 0.47, 95% CI 0.31 to 0.71; P value 0.0004), but in this case with significant statistical heterogeneity (P value 0.0003; I2 = 55%, Analysis 4.1). Most of the studies for the non‐variable antibiotic used a single drug with a principally aerobic spectrum. This analysis also contained significant statistical heterogeneity (P = 0.0003). Many of the studies were of questionable quality due to uncertain randomisation and low power, though no single study stood out in this regard. Eliminating the most skewed studies reduced heterogeneity, but no single elimination was effective in this respect. In four of the studies in this group the constant antibiotic, which was meant to have an aerobic spectrum, also had some anaerobic activity, which differs from Analysis 3.1 (Bergman 1987; Hall 1989b; Renner 1989; Tehan 1989). Elimination of these studies from the analysis (not shown) diminished heterogeneity, but not to an insignificant level.
Antimicrobial prophylaxis using antibiotic with aerobic coverage only versus anaerobic coverage only (Comparison 5)
This comparison of four studies did not demonstrate superiority of aerobic over anaerobic coverage (RR 0.84, 95% CI 0.30 to 2.36). The investigation of heterogeneity in this comparison suggested a poor aerobic antibiotic choice by Lewis 1981. If this study is excluded the heterogeneity disappears and the benefit of aerobic coverage is of borderline statistical significance (RR 0.56, 95% CI 0.30 to 1.06) (Analysis 5.1). The aerobic coverage in Lewis 1981 came from cephradine, a first‐generation cephalosporin with aerobic Gram‐positive coverage, principally for skin and respiratory infections, so like the outlier in (Analysis 2.2) there was clinical heerogeneity due to inaapropriate antibiotic choice.
Antimicrobial prophylaxis administered orally versus intravenous administration (Comparison 06)
Three studies looked at the same antibiotic delivered either intravenously or by mouth without demonstrable advantage of either route alone (RR 2.31, 95% CI 0.60 to 8.83; P = 0.22) (Analysis 6.1).
Antimicrobial prophylaxis administered both orally and intravenously versus intravenous prophylaxis alone (Comparison 07.1)
A statistically significant benefit in favour of combined oral and intravenous dosing, compared to intravenous dosing alone, was shown (RR 0.55, 95% CI 0.43 to 0.71; P = 0.0001) (Analysis 7.1; Summary of findings table 2).
Antimicrobial prophylaxis administered both orally and intravenously versus oral prophylaxis alone (Comparison 07.2)
Again, a statistically significant benefit was shown for combined oral and intravenous dosing, compared to oral prophylaxis alone (RR 0.52, 95% CI 0.35 to 0.76; P = 0.0003) (Analysis 7.2; Summary of findings table 3).
Antimicrobial prophylaxis given before surgery or immediately afterward (Comparison 8)
Two studies gave the same antibiotic just before or just after surgery. No advantage was seen in the comparison (RR 0.67, 95% CI 0.21 to 2.15; P = 0.5) (Analysis 8.1).
Antibiotic choice versus a published gold standard (Comparison 09)
There is no combined analysis for these 43 studies as the purpose here, unlike the other comparisons, was to look for outliers on the right or left of the forest plot. Several stand out: Antonelli 1985 compared two cephalosporins that did not differ significantly in other studies (Jones 1987b). Weaver 1986 and Kling 1989 compared intravenous ceftriaxone and metronidazole to oral neomycin and erythromycin with apparent significant benefit, though other ceftriaxone studies were not so striking (Burdon 1987; Garcia 1989; Hall 1991; Nyam 1995; Zanella 2000), especially when this was compared to other intravenous choices. Also, Schoetz 1990 showed a significant benefit to oral plus intravenous dosing of two established gold‐standard regimens (Medical Letter 2012), intravenous cefoxitin and oral neomycin/erythromycin base, compared to each one alone. Itani 2006 compared ertapenem to cefotetan and found a significant benefit with the former, though no real intention‐to‐treat analysis was performed in this large multicentre study and the per protocol analyses presented included only 63% of those randomised. Importantly, this is the only study to report subsequent infection with Clostridium difficile. Eight patients who received ertapenem acquired C. difficile, together with three from the cefotetan group (Analysis 9.1).
Sensitivity analyses
We undertook several sensitivity analyses, in addition to the two described above, to isolate those studies in each section which were, apparently, of higher quality because of a specified method of randomisation, blinding of outcome assessment and low drop‐out rate. This eliminated over 90% of the included studies and left either one or two studies in each general category for analysis. Power was, of course, diminished, but no conclusions were reversed markedly, though for comparisons Analysis 3.1 and Analysis 4.1 statistical significance disappeared due to small sample sizes.
In no case was the summary assessment of these sensitivity analyses significantly altered from the broader analyses. For studies testing comparisons to gold‐standard options, only one showed a significant benefit (Schoetz 1990), which in fact compared two gold‐standard options combined with each individually, essentially strengthening the efficacy of combined intravenous/oral prophylaxis.
Discussion
Summary of main results
This is an update of the first published version of this review in The Cochrane Library 2009, Issue 1 (Nelson 2009). Our review shows that:
-
antibiotics given before elective or emergency colorectal surgery reduce wound infections;
-
there is insufficient evidence to support the use of more than a single preoperative dose. Prolonged dosing both increases the risk of development of resistant bacteria and Clostridium difficile colitis (Nelson 2011).
-
evidence from the analysis in this review indicates that antibiotic choice should include aerobic bacterial coverage.
-
In addition antibiotic choice should include coverage of anaerobic bacteria. Evidence from these analyses show that coverage of both types of bacteria either with a single agent or combination therapy is more effective than coverage of either aerobic or anaerobic bacteria alone in reducing wound infection
-
both oral and intravenous administration of antibiotics substantially reduce wound infections compared with no antibiotic.
It appears that a combination of both routes of administration will result in the greatest decrease in risk. This raises a quandary since bowel cleansing prior to surgery is not recommended (Guenaga 2011) and the effectiveness of oral antibiotics in an uncleansed colon is not known (see below). No new antibiotic has appeared that warrants further investigation. Among those discussed above, ertapenem provided benefit but in a flawed study (Itani 2006). There is only weak and inconclusive evidence from two small studies which have compared antibiotic administration before or after surgery. That is an important point but one that is not likely to be subjected to a fair test again, i.e., prophylaxis has meant throughout the last 50 years antibiotics given before the surgery starts, before the colon is opened. And it has been found to be effective. Would prophylaxis given immediately after surgery but before infection occurs be as effective?
Overall completeness and applicability of evidence
This systematic review includes 260 randomised controlled trials (RCTs) with 43,451 patients and 68 different antibiotics, including 24 cephalosporins. At first glance it would appear that our goal should be to find the one antibiotic that provides the best outcome for elective colorectal surgery. In the earlier versions of this review it became apparent that a seemingly endless progression of individual comparisons would not lead to that desired result (Song 1998). Many of the RCTs included multiple variables between comparison groups ‐ for instance varying antibiotic type, route of administration, timing and duration of dosage ‐ making a simple interpretation of their results regarding antibiotic preference, as well as comparability to other studies impossible. Apparently, many other studies were performed for the purpose of demonstrating that a particular antibiotic was 'as good as' an existing approved antibiotic for prophylaxis, thus justifying similar regulatory approval for the new antibiotic. Therefore, out of the 260 studies, only 181 trials encompassing 25,314 patients are included in the combined analyses. In most of the studies included in this review pre‐operative cleansing of the colon was undertaken routinely and the more recent avoidance of this part of the pre‐operative routine casts some uncertainty over the applicability of the findings to current clinical practice.
Also, it was suggested, even by the first systematic reviews in this field (Baum 1981), that meta‐analysis of multiple different antibiotics when compared to placebo would yield a homogeneous result, i.e. that the individual antibiotic choice may not be nearly as important as the fact that some antibiotic be used, and that timing, coverage and duration are the most important variables. So our goal in this systematic review turned out to be not to name a single antibiotic for prophylaxis, but to re‐establish the principle of antibiotic prophylaxis, and to determine the best timing, duration, route of administration and overall bacterial coverage that should be used in antibiotic prophylaxis for elective colorectal surgery.
Attention must be paid to antibiotic specificity. The required spectrum of coverage in colorectal surgery is determined by the flora found within the patient's large bowel. This is a copious mixture of both anaerobic and aerobic species (Baeckhed 2005) rather than introduction of contaminants from the patient's skin or the operating room team (Gorbach 1991), so antibiotic choices that cover both anaerobic and aerobic bacteria gave the best results.
This is a large systematic review. It could have been much larger if we had chosen to pursue analysis of all reported outcomes, from those that might have much more to do with surgical technique such as anastomotic or fascial dehiscence, or patient‐related factors such as infections of the lungs or urinary bladder. Instead we focused on a single outcome, which was infection of the abdominal surgical wound. It can be seen from data cited in the Background section that much of what can go wrong in colorectal surgery is due to or associated with infection at this site (Kirkland 1999; Nespoli 2004). Surgical wound infection is reported as an outcome in virtually every antibiotic trial, since the stated purpose of antibiotic prophylaxis is to reduce this incidence. Other outcomes are reported with much less frequency, and also much less precision as they are harder to detect and lack commonly used definitions such as the Centers for Disease Control (CDC) guidelines on infection. In other meta‐analyses, it has been found that inclusion of infectious outcomes that are anatomically distant, or more broadly defined, introduces significant heterogeneity into the analyses and makes calculation of a summary statistic inadvisable (Nelson 2003). Heterogeneity was rare in our calculations.
Baum made three recommendations at the end of his classic report (Baum 1981). Firstly, that it was unethical to perform subsequent studies of antibiotic prophylaxis in colorectal surgery in which the control group received no treatment. Despite this, there have been nine such studies since (see Analysis 1.1). Secondly, in order to keep some organisation in the field, all subsequent studies should measure some factor (antibiotic choice, dose, timing, route) against an established guideline, so that narrowing of the field could take place. This has rarely been done. Of the 260 RCTs included here, 43 made such comparisons but 79 contained pairings that made no clinical sense, or contained so many variables between the two treatment groups that no specific conclusions could be drawn from the results. Thirdly, that the best prophylactic drugs might be 'fringe' antibiotics, i.e. ones that would not be used as a first‐line choice in the treatment of surgical infection. Oral neomycin/erythromycin base fulfilled this suggestion well. The prevalent use of cefoxitin, cefotetan or metronidazole does not. These are clearly efficacious, but one must be concerned about the potential for generating bacterial resistance to these important drugs.
The disappearance of doxycycline from clinical practice is curious. It was only reported extensively in the Scandinavian literature, but appeared to be efficacious. It has several advantages over cephalosporins, which are currently the most commonly used antibiotics in surgical prophylaxis. Doxycycline is not an antibiotic commonly used in the treatment of established surgical infection, nor is it prominently associated with causing C. difficile colitis, and it is not expensive. Doxycycline has not been studied extensively in comparison to other established gold‐standard antibiotic recommendations, but perhaps it should be.
It is tempting when looking back at so many comparisons to infer a transitive effect, i.e. to conclude that if one antibiotic is better than a second, and the second in turn is better than a third, that the first must be better than the third. There are statistical reasons why such a process is called the transitive fallacy and it should be avoided (Baker 2002).
Quality of the evidence
The quality of evidence across the comparisons in this review is high. Our analyses are robust to the exclusion of studies at high risk of attrition and detection bias in terms of both the direction and magnitude of effect. The clinical heterogeneity noted above in terms of the type of surgery and antibiotic used did not translate into substantial statistical heterogeneity. The results of many of the studies in our primary analyses suggest variation more by size rather than direction of effect. We do not consider publication bias or indirectness to impact on our confidence in the results.
Agreements and disagreements with other studies or reviews
Other meta‐analyses have already been published in this field. Among those that have been informative:
-
one looked at antibiotic prophylaxis versus no treatment (Baum 1981), frequently discussed above;
-
the second looked at route of administration of antibiotics (Lewis 2002);
-
Bellows 2011 also looked at route of administration and also provided useful guidance as to the search strategy for this review;
-
Kujath 1984 provided information about many randomised trials from the 1960s and 1970s;
-
Song 1998 provided a global assessment of all antibiotics in a somewhat narrower period from 1984 to 1997 and was the predecessor of this review.
The Lewis 2002 study focused on the comparison of combined oral and intravenous antibiotic prophylaxis versus intravenous prophylaxis alone. The authors added an additional RCT of their own to previously published studies dating from 1979 to 2002. A significant advantage to combined prophylaxis was found in the combined analysis (P value less than 0.0001). An appropriate assessment of the quality of the included studies was performed based upon that assessment, and no RCTs were excluded. Their analysis included 2065 patients. Our analysis agrees with their findings (Analysis 7.1). Significant heterogeneity was not present (P value 0.25; I2 = 18.8%) in the Lewis 2002 analyses.
This current systematic review is the first with the purpose of addressing the broader clinical questions, and to suggest a template for future antibiotic assessment. With these findings we can propose the guidelines outlined above and below.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
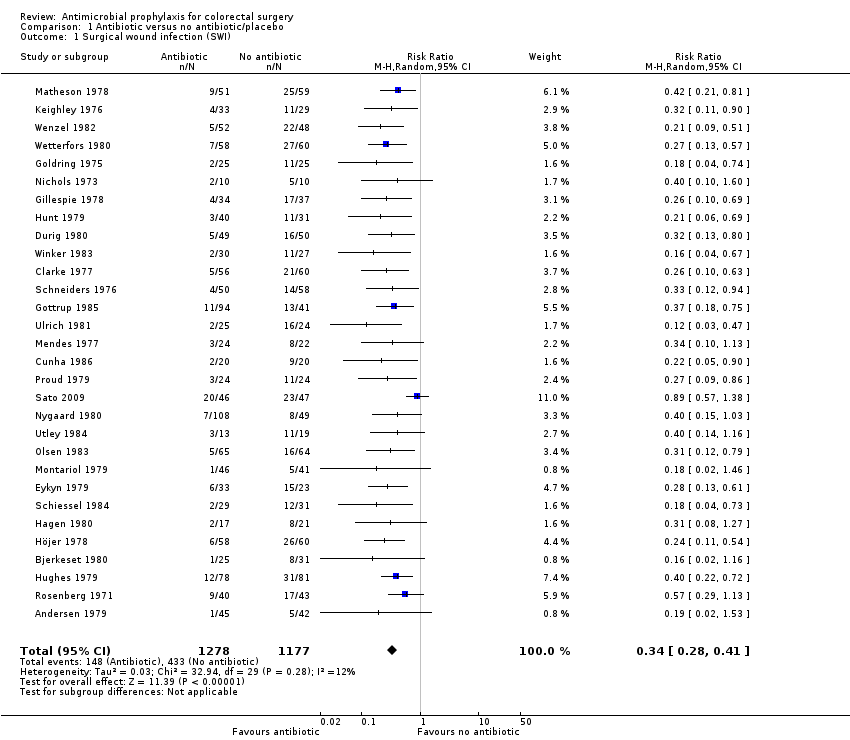
Comparison 1 Antibiotic versus no antibiotic/placebo, Outcome 1 Surgical wound infection (SWI).

Comparison 2 Duration of therapy, Outcome 1 Surgical wound infection (SWI).
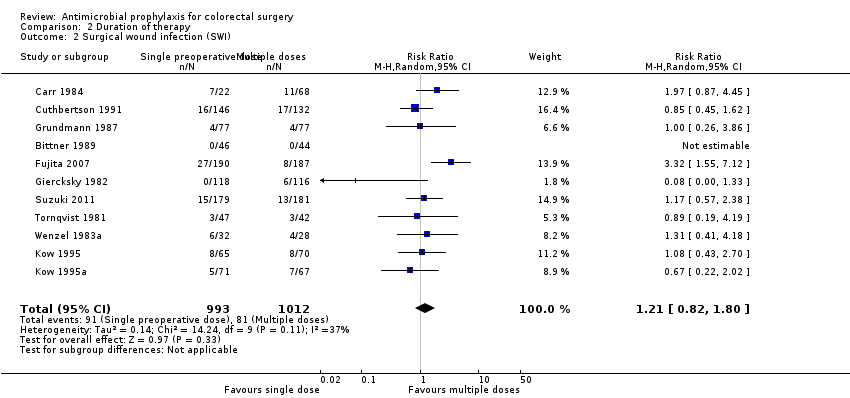
Comparison 2 Duration of therapy, Outcome 2 Surgical wound infection (SWI).

Comparison 3 Additional aerobic coverage, Outcome 1 Surgical wound infection (SWI).

Comparison 4 Additional anaerobic coverage, Outcome 1 Surgical wound infection (SWI).

Comparison 5 Aerobic versus anaerobic cover, Outcome 1 Surgical wound infection.
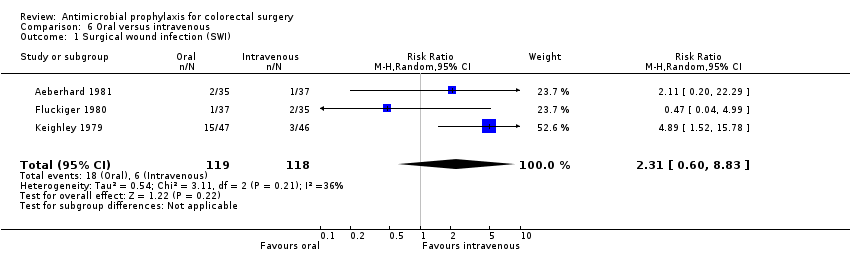
Comparison 6 Oral versus intravenous, Outcome 1 Surgical wound infection (SWI).

Comparison 7 Combined oral and intravenous versus oral or intravenous alone, Outcome 1 Surgical wound infection: oral + iv versus iv alone.

Comparison 7 Combined oral and intravenous versus oral or intravenous alone, Outcome 2 Surgical wound infection: combined oral and iv versus oral alone.

Comparison 8 Antibiotic given pre‐ or postoperatively, Outcome 1 Surgical wound infection.

Comparison 9 Antibiotic choice versus a gold standard, Outcome 1 Surgical wound infection (SWI).
| Antibiotic versus no antibiotic/placebo for colorectal surgery | ||||||
| Patient or population: patients undergoing colorectal surgery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antibiotic versus no antibiotic/placebo | |||||
| Surgical wound infection (SWI) | Study population | RR 0.34 | 2455 | ⊕⊕⊕⊕ | ||
| 368 per 1000 | 125 per 1000 | |||||
| Moderate | ||||||
| 391 per 1000 | 133 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We consider our results not to be affected by either post‐randomisation attrition or clinical heterogeneity. | ||||||
| Combined oral and intravenous compared to oral or intravenous alone for colorectal surgery | ||||||
| Patient or population: colorectal surgery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| oral or intravenous alone | Combined oral and intravenous | |||||
| Surgical wound infection: oral + iv versus iv alone | Study population | RR 0.55 | 2929 | high | ||
| 128 per 1000 | 70 per 1000 | |||||
| Moderate | ||||||
| 230 per 1000 | 126 per 1000 | |||||
| Surgical wound infection: combined oral and iv versus oral alone | Study population | RR 0.52 | 1880 | high | ||
| 79 per 1000 | 41 per 1000 | |||||
| Moderate | ||||||
| 146 per 1000 | 76 per 1000 | |||||
| Study population | not estimable | ( studies) | ||||
| 0 per 1000 | 0 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection (SWI) Show forest plot | 30 | 2455 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.28, 0.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection (SWI) Show forest plot | 34 | 5123 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.93, 1.29] |
| 2 Surgical wound infection (SWI) Show forest plot | 11 | 2005 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.82, 1.80] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection (SWI) Show forest plot | 15 | 1869 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.29, 0.68] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection (SWI) Show forest plot | 19 | 2687 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.30, 0.69] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection Show forest plot | 4 | 546 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.30, 2.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection (SWI) Show forest plot | 3 | 237 | Risk Ratio (M‐H, Random, 95% CI) | 2.31 [0.60, 8.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection: oral + iv versus iv alone Show forest plot | 15 | 2929 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.43, 0.71] |
| 2 Surgical wound infection: combined oral and iv versus oral alone Show forest plot | 9 | 1880 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.35, 0.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection Show forest plot | 2 | 129 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.21, 2.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection (SWI) Show forest plot | 43 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |

