Profilaxis antimicrobiana para la cirugía colorrectal
Appendices
Appendix 1. Search strategies
Search based on search strategy from Bellows 2011.
MEDLINE (OVID)
1. exp Surgical Wound Infection/
2. exp Postoperative Complications/
3. exp Bacterial Infections/
4. exp Infection/
5. exp Sepsis/
6. (postoperative complication* or infection* or sepsis).mp.
7. 1 or 2 or 3 or 4 or 5 or 6
8. exp Anti‐Infective Agents/
9. exp Anti‐Bacterial Agents/
10. (Anti infective or antibiotic* or antimicrobial* or anti bacterial*).mp.
11. exp Colorectal Surgery/
12. exp Colon/
13. exp Rectum/
14. exp Colectomy/
15. exp Colostomy/
16. (colon or rectum or rectal or colorectal or colectomy or colostomy).mp.
17. 8 or 9 or 10
18. 11 or 12 or 13 or 14 or 15 or 16
19. 7 and 17 and 18
20. randomized controlled trial.pt.
21. controlled clinical trial.pt.
22. randomized.ab.
23. placebo.ab.
24. clinical trial.sh.
25. randomly.ab.
26. trial.ti.
27. 20 or 21 or 22 or 23 or 24 or 25 or 26
28. humans.sh.
29. 27 and 28
30. 19 and 29
EMBASE (OVID)
1. exp surgical infection/
2. exp postoperative complication/
3. exp bacterial infection/
4. exp infection/
5. exp sepsis/
6. (postoperative complication* or infection* or sepsis).m_titl.
7. 1 or 2 or 3 or 4 or 5 or 6
8. exp antiinfective agent/
9. exp antibiotic agent/
10. (anti infective or antibiotic* or antimicrobial* or anti bacterial).m_titl.
11. 8 or 9 or 10
12. exp colorectal surgery/
13. exp colon/
14. exp rectum/
15. exp colon resection/
16. exp colostomy/
17. (colon or rectum or rectal or colorectal or colectomy or colostomy).m_titl.
18. 12 or 13 or 14 or 15 or 16 or 17
19. 7 and 11 and 18
20. randomized controlled trial/
21. randomization/
22. controlled study/
23. multicenter study/
24. phase 3 clinical trial/
25. phase 4 clinical trial/
26. double blind procedure/
27. single blind procedure/
28. ((singl* or doubl* or trebl* or tripl*) adj (blind* or mask*)).ti,ab.
29. (random* or cross* over* or factorial* or placebo* or volunteer*).ti,ab.
30. 25 or 22 or 26 or 28 or 21 or 27 or 23 or 20 or 29 or 24
31. "human*".ti,ab.
32. (animal* or nonhuman*).ti,ab.
33. 32 and 31
34. 32 not 33
35. 30 not 34
36. 19 and 35
The Cochrane Library
#1 MeSH descriptor Surgical Wound Infection explode all trees
#2 MeSH descriptor Postoperative Complications explode all trees
#3 MeSH descriptor Bacterial Infections explode all trees
#4 MeSH descriptor Infection explode all trees
#5 MeSH descriptor Sepsis explode all trees
#6 (postoperative complication* or infection* or sepsis):ti,ab,kw
#7 (#1 OR #2 OR #3 OR #4 OR #5 OR #6)
#8 MeSH descriptor Anti‐Infective Agents explode all trees
#9 MeSH descriptor Anti‐Bacterial Agents explode all trees
#10 (anti infective or antibiotic* or antimicrobial* or anti bacterial*):ti,ab,kw
#11 (#8 OR #9 OR #10)
#12 MeSH descriptor Colorectal Surgery explode all trees
#13 MeSH descriptor Colon explode all trees
#14 MeSH descriptor Rectum explode all trees
#15 MeSH descriptor Colectomy explode all trees
#16 MeSH descriptor Colostomy explode all trees
#17 (colon or rectum or rectal or colorectal or colectomy or colostomy):ti,ab,kw
#18 (#12 OR #13 OR #14 OR #15 OR #16 OR #17)
#19 (#7 AND #11 AND #18)
Appendix 2. Data Abstraction Form
ANTIMICROBIAL PROPHYLAXIS IN COLORECTAL SURGERY Ms#_____
Reviewer: ……………………………………………………………………………………….
Study ID (first author, year)……………………………………………………………………...
Source (journal title, year, volume, page numbers) …………..………………………………… …………..………………………………………………………………………………………..
Place of publication JournalBookUnpublished
Country of origin…………………………………………………………………….………….
Language of publication ………………………………………………………………………..
Source of funding ……………………………………………………………………………….
ELIGIBILITY CRITERIA
Is the study an RCT?YesNoUnclear
Does it include patients
undergoing colorectal surgery?YesNoUnclear
Does it examine the effectiveness
of antimicrobial PROPHYLAXIS?Yes NoUnclear
Do the authors report SWIs
as an outcome?YesNoUnclear
(note, if No, to this question but all other
eligibility criteria are met, contact author
to ascertain whether or not information on
SWIs was gathered but not reported in the paper)
Does the study meet the inclusion
criteria?YesNoUnclear
Comments/points for clarification with author …………………………………………………………………………………………………………………………………………………………………………………………………………METHODOLOGY
Generation of allocation sequenceAdequateInadequateUnclear/Not stated
Allocation concealment AdequateUnclear/InadequateNot usednot stated
Outcome assessor blinded YesNoUnclear/ Not possible Not stated
A priori calculation of sample size YesNoUnclear/ Not stated
Clear definition of inclusion/ YesNoUnclear/
exclusion criteria Not stated
Groups comparable at YesNoUnclear/
baseline Not stated
Withdrawal rate < 10% YesNoUnclear/Not stated
Clear explanation of drop‐outs YesNoUnclear/
in each treatment group Not stated
Post randomization drop‐outsYesNo
Divided by group
Adequate definition of SWI YesNoUnclear/Not stated
Aim of study ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
PARTICIPANTS
Number of patients assessed for eligibility ……………………………………………………
Source of recruitment ………………… ……………………………………………………….
Criteria for inclusion ………………… ……………………………………………………. .……………………………………………………………………………………………………
Criteria for exclusion ….………………… …………………………………………………. ……………………………………………………………………………………………………
Surgical procedures:colorectalappendicectomybiliary
electiveemergency
Bowel preparation procedure ……………………………………………………………………………………………………
Additional comments………………………………………………………………………………………………………………………………………………………………………………
| Group 1 | Group 2 | Group 3 | Group 4 | |
| Number of patients randomised | ||||
| Mean age | ||||
| Gender |
INTERVENTIONS
PurposeSingle vs multiple dose
Same antibiotic, different dose
Different antibiotics, one being a gold standard
Different antibiotics, neither guidelined
Extra antibiotics for aerobic coverage
Extra antibiotics for anaerobic coverage
Oral vs. IV vs. both
Topical
Placebo vs. Ab.
Multiple variables separating intervention groups
Other………………………………………………………………….
| Group A | Group B | Group C | |
| Antibiotics used | |||
| Dose | |||
| Time | |||
| Route of Administration | |||
| Duration of administration (hours) |
Additional comments
…………………………………………………………………………
OUTCOMES ASSESSED
Definition of SWI …………………………………………………………………………………………………………………………………………………………………………………………………………
| Group A | Group B | Group C | Group D | |
| Number of drop‐outs |

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
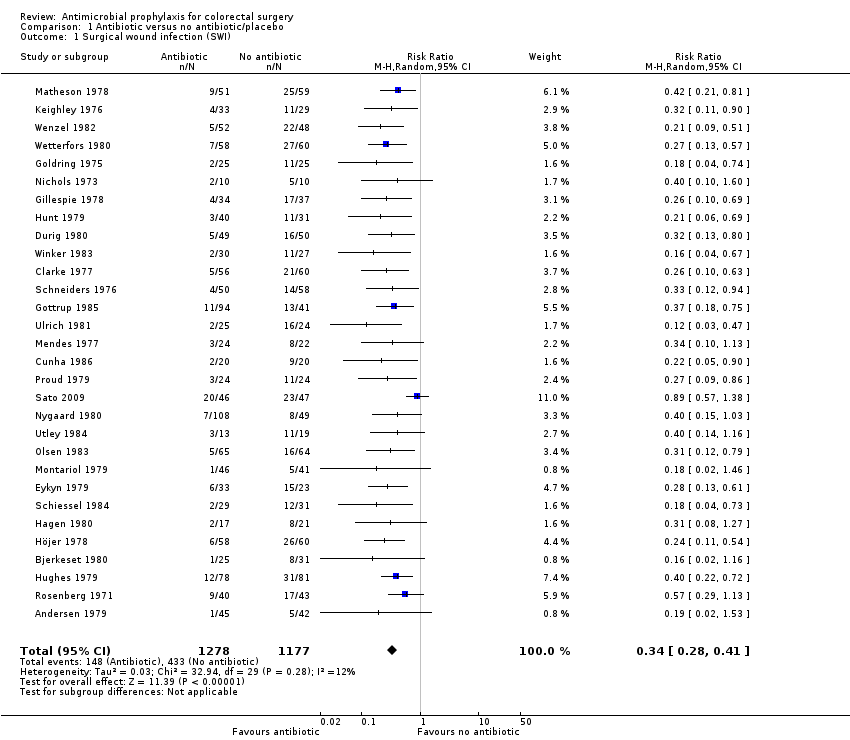
Comparison 1 Antibiotic versus no antibiotic/placebo, Outcome 1 Surgical wound infection (SWI).

Comparison 2 Duration of therapy, Outcome 1 Surgical wound infection (SWI).
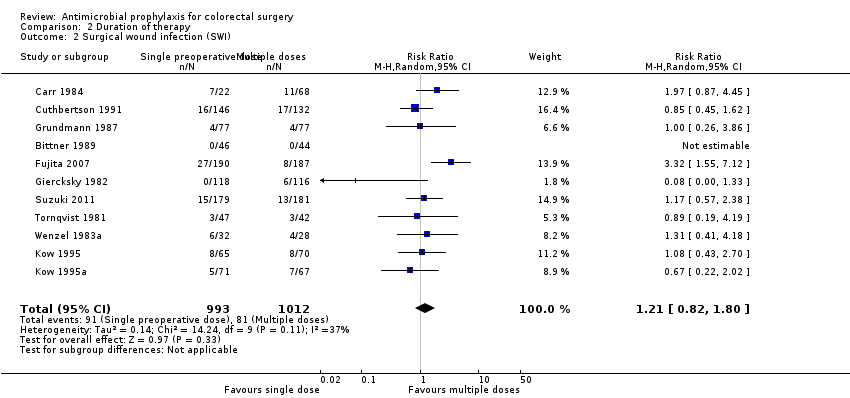
Comparison 2 Duration of therapy, Outcome 2 Surgical wound infection (SWI).

Comparison 3 Additional aerobic coverage, Outcome 1 Surgical wound infection (SWI).

Comparison 4 Additional anaerobic coverage, Outcome 1 Surgical wound infection (SWI).

Comparison 5 Aerobic versus anaerobic cover, Outcome 1 Surgical wound infection.
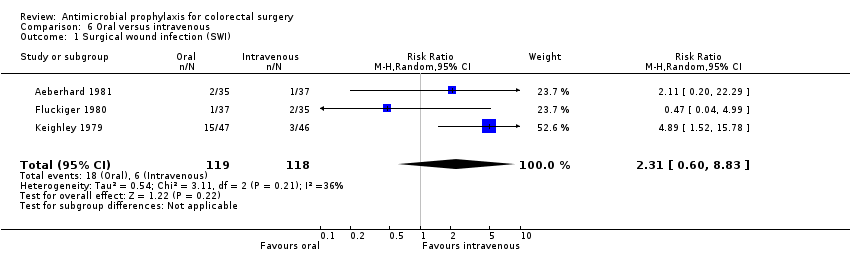
Comparison 6 Oral versus intravenous, Outcome 1 Surgical wound infection (SWI).

Comparison 7 Combined oral and intravenous versus oral or intravenous alone, Outcome 1 Surgical wound infection: oral + iv versus iv alone.

Comparison 7 Combined oral and intravenous versus oral or intravenous alone, Outcome 2 Surgical wound infection: combined oral and iv versus oral alone.

Comparison 8 Antibiotic given pre‐ or postoperatively, Outcome 1 Surgical wound infection.

Comparison 9 Antibiotic choice versus a gold standard, Outcome 1 Surgical wound infection (SWI).
| Antibiotic versus no antibiotic/placebo for colorectal surgery | ||||||
| Patient or population: patients undergoing colorectal surgery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antibiotic versus no antibiotic/placebo | |||||
| Surgical wound infection (SWI) | Study population | RR 0.34 | 2455 | ⊕⊕⊕⊕ | ||
| 368 per 1000 | 125 per 1000 | |||||
| Moderate | ||||||
| 391 per 1000 | 133 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We consider our results not to be affected by either post‐randomisation attrition or clinical heterogeneity. | ||||||
| Combined oral and intravenous compared to oral or intravenous alone for colorectal surgery | ||||||
| Patient or population: colorectal surgery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| oral or intravenous alone | Combined oral and intravenous | |||||
| Surgical wound infection: oral + iv versus iv alone | Study population | RR 0.55 | 2929 | high | ||
| 128 per 1000 | 70 per 1000 | |||||
| Moderate | ||||||
| 230 per 1000 | 126 per 1000 | |||||
| Surgical wound infection: combined oral and iv versus oral alone | Study population | RR 0.52 | 1880 | high | ||
| 79 per 1000 | 41 per 1000 | |||||
| Moderate | ||||||
| 146 per 1000 | 76 per 1000 | |||||
| Study population | not estimable | ( studies) | ||||
| 0 per 1000 | 0 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection (SWI) Show forest plot | 30 | 2455 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.28, 0.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection (SWI) Show forest plot | 34 | 5123 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.93, 1.29] |
| 2 Surgical wound infection (SWI) Show forest plot | 11 | 2005 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.82, 1.80] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection (SWI) Show forest plot | 15 | 1869 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.29, 0.68] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection (SWI) Show forest plot | 19 | 2687 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.30, 0.69] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection Show forest plot | 4 | 546 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.30, 2.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection (SWI) Show forest plot | 3 | 237 | Risk Ratio (M‐H, Random, 95% CI) | 2.31 [0.60, 8.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection: oral + iv versus iv alone Show forest plot | 15 | 2929 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.43, 0.71] |
| 2 Surgical wound infection: combined oral and iv versus oral alone Show forest plot | 9 | 1880 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.35, 0.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection Show forest plot | 2 | 129 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.21, 2.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection (SWI) Show forest plot | 43 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |

