Profilaxis antimicrobiana para la cirugía colorrectal
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: metronidazole 1 g iv, given over 40 min at premedication, with a postoperative dose of 500 mg in the evening and 500 mg 8‐hourly for 2 days Group B: doxycycline 200 mg iv at premedication and 100 mg in the morning for 2 days postoperatively | |
| Outcomes | SWI: swelling and reddening with temperature > 38°C and no other known cause; or purulent secretion from the wound; serous secretion yielding pathogenic bacteria on culture | |
| Notes | SWI 21/81 met; 16/76 doxycycline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 21% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective abdominal surgery* (*see footnotes) | |
| Interventions | Group A: cefuroxime 1.5 g, iv at skin incision and metronidazole 0.5 g iv at anaesthesia, with repeated doses of both after 8 h and 16 h Group B: single dose of cefuroxime 1.5 g iv at skin incision, plus metronidazole 0.5 g at the start of anaesthesia | |
| Outcomes | SWI: discharge of pus | |
| Notes | SWI 1/29 versus 2/19 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: metronidazole 500 mg iv perioperatively plus kanamycin 1 g iv postoperatively Group B: metronidazole 200 mg orally approx 6 doses preoperatively plus kanamycin 1 g orally approx 9 doses | |
| Outcomes | SWI: stratified as superficial or deep | |
| Notes | Translated from German | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: ampicillin plus sulbactam 1.5 g iv preoperatively followed by 2 x 8‐hourly postoperative doses of 750 mg Group B: gentamicin 1.5 mg/kg body weight plus metronidazole 500 mg iv preoperatively and 2 x 8‐hourly postoperative doses | |
| Outcomes | SWI: presence of pus or purulent discharge in the wound; marked cellulitis; serous discharge with positive bacteriological culture | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 10% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | Colostomy closure; n = 30 | |
| Interventions | Duration; cotrimoxazole and ornidazole for 1 or 7 days | |
| Outcomes | SWI | |
| Notes | 1/15 wound infection in each group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Methods | RCT | |
| Participants | eCRS (elective colorectal surgery); n = 96 | |
| Interventions | Mezlocillin versus cefuroxime, both got metronidazole | |
| Outcomes | SWI | |
| Notes | 4 drop‐outs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: cefazolin 1 g iv at premedication, then 4 further doses over next 2 days Group B: cefazolin as for Group A, plus metronidazole 500 mg preoperatively and 2 further doses over the next 2 days | |
| Outcomes | SWI: NA | |
| Notes | Duration of follow‐up not specified but presumed to be until hospital discharge | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Incomplete outcome data (attrition bias) | Low risk | |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: fosfomycin 8 g plus metronidazole 1 g iv given 30 min before skin incision, and 8 g fosfomycin 8 h later Group B: doxycycline 400 mg plus metronidazole 1 g iv 30 min before skin incision, and placebo 8 h later | |
| Outcomes | SWI: discharge of pus | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | 7.5% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Colorectal surgery | |
| Interventions | STUDY 1: short‐term prophylaxis over 48 h Group B: cefamandole iv (dose NA) STUDY 2: Ultra short‐term prophylaxis over 24 h Group B: cefotaxim iv (dose NA) STUDY 3: 1‐shot prophylaxis Group B: lamoxactam iv (dose NA) | |
| Outcomes | SWI: NA from translation | |
| Notes | Translated from German | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Incomplete outcome data (attrition bias) | Low risk | |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | As Anders 1984 above | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Study 2 as outlined in Anders 1984 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Incomplete outcome data (attrition bias) | Low risk | |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | As Anders 1984 and Anders 1984a above | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Study 3 as outlined in Anders 1984 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Incomplete outcome data (attrition bias) | Low risk | |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 100 | |
| Interventions | Neomycin versus bacitracin versus placebo | |
| Outcomes | SWI | |
| Notes | 1/45 versus 5/42 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) | High risk | 13% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | University Melbourne 1986 = Ryan 1986 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Methods | RCT | |
| Participants | Open CRS | |
| Interventions | Timentin versus mezlocillin | |
| Outcomes | SWI | |
| Notes | = University Melbourne 1989 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | 11.5% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: cefoxitin 2 g iv 30 min preoperatively, intraoperatively (for operations over 2 h long) and 8‐hourly for 72 h Group B: cephalothin 2 g iv 2 h preoperatively, 2 g during surgery and 6‐hourly postoperatively for 4 days | |
| Outcomes | SWI: purulent discharge or dehiscence | |
| Notes | Translated from French | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Emergency or elective colorectal surgery | |
| Interventions | Group A: cefoxitin 2 g iv (timing of dose not stated) Group B: piperacillin 4 g iv (timing of dose not stated) | |
| Outcomes | SWI: not defined | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: amoxycillin/clavulanic acid 2 g/200 mg as a single 30‐min infusion at induction of anaesthesia. If surgery expected to last more than 4 h, a perioperative dose of 2 g/200 mg was given within 3 h of incision Group B: cefotetan 2 g as a single 30‐min infusion on the induction of anaesthesia. No further dose | |
| Outcomes | SWI: primary infections subclassified as minor infection in cases of stitch abscess or wound abscess, and major infections in the case of intraperitoneal abscess, peritonitis, bacteraemia or septicaemia of intestinal origin | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | 6% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Colorectal surgery | |
| Interventions | Group A: tinidazole 160 mg iv 80 min prior to operation during anaesthesia Group B: metronidazole 500 mg iv 2 h preoperatively and then 8‐hourly for 3 days | |
| Outcomes | SWI: secondary healing or abscess in the area of the wound or fever | |
| Notes | Translated from German | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | No drop outs |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: metronidazole 1 g iv preoperatively, with 2 further doses of 500 mg at 8 h and 16 h after Group B: erythromycin base 1 g plus neomycin sulphate 1 g preoperatively at 1 pm, 2 pm and 11 pm on the day before operation | |
| Outcomes | SWI: possibly infected (wound inflamed without discharge or draining culture positive serous discharge); definitely infected (presence of a purulent discharge) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 14% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 69 | |
| Interventions | po neomycin/erythromycin +/‐ iv gentamycin/clindamycin | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) | High risk | 14.5% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | eIBD; n = 100 | |
| Interventions | phthalylsulphathiazole + colomycin duration | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Emergency and elective abdominal surgery | |
| Interventions | Group A: metronidazole 500 mg iv plus cephazolin 1 g iv intraoperatively and 2 further doses 6 h and 12 h later Group B: metronidazole 500 mg iv plus cephazolin 1 g iv preoperatively, with 2 further doses 6 h and 12 h later | |
| Outcomes | SWI: discharge of pus | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 24% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: true | |
| Participants | At risk abdominal surgery* | |
| Interventions | Group A: amoxycillin/clavulanic acid (co‐amoxiclav) 250 mg/125 mg iv at induction of anaesthesia Group B: amoxycillin/clavulanic acid 250 mg/125 mg iv at induction of anaesthesia and at 8 h and 16 h later | |
| Outcomes | SWI: discharge of pus | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Both groups: oral neomycin 1 g plus erythromycin 1 g on preoperative day. Cefoxitin 2 g iv before operation and at 6 h and 12 h after initial dose Group A: cefoxitin 1 g iv 6‐hourly for 5 days, beginning 6 h after the fixed postoperative dose Group B: placebo | |
| Outcomes | SWI: purulent drainage, regardless of culture results, or if non‐purulent material contained pathogenic bacteria | |
| Notes | Adverse events collected: only one minor skin rash in each group. and cost unknown | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | Colorectal cancer and diverticular disease surgery; n = 101 | |
| Interventions | Metronidazole oral or iv | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | |
| Incomplete outcome data (attrition bias) | Low risk | 4% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | All patients received tobramycin 1.5 mg/kg iv 1 h preoperatively, repeated 8 h and 16 h later Group A: erythromycin 500 mg iv 1 h preoperatively, repeated 8 h and 16 h later Group B: metronidazole 500 mg iv 1 h preoperatively, repeated 8 h and 16 h later | |
| Outcomes | SWI: classed as major or minor depending on length of hospital stay | |
| Notes | Single centre study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 36% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: cefotetan 2 g iv at time of anaesthesia and 2 further 12‐hourly doses Group B: clindamycin 600 mg plus aztreonam 1 g iv at induction of anaesthesia, 2 further 8‐hourly doses | |
| Outcomes | SWI: purulent discharge with or without culture of pathogenic micro‐organisms | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 11% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: doxycycline 400 mg plus metronidazole 1.5 g iv sometime between 12 minutes to 6 hours preoperatively Group B: doxycycline 400 mg plus placebo | |
| Outcomes | SWI: discharge and positive culture | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Incomplete outcome data (attrition bias) | High risk | 14% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: mezlocillin 4 g plus metronidazole 500 mg iv 20 to 30 min preoperatively Group B: mezlocillin 4 g plus metronidazole 500 mg iv preoperatively and repeated 8‐hourly for 2 days | |
| Outcomes | SWI: differentiated between superficial infections with little secretion, and those with abscess or peritonitis No information on adverse events or cost were available in the translation | |
| Notes | Translated from German | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Incomplete outcome data (attrition bias) | Low risk | |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | CRS; n = 80 | |
| Interventions | Metronidazole oral versus placebo | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) | High risk | 30% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: ticarcillin/clavulanic acid 3 g/100 mg iv infused at induction of anaesthesia Group B: metronidazole 500 mg plus netilicin 80 mg iv infused within 15 min of induction of anaesthesia then 8 h and 16 h later | |
| Outcomes | SWI: purulent discharge from surgical wound or peritoneal cavity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | 7% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | eCRS; n = 34 | |
| Interventions | Piperacillin given iv preoperatively to either 24 h or 5 d post op | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 80 | |
| Interventions | Cephalothin with either metronidazole or erythromycin base | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | 1% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: metronidazole 500 mg plus netilmicin 100 mg iv on induction of anaesthesia and 8 h and 16 h later Group B: doxycycline 200 mg iv 4 h preoperatively and then 100 mg daily for 5 days | |
| Outcomes | SWI: pus present in the wound, or if microbiological culture was positive from a wound | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 10% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Emergency and elective colorectal surgery | |
| Interventions | Group A: ceftriaxone 2 g plus metronidazole 1.5 g iv at start of operation Group B: gentamicin 120 mg plus metronidazole 1.5 g iv at start of operation | |
| Outcomes | SWI: wound sepsis graded as minor or major. Major defined as discharge of pus from a wound opening at least 2 cm in length with fever and anorexia | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | 2.5% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | CRS; n = 87 | |
| Interventions | Phthalysulfathiazole 2 g q 6 h for 96 h preoperatively versus neomycin 1 g and bacitracin 100,000 units q 12 h for 48 h preoperatively; all oral | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | |
| Incomplete outcome data (attrition bias) | High risk | 18% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective radical resection of colorectal cancer | |
| Interventions | Group A: oral gentamicin 80 mg plus metronidazole 400 mg preoperatively, then 8‐hourly for 2 days Group B: gentamicin 80 mg plus metronidazole 400 mg 8‐hourly for 2 days preoperatively, followed by gentamicin 80 mg plus metronidazole 500 mg iv at induction of anaesthesia and 6 h and 12 h later | |
| Outcomes | SWI: not defined | |
| Notes | Translated from Chinese | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: gentamicin 80 mg plus clindamycin 600 mg im 1 h preoperatively and 8 h later Group B: cefoxitin 2 g iv prior to skin incision then 2 h and 6 h after first dose | |
| Outcomes | SWI: not defined | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: not clear | |
| Participants | Emergency elective abdominal surgery | |
| Interventions | Group A: mezlocillin 5 g at induction of anaesthesia plus mezlocillin 2 g 8‐hourly for 48 h Group B: cefuroxime 750 mg plus metronidazole 500 mg at induction of anaesthesia and then 8‐hourly for 72 h | |
| Outcomes | SWI: primary = first discharge from a dry sutured wound being pus; secondary = discharging serum, bile or intestinal contents, subsequently becoming contaminated with micro‐organisms | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | 7% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: metronidazole 500 mg iv preoperatively plus placebo Group B: metronidazole 500 mg iv preoperatively and at 8 h postoperatively plus placebo Group C: metronidazole 500 mg iv preoperatively and at 8 h and 16 h postoperatively plus placebo Group D: metronidazole 500 mg iv preoperatively and at 8 h, 16 h and 24 h postoperatively | |
| Outcomes | SWI: purulent discharge from the main suture line, even if culture was negative No information on adverse events or cost provided | |
| Notes | Duration | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Both groups: metronidazole 1 g iv at induction of anaesthesia and 12 h postoperatively Group A: additional cefuroxime 1.5 g iv 8‐hourly for 2 days Group B: no additional antibiotics | |
| Outcomes | SWI: discharge or pus. Classified as early when found within 10 days after the operation, and otherwise as late. Only primary wound sepsis was recorded No information on adverse events or cost provided | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Incomplete outcome data (attrition bias) | Low risk | 1% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 116 | |
| Interventions | Neomycin + erythromycin versus placebo | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | 167 but trial stopped early due to preliminary results; 116 analysed; no drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective surgery | |
| Interventions | Group A: cefuroxime 750 mg im preoperatively, 750 mg liquid over the fascia before skin closure and 750 mg iv at the end of the operation, repeated 4 times every 6 h Group B: cefuroxime 750 mg iv at the end of the operation, repeated 6 times every 6 h | |
| Outcomes | SWI: not defined | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | Veterans Administration co‐operative study, 4331 eligible, 1751 randomised | |
| Interventions | Neomycin + erythromycin base, 3 doses 1 g each preoperative +/‐ cephalothin 2 iv 3 doses preoperative | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | 37% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 281 | |
| Interventions | Neo. + eryth. 2 g/1 g 3 doses preoperative +/‐ cefoxitin at induction and q 6 h x 2 postoperative | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: cefoxitin 1 to 2 g iv at induction of anaesthesia, intraoperatively and every 6 h for the first postoperative day Group B: cefoxitin, as in Group A, plus neomycin 8 g/day plus erythromycin base 4 g/day in divided doses for 24 h preoperatively | |
| Outcomes | SWI: suppurated and had positive cultures | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | All groups: oral erythromycin plus neomycin administered the day before surgery Group A: cefuroxime 1.5 g plus metronidazole 500 mg 30 to 60 min prior to incision Group B: cefuroxime 1.5 g 30 to 60 min prior to incision Group C: cefoxitin 2 g 30 to 60 min prior to incision and every 6 h for 3 additional doses Group D: cefoxitin 2 g 30 to 60 min prior to incision | |
| Outcomes | SWI: an unsatisfactory response was defined in terms of patients with evidence of wound or systemic infections, including chills, sweating, temperature > 38°C, or purulent drainage, swelling or erythema at the incision site | |
| Notes | In this reference only Groups C & D, seen in comparison Analysis 2.1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Incomplete outcome data (attrition bias) | High risk | 12% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | As Corman 1993 | |
| Participants | ||
| Interventions | Groups A & B from Corman 1993 | |
| Outcomes | SWI seen in comparison Analysis 5.1 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Incomplete outcome data (attrition bias) | High risk | 12% drop out |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: metronidazole 500 mg iv plus cefuroxime 1.5 g iv preoperatively Group B: metronidazole 500mg iv preoperatively | |
| Outcomes | SWI: purulent discharge from the main suture line irrespective of negative bacteriological culture | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | Colostomy closure; n = 40 mean age 33.5. 80% male | |
| Interventions | Group A: oral tinidazole 2 grams single dose 10‐12 hours pre operatively Group B: placebo | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | = U Melbourne 1983; eCRS; n = 248 | |
| Interventions | Tinidazol 2 g preoperative +/‐ cefamandol 1 g preoperative and 2 hours later | |
| Outcomes | SWI | |
| Notes | = U Melbourne 1983 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | 15% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: ticarcillin/clavulanic acid (Timentin) 3.1 g iv commenced before skin incision and given over 30 min Group B: ticarcillin/clavulanic acid 3.1 g iv before skin incision and at 2 h after start of operation | |
| Outcomes | SWI: purulent discharge from the suture line or a non‐purulent discharge that contained pathogenic bacteria. Minor WI: localised inflammation with only a serous discharge | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Incomplete outcome data (attrition bias) | High risk | 17% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: sulbactam 1 g plus ampicillin 1 g iv, 4 doses 6‐hourly, commencing at induction of anaesthesia Group B: cefoxitin 2 g iv 4 doses 6‐hourly commencing at induction of anaesthesia | |
| Outcomes | SWI: major wound sepsis = discharge of pus from wound; minor = non‐purulent, bacteriologically positive wound discharge | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 12% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true | |
| Participants | Emergency and elective colorectal surgery | |
| Interventions | Group A: gentamicin 80 mg iv at anaesthesia and then tid for 48 h Group B: gentamicin 80 mg plus ticarcillin 5 g iv 1 h preoperatively and then tid for 48 h | |
| Outcomes | SWI: NA from translation | |
| Notes | Translated from French | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Incomplete outcome data (attrition bias) | High risk | 15% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: not clear | |
| Participants | Emergency and elective colorectal surgery | |
| Interventions | Group A: mezlocillin 5 g at induction of anaesthesia then 2 g at 8 h and 16 h postoperatively Group B: cefuroxime 1.5 g plus metronidazole 500 mg at induction of anaesthesia then cefuroxime 750 mg plus metronidazole 500 mg at 8 h and 16 h postoperatively | |
| Outcomes | SWI: minor = swelling with purulent or bacteriologically positive discharge but no constitutional upset; severe = pus discharged, the patient suffered constitutional symptoms and discharge from hospital was delayed | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | 7% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | Colorectal cancer; n = 124 | |
| Interventions | Cefminox 2 g versus cefoxitin 1 g iv preoperative and cefoxitin one more dose | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | 25% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | CRS; n = 109 | |
| Interventions | Neomycin + oral or iv metronidazole | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) | High risk | 28% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective abdominal surgery* | |
| Interventions | All colorectal patients: neomycin 1 g plus erythromycin 1 g at 19 h, 18 h and 9 h preoperatively. Patients received additional dose if operation exceeded 2 to 4 h Group A: cefmetazole 2 g iv preoperatively Group B: cefoxitin 2 g iv preoperatively and then 6‐hourly for 2 doses | |
| Outcomes | SWI: drained purulent material spontaneously or as a result of incision | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 21% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true # centres unknown | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: cefazolin 2 g at induction of anaesthesia iv over 20 min. Second dose at 4 h if long operation Group B: no treatment | |
| Outcomes | SWI: NA | |
| Notes | Translated from German | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Incomplete outcome data (attrition bias) | Low risk | 1% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | eCRS; n = 123 | |
| Interventions | Cephaloridine im versus neo/erythro po | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT Single centre | |
| Participants | Elective Colorectal surgery; n = 300 | |
| Interventions | 3 groups A: 1 g oral neomycin and 1 g metronidazole at 15, 19, 23 h day before surgery B: single oral dose at 15 h C: no oral antibiotic; all 3 groups got 1 g iv cefoxitin | |
| Outcomes | SWI, dehiscence, urine tract infection, abd. abscess, blood transfusion, nausea and vomiting | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | eCRS; n = 95 | |
| Interventions | Metronidazole iv versus. placebo | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | 13% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | All patients: neomycin 1 g plus erythromycin 1 g 3 times preoperatively Group A: cefonicid 1 g iv given 30 min to 1 h preoperatively, and 2 g 6‐hourly thereafter for up to 24 h | |
| Outcomes | SWI: discharge of pus | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 14% |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: cefotaxime 1 g iv at premedication, and 1 g with the abdomen open but before visceral procedures. 2 more 1 g doses every 4 h. If the operation lasted for more than 3 h, an additional dose of 1 g was given 3 h after last injection Group B: cefotaxime as in Group A. Metronidazole or ornidazole 1 g iv in 2 injections Group C: metronidazole 750 mg/day preoperatively for 3 days before operation, plus cefotaxime as in Group A | |
| Outcomes | SWI: estimated by combining local wound abscess with abdominal wall discharge | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: neomycin 1 g plus erythromycin 1 g preoperatively 2 h before mannitol and at 18 h and 10 h preoperatively Group B: metronidazole 500 mg plus gentamicin 80 mg iv 2 h preoperatively and 8 h and 16 h after the first dose | |
| Outcomes | SWI: classified as: no signs of sepsis; erythema, swelling or excessive pain or tenderness in wound not opened; wound opened, no pus; pus visible in wound | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: cefotaxime sodium 1 g (4 injections, total 4 g) plus metronidazole 500 mg (3 injections, total 1.5 g) iv for 24 h starting at induction of anaesthesia Group B: ceftriaxone sodium 1 g plus ornidazole 1 g iv at induction of anaesthesia | |
| Outcomes | SWI: discharge of pus or inflammation of serous discharge. Peritonitis, anastomotic leakage | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 15% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | eCRS; n = 72 | |
| Interventions | Metronidazole and kanamycin given either iv or po | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: ceftriaxone 1 g before anaesthetic Group B: clindamycin 0.6 g plus aztreonam 1 g in the morning and evening on the day before operation, and then at anaesthesia on the day of surgery and 8 h and 16 h after preoperative dose | |
| Outcomes | SWI: suppuration of wounds, dehiscence of anastomosis, fever > 38°C beyond 6 days postoperatively | |
| Notes | Translated from Italian | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 21 | |
| Interventions | Neomycin po plus either cefoxitin or ceftizoxime iv | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | A tiny study and 14% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS CA; n = 384 | |
| Interventions | Single versus 3 doses of cefmetazole given pre‐ and +/‐ postoperatively | |
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | 2% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: ceftriaxone 2 g plus metronidazole 500 mg iv 30 min prior to induction of anaesthesia Group B: ceftazidime 2 g plus metronidazole 500 mg iv 30 min prior to induction of anaesthesia and 8‐hourly for 24 h | |
| Outcomes | SWI: not defined | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 100 | |
| Interventions | Cefoxitin versus metronidazole, both iv, pre‐ and postoperatively | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | 14% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: | |
| Participants | Colorectal surgery | |
| Interventions | Group A: pefloxacin 800 mg iv slow infusion plus metronidazole 500 mg iv 1 to 2 h before surgery, followed by metronidazole alone 6 h and 12 h later Group B: netilmicin 200 mg im with metronidazole 500 mg iv 1 to 2 h before surgery and both 6 h and 12 h later | |
| Outcomes | SWI: absence of sterile drainage with intact fascia | |
| Notes | Translated from Italian | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Incomplete outcome data (attrition bias) | Low risk | NO DROP OUTS |
| Blinding of outcome assessment (detection bias) | High risk | YES |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: doxycycline 400 mg plus tinidazole 1.6 g iv infusion over 2 h shortly before operation Group B: doxycycline 400 mg plus placebo | |
| Outcomes | SWI: purulent discharge or fluid discharge yielding positive bacteriological culture | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Incomplete outcome data (attrition bias) | Low risk | 6% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | eCRS; n = 240 | |
| Interventions | Tinidazole +/‐ doxycycline | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) | Low risk | 5% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | eCRS; n = 282 | |
| Interventions | Tinidazole +/‐ doxycycline | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) | Low risk | 5% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | eCRS; n = 71 | |
| Interventions | Kanamycin and metronidazole po versus placebo; n = 71 | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | eCRS; n = 50 | |
| Interventions | Kanamycin and metronidazol versus placebo po; n = 50 | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: doxycycline 0.4 g in 1 litre of saline as an infusion completed 2 h preoperatively Group B: doxycycline 0.2 g preoperatively as for Group A, then doxycycline 0.1 g iv for 3 days postoperatively | |
| Outcomes | SWI: pus or fluid emptied spontaneously or after incision from the wound, or pus recovered from the abdomen at laparotomy, or anastomotic leakage | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Incomplete outcome data (attrition bias) | High risk | 12% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: latamoxef (moxalactam) 2 g iv at induction of anaesthesia Group B: ciprofloxacin 200 mg iv at induction of anaesthesia plus metronidazole 500 mg iv 2 h preoperatively | |
| Outcomes | SWI: not defined | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | 7.5% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: whole gut irrigation alone Group B: whole gut irrigation plus metronidazole 500 mg iv 30 min preoperatively, then 8‐hourly for 3 days Group C: whole gut irrigation plus ampicillin 1 g, plus metronidazole 500 mg iv 30 min preoperatively, then 8‐hourly for 3 days | |
| Outcomes | SWI: superficial accumulation of pus requiring surgical drainage | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Incomplete outcome data (attrition bias) | Low risk | 8% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: metronidazole 500 mg plus mezlocillin 5 g preoperatively Group B: 3 x the same combination of antibiotics at premedication, 1.5 h after skin incision and 6 h later | |
| Outcomes | SWI: not defined | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: doxycyline 200 mg iv 12 h preoperatively, intraoperatively and for 3 days postoperatively Group B: doxycycline as for Group A plus tinidazole 2 g at 12 h and 24 h preoperatively | |
| Outcomes | SWI: presence of pus in wound | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: rifamixin 200 mg tid 3 days preoperatively plus placebo (as for Group B) Group B: gentamycin 100 mg iv 1 h preoperatively over 30 min plus placebo (as for Group A) Group C: both antibiotics | |
| Outcomes | SWI: superficial pus requiring drainage | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Incomplete outcome data (attrition bias) | Low risk | 7.8% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | eCRS; n = 75 | |
| Interventions | Metronidazole po versus placebo; n = 75 | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) | High risk | 49% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: cefotaxime 2 g iv at induction of anaesthesia and 3 h and 9 h later Group B: cefotaxime 2 g plus metronidazole 1.5 g iv at induction of anaesthesia | |
| Outcomes | SWI: discharge of pus from the wound. Anastomotic leakage: air or faeces, discharged through a drain or fistula. Intra‐abdominal abscess: collection of pus detected by either ultrasound‐guided aspiration or spontaneous discharge of pus directly from the peritoneum | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Incomplete outcome data (attrition bias) | High risk | 14% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true | |
| Participants | Emergency and elective abdominal surgery | |
| Interventions | Group A: latamoxef (moxalactam) 1 g iv at induction of anaesthesia Group B: latamoxef 1 g iv at induction of anaesthesia then every 6 h for a further 7 doses | |
| Outcomes | SWI: purulent wound discharge or a serous wound discharge with culture of pathogenic organisms. A WI was classified as major if it resulted in an extension of the hospital stay or required dressings at home for more than 7 days | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Incomplete outcome data (attrition bias) | Low risk | 0.7% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: amoxycillin/clavulanic acid 1 g/200 mg (co‐amoxiclav 1.2 g) immediately before operation and 2 h later Group B: gentamicin 120 mg immediately before operation and 2 h later, plus 1 preoperative dose of metronidazole 1.5 g | |
| Outcomes | SWI: pus in a surgical wound with, or without, constitutional disturbance such as fever, dehiscence, foul smell or prolonged hospital stay | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Incomplete outcome data (attrition bias) | High risk | 10% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true | |
| Participants | Elective abdominal operation (laparotomy)* Single centre | |
| Interventions | Group A: ceftriaxone 1 g plus metronidazole 500 mg iv after induction of anaesthesia Group B: cefamandole 1 g plus metronidazole 500 mg iv after induction of anaesthesia | |
| Outcomes | SWI: purulent or serous wound discharge with culture of pathogenic organisms | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | 4% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: neomycin 1 g plus metronidazole 250 mg tid day before surgery Group B: mezlocillin 5 g iv plus metronidazole 500 mg iv on call | |
| Outcomes | SWI: presence of pus | |
| Notes | Translated from German | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 29% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | eCRS; n = 171 | |
| Interventions | 4 groups: a. metronidazole po for 3 days preoperatively, 1 g then 200 mg plus oral kanamycin b. metronidazole iv preoperatively and then 2 h later, 1 g then 500 mg plus iv kanamycin 12 h and 2 h preoperatively c. metronidazole po as above alone d. metronidazole as above but no kanamycin | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) | High risk | 23% drop‐out in a/b and 18% drop‐out in c/d |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | All groups: antimicrobial treatment started after the induction of anaesthesia, and was continued 8 h and 16 h later Group A: metronidazole 500 mg iv followed immediately by netilmicin 100 mg iv 3 doses Group B: metronidazole 500 mg iv followed immediately by cefuroxime 1.5 g iv 3 doses Group C: metronidazole 500 mg iv followed immediately by saline iv 3 doses | |
| Outcomes | SWI: not defined | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Incomplete outcome data (attrition bias) | High risk | 28% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 153 | |
| Interventions | Cefotetan versus piperacillin | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery 2 centres | |
| Interventions | Group A: cefotetan 2 g iv at induction of anaesthesia Group B: piperacillin 2 g iv 3 doses commencing at induction of anaesthesia | |
| Outcomes | SWI: presence of an abscess or discharging pus from the wound (excluding erythema or serous discharge) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | 9% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | CRS; n = 157 | |
| Interventions | All got neomycin and cephalothin iv pre‐ and postoperatively; 4 groups: a. Metronidazole 750 mg tid po the day before surgery b. Erythromycin 1 g po tid the day before surgery c. Metronidazole 750 mg po tid for 2 days preoperatively d. Metronidazole 1 g iv pre‐ and postoperatively | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | |
| Blinding of outcome assessment (detection bias) | Low risk | No drop‐out |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: latamoxef (moxalactam) 2 g iv 1 h preoperatively and at 4 h and 8 h after first dose Group B: neomycin 1 g orally tid on the day before surgery plus metronidazole 1 g iv 1 h preoperatively followed by 500 mg at 8 h and 16 h after first dose | |
| Outcomes | SWI: inflammation and induration with discharge of pus, irrespective of wound culture results | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | 4% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | eCRS; n = 20 | |
| Interventions | Metronidazole iv in different doses; 500 mg and 150 mg preoperatively | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: oral kanamycin 1 g 4‐hourly for 48 h preoperatively. Cefoxitin 2 g iv, 3 doses given over 3 min at 2 h intervals Group B: oral kanamycin 1 g 4‐hourly for 48 h preoperatively | |
| Outcomes | SWI: purulent discharge | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Incomplete outcome data (attrition bias) | High risk | 18% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: ceftizoxime 2 g plus metronidazole 500 mg iv infusion at induction of anaesthesia Group B: ceftizoxime 2 g plus placebo iv infusion at induction of anaesthesia Both groups received a second dose of ceftizoxime 2 h later | |
| Outcomes | SWI: discharged pus. Minor WI = superficial pus in the incision. Major WI = wound dehiscence or associated with intra‐abdominal or pelvic abscess and/or systemic disturbance | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Incomplete outcome data (attrition bias) | High risk | 13% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | CRS; n = 177 | |
| Interventions | Cephaloridine versus placebo im preoperatively | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) | High risk | 10% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | CRS; n = 71 | |
| Interventions | Tinidazole po preoperatively versus placebo | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | eCRS; n = 142 | |
| Interventions | Doxycycline 200 mg po preoperatively and for 5 d postoperatively versus placebo | |
| Outcomes | SWI | |
| Notes | Same as Höjer 1978a | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) | High risk | 17% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | CRS; n = 116 | |
| Interventions | Doxycycline po for 5 days versus placebo | |
| Outcomes | ||
| Notes | Same numbers and procedure as Hojer 1978 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: cefoxitin 1 g iv at induction, 1 h postoperatively and bid for 2 days Group B: kanamycin 2 g/day orally and erythromycin 1.6 g/day orally divided into 4 doses for 2 days before surgery. Cefoxitin 1 g iv at induction, 1 h postoperatively and bid for 2 days | |
| Outcomes | SWI: Centre for Disease Control definition | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Incomplete outcome data (attrition bias) | Low risk | 2% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | Elective colorectal surgery with stratification for rectal surgery; n = 1002 | |
| Interventions | Ertapenem 1 g versus cefotetan 1 g prophylaxis iv within 60 min of incision | |
| Outcomes | SWIs, deep wound infection, anastomotic leak, C. difficile, mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Incomplete outcome data (attrition bias) | High risk | 23% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: Three doses of cefoxitin 1 g iv commencing 1.5 h preoperatively, administered over 20 to 30 min, were given at 6 h intervals Group B: Two doses of doxycycline 200 mg IV commencing 1.5 h preoperatively. | |
| Outcomes | SWI: wounds requiring drainage. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Incomplete outcome data (attrition bias) | High risk | 27% drop‐out (38) |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 107 | |
| Interventions | All received neomycin 1 g po q 4 h day preoperatively a. Cefotaxime b. Cefotaxime continued for 4 doses postoperatively c. Cefazolin as b | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | 7.5% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Both groups: oral neomycin 1 g every 3 h with a total of 3 g given to all patients Group A: metronidazole 15 mg/kg iv 1 h preoperatively then 7.5 mg/kg 6 h and 12 h later Group B: placebo 3 times, as for Group A | |
| Outcomes | SWI: passage or isolation of pus with a positive culture in the postoperative follow‐up period | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 22% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 94 | |
| Interventions | Piperacillin versus cefoxitin iv pre‐ and early postoperatively | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | 8.5% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: cefotetan 2 g iv 30 to 60 min before initial incision Group B: cefoxitin 2 g iv 30 to 60 min before initial incision then 2 g every 6 h for no more than 24 h postoperatively | |
| Outcomes | SWI: graded 0 to 3 (0 = no erythema or discharge; 3 = infection throughout wound or intra‐abdominal abscess) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 17% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true | |
| Participants | Emergency and elective colorectal surgery | |
| Interventions | Group A: cefuroxime 3 g plus metronidazole 1.5 g iv after induction of anaesthesia Group B: ampicillin 3 g plus metronidazole 1.5 g iv after induction of anaesthesia Group C: ampicillin 1 g plus metronidazole 500 mg iv after induction of anaesthesia and 8 h and 16 h later | |
| Outcomes | SWI: accumulation of pus, either with spontaneous discharge or requiring surgical drainage | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Incomplete outcome data (attrition bias) | Low risk | 8% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Emergency surgical procedures requiring prophylactic antibiotics (abdominal, obstetrics/gynaecology, orthopaedic) | |
| Interventions | Group A: cefoperazone 1 g as a slow iv bolus injection after induction of anaesthesia Group B: cefotaxime 1 g as slow iv bolus injection after induction of anaesthesia An additional 1 g was administered intraoperatively if the procedures lasted longer than 2 drug serum half‐lives, or approximately 2 h | |
| Outcomes | SWI: presence of purulent material drained from the surgical incision or the peritoneal cavity, regardless of bacteriological or laboratory investigation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Incomplete outcome data (attrition bias) | High risk | 17% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective surgical procedures requiring prophylactic antibiotics (abdominal, obstetrics/gynaecology, orthopaedic) | |
| Interventions | Intraluminal antimicrobials were used in all colorectal operations Group A: cefazolin 1 g iv on arrival in the operating room and every 8 h for 24 h Group B: cefoxitin 2 g iv on arrival in the operating room and 2 g every 6 h for 24 h Group C: cefotaxime 1 g iv on arrival in the operating room If operation lasted longer than 2 h, 1 additional dose of 1 g was administered intraoperatively for patients receiving cefazolin or cefotaxime | |
| Outcomes | SWI: presence of purulent material drained from the surgical incision or the peritoneal cavity, regardless of bacteriological or laboratory investigation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Incomplete outcome data (attrition bias) | High risk | 33% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective surgical procedures requiring prophylactic antibiotics (abdominal, obstetrics/gynaecology, orthopaedic) | |
| Interventions | Group A: ticarcillin/clavulanic acid (Timentin) 3.1 g iv slow bolus injection upon induction of anaesthesia for at least 3 days Group B: cefotaxime 1 g iv at induction of anaesthesia then 3 further doses postoperatively | |
| Outcomes | SWI: purulence at wound site | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 13% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | eCRS; n = 40 | |
| Interventions | Tinidazole iv versus ornidazole po pre‐ and early postoperative | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | All patients: metronidazole 1.5 g plus ampicillin 3 g iv during induction of anaesthesia and operation Group A: metronidazole 500 mg plus ampicillin 1 g iv tid for the second and third postoperative days Group B: no further treatment | |
| Outcomes | SWI: accumulation of pus either with spontaneous discharge or requiring surgical drainage | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Incomplete outcome data (attrition bias) | Low risk | 5% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: erythromycin 1 g, neomycin 1 g orally at 1 pm, 2 pm and 11 pm on the before operation. Plus cefazolin 1 g iv with the 'on call' medications and 500 mg perioperatively and postoperatively every 6 h for 4 doses Group B: placebo plus cefoxitin 2 g iv with 'on call' medications, 500 mg perioperatively and 1 g iv after surgery every 6 h for 4 doses | |
| Outcomes | SWI: purulent drainage present | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | D ‐ Not used |
| Incomplete outcome data (attrition bias) | Low risk | 9% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: imipenem 1 g iv at induction of anaesthesia with a further dose 3 h after operation Group B: imipenem 1 g iv at induction of anaesthesia and 3 h postoperatively, then a further 2 500 mg doses at 8 h and 16 h Group C: cefuroxime 1.5 g plus metronidazole 500 mg iv at induction of anaesthesia, then further doses of cefuroxime 0.75 g plus metronidazole 500 mg at 8 h and 16 h postoperatively | |
| Outcomes | SWI: if purulent discharge from the wound occurred, a positive bacteriological culture was obtained, or a deep abscess developed at the site of operation | |
| Notes | Group C was not utilised within this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Incomplete outcome data (attrition bias) | High risk | 15% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | CRS: cancer or IBD; n = 62 | |
| Interventions | Lincomycin pre‐ and 5 d postoperatively versus placebo | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS cancer; n = 93 | |
| Interventions | Metronidazole and kanamycin given either po preoperatively or iv pre‐ and early postoperatively | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | eCRS cancer; n = 73 | |
| Interventions | Metronidazole with either cefuroxime or mezlocillin for 3 doses | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true | |
| Participants | Elective and non‐obstructive colon surgery | |
| Interventions | Group A: neomycin 1 g plus erythromycin 1 g preoperatively at 1 pm, 2 pm and 11 pm the day before the operation. Plus cefazolin 1 g iv 1 h preoperatively and at 6 h and 12 h postoperatively Group B: metronidazole 1 g iv 1 h preoperatively and 500 mg iv at 6 h and 12 h postoperatively | |
| Outcomes | SWI: exhibited redness, induration or discharge of pus | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 34% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: latamoxef (moxalactam) 1 g iv at induction of anaesthesia Group B: cefuroxime 1 g plus metronidazole 500 mg iv at induction of anaesthesia | |
| Outcomes | SWI: purulent discharge. A major infection was defined as a discharge associated with pain and/or pyrexia and positive bacteriology | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | 5% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective and emergency surgery | |
| Interventions | Group A: metronidazole 1 g iv over 30 min, 3 to 4 h preoperatively Group B: doxycycline 0.2 g iv over 30 min, 3 to 4 h preoperatively | |
| Outcomes | SWI: visible pus | |
| Notes | 9 patients in each group received antibiotics for prolonged periods | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Incomplete outcome data (attrition bias) | High risk | 11% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal | |
| Interventions | Group A: metronidazole 1 g iv at induction of anaesthesia Group B: metronidazole 3 g iv at induction of anaesthesia Group C: metronidazole 1 g plus nalidixic acid 3 g slow infusion at induction of anaesthesia | |
| Outcomes | SWI: discharge of pus either spontaneously or by debridement | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Incomplete outcome data (attrition bias) | High risk | 14% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective surgery | |
| Interventions | Group A: neomycin sulphate 1 g plus erythromycin base 1 g preoperatively at 1 pm, 2 pm and 11 pm on the day before operation Group B: metronidazole 1.5 g plus ceftriaxone 2 g iv at induction of anaesthesia | |
| Outcomes | SWI: discharge of pus | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 14% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: metronidazole 500 mg iv at induction, 9 pm and 6 am Group B: metronidazole 500 mg iv as for Group A plus tobramycin 80 mg iv | |
| Outcomes | SWI: not defined | |
| Notes | Translated from German | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Incomplete outcome data (attrition bias) | Low risk | 7.6% drop‐out (8) |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | eCRS; n = 500 | |
| Interventions | Kanamycin and erythromycin po +/‐ iv cefmetazole | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | |
| Incomplete outcome data (attrition bias) | Low risk | 3.2% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true | |
| Participants | Emergency and elective abdominal surgery* | |
| Interventions | Group A: cefoxitin 2 g iv at induction of anaesthesia Group B: cefotaxime 1 g plus metronidazole 500 mg at induction of anaesthesia Group C: cefoxitin 2 g at induction of anaesthesia then at 6 h and 12 h postoperatively Group D: cefotaxime 1 g plus metronidazole 500 mg at induction of anaesthesia followed by 2 more doses of cefotaxime at 6 h and 12 h postoperatively | |
| Outcomes | SWI: presence of purulent discharge from the wound or a serous discharge with a positive culture of pathogenic organisms | |
| Notes | Duration of therapy in comparisons Analysis 2.1 and Analysis 2.2 of Groups A & C only: cefoxitin. Fore Groups B & D see below. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 32% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Comparison of Groups B & D cefotaxime and metronidazole from the above reference Kow 1995 for duration in Analysis 2.1 and Analysis 2.2 | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: co‐amoxiclav 1.2 g iv on call to the operating theatre and 2 more doses postoperatively every 8 h Group B: cefotaxime 500 mg plus metronidazole 500 mg as an iv infusion over 15 min on call to operating theatre and 2 more 8‐hourly doses postoperatively | |
| Outcomes | SWI: pus discharge from the surgical wound or pus accumulation in the wound that required drainage. Serous discharge with positive bacteriological culture and marked cellulitis that warranted systemic antibiotic treatment were also taken as signs of definite infection | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | 7% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | eCRS; n = 40 | |
| Interventions | Tinidazole 800 mg pre 2 and 6 h versus ornidazole 500 mg 2 and 6 h plus 4 days after | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 48 | |
| Interventions | Tinidazole at different dose intervals, 8 h and 12 h | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | |
| Allocation concealment (selection bias) | High risk | |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: neomycin 1 g plus erythromycin 1 g preoperatively at 1 pm, 2 pm and 11 pm on the day before the operation Group B: metronidazole 500 mg plus gentamicin 2 mg/kg body weight iv over 30 min before operation Group C: both preoperative and iv antibiotics as in Group A and Group B | |
| Outcomes | SWI: purulent discharge. Wounds with serous discharge which gave positive bacteriological cultures and wounds with serous discharge after the patients had returned home so cultures could not be taken were also included in infected group | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | 4% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: penicillin 2 ml IU im plus streptomycin 500 mg im in the evening before surgery, then penicillin tid for 6 days and strptomycin twice daily for 4 days Group B: cefotaxime 2 g iv at the induction of anaesthesia and at 3 h and 6 h later If the wound was heavily contaminated metronidazole was given tds for 3 days in both groups | |
| Outcomes | SWI: superficial accumulation of pus requiring surgical drainage | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | 9% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: kanamycin 4 g/day iv in 4 equally‐divided doses over 3 days prior to surgery, plus metronidazole 1 g/day in 4 equally‐divided doses Group B: cephradine 2 g iv at induction plus 4 h metronidazole 500 mg infusion Group C: as for Group A plus gentamicin 2 mg/kg im at time of premedication followed by cephradine 2 g iv at induction | |
| Outcomes | SWI: minor abscess requiring neither drainage nor prolonged hospital stay, or major abscess requiring drainage or prolonged hospitalisation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 57 | |
| Interventions | Doxycycline versus pen and strep | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 79 | |
| Interventions | Cephaloridine 2 g iv versus neo/erythro 1 g at 1, 2, 11 PM the day before | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 130 | |
| Interventions | Cephradine iv versus erythro/met po | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | |
| Allocation concealment (selection bias) | High risk | |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 101 | |
| Interventions | Cefazolin iv versus erythromycin/metronidazole po | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | |
| Allocation concealment (selection bias) | High risk | |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: erythromycin base 1 g plus neomycin sulphate 1 g preoperatively at 1 pm, 2 pm and 11 pm on the day before operation Group B: erythromycin base as in Group A, plus metronidazole 750 mg preoperatively tid for 2 days before operation | |
| Outcomes | SWI: pus drained from the wound; a sample of the discharge was then obtained for culture | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Incomplete outcome data (attrition bias) | Low risk | 5% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | All patients received amikacin 1 g and metronidazole 1 g iv on way to operating theatre Group A: neomycin 2 g and metronidazole 2 g orally 7 pm and 11 pm Group B: placebo | |
| Outcomes | SWI: Centre for Disease Control definitions | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Incomplete outcome data (attrition bias) | Low risk | 4% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Prophylactic treatment began 1 to 1.5 h before operation. All patients: metronidazole 500 mg iv infused over 20 min. After operation it was repeated 3 times every 8 h Group A: 5.5% glucose solution 100 ml given immediately after each metronidazole infusion Group B: fosfomycin 2 g in 100 ml 5.5% glucose was given iv after each of the 4 metronidazole doses | |
| Outcomes | SWI: discharge of pus, classified as major if the patient was ill, and as minor when symptoms were only trivial and local | |
| Notes | Adverse event collected but not reported. No cost data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: true # centres not stated | |
| Participants | Colorectal surgery | |
| Interventions | Group A: mezlocillin 5 g plus metronidazole 500 mg in 1 short iv during anaesthesia Group B: amoxicillin/clavulanic acid 2 g/0.2 g in 1 short iv during anaesthesia | |
| Outcomes | SWI: not defined | |
| Notes | Translated from German | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 31% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: cefotaxime 3 g iv with anaesthesia Group B: cefotaxime 3 g iv with anaesthesia and 2 g 8 h and 16 h later | |
| Outcomes | SWI: not defined | |
| Notes | Translated from German | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Emergency and elective colorectal surgery | |
| Interventions | Group A: gentamicin 80 mg plus lincomycin 600 mg im 2 h preoperatively and every 8 h postoperatively for 3 days Group B: gentamicin 80 mg plus clindamycin 600 mg im 2 h preoperatively and every 8 h postoperatively for 3 days Group C: gentamicin 80 mg plus metronidazole 500 mg 2 h preoperatively and every 8 h postoperatively for 3 days | |
| Outcomes | SWI: a positive culture of any discharge from the wound | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: not clear | |
| Participants | Emergency and elective abdominal surgery* | |
| Interventions | Group A: ceftriaxone 1 g iv at induction of anaesthesia Group B: ampicillin 2 g plus metronidazole 1.5 g iv at induction of anaesthesia | |
| Outcomes | SWI: cicatricial infection, rupture or cleavage of the skin with discharge of pus | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | 7% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: true | |
| Participants | Emergency and elective colorectal surgery | |
| Interventions | Group A: ceftriaxone 2 g iv plus metronidazole 1 g suppository on call to operating theatre Group B: ceftriaxone 2 g iv plus glycerol suppository on call to theatre Group C: cephazolin 1 g iv plus metronidazole 1 g suppository on call to theatre | |
| Outcomes | SWI: presence of erythema accompanied by deep or superficial pus | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | eCRS; n = 126 | |
| Interventions | Gent., vanc. and Mycostatin given for 24 h or 60 h preoperatively q 6 h | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) | High risk | 34% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | eCRS; n = 100 | |
| Interventions | Neomycin plus 1 of 3 cephalosporins: cefazolin, cefositin, ceftizoxime | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | 8% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: gentamicin 80 mg iv plus clindamycin 600 mg iv 20 min prior to induction, both for 6 doses at 8‐hourly intervals Group B: gentamicin 80 mg iv plus metronidazole 500 mg iv 20 min prior to induction, both for 6 doses at 8‐hourly intervals | |
| Outcomes | SWI: NA from translation | |
| Notes | Translated from French | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 10% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | eCRS; n = 120 | |
| Interventions | Neomycin and metronidazole q 8 h for 2 days preoperatively versus placebo | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) | Low risk | 8% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: ceftriaxone 2 g plus tinidazole 500 mg iv at induction of anaesthesia Group B: netilmicin 150 mg or tobramycin 80 mg plus tinidazole 500 mg iv during induction of anaesthesia | |
| Outcomes | SWI: suppuration of the wound or positive bacterial culture of the wound | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 21% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: not clear | |
| Participants | Emergency and elective colorectal surgery | |
| Interventions | Group A: gentamicin 120 mg plus metronidazole 500 mg iv at induction of anaesthesia. Further doses of gentamicin 80 mg and metronidazole 500 mg at 8 h and 16 h postoperatively Group B: ciprofloxacin 1 g preoperatively plus metronidazole 500 mg iv at induction of anaesthesia. Further doses of metronidazole 500 mg iv at 8 h and 16 h postoperatively Group C: gentamicin 120 mg plus metronidazole 500 mg iv at induction of anaesthesia. Further doses of gentamicin 80 mg tid and metronidazole 500 mg tid for 3 days Group D: ciprofloxacin 1 g 1 h preoperatively plus metronidazole 500 mg iv at the induction of anaesthesia., followed by preoperative ciprofloxacin 750 mg bid and metronidazole 500 mg iv tid for 3 days | |
| Outcomes | SWI: presence of pus, either discharging spontaneously or requiring drainage | |
| Notes | Gentamicin only in this comparison, ciprofloxacin in McArdle 1995 A | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | 4% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | CRS | |
| Interventions | Details in McArdle 1995. Ciprofloxacin in this comparison | |
| Outcomes | SWI | |
| Notes | Gentamicin in McArdle 1995 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | 4% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: metronidazole 500 mg plus gentamicin 120 mg iv at induction of anaesthesia and at 8 h and 16 h after operation Group B: latamoxef (moxalactam) 1 g iv at induction of anaesthesia and at 8 h and 16 h after surgery Dosages were reduced by one‐third in patients who were more than 80 years old, and in those weighing less than 50 kg | |
| Outcomes | SWI: discharge of pus | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | 8% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT? | |
| Participants | CRS; n = 50 | |
| Interventions | Cefazolin +/‐ metronidazole preoperatively | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) | Low risk | 4% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | eCRS; n = 103 | |
| Interventions | Cefoxitin versus metronidazole and gentamycin | |
| Outcomes | SWI | |
| Notes | Duration not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | 3% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 109 | |
| Interventions | Mezlocillin +/‐ metronidazole | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out? |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | = University 1987 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Methods | RCT | |
| Participants | eCRS; n = 132 | |
| Interventions | Mandol versus gentamicin/clindamycin/penicillin versus placebo with all groups getting neomycin and antibiotic peritoneal lavage | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | 14% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 120 | |
| Interventions | Latamoxef iv versus mezlocillin and metronidazole po | |
| Outcomes | ||
| Notes | All got 6 days of TPN postoperatively | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: ceftizoxime 2 g Group B: mezlocillin 5 g Group C: mezlocillin 5 g plus metronidazole 500 mg Group D: mezlocillin 5 g plus metronidazole 500 mg tid for 3 days Group E: latamoxef 2 g | |
| Outcomes | SWI: not defined | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | CRS; n = 46 | |
| Interventions | 6 antibiotics versus placebo | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear Number of centres not stated | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: ampicillin 2 g plus sulbactam 1 g iv on anaesthesia and a further 2 doses every 6 to 8 h Group B: cefoxitin 2 g iv on anaesthesia and a further 2 doses every 6 to 8 h Group C: piperacillin 4 g plus metronidazole 500 mg on anaesthesia and a further 2 doses every 6 to 8 h | |
| Outcomes | SWI: definition set out by Centre for Disease Control | |
| Notes | Translated from German. Cefoxitin is the gold standard therapy (Medical Letter 2004) in this comparison to Group A, ampicillin | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | 4% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: ampicillin 2 g plus sulbactam 1 g iv on anaesthesia and a further 2 doses every 6 to 8 h Group B: cefoxitin 2 g iv on anaesthesia and a further 2 doses every 6 to 8 h Group C: piperacillin 4 g plus metronidazole 500 mg on anaesthesia and a further 2 doses every 6 to 8 h | |
| Outcomes | SWI: definition set out by Centre for Disease Control | |
| Notes | Translated from German. Cefoxitin is the gold standard therapy Medical Letter 2012, in this comparison to Group C, piperacillin | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | 4% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Emergency and elective abdominal surgery | |
| Interventions | Group A: co‐amoxiclav 1.2 g at induction of anaesthesia and 8 h and 16 h postoperatively Group B: mezlocillin 5 g at induction of anaesthesia and 8 h and 16 h postoperatively | |
| Outcomes | SWI: WI, no infection, stitch infection, superficial infection, deep infection | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 14% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear 3 centres | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: alatrofloxacin 200 mg iv preoperatively Group B: cefotetan 2 g iv preoperatively | |
| Outcomes | SWI: minor = red, major = pus | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 39% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | eCRS; n = 70 | |
| Interventions | Metronidazole +/‐ cefuroxime iv preoperatively | |
| Outcomes | SWI | |
| Notes | The recommended UK prophylaxis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Drugs administered as a single dose at induction of anaesthesia. Metronidazole was infused over 20 min and cefuroxime was given as an iv injection Group A: cefuroxime 1.5 g plus metronidazole 500 mg Group B: metronidazole 500 mg | |
| Outcomes | SWI: purulent discharge only. Moderate = pus with constitutional upset; severe = requiring active surgical intervention | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | 9% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery Follow up not stated | |
| Interventions | Group A: bacimycin Group B: doxycycline 200 mg iv 12 to 18 h preoperatively Group C: doxycycline 200 mg as for Group B plus tinidazole 2 g iv 12 to 18 h preoperatively | |
| Outcomes | SWI: not defined | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Incomplete outcome data (attrition bias) | Low risk | 7% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | All patients: metronidazole 500 mg plus gentamicin 80 mg at induction of anaesthesia then every 8 h for 2 days Group A: local injection of 80 ml metronidazole (500 mg/100 ml) and gentamicin 2 ml (80 mg/ml) into the muscular and subcutaneous layer of the perineal wound, during and after closure of that wound Group B: no locally injected antibiotics | |
| Outcomes | SWI: presence of pus | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 17% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true | |
| Participants | Elective abdominal surgery | |
| Interventions | Group A: gentamicin 80 mg plus metronidazole 500 mg iv at start of operation and 6 h later Group B: gentamicin 80 mg plus metronidazole 500 mg iv at start of operation and every 8 h for 2 days | |
| Outcomes | SWI: presence of pus | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | 9% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 60 | |
| Interventions | Kan/gent/met/cepharidine versus cepharidine/metronidazole | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 107 | |
| Interventions | Neomycin and tetracycline 36 h preoperatively x 3 versus placebo | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | 19% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 100 | |
| Interventions | Gent and met versus met | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true Single centres | |
| Participants | Elective abdominal surgery | |
| Interventions | All patients: cefotaxime 2 g iv plus metronidazole 500 mg iv pre‐ or intraoperatively, then every 8 h for 3 days Group A: cefotaxime 2 g applied topically to the subcutaneous layer at the time of wound closure Group B: metronidazole either 500 mg or 1.5 g in single or divided doses | |
| Outcomes | SWI: major = fever, purulent discharge with erythema and necrotic slough; minor = no surrounding erythema, slough or constitutional disturbances | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | 2% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: aztreonam 1 g iv plus metronidazole 500 mg iv at induction of anaesthesia followed by 2 more doses every 8 h Group B: cefotaxime 1 g plus metronidazole 500 mg iv at induction of anaesthesia followed by 2 more doses every 8 h | |
| Outcomes | SWI: 1 = the discharge of pus; 2 = the presence of serous discharge with the isolation of pathogenic bacteria from the culture; 3 = abnormal erythema and induration requiring drainage and antibiotic therapy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | 8% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | CRS; n = 260 | |
| Interventions | Gent and met versus ceftriaxone iv preoperatively | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) | High risk | 25% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: cefotetan 2 g iv at induction of anaesthesia and at 12 h postoperatively Group B: cefotetan 2 g at induction of anaesthesia and at 12 h postoperatively plus metronidazole 500 mg by slow iv infusion at induction of anaesthesia and at 8 h and 16 h postoperatively | |
| Outcomes | SWI: discharge of pus from the wound, wound dehiscence, or the discharge of serous fluid from which organisms were isolated on bacterial culture | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 14% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true | |
| Participants | Elective colonic surgery | |
| Interventions | Group A: cefoxitin 2 g iv 30 min to 1 h before incision, repeated 2 h later Group B: clindamycin 600 mg plus gentamicin 80 mg iv 1 h before operation, repeated 8 h and 16 h later | |
| Outcomes | SWI: not defined | |
| Notes | Translated from French | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | 9% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: amoxicillin/clavulanic acid 1.2 g iv at induction and 8 h and 16 h later Group B: gentamycin 80 mg iv plus clindamycin 600 mg iv at induction and 8 h and 16 h later | |
| Outcomes | SWI: quantitative score used | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Incomplete outcome data (attrition bias) | Low risk | 9% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: aztreonam 1 g plus clindamycin 900 mg at induction of anaesthesia and 8 h and 16 h later Group B: gentamicin 80 mg plus clindamycin 900 mg at induction of anaesthesia and 8 h and 16 h later | |
| Outcomes | SWI: discharge of pus, serous discharge with a positive culture or erythema and/or induration requiring antibiotic therapy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | 8% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | CRS cancer; n = 120 | |
| Interventions | Tobramycin with either metronidazole or clindamycin, all iv pre‐ and postoperatively to 7 doses q 8 h | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) | High risk | 17% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective abdominal surgery | |
| Interventions | Group A: ceftriaxone 1 g Group B: cefoxitin 4 to 6 g, 3 doses with or without metronidazole | |
| Outcomes | SWI: not defined | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 20 | |
| Interventions | Neomycin/erythromycin versus placebo | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery; n = 171, 78% colorectal cancer mean age 66 (range 56‐74) | |
| Interventions | All patients: placebo used in both groups, starting 2 days before the operation Group A: bacitracin 250 mg plus neomycin 250 mg preoperatively tid for 2 days, plus metronidazole 500 mg preoperatively tds, plus ampicillin 1 g 1 h preoperatively Group B: fosfomycin 8 g plus metronidazole 1 g 1 h before operation | |
| Outcomes | SWI: presence of pus or discharge resulting in a positive bacteriological culture. Perineal sepsis was defined as discharge of pus from the perineal wound or drain culture | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 13% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | All drugs were administered iv within 2 h preoperatively Group A: tinidazole 1600 mg plus doxycycline 400 mg Group B: tinidazole 1600 mg plus placebo (vitamin solution) | |
| Outcomes | SWI: presence of pus or discharge resulting in a positive bacteriological culture. Perineal sepsis was defined as discharge or pus from the perineal wound or drain channel | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | 5% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: amoxicillin/clavulanic acid 1 g/200 mg (1.2 g co‐amoxiclav) at induction of anaesthesia and at 8 h postoperatively Group B: ceftriaxone 1 g plus metronidazole 500 mg at induction of anaesthesia and at 12 h postoperatively | |
| Outcomes | SWI: abscess or wound discharging pus | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: doxycycline 200 mg iv perioperatively and postoperatively for 4 days Group B: as for Group A plus doxycycline 200 mg ip Group C: placebo | |
| Outcomes | SWI not defined | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Incomplete outcome data (attrition bias) | Low risk | 1% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | Colorectal surgery | |
| Interventions | The first dose was given with the start of anaesthesia. Both groups received metronidazole 500 mg iv every 8 h for 24 h Group A: ciprofloxacin 200 mg iv, bid every 12 h Group B: cephazolin 2 g iv, bid for 3 days | |
| Outcomes | SWI: not defined | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | 1.4% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: tinidazole 2 g perorally 12 h before operation Group B: tinidazole 2 g plus 200 mg oral doxycycline 12 h before operation Group C: oral doxycycline 200 mg 12 h before operation, 200 mg doxycycline iv immediately after operation and in the following 3 days Group D: tinidazole 2 g plus 200 mg oral doxycycline 12 h before operation, 200 mg doxycycline iv immediately after operation and in the following 3 days | |
| Outcomes | SWI: pus draining from incision | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 19% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 169 | |
| Interventions | Metronidazole versus placebo | |
| Outcomes | SWI | |
| Notes | Ampicillin topical in fascia and subcutaneously | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | 20% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: imipenem plus cilastatin 1 g at anaesthesia Group B: cefuroxime 1.5 g iv plus metronidazole 500 mg iv at induction of anaesthesia, 2 further 8‐hourly doses | |
| Outcomes | SWI: purulent discharge with or without culture of pathogenic micro‐organisms | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 60 | |
| Interventions | Metro with either ceftriaxone 2 g preoperatively or ceftazidime 2 g pre‐ and q 8 h postoperatively x 3 | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 74 | |
| Interventions | Cephalothin 2 g, cefoxitin 2 g and metronidazole 500 mg preoperatively and to 64 h or 72 h for metronidazole postoperatively | |
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | CR cancer n = 306 | |
| Interventions | Cefotetan given for 3 d or 5 d | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | 17% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | CRS; n = 60 | |
| Interventions | Metronidazole with low and high‐dose cefotiam | |
| Outcomes | SWI | |
| Notes | Same as Peiper 1997 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: cefotiam 1 g iv 30 min preoperatively plus metronidazole 500 mg iv Group B: cefotiam 2 g iv 30 min preoperatively plus metronidazole 500 mg iv | |
| Outcomes | SWI: Centre for Disease Control standards | |
| Notes | Same as Peiper 1996 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: cefoxitin 1 g iv at the start of operation and 3 h, 6 h and 12 h after initial dose Group B: cefotetan 2 g iv at start of operation | |
| Outcomes | SWI: drained purulent or serous material | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 11% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | Elective colorectal surgery; Cancer patients: 87% | |
| Interventions | Group A: cefotetan 2 g rapid iv at induction of anaesthesia plus thymostimulin 70 mg im for 7 days starting 48 h preoperatively Group B: cefotetan 2 g rapid iv at induction of anaesthesia | |
| Outcomes | SWI: not defined | |
| Notes | Immunomodulation protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 11% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal and biliary surgery | |
| Interventions | Group A: metronidazole 500 mg plus tobramycin 80 mg at start of operation, then at 8 h and 16 h later, plus penicillin 2 milli‐units at start of operation followed by 4 further 4‐hourly doses Group B: cefoxitin 2 g at start of operation and 2 more 6‐hourly doses | |
| Outcomes | SWI: showing abnormal reddening and swelling out of proportion to the expected, or if frank suppuration occurred | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | 5% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: cefoxitin as iv bolus 30 min preoperatively and then at 6 h and 12 h postoperatively Group B: cefoxitin as for Group A, plus tinidazole 2 g at 2 h prior to surgery, plus neomycin 1 g at 19 h, 18 h and 9 h prior to surgery | |
| Outcomes | SWI: drainage of serous purulent material with or without culture of pathogen microorganisms from a discharging abdominal wound | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | CRS; n = 96 | |
| Interventions | Cefazolin versus metronidazole | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: quasi‐randomised | |
| Participants | Elective colorectal surgery | |
| Interventions | All patients underwent a 3‐day mechanical bowel preparation and received erythromycin base 1 g plus neomycin 1 g preoperatively at 1 pm, 2 pm and 11 pm on the day before the operation Group A: cefamandole 1 g iv 1 h preoperatively, then 6‐hourly for a total of 4 doses Group B: no iv antibiotics | |
| Outcomes | SWI: discharge of pus | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: cefotaxime 2 g iv at induction of anaesthesia Group B: cefotaxime 2 g plus ornidazole 500 mg at induction of anaesthesia and 2 further doses of cefotaxime 8 h and 16 h later, plus further dose of ornidazole 12 h later | |
| Outcomes | SWI: not defined | |
| Notes | Translated from French | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Emergency and elective abdominal surgery | |
| Interventions | All colorectal patients: either neomycin plus metronidazole 24 h preoperatively, or single suppository of metronidazole 1 g approx 2 h preoperatively Group A: latamoxef 1 g/20 ml solution as single iv injection at induction of anaesthesia Group B: co‐amoxiclav 1.2 g/20 ml solution as single iv injection at induction of anaesthesia | |
| Outcomes | SWI: discharge of pus. Major infection caused constitutional disturbances including pyrexia and delayed patient's discharge from hospital. Minor infections did neither. Serous and haemoserous discharges were not counted as infections | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | 1% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | All patients: single preoperative or intraoperative dose of an antibiotic effective against faecal bacteria Group A: metronidazole iv at premedication Group B: neomycin 1 g preoperatively every 6 h plus metronidazole 200 mg every 8 h for 24 h, plus parenteral preoperative dose of metronidazole 1 g rectal or 500 mg iv | |
| Outcomes | SWI: major = causing pyrexia and delaying patient's discharge from hospital | |
| Notes | No data on adverse events or cost | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective abdominal surgery | |
| Interventions | Group A: cephazolin 1 g iv on call to the operating theatre and 2 further 6‐hourly doses postoperatively Group B: latamoxef (moxalactam) 1 g iv on call to the operating theatre and 2 further 6‐hourly doses postoperatively | |
| Outcomes | SWI: purulent discharge and isolated pathogen | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 20% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective abdominal surgery | |
| Interventions | Patients undergoing colorectal operation also received oral antibiotic bowel preparation Group A: cefmetazole 2 g preoperatively and 2 further 8‐hourly doses Group B: cefoxitin 2 g preoperatively and 2 further 6‐hourly doses | |
| Outcomes | SWI: not defined | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 31% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective abdominal surgery | |
| Interventions | Group A: amoxicillin/clavulanic acid 1 g/200 mg (co‐amoxiclav 1.2 g) iv at induction of anaesthesia Group B: co‐amoxiclav 1.2 g injected subcutaneously along the line of the proposed abdominal incision, using a spinal needle Metronidazole was used in the majority of colorectal patients | |
| Outcomes | SWI: discharge of pus. Major caused fever and delaying of patient's discharge from hospital. Minor required dressings only. Late occurred after patients had left hospital | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 13% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | eCRS; n = 67 | |
| Interventions | Metronidazole and kanamycin preoperatively for 3 d Metronidazole alone for 3 d preoperatively Placebo | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 49 | |
| Interventions | Metronidazole versus latamoxef iv preoperatively | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | Elective colorectal surgery | |
| Interventions | All patients: metronidazole 500 mg bd for 3 days with the first dose administered immediately prior to operation Group A: cefotaxime 1 g 1 h preoperatively Group B: cefotaxime 1 g 1 h preoperatively and 8 h and 16 h postoperatively | |
| Outcomes | SWI: not defined | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 21% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 169 | |
| Interventions | Piperacillin versus latamoxef | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 108 | |
| Interventions | Ceftriaxone 1 shot iv versus cephalothin and metronidazole pre‐ and q 8 h postoperatively x 3 | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: metronidazole 400 mg 8‐hourly plus neomycin 1 g preoperatively 6‐hourly for 48 h prior to operation with the last dose given 8 h and 12 h preoperatively. In addition, piperacillin 2 g iv at induction of anaesthesia followed by 3 further 8‐hourly doses Group B: piperacillin 2 g iv at induction of anaesthesia and 3 further 8‐hourly doses Group C: metronidazole 500 mg plus cefuroxime 1.5 g at induction of anaesthesia followed by 3 further doses of metronidazole and 2 doses of cefuroxime 750 mg | |
| Outcomes | SWI: presence of purulent pus in wound appearing spontaneously or on incision | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 16% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: clindamycin 0.6 g plus aztreonam 1 g iv 30 min preoperatively and 8 h and 16 h postoperatively Group B: clindamycin 0.6 g plus gentamicin 80 mg iv 30 min preoperatively and 16 h postoperatively | |
| Outcomes | SWI: purulent discharge from the surgical wound or a serous discharge with a positive microbiological culture | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 233 | |
| Interventions | Metronidazole a. Given for 1 day b. Given for 1 day with ampicillin c. Given for 3 days d. Given for 3 days with ampicillin | |
| Outcomes | SWI | |
| Notes | 1‐day data here for aerobic comparison | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | As above, with 3‐day data here in aerobic comparison | |
| Methods | RCT | |
| Participants | eCRS; n = 421 | |
| Interventions | Metronidazole +/‐ ampicillin or doxycycline sorted in a non‐random manner | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: cefoxitin 2 g iv 15 min preoperatively and at 4 h and 10 h after the first dose Group B: ampicillin 1 g plus metronidazole 500 mg iv 15 min preoperatively and every 6 h (ampicillin) and 8 h (metronidazole) for a total of 72 h | |
| Outcomes | SWI: abscess or discharge of pus | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 11% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | CRS; n = 150 | |
| Interventions | Phthalylsulfathiazole +/‐ neomycin and placebo (3 groups) | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | 15% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true | |
| Participants | Emergency and elective colorectal surgery | |
| Interventions | Group A: cefotaxime 1 g plus metronidazole 500 mg iv infusion after induction of anaesthesia and 5 to 10 min before opening of peritoneum Group B: cefuroxime 1.5 g plus metronidazole 500 mg iv infusion after induction of anaesthesia and before peritoneal opening, followed by 2 further iv doses of cefuroxime 750 mg plus metronidazole 500 mg at 8 h and 16 h postoperatively | |
| Outcomes | SWI: abdominal incision discharged pus | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 11% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | eCRS; n = 302 | |
| Interventions | Ticarcillin iv preoperatively and a postoperative dose versus tinidazole po PM before | |
| Outcomes | SWI | |
| Notes | = Anon 86 and U Melbourne 1986 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | 17% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 100 | |
| Interventions | Cefotetan versus placebo | |
| Outcomes | SWI | |
| Notes | Note late date. Also astronomical wound infection rate ˜50% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | 7% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true | |
| Participants | Emergency and elective abdominal surgery | |
| Interventions | For elective ileo colorectal operations patients were given preoperative metronidazole suppositories with or without oral neomycin Group A: latamoxef 1 g iv at induction of anaesthesia Group B: peritoneal and parietal irrigation with 1 litre of saline containing 1 g tetracycline at the conclusion of the operation | |
| Outcomes | SWI: discharge of pus | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 21% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: intraluminal antibiotics; neomycin 1 g/litre plus bacitracin 50,000 IU/litre, plus clindamycin 900 mg/litre were added to the last 3 litres of irrigation fluid Group B: parenteral antibiotics; mezlocillin 4 g plus oxacillin 2 g iv on induction of anaesthesia then at 8 h and 16 h postoperatively Group C: no antibiotics | |
| Outcomes | SWI: inflammation of the wound with discharge of pus occurring within 4 weeks from surgery | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 18% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | eCRS; n = 108 | |
| Interventions | Placebo versus neomycin and bacitracin po for 4 preoperatively days | |
| Outcomes | SWI | |
| Notes | Birth year randomisation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | |
| Allocation concealment (selection bias) | High risk | |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: neomycin 1 g plus erythromycin base 1 g preoperatively at 1 pm, 2 pm, 11 pm on the day before operation Group B: neomycin 1 g plus erythromycin base 1 g preoperatively at 1 pm, 2 pm, and 11 pm on the day before the operation and cefoxitin 2 g iv within 60 min before incision, and at 6 h and 12 h thereafter | |
| Outcomes | SWI: purulent wound drainage; the exudate either spontaneously drained or was expressed after the removal of skin staples over a clinically suspect area of the wound | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 12% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective abdominal surgery | |
| Interventions | Group A: cefotaxime 1 g iv or im 30 to 90 min preoperatively Group B: cefoxitin 2 g iv or im 30 to 90 min preoperatively and 6‐hourly for no more than 24 h postoperatively | |
| Outcomes | SWI: if the incision or peritoneal cavity drained purulent material, it was considered infected, regardless of bacteriological or laboratory testing | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 14% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | CRS; n = 91 | |
| Interventions | Cefmetazol versus flomoxef iv q 3 h for as long as surgery lasted | |
| Outcomes | SWI | |
| Notes | Variable duration | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: cefotetan 2 g at induction of anaesthesia and at 12 h postoperatively Group B: cefuroxime 1.5 g plus metronidazole 500 mg at induction of anaesthesia and cefuroxime 750 mg plus metronidazole 500 mg at 8 h and 16 h postoperatively | |
| Outcomes | SWI: 1 = no infection; 2 = erythema; 3 = purulent or bacteriologically positive discharge; 4 = severe WI with or without dehiscence | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 17% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 34 | |
| Interventions | Cephalothin versus cefamandol preoperatively and 12 more doses q 4 h | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | CRS; n = 194 | |
| Interventions | Metronidazole iv versus doxycycline iv | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | 24% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: neomycin 1 g plus erythromycin base, 1 g preoperatively at 1 pm, 2 pm, and 11 pm an the day before the operation, plus iv placebo Group B: cefoxitin 1 g iv at induction of anaesthesia and at 6 h and 12 h following the first dose, plus preoperative placebo Group C: both preoperative and iv antibiotics | |
| Outcomes | SWI: wound with visible pus or with a positive culture | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 14% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: piperacillin 4 g iv as a single bolus dose at induction of anaesthesia Group B: piperacillin 4 g plus sulbactam 2 g iv as bolus dose at induction of anaesthesia | |
| Outcomes | SWI: WI intra‐abdominal infection or septicaemia occurring within 42 days of operation were considered to be evidence of failure of antibiotic prophylaxis No information on adverse events or cost provided | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Incomplete outcome data (attrition bias) | High risk | 14% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: mezlocillin 5 g iv at start of operation Group B: cefuroxime 1.5 g iv plus metronidazole 500 mg iv at start of operation, followed by 2 doses of cefuroxime 750 mg plus metronidazole 500 mg iv 8 h and 16 h later | |
| Outcomes | SWI: failure of primary healing in any portion of the wound, or when there was wound discharge | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | 5% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 370 | |
| Interventions | Flomoxef for 1 or 3 days from preoperative to 3rd day po | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | 2.7% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 100 | |
| Interventions | Cefmetazole iv +/‐ po kanamycin and metronidazole x 3 the day before surgery | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | 17% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | All patients: single dose of piperacillin 4 g iv at induction of anaesthesia Group A: ciprofloxacin 500 mg bd preoperatively on the day before operation Group B: no oral antibiotics | |
| Outcomes | SWI: not defined | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 14% |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective gynaecological or general surgery | |
| Interventions | First dose given at induction of anaesthesia and then 8 h and 16 h after the first dose Group A: co‐amoxiclav 1.2 g iv plus placebo suppository Group B: cephradine 1 g iv plus metronidazole 1 g suppository | |
| Outcomes | SWI: 1 = moderate or severe erythema; 2 = purulent discharge; 3 = serous discharge from which an organism was isolated; 4 = serous discharge associated with either erythema or other factors indicative of infection e.g. pyrexia | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 16% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: latamoxef 1 g iv at induction of anaesthesia and at 6 h and 12 h postoperatively Group B: cefazolin 1 g plus metronidazole 500 mg iv at induction of anaesthesia and at 6 h and 12 h postoperatively | |
| Outcomes | SWI: not defined | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | eCRS; n = 196 | |
| Interventions | Doxycycline dosing and timing: 1. 200 mg preoperatively 2. 600 mg preoperatively 3. 600 mg postoperatively 4. 200 mg preoperatively and daily postoperatively for 3 days | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | 15% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery; n = 50 | |
| Interventions | Group A: metronidazole 500 mg plus amikacin 500 mg iv 2 h preoperatively and every 8 h (metronidazole) or every 12 h (amikacin) for 2 days Group B: ornidazole 1 g plus ceftriaxone 2 g iv 2 h preoperatively and every 24 h postoperatively for 2 days | |
| Outcomes | SWI: obvious collection of pus which either drained spontaneously or on incision | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear #centres not stated | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: piperacillin 4 g plus tinidazole 0.8 g iv before operation Group B: piperacillin 4 g plus tinidazole 0.8 g iv before operation and every 8 h (piperacillin) and 12 h (tinidazole) for 24 h postoperatively | |
| Outcomes | SWI: not defined | |
| Notes | Translated from German | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: clindamycin phosphate 600 mg im Group B: placebo | |
| Outcomes | SWI: defined as grade 1 = bacteriological infection; no prolonged hospital stay; grade 2 = clinically important infection with presence of pus | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Incomplete outcome data (attrition bias) | Low risk | 8% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: ticarcillin/clavulanic acid (Timentin) 3.1 g iv before skin incision and at 2 h after the first dose Group B: tinidazole 2 g preoperatively at 10 pm on the night before operation | |
| Outcomes | SWI: presence of pus or recovery of bacteria of pathogenic potential from a wound exudate | |
| Notes | = McLeish | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 12.6% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: ticarcillin/clavulanic acid (Timentin) 3.1 g iv before skin incision and at 2 h after the first dose and completed after 30 min Group B: mezlocillin 2 g iv before skin incision and completed after 30 min | |
| Outcomes | SWI: purulent discharge from the suture line, or non‐purulent discharge that contained pathogenic bacteria | |
| Notes | = Ross 1989 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | High risk | 15% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: not clear | |
| Participants | Colorectal operations | |
| Interventions | Group A: cefoxitin 6 g given as 3 x 2 g iv doses, the first before skin incision Group B: placebo | |
| Outcomes | SWI: moderate = superficial inflammation of wound with purulent discharge; severe = deep purulent infection with an obvious inflammatory reaction and pus | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | CRS; n = 1265 | |
| Interventions | 4 independent studies, very difficult to summarise: 1. mechanical variables only n = 202 2. n = 326 a. Normal prep with tetracycline and neomycin pre‐ and postoperatively penicillin G b. Normal prep with metronidazole pre‐ and postoperatively penicillin G c. Senna (b) antibiotics d. Mannitol lavage with (b) antibiotics 3. n = 335 a. Senna with 2(b) antibiotics b. Senna + Betadine enemas and 2(b) antibiotics c. 3B prep and ceftriaxone pre‐ and to 24 h postoperatively d. 3B prep and ceftriaxone + metronidazole 4. n = 402 a. Betadine enemas; ceftriaxone and ornidazole b. Betadine enemas; ceftriaxone and metronidazole c. "serum physiologique"; ceftriaxone and metronidazole | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | 8.4% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 91 | |
| Interventions | Metronidazole +/‐ neomycin po | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) | High risk | 20% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | CRS; n = 100 | |
| Interventions | Neomycin + sulfaguanidine preoperatively versus metronidazole pre‐ and to 24 h after iv versus metronidazole and gentamycin pre‐ and for 2 d postoperatively iv | |
| Outcomes | SWI | |
| Notes | Multiple variables between groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | CRS; n = 91 | |
| Interventions | Neomycin/erythromycin versus neomycin alone versus neomycin/phthalylsulfathiazole all preoperatively | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: doxycycline 200 mg iv 12 h preoperatively and daily for 6 days Group B: doxycycline as for Group A plus tinidazole 2 g 12 h preoperatively and on days 4, 5 and 6 | |
| Outcomes | SWI: not defined | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Incomplete outcome data (attrition bias) | High risk | 16% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 239 | |
| Interventions | Piperacillin 2 g preoperatively and at 8 and 16 h versus netilmycin preoperatively and 8 and 18 h and metronidazole preoperatively and at 12 h | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | 11% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 77 | |
| Interventions | Kanamycin 1 g po for 2 d preoperatively +/‐ erythromycin 750 mg as kanamycin | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | RCT | |
| Participants | eCRS; n = 137 | |
| Interventions | Metronidazole versus doxycycline po continued postoperatively iv | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: neomycin sulphate 1 g plus erythromycin base 1 g preoperatively 1 pm, 2 pm and 11 pm on the day before the operation Group B: ceftriaxone 2 g plus metronidazole 1.5 g slow iv infusion at the start of the operation | |
| Outcomes | SWI: discharge of pus. Major infection was defined as purulent discharge associated with: fever; foul smelling discharge; dehiscence; hospital stay greater than 14 days; or leukocytosis | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: gentamicin 80 mg plus metronidazole 500 mg iv starting at premedication and then 8‐hourly for 24 h Group B: metronidazole 500 mg iv 8‐hourly for 24 h starting at premedication | |
| Outcomes | SWI: presence of pus in the wound | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Methods | RCT | |
| Participants | eCRS; n = 100 | |
| Interventions | Mezlocillin 2 g pre‐ and 5 d postoperatively versus placebo | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 95 | |
| Interventions | Mezlocillin 2 g +/‐ metronidazole 500 mg pre‐ and to 2 d postoperatively iv q 8 h | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | 2% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 32 | |
| Interventions | From Wenzel 1983 1 additional group of 1 shot mezlocillin 2 g preoperatively | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: ornidazole 1 g iv plus gentamicin 80 mg iv 45 min preoperatively Group B: ornidazole plus gentamicin as for Group A, followed by 3 further 12‐hourly doses of ornidazole 500 mg and 3 further 8‐hourly doses of gentamicin 80 mg | |
| Outcomes | SWI: oedematous and/or red wound with a purulent secretion | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true Single centre | |
| Participants | Colorectal surgery | |
| Interventions | Group A: doxycycline 200 mg orally 4 to 6 h preoperatively and 100 mg iv bid postoperatively Group B: placebo | |
| Outcomes | SWI: not defined | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Methods | RCT | |
| Participants | eCRS; n = 64 | |
| Interventions | Gentamycin im preoperatively +/‐ metronidazole po and by suppository 24 preoperatively to 7 d postoperatively | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) | High risk | 28% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: not clear | |
| Participants | Colorectal surgery | |
| Interventions | Group A: cefoxitin 2 g iv at induction to anaesthesia and 2 h later Group B: placebo | |
| Outcomes | SWI: presence of pus. Classified as either superficial or deep | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: not clear | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: ceftriaxone 2 g short iv infusion Group B: 3 doses cefotiam 1 g, 2 doses gentamicin 80 mg, plus 2 doses metronidazole 500 mg | |
| Outcomes | SWI: not defined | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised study but the method of randomisation was not specified |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Incomplete outcome data (attrition bias) | Low risk | n = 60 |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | RCT | |
| Participants | eCRS; n = 154 | |
| Interventions | Metro + tobramycin po 3 d preoperatively versus cefmetazol preoperatively and 3 h later and versus cefmetazol plus tobramycin luminal wash | |
| Outcomes | SWI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | 11% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | = AhChong 1994 | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A cefepime 2 g iv 1 h before surgery over 15 to 30 min plus metronidazole 500 mg iv using same intravenous line Group B: ceftriaxone 2 g iv 1 h before surgery over 15 to 30 min plus metronidazole as for Group A | |
| Outcomes | SWI: primary site infection | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Incomplete outcome data (attrition bias) | Low risk | 2% drop‐out |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomisation: true single centre | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: gentamicin 4.5 mg/kg iv preoperatively plus metronidazole 500 mg iv preoperatively and placebo at 8 h, 16 h and 24 h Group B: gentamicin 1.5 mg/kg iv preoperatively plus metronidazole 500 mg iv preoperatively and at 8 h, 16 h and 24 h | |
| Outcomes | SWI: Centre for Disease Control definitions | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Incomplete outcome data (attrition bias) | High risk | 11% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Randomisation: true | |
| Participants | Elective colorectal surgery | |
| Interventions | Group A: cephazolin 2 g plus ornidazole 1 g iv on anaesthesia Group B: cephazolin 2 g iv on anaesthesia | |
| Outcomes | SWI: presence of pus or abscess | |
| Notes | Translated from German | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Incomplete outcome data (attrition bias) | Low risk | 5% drop‐out |
| Blinding of outcome assessment (detection bias) | Low risk | |
*Indicates data for all surgical procedures included in a trial (i.e. where data for elective colorectal surgery cannot be separated out from other procedures).
Age presented as mean unless otherwise stated.
Abbreviations
> = greater/more than
abd = abdominal
approx = approximately
bid = 2 times a day
CA = cancer
CRS = colorectal surgery
d = day(s)
eCRS = elective colorectal surgery
Erythro = erythromycin
gent = gentamycin
h = hour(s)
IBD = inflammatory bowel disease
im = intramuscular
ip = intraperitoneal
IU = international units
iv = intravenous
kan = kannamycin
max = maximum
met = metronidazole
min = minute(s)
NA = not available
Neo = neomycin
NS = not significant
po = oral
q = each
pen = penicillin
RCT = randomised controlled trial
sc = subcutaneous
SD = standard deviation
Strrep = streptomycin
SWI = surgical wound infection
tds/tid = 3 times a day
TPN = total parenteral nutrition
vanc = vancomycin
WI = wound infection
y = year(s)
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| NRT | |
| Non‐antibiotic | |
| Non‐antibiotic study: antiseptic suture | |
| Review | |
| Topical antibiotic | |
| Oral antibiotics given in non‐random manner | |
| Only qualitative data | |
| NRT | |
| Mechanical bowel prep study | |
| Review of topical ampicillin | |
| Non‐randomised study | |
| Does not report SWI | |
| Review | |
| Not a RCT: review | |
| Review | |
| Includes trauma | |
| Partial publication of Mozzillo 1989 | |
| Not clearly RCT | |
| Tables do not equal text | |
| Abstract | |
| Merged Polk 1986 and Jagelman 1987 | |
| NRT | |
| Unclear methods and data presentation | |
| Appendicitis cases included | |
| Topical; peritoneal lavage | |
| See Grundmann 1987 | |
| Letter | |
| NRT | |
| Mechanical bowel prep study | |
| Unclear methods and data presentation | |
| Topical ampicillin | |
| Unit of analysis not the individual patient | |
| NRT | |
| NRT | |
| Same as Höjer 1978 | |
| Topical ampicillin added in non‐random manner | |
| Wicking and packing wounds routine | |
| Non‐random distribution of antibiotics | |
| Non‐random duration of treatment | |
| Abstract | |
| Topical; fascial injection | |
| Abstract | |
| Abstract | |
| SWI not reported | |
| NRT | |
| NRT | |
| Review | |
| Abstract only | |
| NRT | |
| Unclear methods and data presentation | |
| NRT | |
| Did not meet criteria with regard to surgery type | |
| Trauma surgery | |
| Abstract | |
| Topical antibiotic | |
| Tiny groups: 6 | |
| NRT | |
| NRT | |
| Bacteriology for Andaker 1992 | |
| Not limited to CRS. Results not separately reported for CRS | |
| NRT | |
| Review | |
| Review | |
| NRT | |
| Letter | |
| Topical Rx | |
| Topical Rx | |
| Topical Rx | |
| Topical Rx | |
| Non‐antibiotic therapy | |
| NRT | |
| Non‐antibiotic Rx | |
| Topical Rx | |
| Data on SWI not available by group | |
| Perineum only | |
| Topical therapy | |
| Topical Rx | |
| 40 patients some of whom only had cholecystectomy | |
| NRT | |
| Bacteriology | |
| NRT | |
| Japanese?? | |
| SWI not reported as outcome | |
| Non random antibiotic administration in Group B | |
| NRT | |
| Abstract | |
| Emergency operations | |
| SWI not separately reported | |
| Review | |
| Review | |
| NRT | |
| Non‐surgical prophylaxis | |
| Mechanical bowel prep study | |
| Variation in numbers randomised to colorectal surgery inconsistent between publications and no clear separation of elective from emergency colorectal procedures with established infection | |
| Cost study of Woodfield 2003 |
Abbreviations
NRT = non randomized trial
RCT = randomised controlled trial
SWI = surgical wound infection
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting full text |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting translation |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection (SWI) Show forest plot | 30 | 2455 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.28, 0.41] |
| Analysis 1.1  Comparison 1 Antibiotic versus no antibiotic/placebo, Outcome 1 Surgical wound infection (SWI). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection (SWI) Show forest plot | 34 | 5123 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.93, 1.29] |
| Analysis 2.1  Comparison 2 Duration of therapy, Outcome 1 Surgical wound infection (SWI). | ||||
| 2 Surgical wound infection (SWI) Show forest plot | 11 | 2005 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.82, 1.80] |
| Analysis 2.2  Comparison 2 Duration of therapy, Outcome 2 Surgical wound infection (SWI). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection (SWI) Show forest plot | 15 | 1869 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.29, 0.68] |
| Analysis 3.1  Comparison 3 Additional aerobic coverage, Outcome 1 Surgical wound infection (SWI). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection (SWI) Show forest plot | 19 | 2687 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.30, 0.69] |
| Analysis 4.1  Comparison 4 Additional anaerobic coverage, Outcome 1 Surgical wound infection (SWI). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection Show forest plot | 4 | 546 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.30, 2.36] |
| Analysis 5.1  Comparison 5 Aerobic versus anaerobic cover, Outcome 1 Surgical wound infection. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection (SWI) Show forest plot | 3 | 237 | Risk Ratio (M‐H, Random, 95% CI) | 2.31 [0.60, 8.83] |
| Analysis 6.1  Comparison 6 Oral versus intravenous, Outcome 1 Surgical wound infection (SWI). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection: oral + iv versus iv alone Show forest plot | 15 | 2929 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.43, 0.71] |
| Analysis 7.1  Comparison 7 Combined oral and intravenous versus oral or intravenous alone, Outcome 1 Surgical wound infection: oral + iv versus iv alone. | ||||
| 2 Surgical wound infection: combined oral and iv versus oral alone Show forest plot | 9 | 1880 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.35, 0.76] |
| Analysis 7.2  Comparison 7 Combined oral and intravenous versus oral or intravenous alone, Outcome 2 Surgical wound infection: combined oral and iv versus oral alone. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection Show forest plot | 2 | 129 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.21, 2.15] |
| Analysis 8.1  Comparison 8 Antibiotic given pre‐ or postoperatively, Outcome 1 Surgical wound infection. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection (SWI) Show forest plot | 43 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 9.1  Comparison 9 Antibiotic choice versus a gold standard, Outcome 1 Surgical wound infection (SWI). | ||||

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
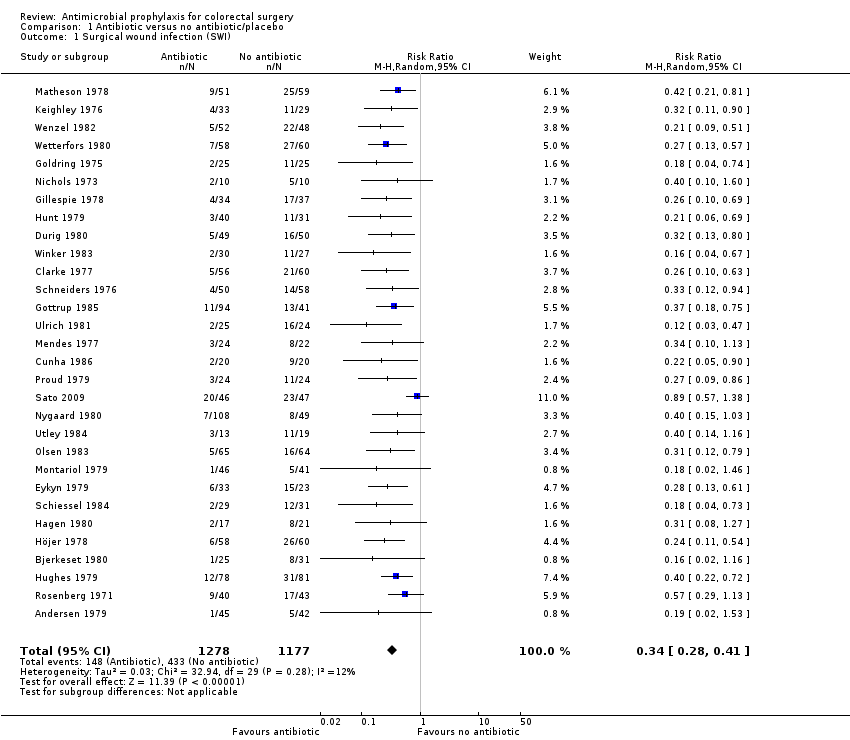
Comparison 1 Antibiotic versus no antibiotic/placebo, Outcome 1 Surgical wound infection (SWI).

Comparison 2 Duration of therapy, Outcome 1 Surgical wound infection (SWI).
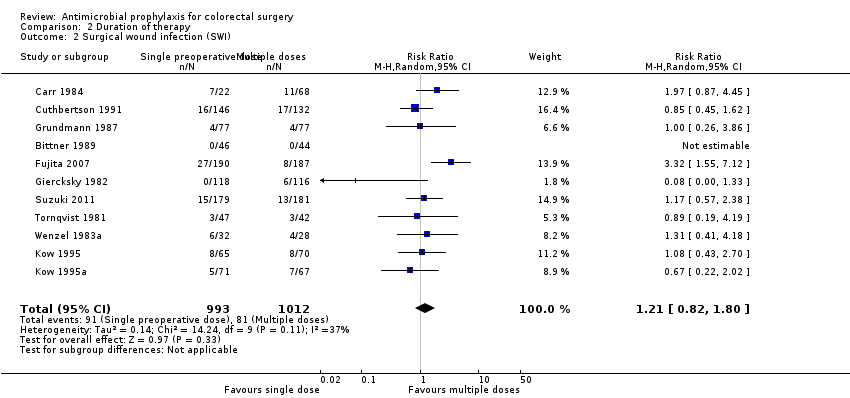
Comparison 2 Duration of therapy, Outcome 2 Surgical wound infection (SWI).

Comparison 3 Additional aerobic coverage, Outcome 1 Surgical wound infection (SWI).

Comparison 4 Additional anaerobic coverage, Outcome 1 Surgical wound infection (SWI).

Comparison 5 Aerobic versus anaerobic cover, Outcome 1 Surgical wound infection.
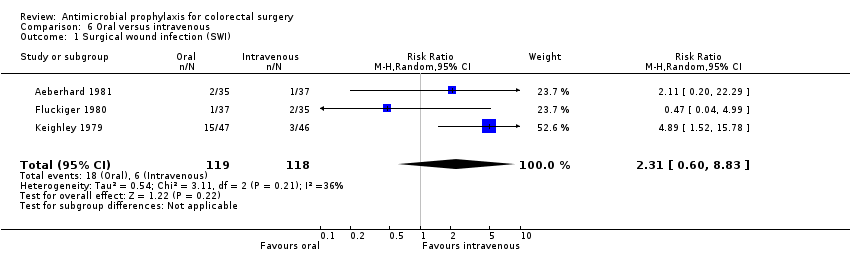
Comparison 6 Oral versus intravenous, Outcome 1 Surgical wound infection (SWI).

Comparison 7 Combined oral and intravenous versus oral or intravenous alone, Outcome 1 Surgical wound infection: oral + iv versus iv alone.

Comparison 7 Combined oral and intravenous versus oral or intravenous alone, Outcome 2 Surgical wound infection: combined oral and iv versus oral alone.

Comparison 8 Antibiotic given pre‐ or postoperatively, Outcome 1 Surgical wound infection.

Comparison 9 Antibiotic choice versus a gold standard, Outcome 1 Surgical wound infection (SWI).
| Antibiotic versus no antibiotic/placebo for colorectal surgery | ||||||
| Patient or population: patients undergoing colorectal surgery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antibiotic versus no antibiotic/placebo | |||||
| Surgical wound infection (SWI) | Study population | RR 0.34 | 2455 | ⊕⊕⊕⊕ | ||
| 368 per 1000 | 125 per 1000 | |||||
| Moderate | ||||||
| 391 per 1000 | 133 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We consider our results not to be affected by either post‐randomisation attrition or clinical heterogeneity. | ||||||
| Combined oral and intravenous compared to oral or intravenous alone for colorectal surgery | ||||||
| Patient or population: colorectal surgery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| oral or intravenous alone | Combined oral and intravenous | |||||
| Surgical wound infection: oral + iv versus iv alone | Study population | RR 0.55 | 2929 | high | ||
| 128 per 1000 | 70 per 1000 | |||||
| Moderate | ||||||
| 230 per 1000 | 126 per 1000 | |||||
| Surgical wound infection: combined oral and iv versus oral alone | Study population | RR 0.52 | 1880 | high | ||
| 79 per 1000 | 41 per 1000 | |||||
| Moderate | ||||||
| 146 per 1000 | 76 per 1000 | |||||
| Study population | not estimable | ( studies) | ||||
| 0 per 1000 | 0 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection (SWI) Show forest plot | 30 | 2455 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.28, 0.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection (SWI) Show forest plot | 34 | 5123 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.93, 1.29] |
| 2 Surgical wound infection (SWI) Show forest plot | 11 | 2005 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.82, 1.80] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection (SWI) Show forest plot | 15 | 1869 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.29, 0.68] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection (SWI) Show forest plot | 19 | 2687 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.30, 0.69] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection Show forest plot | 4 | 546 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.30, 2.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection (SWI) Show forest plot | 3 | 237 | Risk Ratio (M‐H, Random, 95% CI) | 2.31 [0.60, 8.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection: oral + iv versus iv alone Show forest plot | 15 | 2929 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.43, 0.71] |
| 2 Surgical wound infection: combined oral and iv versus oral alone Show forest plot | 9 | 1880 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.35, 0.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection Show forest plot | 2 | 129 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.21, 2.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical wound infection (SWI) Show forest plot | 43 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |

