Support for healthy breastfeeding mothers with healthy term babies
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT, 3‐arm trial with individual randomisation. | |
| Participants | The study was carried out in the Tema area of Ghana (sub‐Saharan Africa). Women were recruited in prenatal clinics in 2 hospitals (1 government and 1 private). The study hospitals served urban areas (an industrial city and a commercial town). High baseline prevalence of breastfeeding. In Ghana, the median duration of breastfeeding was reported as being 22 months and 53.4% of women with babies less than 6 months breastfeeding exclusively. It was reported that "almost all" mothers initiated breastfeeding. 231 women randomised (136 eligible at the beginning of the intervention period). Inclusion criteria: pregnant women in the last trimester planning delivery in the study hospitals and to stay in study area for 6 months after delivery. After delivery: singleton babies with normal birthweight (> 2500 g) and Apgar scores 6 or more at 1 and 5 min. Exclusion criteria: multiple birth, low Apgar score or planning to move out of area. Maternal education: 38% of the women had only primary level or no formal education; 90% of the women were married or living with a partner; 46% of the women were primiparous; 73% of the women had vaginal birth; 24% lived in households with access to a car; 74% were described as Trader/Artisan. | |
| Interventions | All 3 groups (Intervention group 1, Intervention group 2 and control group) were allocated to 2 educational group sessions during pregnancy by trained nurses and 9 proactive home visits by trained nurse counsellors at 1, 2, 4, 6, 8, 12, 16, 20 and 24 weeks postpartum. These were additional to standard care. The content of the sessions differed between the 3 groups. 63% of group 1, 73% of group 2 and 65% of control group women received all 9 scheduled home follow‐up visits. Control group: 85 randomised, 49 followed up. Content of sessions was general health and childcare topics such as immunisation, HIV/AIDS, nutrition and family planning. Intervention group 1: 74 randomised, 43 followed up. Content of sessions was breastfeeding and exclusive breastfeeding. The 2 educational sessions, of approximately 20 minutes each, were given by trained local nurses with experience of breastfeeding to groups of 2‐4 women during the 3rd trimester. At the postpartum home visits women received individual counselling and nurses were advised to respond to concerns. Materials were developed from WHO/UNICEF breastfeeding counselling training manual. Intervention group 2: 72 randomised, 44 followed up. Content of the pregnancy sessions was general health and childcare as for control group. Content of the postpartum home visits was breastfeeding and exclusive breastfeeding as for intervention group 1. | |
| Outcomes | Breastfeeding status at 1, 2, 3, 4, 5, and 6 months exclusive breastfeeding up to 6 months, infant morbidity and growth. | |
| Notes | We have not included data from this study in the review due to high levels of attrition (> 25% loss to follow‐up). Most data were reported in graphs and difficult to interpret. Several measures of exclusive breastfeeding were reported; at 1 and 6 months women were asked about breastfeeding since birth, during previous month and on previous day. In this review we have reported figures for exclusive breastfeeding since birth for both time points. Figures in the paper were expressed as percentage of women still exclusively breastfeeding; in order to use the data we used subtraction to calculate a figure for women who had stopped breastfeeding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Randomization was achieved by writing numbers 1 to 3 on folded pieces of paper." |
| Allocation concealment (selection bias) | High risk | "The numbers were not viewed by either study staff or mothers and the pieces of paper looked the same on the outside. Before offering papers to mothers, they were shuffled in the interviewer’s palm.” “The randomisation scheme used was not a formal one. It was one that could be conducted easily in the field. Despite this, it functionally produced balanced groups with no evidence of bias.” |
| Blinding (performance bias and detection bias) | High risk | Participants: "were not aware of their group allocation or of differences in the content of the health education. " However, women in all 3 intervention arms received an intervention. Women would be aware that they had received an intervention although they may not have been aware of what women in other groups received. Staff: "It was impossible to keep counsellors unaware of study design.... research assistants [collecting outcome data] were aware of mothers group allocation." |
| Incomplete outcome data (attrition bias) | High risk | 231 women randomised during the 3rd trimester. At delivery 95 women were excluded as they were no longer eligible (41% lost before the intervention). A further 13 women were lost to follow‐up during the intervention period. 123 completed the final follow‐up at 6 months (i.e. 53% of the original randomised sample but 90% of those still eligible at delivery). Results were reported in graphs and percentages and it was not clear how many women commenced breastfeeding, so group denominators are not clear. Loss to follow‐up appeared balanced across groups. |
| Selective reporting (reporting bias) | Unclear risk | Failure to provide denominators for results means that they are very difficult to interpret. |
| Other bias | Unclear risk | Women in the 3 arms of the trial appeared similar at baseline. Analysis was according to group allocation. |
| Methods | RCT (n = 66) single site, a Baby‐Friendly hospital, March to July 2008. | |
| Participants | Urban state maternity hospital in Turkey. Background rates of breastfeeding initiation: high. Inclusion criteria: primaparous, live vaginal birth, healthy term singleton infant, lives in study area, speaks Turkish, no history of chronic diseases, non‐smoker, intends to breastfeed. Exclusion criteria: infant birthweight < 2500 g, Apgar score ≤ 7, congenital anomalies, serious disease or needing intensive care. Baseline prevalence of "ever breastfed" in Turkey: 96.7% (WHO Global data bank 2010, accessed 6.10.2011). | |
| Interventions | At this Baby‐Friendly hospital, a standard breastfeeding education session lasting 20‐30 minutes was provided to all mothers before standard discharge home at 24 hours after the birth. The session included the topics covered by the 18h WHO/Unicef training. The intervention group received standard breastfeeding support plus support from trained lay supporters who had undergone WHO/UNICEF 18 h training. The intervention was a single home visit on day 3 after the birth (in hospital), by 2 lay breastfeeding, that lasted about 30 minutes and covered the same topics as routine support. | |
| Outcomes | Exclusive breastfeeding at 2 and 6 weeks and 6 months postpartum; breastfeeding duration (any/exclusive) to 18 months; breastfeeding knowledge scores at 2 and 6 weeks postpartum. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Low risk | 82% follow‐up at 18 months. Reasons for loss were explained and were balanced across groups. |
| Selective reporting (reporting bias) | Low risk | Not apparent. |
| Other bias | Low risk | Groups appeared similar at baseline. |
| Methods | Primary care facilities. Recruitment over 5 months, n = 169. | |
| Participants | 3 hospitals in the city of Pelotas, in southern Brazil. Background rates of breastfeeding initiation: high. Ethnic composition not described. Inclusion criteria: term healthy baby, family income at least US $500 per month (no economic constraints to baby's growth), mother intends to breastfeed and does not smoke. Baseline prevalence of breastfeeding in Brazil in the first 30 days = 88%. | |
| Interventions | Control: attended paediatric clinics where general advice on advantages of breastfeeding may have been offered, but specific lactation counselling was not provided. Intervention: hospital visit, home visits at 5, 15, 30, 45, 90 and 120 days, and 24 hour telephone hotline for help or to arrange visits. 2 members of the lactation support team had received the 40 h WHO lactation support training course. | |
| Outcomes | Breastfeeding pattern and duration up to age 4 months. Breastmilk intake for a subgroup of 68 infants at 4 months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated code. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding (performance bias and detection bias) | Unclear risk | It was stated that different field‐workers delivered the intervention and collected outcome data; but it was not clear whether this attempt at blinding was successful. |
| Incomplete outcome data (attrition bias) | High risk | 188 women were randomised. 21 were excluded after 2 weeks as they had introduced formula. A further 26 withdrew (some data were available for some of these women). 141 women completed the trial (75%). |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Excluding women who introduced formula within 2 weeks of randomisation is likely to have introduced bias although similar numbers were excluded from both groups (9 women lost from the intervention group for this reason and 11 women from the control group and an additional control was withdrawn for smoking). |
| Methods | RCT, 2 arms with individual randomisation. | |
| Participants | Hartford area of Connecticut, USA in a hospital providing care for predominantly Latina low‐income women. Inclusion criteria: age 18 years or older; gestational age less than 32 weeks at first approach; healthy, considering breastfeeding, planning delivery at study hospital and resident in area for 3 months after the birth, 185% of the federal poverty level, available for telephone contact and willing to participate. Exclusion criteria: Mothers ‐ medical conditions such as diabetes or hypertension; drug use that could impair breastfeeding. Infants ‐ preterm, low birthweight (< 2500 g) baby, any complications requiring admission to special care, Apgar score < 7 at 1 and 5 minutes. Participant characteristics: at baseline: intervention n = 63; control n = 72. Age: intervention 68%; control 66%; Married/cohabiting: intervention 40%; control 26%; Hispanic race: intervention 81%; control 64%; Education < high school: intervention 31%; control 38%; Received welfare: intervention 31%; control 38%; Primiparous: intervention 92%; control 89%; Planned breastfeeding duration:‐ Less than 6 months: intervention 20%; control 46%; 6‐12 months or longer: intervention 80%; control 54%. | |
| Interventions | Control group received what would have been standard care for private patients (these women may not have normally qualified to receive this care as many were participating in welfare programmes). This consisted of: breastfeeding support line open to mothers after delivery staffed by lactation specialist. Usual inpatient care and support for breastfeeding was provided by hospital staff. Intervention group received in addition 3 prenatal home visits, daily in‐hospital visits and 9 postpartum home visits from peer counsellors, scheduled 3 in first week, 2 in second week and 1 in each week 3‐6. Women could also phone peer counsellors. Peer counsellors were mothers from the area with experience of successful breastfeeding and training from lactation consultant. | |
| Outcomes | Infant feeding practices (weekly for first month) breastfeeding and exclusive breastfeeding. Infant morbidity (diarrhoea and ear infection). Breastfeeding outcomes measured in 3 different ways – over the past 24 hours, over the past week and since the birth (ever given). | |
| Notes | We have not included data from this study in the review due to high levels of attrition (> 25% loss to follow‐up). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | SPSS software was used to randomly assign subjects to study groups. |
| Allocation concealment (selection bias) | Low risk | "Recruited subjects were entered into the database at the end of every week” and then random allocation by computer software.
|
| Blinding (performance bias and detection bias) | Unclear risk | Blinding not attempted, women, staff and outcome assessors were likely to have been aware of group assignments. |
| Incomplete outcome data (attrition bias) | Unclear risk | 182 women were recruited and randomised. 162 were still eligible at delivery and 135 completed the trial (84% of those still eligible at delivery and 74% of the total randomised). |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Groups appeared similar at baseline although women in the control group were more likely (46%) to plan to breastfeed for less than 6 months than women in the intervention group (20.4%). This difference in breastfeeding intentions mean that results are more difficult to interpret. |
| Methods | RCT 2‐arm trial. Single‐site study, n = 900. | |
| Participants | Urban setting in Brazil: in‐patient maternity unit. Background rates of breastfeeding initiation: High. Ethnic composition not described. Inclusion criteria: family income less than twice the minimum Brazilian wage; hospital stay less than 5 days; wanting to breastfeed: living within the city of Pelotas. Baseline prevalence in Pelotas (1993) for any breastfeeding: 85% at 1 month, 66% at 3 months and 38% at 6 months. | |
| Interventions | In usual care, a social assistant would not normally make routine home visits but would visit only when requested to do so by the hospital team. Intervention: 3 home visits at 5, 10 and 20 days postpartum by a social assistant or nutritionist. The visitor was required to have a personal history of successfully breastfeeding a child and received training in breastfeeding physiology and common breastfeeding problems and their solutions. | |
| Outcomes | Breastfeeding at monthly intervals to 6 months and median duration of breastfeeding. Time to introduction of artificial feeds. Difficulties encountered during breastfeeding and reasons for weaning also recorded. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) | High risk | The nurse collecting outcome data was not aware of previous contacts but the authors state that s/he may have been made aware of group assignment as women were likely to talk about the intervention. |
| Incomplete outcome data (attrition bias) | Low risk | 900 randomised, approximately 8% lost to follow‐up in the interventions and control groups. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | No baseline imbalance apparent. Assessment of risk of bias was made from translation notes. The original paper is in Portuguese. |
| Methods | Randomised controlled trial, 3‐arm trial with individual randomisation. | |
| Participants | Recruited from Maternity Teaching Hospital in Damascus, Syria. Background rates of breastfeeding initiation: High. Inclusion criteria: consenting women who delivered a healthy newborn whether by vaginal delivery or caesarean section, who lived within 30 km from hospital, and who were available for the follow‐up for the coming 6 months. Exclusion criteria (infants): premature, low birthweight (< 2500 g), with apparent congenital anomalies. Participant characteristics: age not clear. Approximately 37% primiparous, 90% had normal labour, more than 99% of the women married. Home conditions were described as bad (number of rooms, poor sanitation or water, etc) in 28.5% of control group and approximately 20% of the intervention groups. Few of the women (approximately 5%) worked outside the home. | |
| Interventions | Control group received standard care in Syria (no postnatal visits). Intervention group 1 (301 randomised): 4 structured home visits from trained midwives at 1, 3 and 7 days and 4 weeks after the birth. Midwives examined mothers and infants and provided and advice and support on a range of healthcare issues including breastfeeding support and education. Intervention group 2 (301 randomised) a single postnatal visit from a trained midwife at 3 days which included advice and education on breastfeeding. | |
| Outcomes | Primary outcomes: maternal postpartum morbidities, postnatal care uptake, contraceptive uptake and type, infant morbidities, infant immunisation according to the national schedule at 3 months and Infant feeding, namely exclusive breastfeeding during the first 4 months of life. Secondary outcomes: women’s perceptions of their health, impressions about the home visit and perceptions of its quality. | |
| Notes | Some baseline imbalance, women in the control groups were more likely to have poor home conditions and were less likely to have received antenatal care. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised into blocks to either of the intervention groups (4 home visits or 1 home visit) or to the control group (no home visits). Randomisation was in blocks of 7 where a caseload of 21 eligible deliveries per day was assumed, based on the average daily number of deliveries in the hospital (ranging from 30 to 35) after excluding non‐eligible cases. |
| Allocation concealment (selection bias) | Low risk | Numbered, opaque and sealed envelopes..” Group allocation was carried out by a senior midwife not involved in the rest of the study. |
| Blinding (performance bias and detection bias) | High risk | Participants and caregivers were not blinded. For outcome assessors it states that the interviewers carrying out outcome assessment were not informed of groups but would be aware of which group women were in from the interviews. |
| Incomplete outcome data (attrition bias) | Low risk | 903 women met the inclusion criteria. After randomisation (301 in each arm), 27 women were excluded (18 due to lack of address detail and 9 refusals). A total of 876 women were followed up in the 3 study groups: Group A (285 women); Group B (294 women) and Group C (297 women). Incomplete data were addressed. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Some baseline imbalance, women in the control groups were more likely to have poor home conditions and were less likely to have received antenatal care. Outcome data were collected at 4 months, but it is likely that there may have been recall bias for some outcomes, e.g. breast engorgement – women in the intervention groups will have discussed this and maybe it was recorded at the time it occurred, women in the control group will not have been asked until 4 months postpartum. Outcome data were collected for a large number of variables so any differences may have occurred by chance. |
| Methods | Cluster‐randomised study with 8 sites, n = 1115. | |
| Participants | 8 village communities located 3‐5 km from the main highway in Haryana, India. Background rates of breastfeeding initiation: High. Inclusion criteria: born in a study village within 9 months of start of intervention. Baseline breastfeeding prevalence stated to be high. | |
| Interventions | At the control sites, the research team provided routine services, in which, according to national policy, workers are required to advise exclusive breastfeeding for 4‐6 months. Health and nutrition workers in the intervention communities received training based on Integrated Management of Childhood Illnesses Training Manual on Breastfeeding Counseling (WHO 1997). Messages ‐ feed only breast milk for first 6 months of life; breastfeed the infant day and night, at least 8 times in 24 h; possible adverse effects of other foods and fluids given to breastfeeding infants ‐ given to mothers at birth, monthly home visits, immunisation clinics and neighbourhood meetings. | |
| Outcomes | Feeding at 3 months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Communities were paired on the basis of similar scores for socio‐economic, mortality and morbidity indicators. 1 of each pair was allocated to the intervention using a random numbers table. 8 areas randomised (4 to each condition). |
| Allocation concealment (selection bias) | Low risk | Statistician independent of project carried out randomisation. |
| Blinding (performance bias and detection bias) | High risk | Women, staff and outcome assessors all aware of intervention. |
| Incomplete outcome data (attrition bias) | Unclear risk | Reasons for drop‐out recorded. 1151 births within the study period (not clear how many in each area). 588 families received the intervention and 527 no intervention. 895 completed 3 months follow‐up (80%) and 880 6 months (79%). |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Areas were paired but it was not clear whether or not this achieved similar baseline characteristics between groups. Results were reported to have been adjusted for clustering. |
| Methods | RCT, 2 arms randomised, individual randomisation (although the study also included a non‐randomised comparison group). | |
| Participants | 100 breastfeeding mothers randomised; recruited 3 days after the birth. Inclusion criteria: married, primiparous with healthy infants born at a maternity hospital in Nova Scotia, Canada. Exclusion criteria: infants with birthweight < 2500 g, with Apgar scores less than 5, twins, women having operative deliveries, women who did not speak English. | |
| Interventions | Women in both groups received a pamphlet on breastfeeding. Women in the control group received usual care (not specified). Intervention: weekly telephone calls beginning 10 days after the birth made by a nurse interviewer, offering support and advice and referral if necessary. Calls lasted 5‐10 minutes and were described as friendly. Women received up to 3 calls up to 6 weeks postpartum. Calls ceased when women discontinued breastfeeding. | |
| Outcomes | Interviews at 6 weeks postpartum. Women were asked about infant behaviour and infant feeding and breastfeeding duration. | |
| Notes | We have not included data from this study, because results in this paper were not reported in a form in which we could use them in the review. Most of the results were not reported according to randomisation group (rather authors described factors and associations with e.g. breastfeeding). Breastfeeding in the randomised groups at 6 weeks was not reported and it was not possible to contact the authors to obtain this information. It was stated that average breastfeeding duration was 28.6 days in the intervention group vs 21.0 days for controls, but no standard deviations were reported. It was not clear when or how breastfeeding duration data were collected; if at the 6 week postpartum interviews this suggests that figures for average breastfeeding duration only apply to those women who had discontinued breastfeeding and denominators are therefore not clear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Described as “randomly assigned”. |
| Blinding (performance bias and detection bias) | Unclear risk | The interviewer who recruited women also carried out the intervention. The interviewer carrying out outcome assessment was reported not to be aware of the initial feeding choice (but may have been made aware of the intervention allocation by women). |
| Incomplete outcome data (attrition bias) | Unclear risk | Rates of follow‐up at 6 weeks were high (97%). However, denominators for breastfeeding duration results were not reported. |
| Selective reporting (reporting bias) | Unclear risk | Most results were not reported by randomisation group and are difficult to interpret. |
| Other bias | Unclear risk | Unclear ‐ no baseline characteristics table for randomised groups. |
| Methods | RCT, 2‐arm trial, n = 382 women randomised. | |
| Participants | From 2 prenatal care centres in the Bronx, New York (reported to be the US county with the highest poverty rate). Background rates of breastfeeding initiation: Low. Inclusion criteria: speaks English or Spanish, singleton or twin pregnancy <24 weeks (twins subsequently excluded), intending to keep infant and attend for prenatal and postnatal care at centre and affiliated hospital, telephone contact numbers available. Exclusion criteria: HIV positive status, chronic disease with medication not compatible with breastfeeding, diabetes, serious illness, or breast reduction surgery. Participant characteristics: 57% Hispanic, 36% African American, 62% multiparous (70% of these had previous breastfeeding experience), mean age 25 years (SD 6.23), 51.5% married or living with a partner, 57% receiving Medicaid. | |
| Interventions | Control group (194 randomised): Women in the control group had no contact with the lactation consultant. Standard care varied between the sites and neither site followed an established protocol for breastfeeding. Women enrolled in women and child nutrition programmes (WIC) had the opportunity to visit a breastfeeding coordinator. Intervention group (188 randomised). The intervention was by a trained lactation consultant. Women recruited before they were 24 weeks pregnant had 2 prenatal lactation consultant visits scheduled. During late pregnancy there was telephone contact, and hospital and home visits and telephone support (up to 12 months postpartum) were planned for the postnatal period. In the postnatal period 25% of the intervention group received at least 1 hospital contact; approximately 50% had telephone and/or home visits; but 36% received no home or hospital visits and no telephone support. | |
| Outcomes | Infant health outcomes. Duration of breastfeeding and exclusive breastfeeding was presented mostly in graphical form and was difficult to interpret. Breastfeeding was categorised on a 7‐point scale from 7 exclusive breastfeeding (which was defined as no other milk or food, but infants may have received water and other liquids) through to exclusive formula, between there were various “intensities” of breastfeeding (e.g. more than 50% breast milk). This means results are complicated and not easy to interpret. Women were followed up to 12 months and detailed (graphical) weekly data are reported for weeks 1‐26 postpartum. | |
| Notes | Results estimated from graphs. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The project’s biostatistical office generated and maintained a list of random codes for subjects... undisclosed blocking factor and stratification according to center”. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes, numbered and opened sequentially. |
| Blinding (performance bias and detection bias) | High risk | “Neither the RA [research assistant] collecting breastfeeding outcome data nor the LC [lactation consultant] providing the intervention were blind with respect to treatment group.” |
| Incomplete outcome data (attrition bias) | Unclear risk | Women were recruited in the antenatal period. 382 women were randomised. Loss to follow‐up included 10 women who miscarried or terminated the pregnancy. 304 women were followed up into the postnatal period (80% of those randomised). There was further missing data for longer term follow‐up. Loss to follow‐up was balanced across groups. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | The intervention did not appear to be standardised and many women in the intervention group (36%) did not receive any postnatal visits. |
| Methods | Single‐site study. Duration not stated, n = 115. RCT. 2‐arm trial with individual randomisation. | |
| Participants | Urban USA ‐ ambulatory care centre and in‐patient maternity unit. Background rates of breastfeeding initiation: Low. Baseline prevalence of breastfeeding at birth in national WIC sample = 33% (1991). Inclusion criteria: English speaking; nulliparous. Exclusion criteria: separated from child at birth; preterm delivery; child in NICU longer than 72 hours. Ethnic composition: described as 71% white. 90% of participants were eligible for WIC programmes for those on low income. Study population not limited to those intending to breastfeed. | |
| Interventions | Control group were offered optional prenatal breastfeeding classes, postpartum breastfeeding instruction by nurses and physicians and out‐patient follow‐up by nurses and physicians in the paediatric ambulatory department. Intervention: package of: 2‐4 prenatal sessions with lactation consultant (10‐15 minutes each); telephone call 48 hours after discharge; visit to lactation clinic at 1 week postpartum (staffed by paediatrician or lactation consultant); contact with lactation consultant at each health supervision visit until weaning or 1 year; professional education of nursing and medical staff. | |
| Outcomes | Rates of breastfeeding at 2 months and median duration of breastfeeding. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sample stratified by age with block randomisation in blocks of 8. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) | High risk | Outcome assessment not independent of intervention. Outcome data were collected by questionnaire administered by the lactation consultant who was not blind to group allocation. |
| Incomplete outcome data (attrition bias) | Unclear risk | Follow‐up 94%. It appeared that 115 women were randomised. It was stated that 7 in the intervention were excluded as they did not receive the intervention. 8 women in the control group were subsequently excluded from the analysis for at least some outcomes as the treatment they received deviated from protocol. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Potential confounders: women were excluded from intervention group following randomisation if they had received fewer than 2 prenatal lactation consultations; ITT analysis not performed (8 women in control group who met lactation consultant excluded); intervention included input by staff caring for both intervention and control groups. |
| Methods | RCT, 2‐arm trial with individual randomisation (n = 339) with add‐on qualitative study. | |
| Participants | Denver, USA; a clinic providing care for a predominantly Hispanic, medically underserved population. Background rates of breastfeeding initiation: Low. Inclusion criteria: women aged 18 years or older, primiparous with healthy, term, singleton baby who were willing to consider breastfeeding. Exclusion criteria: primary language not English or Spanish, medical complication that interfered with breastfeeding, hospital stay longer than 72 hours following vaginal births or longer than 96 hours following caesarean section, baby with medical problems, admitted to NICU or had a hospital stay longer than 72 hours. Participant characteristics: Mean age 22 years; 88% Hispanic or Latino; 77% vaginal delivery. Planned to breastfeed only: Intervention group 50%, control group 55% (other women planned to combine breastfeeding with formula). More than 60% were participating in WIC programmes at 1 month and 74% of these women were provided with formula at WIC clinics. | |
| Interventions | Control: usual hospital care (pamphlets on breastfeeding, a breast pump, lanolin cream and a water bottle); usual discharge care (commercial discharge packs) and scheduled healthcare visits at 3‐5 days and at 2 weeks at the local community health centre. Intervention: telephone support daily, from the day following hospital discharge until 2 weeks postpartum, from trained nurses following a specific protocol covering advantages and disadvantages of breastfeeding, cultural issues, technique, problems and with referral for any lactation or medical problems. | |
| Outcomes | Any breastfeeding or predominantly breastfeeding. Maternal satisfaction, healthcare utilisation, reasons for stopping breastfeeding. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block random allocation. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque sealed envelopes. |
| Blinding (performance bias and detection bias) | Unclear risk | No blinding for participants or caregivers and not described for outcome assessors. |
| Incomplete outcome data (attrition bias) | Unclear risk | 341 women were randomised. At 1 month there approximately 8% loss to follow‐up. By 6 months 27% loss. 73% were described as included in the analyses; women in the intervention group that did not receive the intervention as planned were not included. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Groups appeared similar at baseline. |
| Methods | Study methods were not clear. This appeared to be a cluster‐randomised trial in 35 clinics.The intervention was carried out with healthcare workers. Results are for women attending intervention and control clinics before and after the intervention period. | |
| Participants | Setting: family healthcare teams from Montes Claros city in South East Brazil. Baseline prevalence of breastfeeding initiation in country/setting: not clear. 1423 women (unclear). Follow‐up for 12 months. Inclusion criteria: mothers with children between 0 and 2 years old registered with the family health teams. Approximately 20% under 20 years, 38% primiparous, 27% vaginal deliveries, 90% more than 4 years education. | |
| Interventions | Control: healthcare teams (n = 15 ‐ unclear) in control clinics did not receive the training Intervention: 20 healthcare teams received staff training to promote breastfeeding, based on the Baby Friendly Hospital Initiative. Duration of the intervention unclear; there was an initial interview before the study and a second interview 12 months after the start of training. | |
| Outcomes | Number of exclusive breastfeeding days; survival curves. | |
| Notes | We have not included data from this study. Data were not reported in a way in which we could incorporate results into the review. Authors report the number of days not the number of participants for exclusive breastfeeding. It is reported that the median duration of exclusive breastfeeding was 106 days before and 107 days after the intervention period for the control group. For the intervention group the median duration of exclusive breastfeeding was reported to be104 days before and 125 days after the intervention period; the difference was reported to be statistically significant. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described; it was reported that half of the women were assigned to the Intervention group and the other half to the control group |
| Allocation concealment (selection bias) | Unclear risk | Not described; 20 intervention clinics and 15 control (not clear). |
| Blinding (performance bias and detection bias) | High risk | Clinics assigned to intervention and control (blinding not attempted?). |
| Incomplete outcome data (attrition bias) | Unclear risk | Not clear. Authors reported that dropouts were negligible because all children registered were contacted with the help of community health agent. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Data extraction from translation (original paper in Portuguese). Cluster trial with no apparent adjustment for design effect |
| Methods | RCT 2‐arm trial. Individual randomisation (n = 219). Recruitment July 2000‐August 2002 at an urban US hospital with BFI accreditation. | |
| Participants | Urban US hospital prenatal clinic serving a low‐income, predominantly Latina population. Background rates of breastfeeding initiation: Low. Antenatal inclusion criteria: low‐income women at least 18 years old, at 26 weeks' gestation or less, considering breastfeeding, not yet enrolled in peer counselling programme, resident in hospital area, available for telephone follow‐up. Postnatal inclusion criteria: healthy, full term singleton infants, no congenital abnormalities, no maternal history of HIV and no admission to NICU. After birth, n = 165 women remained in the study, 90 in the intervention group and 75 controls Participant characteristics: Ethnic composition 80% Hispanic (61% Puerto Rican origin), 9% African American, 3% white, 8% other. | |
| Interventions | Control: routine breastfeeding education offered by the study hospital, and the same breastfeeding services as privately paying women. A small amount of exposure to peer counsellors among the control group was reported. Intervention: 1 prenatal home visit, daily visits during postpartum hospitalisation, home visit within 24 hours and at least 2 more home visits as requested, and telephone/pager contact. Intervention from peer counsellors with 30 hours classroom training that covered LLLI Peer Counseling Program and Hispanic Health Council's curriculae. Peer counsellors had to score 85% in a written exam and work for 3‐6 months with experienced peer counsellors to demonstrate competence before working independently with clients. Peer counsellors had 1 hour per month continuing education and were paid for their work. | |
| Outcomes | Breastfeeding rates at birth and 1, 3 and 6 months postpartum. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By computer programme. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) | Unclear risk | It was not stated whether there was any attempt to blind outcome assessors. |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up appeared reasonably balanced although there was more loss from the control group. Reasons for loss to follow‐up stated. 219 were randomised, 72% followed up at 1 month, 70% at 3 months and 66% at 6 months. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Groups appeared similar at baseline. It was reported that many women in the intervention group received less than half of the planned visits. |
| Methods | Quasi‐randomised trial with sequential allocation to 3 study groups. | |
| Participants | 180 women (not clear) attending a hospital in Southern Taiwan. Inclusion criteria: breastfeeding at hospital discharge, term, healthy infant, able to read Chinese. (Hospital discharge at approximately 5 days.) | |
| Interventions | Control: usual care. Phone support intervention: weekly phone calls for 2 weeks after hospital discharge then at 4 and 8 weeks postpartum by maternity nurse. The calls were to increase women’s self confidence. Home visits intervention: same schedule as phone support group with visits at home by the maternity nurse. | |
| Outcomes | Breastfeeding duration and analysis of factors affecting duration of breastfeeding. | |
| Notes | We have not included data from this study in the review as data were not reported in a way that allowed us to enter them into RevMan 2011 for meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequentially to 1 of 3 groups.
|
| Allocation concealment (selection bias) | High risk | In sequence (could be anticipated and changed by the person carrying out randomisation). |
| Blinding (performance bias and detection bias) | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not clear. 180 women were followed up. It was not clear whether this number was randomised. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Baseline characteristics of 3 groups similar. |
| Methods | RCT, 2‐arm trial with individual randomisation | |
| Participants | The study was carried out in 2 hospitals serving urban areas and neighbouring small towns in the interior of the State of Pernambuco, north‐eastern Brazil. Background rates of breastfeeding initiation: High. Inclusion criteria: singleton infants. Exclusion criteria: infants with congenital anomalies or serious illness necessitating intensive care and those whose mothers had serious disease or mental illness or were planning to leave the area within 6 months. Approximately 60% had income lower than the minimum wage; 33% did not have access to a flush toilet, approximately 35% of the mothers were less than 20 years, 39% primiparous, approximately 28% CS. | |
| Interventions | Maternity staff in both hospitals received (90%) the 18h UNICEF/WHO Baby Friendly Hospital Initiative training course. All participants in the intervention and control groups received their hospital postnatal care from these Baby‐Friendly trained staff. In addition, women in the intervention group received ten postnatal home visits (mean duration 30 minutes); 4 during the first month, 2 during the second month and 1 per month during the 3rd to 6th months. Each mother was given an illustrated booklet. At each visit the home visitors observed the positioning of the infant at the breast, flow of milk and the baby’s satisfaction; encouraged exclusive breastfeeding for 6 months and continued breastfeeding for at least 2 years and used the booklet as a basis for discussions of key topics relevant to the infant’s age. If there were any difficulties home visitors could not resolve they referred the mother for more specialist help at the hospital. If other family members were present, their attitude towards exclusive breastfeeding was assessed and their support was sought, including help with household chores. | |
| Outcomes | The main outcome is exclusive breastfeeding. Data collected prospectively at 1, 10, 30, 60, 90, 120,150 and 180 days after birth. Any breastfeeding at same time points. The type of other fluids introduced was also recorded at each time point. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised in blocks of 10 per town by use of a random numbers table. The random numbers were generated by the project manager, and enrolment and group assignment were made by 2 maternity‐based research assistants. |
| Allocation concealment (selection bias) | Unclear risk | Concealment was achieved by drawing numbers from envelopes at the time of assignment. |
| Blinding (performance bias and detection bias) | High risk | Data were collected in the trial by 4 researchers who were not aware of group allocation and were unconnected with the delivery of the interventions. Mothers in the trial were not close neighbours, so discussion with other mothers is unlikely, but we did not formally assess whether masking was maintained. For participants they were not blinded and they did not formally assess whether masking was maintained. For those delivering the intervention (caregivers), they would be aware of group assignment. For the outcome assessors, they were described as blinded but not clear if blinding was effective. |
| Incomplete outcome data (attrition bias) | Low risk | 350 women were randomised, 175 in each group. 20 women (6%) were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | The random numbers were generated by the project manager and so this leads to bias. Results were presented in graphs and aggregated results were not simple to interpret. |
| Methods | RCT (methods not clear) 2 arm trial with individual randomisation. | |
| Participants | From maternity ward of the Hospital de Clinicas de Porto Alegre in Brazil, a university general hospital with Baby Friendly accreditation. Background rates of breastfeeding initiation high, however median duration of exclusive breastfeeding 29 days. Inclusion criteria: mothers living in the city of Porto Alegre, healthy non twin newborns with a birthweight equal or greater than 2500 g. Exclusion criteria: mother‐infant pairs that were unable to stay together due to a health concern in either the mother or infant. Mothers 20 years old or more 76%, vaginal delivery 72%, white mothers 70%, more than 8 years education 64%, living with partner 83%. | |
| Interventions | Control: standard hospital care met Baby‐Friendly standards. The control group appear not to have received home visits. Intervention: In hospital, 2 nurses reinforced the orientation about breastfeeding technique routinely given to mothers, following the WHO breastfeeding counselling principles, in a 30‐minute session with no more than 2 mother‐infant pairs. Topics included comfortable and proper mother and infant positioning, correct attachment of the child to the breast and manual milk expression. Pictures, dolls and a model breast were used for demonstrating proper breastfeeding technique. The intervention group also received 2 home visits from the same nurse, when the child reached 7 and 30 days of age. Infant feeding patterns, positioning, attachment, milk expression and breastfeeding problems were discussed, and breast examination performed. | |
| Outcomes | The primary outcome is the number of mothers who breastfed and exclusively breastfed on maternity ward and at 30 days. The secondary outcome is the frequency of breastfeeding related problems. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Following interviews and feeding assessments, mother‐infant pairs were randomly assigned by pulling coloured balls from a bag indicating either the control or experimental group. After the number of mothers calculated for the experimental group were selected, all women eligible for the study were added to the control group until the sample was complete. |
| Allocation concealment (selection bias) | High risk | By drawing coloured balls from bags – this could be changed and it was not clear that all women in the control group were randomly allocated. |
| Blinding (performance bias and detection bias) | Unclear risk | The researchers responsible for the breastfeeding evaluations did not participate in the intervention and were blinded to the group to which the mother infant pairs had been assigned”. Not clear if blinding was effective. |
| Incomplete outcome data (attrition bias) | Unclear risk | 233 women were eligible, 211 followed up. (It was not clear how many were randomised.) |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Groups were described as similar at baseline although it appeared that more women in the control group that had had previous breastfeeding experience were more likely to feed for 6 months (65%) compared to women in the intervention group (47.5%). Unequal numbers in the intervention and control group. The groups were not balanced (74 in the intervention group and 137 controls). It was not clear that all the women in the control group were randomly allocated. |
| Methods | Single‐site study recruiting over 10 months, n = 258. RCT, 2‐arm trial with individual randomisation. | |
| Participants | Women at home in Toronto, Canada. Background rates of breastfeeding initiation: Intermediate. Inclusion criteria: English speaking; primiparous; 16 years or over; single full‐term baby. Intending to breastfeed. Predominantly educated, Caucasian and over 25 years with income over $40,000/year. | |
| Interventions | Control: not described Intervention: telephone support by briefly‐trained volunteers (2.5 hour session) who had personal breastfeeding experience for at least 6 months. First contact within 48 hours of hospital discharge and then as required. Mean number of contacts in those completing log‐books = 5.4. Mean duration of telephone contact = 16.2 min. 97% of contacts by telephone. 3% at home. | |
| Outcomes | Breastfeeding (any or exclusive) at 1, 2 and 3 months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly generated numbers were provided by a statistician who was not involved in the recruitment process. |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered, sealed opaque envelopes. |
| Blinding (performance bias and detection bias) | Unclear risk | “A research assistant blinded to group allocation telephoned the participants to collect data regarding current infant feeding status, breast problems encountered and health services used.” Not clear if attempted blinding of outcome assessor was successful. |
| Incomplete outcome data (attrition bias) | Low risk | Very little loss to follow‐up. 258 women randomised and 2 women lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Low risk | No apparent differences between groups at baseline. |
| Methods | RCT, 2 arms with individual randomisation (n = 78). | |
| Participants | Setting: 2 hospitals in Rochester, NY, USA. Background rates of breastfeeding initiation: Low. Inclusion criteria: Maternal age < 20 years, uncomplicated postpartum, breastfeeding singleton infant born at gestational age > 36 weeks and weight > 2000 g, mothers and infants discharged home together. Exclusion criteria: maternal contraindications to breastfeeding (HIV, active substance abuse), postpartum transfusion or intensive care; infants in intensive or special care unit > 6 hours, infants with anomalies that interfered with breastfeeding (e.g. cleft lip or palate). Participant characteristics: Mean age 18.3 years, approximately half were African Americans, approximately one‐third had private or health maintenance organisation insurance, the rest were on Medicaid or Medicaid HMO, more than 80% were first time mothers and gave birth vaginally. | |
| Interventions | Usual care included access to paediatric care providers and hospital lactation consultants.The control group did not receive telephone peer support. Intervention group: telephone support from trained peer supporters (teen mothers who had breastfed for more than 4 weeks). Peer supporters telephoned the new teen mothers at 2, 4, 7 days and 2, 3, 4 and 5 weeks post discharge. Peers introduced themselves and talked about the breastfeeding experience. No specific discussion topics were assigned. Peers offered their telephone numbers so that new mothers could call for support. They were advised to refer anyone with a problem to telephone resources for breastfeeding information or to their physician. Peers and women received gift voucher incentives to complete assessments and training. | |
| Outcomes | Primary outcome: ‘any breastfeeding’ duration, as measured by the age in days at complete breastfeeding cessation. Secondary outcome: exclusive breastfeeding duration, as measured by the time to the first introduction of any other supplement (water, juice, vitamins or formula). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers.
|
| Allocation concealment (selection bias) | Low risk | “The assignment was recorded in a sealed and numbered envelope. Envelopes were sequentially opened.” |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding attempted but not clear if it was successful. Participants: “In order to blind subjects to the study hypothesis, recruiters explained that this study was about: how young mothers who breastfeed in the hospitals feed their babies at home; how young mothers make feeding decisions and who helps them make those decisions.” Outcome assessor: “a single research assistant conducted all the telephone interviews, using standardised, closed ended questionnaires. The interviewer had no knowledge of the study hypothesis or design.” |
| Incomplete outcome data (attrition bias) | High risk | 78 randomised (38 intervention, 40 control). In control group‐ 5 dropouts before first interview; 2 dropouts before 8‐week interview; 9 dropouts between 8 and 21 weeks. In intervention group‐ 6 dropouts before first interview; 3 dropouts before 8‐week interview; 7 dropouts between 8 and 37 weeks. Overall, 11 women dropped out immediately after recruitment (14%). By 8 weeks 21% lost to follow‐up. 46/78 (61%) were successfully followed up to complete breastfeeding cessation (22 intervention and 24 control). |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Of the 5 adolescents who completed peer support training, there was only 1 that remained involved for the entire duration of the study. There was very poor compliance with possibly only half of the intervention group receiving the planned intervention. The analysis is presented in diagrams that are not simple to interpret. Study results published in 2010, data collection 1996‐7. |
| Methods | RCT 2‐arm trial. Women randomised. Single‐site study. Mothers recruited March 2000‐December 2001, n = 605. | |
| Participants | Urban Italy. Background rates of breastfeeding initiation: Intermediate. Inclusion criteria: mothers in public maternity ward in Rome, intending to breastfeed. Exclusion criteria: mothers who did not speak Italian, had no phone, breastfeeding medically contraindicated, baby in SCBU. Ethnic composition not defined. Baseline national breastfeeding initiation rate 70%. | |
| Interventions | Control: standard care (not described). Intervention: home visit and telephone contact. Home visit, from 1 of the 6 midwives from the maternity ward of the study hospital, took place within 7 days of hospital discharge. Telephone breastfeeding counselling session provided by the same midwife. These midwives had attended the UNICEF 18 h intensive training course on breastfeeding techniques and management. | |
| Outcomes | Any breastfeeding up to 60 days. | |
| Notes | Extra information about reported numbers requested and received from author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sample was stratified "for age and parity, and finally randomly assigned to either the intervention or control group". |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) | High risk | Outcome assessment not blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | 605 women randomised. Full data were available for 278 women (46%) and partial data available for a further 264 (44%). Follow‐up rates for breastfeeding outcomes collected up to 180 days but after 60 days follow‐up rates were less than 75% so only outcomes up to 60 days are included in this review. Reasons for drop‐out reported by group. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Baseline characteristics similar and no apparent differences between those who refused intervention and those who received it, see Table 1. |
| Methods | Longitudinal study. 2 arms. Cluster‐randomised trial. 10 Swedish municipalities randomised. | |
| Participants | Setting: Antenatal Centres and Child Health Centres in 10 municipalities in Southwest Sweden. Background rates of breastfeeding initiation: High. Inclusion criteria: Swedish speaking mothers who gave birth to singleton, healthy term infants spontaneously, by vacuum extraction or by caesarean section. Participant characteristics: mean age approx 27 years, married 61‐69%, vaginal delivery 70‐75%, university educated 36%. | |
| Interventions | Control group: offered standard family classes, usually discontinued at birth. Intervention group: The intervention included continuity of care at the antenatal and child centres, and a process oriented training program of 7 sessions for health professionals. The staff training included reflection on personal experience of breastfeeding and breastfeeding counselling, management and promotion. Staff were encouraged to develop a common breastfeeding policy between the antenatal and child health centres. The family classes were also kept together before and after childbirth. | |
| Outcomes | Maternal perceptions of the relationship with the infant, maternal feelings for the infant and duration of exclusive/any breastfeeding. | |
| Notes | 10 centres randomised. A total of 540 women took part in the study (intervention group 206 women; 2 control groups 162+172 = 334 women). Data collection took place at different times for the 2 control groups. We have included data from 378 women; the intervention group (206 women) and 1 control group (172 women), from which data were collected at the same time as from the intervention group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The 10 largest municipalities were classified in pairs that were similar in size and had similar figures of breastfeeding duration. The municipalities were randomised pair wise to either an intervention or control group. |
| Allocation concealment (selection bias) | Unclear risk | Does not say. |
| Blinding (performance bias and detection bias) | High risk | Does not appear to be any blinding. |
| Incomplete outcome data (attrition bias) | Unclear risk | The sample included women cared for in intervention clinics and then 2 control groups. However, data collection in 1 of the control groups was carried out before the intervention period so in the analyses we have included only the control group were collected simultaneously with the intervention group (total 540 women, 378 included in analysis). Response rates at 3 days 84% and 93% in the intervention and control groups, by 9 months postpartum 64% and 73%. There was no adjustment for cluster design. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | There was no baseline imbalance apparent, although duration of exclusive breastfeeding was presented as a baseline characteristic. |
| Methods | Study design: RCT. 2‐arm trial with individual randomisation. | |
| Participants | Setting not clear: women expecting to give birth in an urban maternity unit, Canada. 120 women recruited in late pregnancy. | |
| Interventions | Control: usual care in hospital with assistance from nurses who had received breastfeeding education. Intervention: In addition to usual care, prenatal breastfeeding class and postnatal drop‐in breastfeeding session. Telephone follow‐up by nurse at 2, 6 and 12 weeks postpartum. | |
| Outcomes | Exclusive breastfeeding at 1 and 3 months and any breastfeeding at 3 and 6 months. | |
| Notes | We have not included data from this study in the review due to high levels of attrition (> 25% loss to follow‐up). Recruitment to the study took place during pregnancy and by 1 month postpartum there was high loss to follow‐up and loss was not balanced across groups. At 1 month 42% of controls and 22% of the intervention group were not available for follow‐up. The high level and unbalanced attrition means that results from this study are difficult to interpret. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Described as “randomly assigned”. |
| Blinding (performance bias and detection bias) | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) | High risk | Recruitment to the study took place during pregnancy and by 1 month postpartum there was high loss to follow‐up and loss was not balanced across groups. At 1 month 42% of controls and 22% of the intervention group were not available for follow‐up. The high level and unbalanced attrition means that results from this study are difficult to interpret.
|
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Other bias: there was very little information on study methods and most of the results in the paper are not reported by randomisation group. |
| Methods | Single‐site RCT (n = 343) recruiting over 17 months. (4‐arm trial with factorial design). | |
| Participants | Urban USA: in‐patient maternity unit. Background rates of breastfeeding initiation: Low. Inclusion criteria: breastfed once in hospital; able to speak Spanish or English; baby needed less than 48 hours on NICU; able to be contacted by telephone after discharge. Participant characteristics: 57% primiparous. Ethnic composition: black 65%, Hispanic 19%, white 13%, other 4%. Socio‐economic status defined by: < 100% poverty level ‐ 69%; 100%‐200% poverty level ‐ 21%; > 200% poverty level ‐ 10%. Mean age of participants 25.7 years. | |
| Interventions | Routine care consisted of postpartum staff nursing contacts (including discharge teaching session on infant care), infrequent breastfeeding classes, written information on breastfeeding management and the opportunity to access a midwife‐run telephone advice line. Intervention: (1) Postpartum breastfeeding counselling in hospital by trained counsellor (20‐40 minutes) and by telephone at 5, 7, 14, 21, 28, days and 6, 8 and 12 weeks. 24 hour advice by pager. | |
| Outcomes | Exclusive breastfeeding at 1, 2, 3 and 4 months. Any breastfeeding at 4 months. Median duration of breastfeeding. Time to introduction of formula or solids. Rehospitalisation of infants. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised block design (block size 8) with computer‐generated list of random numbers. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque sealed envelopes. |
| Blinding (performance bias and detection bias) | Unclear risk | For follow‐up at 4 months it was stated that the investigator was not aware of group assignment; however, it was not clear whether outcome assessment blinding was effective (outcome assessment by telephone interview). |
| Incomplete outcome data (attrition bias) | Unclear risk | Participants received a fee to minimise sample attrition. 343 women were recruited. There were a small number of protocol deviations (7 women received the wrong type of discharge pack and were analysed according to treatment received rather than by randomisation group). 19 women were lost to follow‐up. Attrition and reasons for attrition were described as similar across groups. follow‐up 94%. Appropriate randomisation procedures. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Low risk | No baseline imbalance apparent. |
| Methods | Single‐site study recruiting over 7 months, n = 134. | |
| Participants | Urban Iran. Background rates of breastfeeding initiation: High. Inclusion: Women without breastfeeding experience or chronic disease giving birth normally at term to a healthy baby 2.5 kg or over. | |
| Interventions | Control: standard care not described. Intervention: nutritionist trained using WHO Breastfeeding Counseling training course (40 hours). Contact in hospital immediately after birth, between 10 and 15 days, after 30 days and monthly to the 4th month at home or in a lactation clinic. | |
| Outcomes | Exclusive breastfeeding at 1, 2, 3 and 4 months. Mean number of days illness with diarrhoea. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation by day of the week of birth. |
| Allocation concealment (selection bias) | High risk | Allocation could be anticipated in advance and different days of the week may have had different characteristics (e.g. staff on duty). |
| Blinding (performance bias and detection bias) | High risk | Women were not told directly which group they had been assigned to but would be aware of whether or not they had received the intervention. It was not stated whether or not there was any attempt to blind outcome assessment. |
| Incomplete outcome data (attrition bias) | Low risk | 134 randomised and 120 followed up. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Low risk | No baseline imbalance apparent. |
| Methods | RCT with individual randomisation. 2‐arm trial, n = 586, 292 assigned to intervention group and 294 to control group. | |
| Participants | Study conducted at a University teaching hospital and affiliated community health centres. Recruitment January 1997‐September 1998 Urban Quebec, Canada. Background rates of breastfeeding initiation: Intermediate. Inclusion criteria: mothers participating in hospital short‐stay programme. Ethnic and socio‐economic composition of sample not reported. Baseline prevalence of breastfeeding initiation in Canada (excluding territories) 1994‐5 = 73%. | |
| Interventions | Control: usual care was a 48‐hour postpartum contact and 1 postpartum hospital clinic visit (day 3) following a standard plan of care and lasting up to 45 minutes. Referral for continued care was available. Intervention: Home visit from community nurse 3‐4 days postpartum. Nurses were Baccalaureate prepared, had minimum 3 years clinical experience in maternal‐child health, and had attended training to ensure assessment skills of maternal‐newborn and breastfeeding support. Contact with the nurse continued if required. | |
| Outcomes | Breastfeeding frequency and infant weight gain assessed at 2 weeks postpartum. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation (block size 8) stratified by parity, by computer‐generated random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) | Unclear risk | It was reported that outcome data were collected by blind investigators. It was not clear whether planned blinding was effective, although investigators apparently asked women "not to divulge their group status". |
| Incomplete outcome data (attrition bias) | Unclear risk | 586 randomised. 21 protocol deviations but analysis according to randomisation. 499 completed trial and provided information on primary outcome (15% attrition). Some further missing data for some outcomes. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Low risk | Groups described as similar at baseline. |
| Methods | RCT 2‐arm trial with individual randomisation. Study conducted in 32 general practices in the UK. Recruitment April 1995‐August 1998, n = 720, 363 assigned to intervention group and 357 to control group. | |
| Participants | Urban South‐East England. Background rates of breastfeeding initiation: Intermediate. Inclusion criteria: mothers considering breastfeeding who had not breastfed a previous child for 6 weeks. Exclusion criteria: planning to contact a breastfeeding counsellor, address considered unsafe to visit, baby born before 36 weeks' gestation. Ethnic composition of sample: 59% white (UK) participants, 11% white (other) participants, 16% African or Caribbean, 8% Indian subcontinent, 6% other. Socio‐economic status on RG classification: 10% I, 26% II, 19% IIINM, 26% IIIM, 12% IV, 3% V, 5% other. First baby: 74%. National baseline prevalence 66% breastfeeding at birth. | |
| Interventions | Control: standard care (UK standard care includes postnatal home visits from midwives and health visitors) Intervention: women were allocated to receive 1 antenatal visit from a National Childbirth Trust trained breastfeeding counsellor, who offered postnatal support by telephone or further visits if the mother requested this after the birth. | |
| Outcomes | Prevalence of any breastfeeding to 6 weeks; duration of any breastfeeding to 4 months; time to introduction of formula feeds; maternal satisfaction and common feeding problems; mothers' perspectives on support from counsellors; association between counselling uptake and feeding behaviour. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted block design stratified by GP practice and parity, randomisation schedule prepared by statistician. |
| Allocation concealment (selection bias) | Low risk | Numbered sealed envelopes. |
| Blinding (performance bias and detection bias) | Unclear risk | Reported that responses to follow‐up questionnaires were coded by blind assessors. |
| Incomplete outcome data (attrition bias) | Low risk | 720 women recruited and randomised. 97% available for follow‐up at birth, 93% at 6 weeks and 86% at 4 months. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Groups were similar at baseline although more women in the intervention group (16) than the control group (6) were undecided about breastfeeding intention at the antenatal assessment. It was reported that a sensitivity analysis was carried out to adjust for this possible confounder. |
| Methods | Cluster‐randomised trial. 4 clinics were “randomly assigned" to 4 different conditions. | |
| Participants | Setting: 4 WIC clinics in Baltimore USA. Women were predominantly African American (>90%). 548 women attending study clinics enrolled at between 6 and 24 weeks’ gestation. Women were WIC eligible with singleton pregnancies, planning to keep the baby and to stay in study areas. | |
| Interventions | The study was carried out in 4 clinics. Each clinic offered a different intervention. Clinic 1: standard care (usual breastfeeding promotion by clinic staff). Clinic 2: standard care plus motivational video (encouraging breastfeeding) playing repetitively in the clinic waiting area. Clinic 3: peer support by a mother who had breastfed and undertaken training. Peer supporters contacted pregnant women and discussed breastfeeding. Women were offered a 1‐hour group breastfeeding support session in the WIC clinic before the birth. After the birth peer counsellors contacted women and remained in contact with breastfeeding women (phone or visits) until 16 weeks after the birth. Clinic 4: standard care plus video plus peer support. | |
| Outcomes | Infant feeding method at 8 and 16 weeks postpartum and maternal work status. | |
| Notes | We were not able to include data from this study in the review due to very high levels of attrition. The study was at high risk of bias. This was a cluster trial with 4 clinics each allocated to a different intervention and with no adjustment for study design effect. Women were recruited in the antenatal period 548 women were enrolled on the study but information was only available for 273 women at 7 to 10 days postpartum (50%); of the 275 women lost to follow‐up 31% (74) were excluded due to pregnancy complications, the remaining 73% (201 women) refused or could not be contacted – these women represent 37% of the original randomised sample. It was not clear whether loss was similar in the 4 clinics. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster trial. 4 clinics; method of randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described.
|
| Blinding (performance bias and detection bias) | Unclear risk | Not attempted. Randomisation at clinic level may have reduced contamination. |
| Incomplete outcome data (attrition bias) | High risk | 548 women were enrolled on the study but information was only available for 273 women at 7 to 10 days postpartum (50%); of the 275 women lost to follow‐up 31% (74) were excluded due to pregnancy complications, the remaining 73% (201 women) refused or could not be contacted – these women represent 37% of the original randomised sample. It was not clear whether loss was similar in the 4 clinics. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Baseline characteristics imbalance for educational status, employment and parity ‐ although these were adjusted for in the analysis. |
| Methods | Quasi‐RCT, 2‐arm trial with individual randomisation. Single‐site study recruiting over 10 months, n = 97. Follow‐up 90%. Quasi‐randomised (coin toss with women sharing same room allocated by the same toss). | |
| Participants | Urban USA ‐ in‐patient maternity unit. Background rates of breastfeeding initiation: Low. WIC breastfeeding prevalence at birth 1991 = 33%. Inclusion criteria: women eligible for WIC programme services for those on low incomes; women intending to breastfeed. Participant characteristics: Approximately one‐third were primiparous. Ethnic composition described as 54% black. Mean age 25.4 years. | |
| Interventions | Control: routine postnatal teaching on infant care and feeding by obstetrical nursing staff. Intervention package of: face‐to‐face meeting in hospital with lactation counsellor (a registered nurse) after birth lasting 30‐45 minutes ‐ educational booklet given; telephone contacts on days 2, 4, 7, 10 and 21; a telephone help‐line staffed by a nurse or paediatrician; back up support for those with problems from a lactation clinic. | |
| Outcomes | Rates of breastfeeding at 6 weeks and 3 and 6 months. Median duration of breastfeeding. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Coin toss at the point of randomisation. |
| Allocation concealment (selection bias) | High risk | Coin toss at the point of randomisation, so allocation could be altered. If 2 women occupied the same room they were allocated to the same group. |
| Blinding (performance bias and detection bias) | High risk | Some data were derived from medical records but telephone outcome assessment not blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | 97 women randomised, by 6 weeks 4 control group women could not be contacted (> 90% follow‐up but loss not balanced across groups). |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Groups appeared similar at baseline. |
| Methods | Community‐based cluster‐randomised study (40 adjacent areas randomised). Recruitment over 10 months, n = 726. | |
| Participants | Dakka, Bangladesh. Background rates of breastfeeding initiation: High. Mainly lower‐middle and low socio‐economic status. Women aged 16‐35 with 3 children or fewer (or 6 pregnancies or fewer) and no serious illness. Multiple births; children with congenital abnormalities, and those weighing less than 1800 g were excluded. National baseline prevalence reported in paper to be similar to control group rates. UNICEF quotes higher rates ‐ 53% exclusive breastfeeding at 0‐3 months. | |
| Interventions | Peer counselling by women with personal breastfeeding experience trained over 40 hours with the WHO/UNICEF Breastfeeding Counseling course. Paid honorarium. Supervised caseload of 12‐25 mothers. 15 home visits: 2 in last trimester/4 in month 1/2‐weekly in months 2‐5. Duration of visits 20‐40 minutes. | |
| Outcomes | Exclusive breastfeeding at birth, 4 days, 4 weeks, 2, 3, 4 and 5 months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random number tables. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) | High risk | Women and counsellors aware of group assignment and interviewers collecting outcome data would also be aware of assignment. |
| Incomplete outcome data (attrition bias) | Unclear risk | 40 areas randomised (20 intervention, 20 control) 726 women randomised. 653 available to follow‐up at delivery (90%). 573 available at 5 months (79%). Loss appeared balanced across groups. No ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Low risk | No differences in baseline characteristics apparent. Staed that results were based on individual level analysis but with adjustment for cluster level of randomisation. |
| Methods | Study design: RCT with 3 arms | |
| Participants | 49 women giving birth in a small community hospital in the USA planning to breastfeed for at least 6 weeks and breastfeeding for the first time. All women had healthy babies. Women were described as married and middle class aged 17–31. | |
| Interventions | 3 groups. Control: 18 randomised 12 followed up (not clear). Intervention group 1: 15 randomised 13 followed up (not clear): usual care plus an educational session. Intervention group 2: 16 randomised 15 followed up (not clear): usual care plus education plus daily visits by nurse while in hospital and telephone support 2 days after discharge and 1 week later and further support if necessary (up to 5 weeks postpartum). | |
| Outcomes | Unclear. Breastfeeding at 6 weeks and breastfeeding problems. | |
| Notes | We have not included data from this study in the review due to methodological weakness and high and unbalanced levels of attrition. More than 30% of the control group were lost to follow‐up and results were therefore difficult to interpret. Most results were not reported according to randomisation group and the only result on breastfeeding duration was approximate. “Approximately 50% of the control group and 50% of the group which received the teaching unit were still nursing at six weeks. Of the group who received the teaching plus support 80% were still nursing at 6 weeks.” | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Described as “randomly assigned”. |
| Blinding (performance bias and detection bias) | Unclear risk | No blinding although outcome assessment was not by the same nurse as the 1 delivering the intervention. |
| Incomplete outcome data (attrition bias) | Unclear risk | There was high attrition in this small study. More than 30% of the control group were lost to follow‐up and results were therefore difficult to interpret. |
| Selective reporting (reporting bias) | Unclear risk | Most results were not reported by randomisation group. |
| Other bias | Unclear risk | No baseline characteristics reported. |
| Methods | Cluster‐RCT with prospective mixed method embedded case studies to evaluate implementation processes; 2 arms. 14 localities randomised; recruitment 2002‐4. | |
| Participants | Setting: women registered at GP practices in 14 localities (of 66) in Scotland. Background rates of breastfeeding initiation: Low. In Scotland, only 44% of babies received any breast milk at 6 weeks in 2005. Clusters randomised n = 14, birth records supplying data n = 9747 in intervention group and n = 9111 in control group. Inclusion criteria: pregnant women and breastfeeding mothers. Exclusion criteria: not stated. In intervention localities 25.2% of the populations were in the most deprived social groups, compared with 32.1% in the control localities. Mean age of mothers at the first child health record was 28‐29 years. In 7 areas (3 intervention, 4 control) women gave birth at Baby‐Friendly hospitals. | |
| Interventions | Control: control localities received no additional intervention; however, breastfeeding support groups existed in some control areas. Intervention: this was a policy intervention aimed at locality areas rather than at individual women. The intervention was a policy aim to double the number of local breastfeeding support groups and to make weekly support groups open to all pregnant women and breastfeeding mothers. The local breastfeeding support groups were to be facilitated by health professionals taking a woman‐centred approach and aiming to provide breastfeeding support and social interaction for women. | |
| Outcomes | Primary outcome: number of babies receiving any breast milk at 6‐8 weeks, as reported in routinely collected data for the 2 pre‐trial years and 2 trial years. Secondary outcomes: any breastfeeding at birth, 5‐7 days and 8‐9 months, and maternal satisfaction. Results were not presented in a way which allowed us to enter them into data and analysis tables but we have summarised findings in the text. | |
| Notes | When we updated our search in October 2011, Hoddinott 2009 was the only evaluation we found a) of a policy‐level intervention; b) of breastfeeding in groups; c) that used routinely collected locality‐level outcome data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster‐RCT; 14 localities randomised. Localities varied in size, baseline breastfeeding rates and numbers of pre‐existing groups and how pregnancy and postnatal care were organised. Localities were matched on breastfeeding rates and existing support groups “An independent statistician used random number tables to randomise locality pairs to either intervention or control”. |
| Allocation concealment (selection bias) | Low risk | Researchers analysing primary and secondary outcomes were blind to allocation, ensured by coding of localities. |
| Blinding (performance bias and detection bias) | Unclear risk | Cluster‐randomised trial so women may not have been aware of the study although they would be aware of the intervention. Researchers analysing primary and secondary outcomes were reported to be blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | According to flow chart no clusters discontinued the intervention or were lost to follow‐up and there was follow‐up of national data in all localities included in the trial. The amount of data missing for women varied for different outcomes (e.g. birth and 6 week postpartum records were available for most of the eligible population but child health records at 8‐9 months available for approximately a quarter of children). |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Analysis took into account aspects of design effect. It appeared that there were some differences in the localities at baseline. Control localities may have had higher levels of social deprivation |
| Methods | RCT with 2 arms, n = 522 women randomised. | |
| Participants | A large metropolitan hospital in Houston, Texas, USA, serving predominantly immigrant Hispanic women (85% monolingual Hispanic). Background rates of breastfeeding initiation high in this study population. Inclusion criteria: mothers of low‐risk infants, mixed feeding in hospitals, had telephones and access to transportation. Exclusion criteria: Infants with elevated risk for hyperbilirubinaemia (preterm, discharged at < 48 hours old, jaundice within 24 hours of birth, Rh‐incompatibility, cephalohematoma, positive Coombs test, family history of disorders of red blood cell enzyme defects, or defects of red blood cell shape and size). Participant characteristics: Mean maternal age intervention group 26.8, control group 27.1; mean parity intervention group 1.5, control group 1.5; mothers born in the USA intervention group 2.8%, control group 1.1% (most of the women were born in Mexico or Central America). | |
| Interventions | Control group: received usual care, which included bedside breastfeeding assistance before hospital discharge and the phone number of the hospital breastfeeding clinic with instructions to call if needed. Intervention group: mothers were given an appointment to visit the hospital‐based breastfeeding clinic at 3 to 7 days postpartum. At the breastfeeding clinic, peer counselling sessions included a breastfeeding history, breast examination, infant oral‐motor assessment, measurement of infant weight, evaluation of latch and milk transfer, and discussion of the mother's concerns and support system. Additional visits and telephone consultations were provided if deemed necessary by the mother and the clinic staff. Women who missed appointments received a telephone call. | |
| Outcomes | Primary outcome: exclusive breastfeeding at 1 month. Secondary outcomes: volume of formula given by mothers who were mixed feeding and incidence of breastfeeding problems. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated by random number table.
|
| Allocation concealment (selection bias) | Low risk | After participants had given informed consent, the group was determined using opaque, sealed envelopes containing assignments generated by random table number. The envelope was opened by the mother. |
| Blinding (performance bias and detection bias) | Unclear risk | Participants were not blinded as the envelope was opened by the mother. Caregivers were also not blinded. For outcome assessors, outcomes were determined by telephone survey at 4 weeks postpartum by interviewers blinded to group assignment. |
| Incomplete outcome data (attrition bias) | Low risk | 522 randomised (255 in intervention group and 267 in control group. 55 women were lost to follow‐up at 4 weeks. (10.5%). Loss was balanced in the 2 groups. Issues around incomplete data were addressed. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Low risk | There were slight baseline differences between control group and experimental group. Women in the intervention group were more likely to have an emergency caesarean, and were taller. Due to low compliance with the intervention secondary analysis was carried out, but we have reported data from the primary analysis (unadjusted). |
| Methods | Quasi‐randomised trial. Recruitment location/duration not stated, n = 38. | |
| Participants | UK white, working‐class women 19‐32 years, living with partner and intending to breastfeed. Background rates of breastfeeding initiation: Intermediate: prevalence of breastfeeding 1985 = 64% at birth and 26% at 4 months. | |
| Interventions | Control: 1 antenatal home visit and 1 postnatal hospital visit. Intervention: 3 antenatal home visits/1 hospital visit/1 'immediate' home visit and 1 or 2 further home visits 'in the early weeks'; face‐to‐face and telephone support by single lay supporter (mother/previous breastfeeding experience). No indication of training. | |
| Outcomes | Breastfeeding at 3 months. Partial breastfeeding grouped with formula feeding as 'breastfeeding failure'. | |
| Notes | Moderate‐to‐high risk of bias. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternate. |
| Allocation concealment (selection bias) | High risk | Alternate allocation. |
| Blinding (performance bias and detection bias) | High risk | Not attempted. |
| Incomplete outcome data (attrition bias) | Low risk | 38 women included. It appeared that all were followed up. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Very little information on study methods. |
| Methods | Quasi‐randomised trial, individual randomisation. Single‐site study. Recruitment period 18 months, n = 678. | |
| Participants | UK ‐ maternity department of district general hospital. Background rates of breastfeeding initiation: Intermediate. Inclusion criteria: all women who attempted at least 1 breastfeed. Exclusion criteria: birth of child overlapped intervention and control periods. 55% of the sample were primiparous. Ethnic composition not stated. Socio‐economic status defined by UK census categories (I and II 22%, III 46%, IV and V 13%). | |
| Interventions | Control: not specified. Intervention: individual support and problem solving by lactation nurse in hospital and at home. Duration of the intervention not specified. | |
| Outcomes | Breastfeeding rates at 4 weeks, 3, 6 and 12 months. Satisfaction with care and intention to breastfeed next pregnancy. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternating 2 week periods (i.e. 2 week intervention recruitment period, 2 week control recruitment period). |
| Allocation concealment (selection bias) | High risk | The randomisation method did not achieve balanced group size; 228 in the intervention group vs 355 controls. |
| Blinding (performance bias and detection bias) | Unclear risk | Stae that 12 months follow‐up was by an independent interviewer. |
| Incomplete outcome data (attrition bias) | Unclear risk | 678 women randomised and 649 available to follow‐up (96%) Potential confounder: Late exclusion of 66 women because of overlap of recruitment periods and group sizes were uneven. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | The method of recruiting intervention and control women appeared different; possibly face‐to‐face for intervention group and records/ not clear for the control group. |
| Methods | Not clear; described as “case control randomized trial”. 2 arms. | |
| Participants | Setting: Tehran, Iran; mothers and babies recruited in a Baby Friendly accredited hospital. The intervention included a programme of home visits. 120 women (baseline characteristics not described). Inclusion criteria: women giving birth to singletons by caesarean section only. Exclusion criteria: infants with congenital abnormalities or serious illness necessitating intensive care and mothers who had a serious illness or were planning to leave the area within 6 months. Infants weighing less than 2500 g at birth were also excluded. | |
| Interventions | Intervention: 4 postnatal home visits (not clear) Control group: standard care (not clear) | |
| Outcomes | Follow‐up interviews by telephone on days 90, 120, 150 and 180. Results were not reported in a way in which we can include them in the review. Authors report that "the patterns of exclusive breastfeeding in the 2 groups for days 3 to 180 differed significantly (P < 0.0001) with a mean aggregated of 67.72% among the group assigned home visits compared with 31.78% for the group assigned none". | |
| Notes | We have not included data from this study in the review. Results were difficult to interpret and data were not reported in a way that allowed us to include them in meta‐analyses. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) | High risk | No blinding of participants/professionals reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not clear. 120 women were recruited but it was not clear how many were followed up. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Does not state what was included in the telephone interview. Results reported on exclusive breastfeeding (not reported in a form we can use in the review). |
| Methods | RCT (n = 140) with 2 groups, Aug 2008‐Apr 2009. | |
| Participants | Recruitment from postnatal wards of 2 hospitals in South Jordan. Inclusion criteria: primiparous women following vaginal delivery with term infants. Exclusion criteria: women who lived outside the study area or who could not be contacted by phone. Prevalence of "ever breastfed" in country: 93% (WHO Global Data Bank on Breastfeeding, accessed 12 Oct 2011). Paper states that traditionally most women initiate breastfeeding and breastfeed for up to 2 years, with 32% fully breastfeeding for more than 6 months. | |
| Interventions | Usual care: women were given an appointment for 6 weeks after discharge to attend the maternal and child health services for support and follow‐up. Paper states most women did not return for these appointments and were not receiving any postnatal care. Control group women did not receive postnatal home visits from a midwife or a child health nurse. Intervention group women received a 1‐hour education session approximately 2 hours after the birth. The session included demonstrations of breastfeeding. Mothers were encouraged to ask questions and were given a pamphlet on breastfeeding. At 2 and 4 months postpartum women were contacted by phone by the same researcher/nurse. The purpose of calls was to offer support, monitor breastfeeding practices and identify any problems. | |
| Outcomes | Primary outcomes were exclusive breastfeeding at 6 months and breastfeeding knowledge. Secondary outcome was infant hospital admissions for diarrhoea and vomiting or respiratory tract infections. | |
| Notes | Due to high levels of attrition (36%) we have not included outcome data from this study in the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Low risk | Paper states randomisation occurred by women selecting 1 envelope from a group of sealed opaque envelopes. |
| Blinding (performance bias and detection bias) | High risk | No blinding described and it appears that the same researchers that collected data also carried out recruitment and delivered the intervention. |
| Incomplete outcome data (attrition bias) | High risk | Serious loss to follow‐up. At 6 months, follow‐up was 62.5% in the intervention group and 66.2% in the control group. |
| Selective reporting (reporting bias) | Unclear risk | Not apparent. |
| Other bias | Unclear risk | Data collection procedures varied at 6 months. Some women were visited at home while others were telephoned. It was not clear how many women in the control and intervention groups were telephoned vs visited. The different data collection procedures may have affected responses. |
| Methods | Cluster‐randomised study with 10 sites, divided into 2 groups, which had similar numbers of births and breastfeeding rates. Recruitment December 2000‐December 2002, n = 781, 408 women in sites assigned to the intervention and 373 in sites assigned to the control group. | |
| Participants | Child healthcare centres in Limbourg province, Netherlands. Background rates of breastfeeding initiation: High. Inclusion criteria: mothers applying for maternity care at any of the 10 centres. Exclusion criteria: birthweight < 2000 g. Ethnic composition not defined. Baseline prevalence of breastfeeding initiation 80% in the Netherlands in 2002. | |
| Interventions | Control: not specified. Intervention: programme with 3 elements: structured health counselling by maternity and child healthcare nurses and physicians; booklet to transfer information between caregivers and between mother and caregivers and used at each consultation; lactation consultancy available via caregiver faxing consultant with details of problem (consultant would then contact the caregiver or mother within 24 hours of receiving the fax). | |
| Outcomes | Exclusive and complementary breastfeeding rates at 3 months; determinants of breastfeeding at 3 months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By coin flip. |
| Allocation concealment (selection bias) | Low risk | Clusters were randomised after sites were paired for similarity of breastfeeding rates and the number of births in each centre. |
| Blinding (performance bias and detection bias) | High risk | Oucome assessors not reported as blinded. |
| Incomplete outcome data (attrition bias) | Low risk | 20 centres were randomised. 781 women. Data available for 701 for the first follow‐up (90%) and 683 (87%) at 6 months postpartum. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Low risk | Analyses adjusted for cluster effect by multi‐level analysis. No baseline imbalance apparent. |
| Methods | Multi‐site cluster‐randomised study. Recruitment period 19 months, n = 17,046. | |
| Participants | Urban and rural sites within Belarus. Background rates of breastfeeding initiation: High. Inclusion criteria: intention to breastfeed, healthy mother, child 2500 g or more at term, Apgar 5 or more at 5 minutes. Baseline breastfeeding prevalence 50% at 3 months. | |
| Interventions | Control: staff did not receive the training. Intervention: WHO/UNICEF BFI training for all staff dealing with mothers and babies in hospitals and community polyclinics. Infants seen monthly for polyclinic well‐child visits and whenever ill. | |
| Outcomes | Any breastfeeding at 3, 6, 9 and 12 months. Incidence of respiratory, gastro‐intestinal and atopic eczema in first year. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster‐randomised trial with double randomisation procedure. Random number tables were used to ascribe numbers to sites and higher/lower numbers were used to allocate sites to A or B interventions. Then later, in public, a coin flip was used to determine whether A or B would be intervention or control sites. |
| Allocation concealment (selection bias) | Low risk | 2‐stage randomisation procedure. |
| Blinding (performance bias and detection bias) | Unclear risk | Clinical outcomes were recorded by staff caring for women but an audit was also carried out. |
| Incomplete outcome data (attrition bias) | Unclear risk | 34 sites randomised, 2 of which refused to carry out allocated intervention and 1 clinic falsified outcome data and was excluded. 31 sites contributed data. In addition, follow‐up data were missing for 3.3% of women. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | A steering group ensured that "control sites did not institute any changes that would render their maternity hospitals or polyclinics more baby friendly". Analysis took account of cluster design. Groups appeared similar at baseline. |
| Methods | Cluster‐randomised, 2 community based trial. 22 municipalities randomised to intervention and control clusters. | |
| Participants | Western Denmark, urban and agricultural areas. Background rates of breastfeeding initiation: high. The 5 hospitals serving the area had adopted Unicef/ WHO Baby Friendly Hospital Initiative standards, and 3 of the 5 were accredited at the time of the study. Inclusion criteria: Danish mothers who lived in the 22 municipalities and gave birth to a single child with gestational age of 37 completed weeks or more. Participant characteristics: 36% primiparous, 7.5% multiparous with previous short breastfeeding experience | |
| Interventions | Usual care included hospital care at hospitals working to Baby‐Friendly standards, and an existing Health Visitor service in all municipalities. Control cluster: Health Visitors' usual practice consisting of 1 or more non‐standardised visits. Intervention cluster: 1 to 3 structured home visits within the first 5 postnatal weeks from Health Visitors with additional training. Main topics for the first visit were effective breastfeeding technique and learning to know the baby; for the second visit, self‐regulated feeding and interpretation of the baby's cues; for the third visit, sufficient milk and interaction with the baby. Mothers were also given a booklet about how to breastfeed and how to read the baby's cues. | |
| Outcomes | Duration of exclusive breastfeeding and mother’s satisfaction with breastfeeding | |
| Notes | The authors did not adjust for cluster design effect. In our data and analysis tables we have adjusted the sample size and event rates to take account of the design effect. We calculated an effective sample size by dividing figures by the design effect – calculated using the ICC for breastfeeding cessation given in the paper: ICC = 0.02. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | To ensure that the health visitors that were in regular contact with each other were randomised to the same breastfeeding procedure the 22 municipalities were chosen as clusters. The 22 clusters were stratified according to their number of births the year before the trial, and within 3 strata, 11 municipalities were randomised to the intervention group and 11 to the comparison group. The randomisation was computerised and done independently of the investigators. |
| Allocation concealment (selection bias) | Low risk | As for the sequence generation. |
| Blinding (performance bias and detection bias) | Unclear risk | Participants and caregivers were not blinded. Outcome assessors were blinded‐ the identity of the health visitors was blinded to the investigators. It was not clear whether this partial blinding was effective. |
| Incomplete outcome data (attrition bias) | Unclear risk | 22 municipalities were randomised. 2186 women had babies during the study time period. 1760 women followed up (figure showing trial attrition not printed out with paper – to check). |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Reported that there were no significant differences between groups at baseline. This was a cluster‐randomised trial and authors state they did not make allowance for clustering in the sample size calculation as the cluster effect was expected to be small. Elsewhere in the paper an ICC value is provided which the authors say indicate that cluster effect was small. It was not clear that cluster design effect was taken into account in any of the analyses. |
| Methods | RCT, 2 arms with individual randomisation. Recruitment: October 2001 to May 2002. | |
| Participants | Setting: the maternity section at the Chambery Teaching hospital in Chambery, France. Background rates of breastfeeding initiation: Intermediate. In the study hospital, breastfeeding prevalence rates were 70.8% in hospital and 58.1% at 1 month of infant age. Participants randomised = 231. Inclusion criteria: mothers of healthy singleton infants (gestational age: > 37 completed weeks), breastfeeding on the day of discharge and consented to participate in the study. Exclusion criteria: infants admitted to neonatal unit, mothers transferred to intensive care unit, mothers <18 years old, living outside the area, unable to speak French, or unable to complete follow‐up monitoring because of psychosocial problems such as homelessness. Participant characteristics: age, mean, SD: intervention: 29.3 (4.1); control: 29.7 (4.8); education more than high school graduate: intervention: 87 (75.0); control 84 (73.0); white collar worker: intervention: 92 (79.3); control 87 (75.6); primiparous: intervention: 58 (50.0); control: 63 (54.8). | |
| Interventions | Control group (n = 115) standard care: mothers received verbal encouragement from maternity ward staff to maintain breastfeeding. On discharge, the infant was examined by the paediatrician working in the department, for a general health assessment and an evaluation for evidence of successful breastfeeding behaviour. The mothers were also provided with the telephone number of a peer support group that they could call to ask questions and request help. The post discharge follow‐up monitoring consisted of routine, preventive, outpatient visits in a primary care physician’s office at 1, 2, 3, 4, 5 and 6 months of infant age. Intervention group (n = 116): in addition to standard care, mothers were invited to an outpatient visit in a primary care physician’s office within 2 weeks after the birth to see a primary care doctor who had received special breastfeeding education. Topics covered at this visit included general health assessment, lactation physiology, feeding position and latch on assessment, management of common lactation problems (nipple pain, nipple cracks, sore nipples, mastitis, and maternal concern regarding low milk supply), management of infant problems (insufficient weight gain, breastfeeding jaundice, diarrhoea and dehydration), maternal medication use while breastfeeding and sources of support. The physicians' training programme was delivered through lectures, panel discussions, role playing exercises and printed educational materials. | |
| Outcomes | The primary outcome was exclusive breastfeeding at 4 weeks. Exclusive breastfeeding was defined as giving maternal milk as the only food source, with no other liquids or foods. The secondary outcomes were any breastfeeding at 4 weeks, median duration of breastfeeding, breastfeeding difficulties and maternal satisfaction with the infant feeding experience. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The allocation sequence was generated by the statistical adviser of the study with random permuted blocks with a block size of 8. |
| Allocation concealment (selection bias) | Low risk | The randomisation assignments were unknown to any of the investigators and were concealed in consecutively numbered, sealed, opaque envelopes. |
| Blinding (performance bias and detection bias) | High risk | No blinding. The trial was described as open, although questionnaires to mothers were used to reduce the risk of observer bias, and reduce outcome assessment bias. |
| Incomplete outcome data (attrition bias) | Low risk | (1080 women assessed for eligibility, 849 deemed not eligible). 231 women randomised, outcome data were available for all but 5 of the woman randomised, and a sensitivity analysis was carried out where the most conservative values were assumed for those women lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | The majority of women assessed were not eligible for inclusion in this trial and so the results may not be generalisable. |
| Methods | Participants recruited from 8 public health maternity units. Duration of recruitment 6 months, n = 1003. RCT 2‐arm trial with individual randomisation. | |
| Participants | Urban Brazil. Background rates of breastfeeding initiation: High. Inclusion criteria: healthy babies, weighing < 3000 g. Exclusion criteria: twins, important health problems in mother or child. | |
| Interventions | Peer counsellor home visits lasting 30‐40 minutes at 5, 15, 30, 60, 90 and 120 days. Counsellors from same social group as women they supported, had personal experience of breastfeeding and had been associated with maternity unit milk bank for a minimum of 5 years. Trained with adapted WHO breastfeeding counselling course (20 hours). Paid $4 per visit. Each counsellor supported 25 mothers. | |
| Outcomes | Rates of exclusive, predominant, partial and artificial feeding at 4 months. | |
| Notes | This is the only study in this review that targeted babies with birthweight below 3000 g. We considered excluding it from this review as the paper does not state the babies had to be born at term and does not specify a lower limit for birthweight. However as the babies had to be "free of important health problems" we considered them to be healthy and therefore included this study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table. |
| Allocation concealment (selection bias) | Low risk | Study secretary opened a sealed envelope that contained the study code. |
| Blinding (performance bias and detection bias) | Unclear risk | Interviewers collecting outcome data had had no previous contact with the women and were described as being unaware of the objectives of the research. It was not clear if blinding was effective. |
| Incomplete outcome data (attrition bias) | Unclear risk | 1003 women randomised. 14% lost to follow‐up by the end of 4 months. Reasons for loss to follow‐up were not described but the loss appeared balanced across the 2 groups. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Low risk | No baseline imbalance apparent. |
| Methods | RCT 2‐arm trial with individual randomisation Single site study. Duration of recruitment not stated, n = 270. | |
| Participants | Urban Canada ‐ maternity unit of regional general hospital. Background rates of breastfeeding initiation: Intermediate. Baseline prevalence (1984) = 69% breastfeeding initiation (75% stopping by 6 months). Inclusion criteria: intending to breastfeed; English speaking. Exclusion criteria: multiple births; birthweight < 2500 g; birth before 37 weeks. 41% were primiparous. Ethnic composition not described. Socio‐economic status defined by Blishen scale for husband's occupation (62% groups 2‐3). | |
| Interventions | Control: postpartum home visit by public health nurse who gave breastfeeding advice determined largely by the questions and concerns of the mother. Intervention: combination of home visit by breastfeeding consultant within 5 days of hospital discharge (duration 2 hours) and telephone calls by the consultant weekly for 1 month and monthly from 2‐6 months. | |
| Outcomes | Duration of breastfeeding. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described "we randomly allocated 270 new mothers". |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) | Unclear risk | It was stated that the interviewer collecting outcome data was not aware of group allocation. |
| Incomplete outcome data (attrition bias) | Low risk | There appeared to be little loss to follow‐up. 270 women were randomised and questionnaire data from 256 (5% attrition). 3 women were lost from the intervention group vs 11 controls. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Little information on methods. Possible confounders: significant differences in baseline characteristics were present for parity (P = 0.02) and intention to return to work (P = 0.05). |
| Methods | Randomised controlled trial with 2 groups. Recruitment March 2000‐October 2001. | |
| Participants | Large University teaching hospital in Victoria, Australia. Background rates of breastfeeding initiation: High. Participants were women intending to breastfeed their term infants, stratified by tertiary education and parity. Baseline prevalence of breastfeeding in Australia = 83% at hospital discharge. | |
| Interventions | Control: Standard Midwifery Support (SMS) n = 424. Received routine midwifery support and information as per the hospital protocol. The study hospital was working towards Baby‐Friendly accreditation during data collection (achieved in 2004). Intervention: Extended Midwifery Support (EMS) n = 425. Received an in‐hospital postnatal education session. Post‐discharge, they were offered home support visits with a research midwife once per week and telephone contact at least twice per week for 6 weeks. | |
| Outcomes | Any breastfeeding and exclusive breastfeeding at 6 months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sample stratified by educational level and parity. Methods not clear. |
| Allocation concealment (selection bias) | Unclear risk | Paper states "Women were asked to select an envelope from a group of at least six sealed, opaque envelopes, replenished in blocks of 12. The envelopes contained the allocation to either the intervention or control group". |
| Blinding (performance bias and detection bias) | High risk | Not mentioned. |
| Incomplete outcome data (attrition bias) | Unclear risk | 849 women randomised. Loss to follow‐up was reported by group at 2 months (intervention 83/425, 19.5% vs control 124/424, 29.2%) and at 6 months (intervention 8/425, 1.9% vs. control 4/424, 0.9%). |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Abstract does not include details of allocation concealment, outcome assessment or loss to follow‐up. Outcomes included in the abstract are reported by ITT. |
| Methods | RCT with 2 arms, individual randomisation. | |
| Participants | Setting: study carried out in Canada.101 women randomised. Inclusion criteria: live, singleton, term or near term infant delivered in 12 hours before recruitment. Women aged at least 21 residing in defined study area, intending to breastfeed and had satisfactory home circumstances (assessed by postpartum nurses). Exclusions: non‐English speaking women, caesarean delivery, postpartum complications, infants with congenital abnormalities or morbidity. | |
| Interventions | Control: planned hospital discharge 48‐60 hours postpartum (usual care) with hospital based support for breastfeeding. Intervention: planned early discharge from hospital (24‐36 hours postpartum) and up to 3 home visits by community nurse lactation consultants. Content of support unclear. The study aimed to compare of breastfeeding support in home and hospital settings. | |
| Outcomes | Exclusive breastfeeding at 5‐10 days postpartum and satisfaction with care. | |
| Notes | We have not included data from this study in the review. Outcomes were not assessed at the same time in intervention and control groups (mean day of follow‐up was 8.4 days in the intervention group vs 7.8 days for controls) and there was high attrition (26% overall, with 33% loss to follow‐up in the control group). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation. |
| Allocation concealment (selection bias) | Low risk | Central randomisation by staff not concerned with the study. |
| Blinding (performance bias and detection bias) | Unclear risk | Interviewers were reported to be initially blinded but this was not sustained as mothers revealed allocation. |
| Incomplete outcome data (attrition bias) | Unclear risk | Outcomes were not assessed at the same time in both groups and there was high attrition (26% overall, with 33% loss to follow‐up in the control group). |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | "At baseline, no differences in maternal age, parity or gestational age were found in the two groups." |
| Methods | Pilot RCT (n = 150), March‐July 2008. | |
| Participants | Recruitment from 1 hospital in Northwestern Ontario, Canada, the sole provider of maternity care for the city and regional referral centre. Background rates of breastfeeding initiation for Canada: Intermediate, however, baseline prevalence of "ever breastfed" in Ontario 90.6% (WHO Global Data Bank on Infant and Young Child Feeding accessed 12 Oct 2011). Inclusion criteria: English speaking, primiparous, planning on breastfeeding single, healthy, term infants. Exclusion criteria: conditions that could significantly interfere with breastfeeding such as serious illness, infant with congenital anomaly or admitted to special care. | |
| Interventions | Control: standard in‐hospital and community postpartum care, which included a visit by a public health nurse after hospital discharge. Intervention: standard in‐hospital and community postpartum care plus a 1‐to‐1 self‐efficacy intervention from the researcher (a Registered Nurse with practice, education, and research experience working with breastfeeding mothers). The intervention included assessment of the mother’s breastfeeding goals and breastfeeding self‐efficacy and her general physiologic and affective state; strategies to increase breastfeeding self‐efficacy; evaluation, and planning the next session. There were 3 contacts, 2 face‐to‐face in hospital on days 1 and 2 after the birth, and 1 phone call up to 7 days after hospital discharge. | |
| Outcomes | Feasibility, compliance, and acceptability of the intervention. Breastfeeding confidence (self‐efficacy scores), any and exclusive breastfeeding at 4 and 8 weeks. | |
| Notes | Paper states "Observation of breastfeeding at 1 of the 2 in‐hospital sessions was planned, to try to maximise performance accomplishment (successful breastfeeding)”. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Group allocations generated by an experienced researcher not involved in the trial. |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered, sealed opaque envelopes. |
| Blinding (performance bias and detection bias) | Unclear risk | Participants and caregiver: not blinded (due to nature of the intervention). Outcome assessor: outcomes at 4 and 8 weeks postpartum were collected during phone interview by research assistant reported to be blind to group allocation. |
| Incomplete outcome data (attrition bias) | Low risk | Outcomes measured at 8 weeks in 134/150 (89%). Loss to follow‐up was balanced across groups. For breastfeeding outcomes we have carried out an ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | No baseline differences between groups apparent. |
| Other bias | Unclear risk | Baseline characteristics were similar. |
| Methods | Single‐site study. Duration of recruitment not stated, n = 200. Follow‐up 97%. Quasi‐randomised trial (drawing numbered tickets). | |
| Participants | Urban Canada. Background rates of breastfeeding initiation: Intermediate. Antenatal meetings in a community health district. Inclusion criteria: women who wish to breastfeed and who have not previously breastfed. 97% of subjects were primiparous. Ethnic composition not stated. 57% had received education to college or university level. No specific socio‐economic classification used. | |
| Interventions | Home visit by volunteer during last month of pregnancy followed by telephone contacts weekly for 6 weeks and then 2 weekly to 5 months or until weaning. Volunteers were women who had breastfed themselves and had received 3 training sessions of 3 hours duration followed by on‐going monthly supervision sessions. Average caseload 1‐3 cases at any one time. | |
| Outcomes | Breastfeeding rates at 1, 2, 3, 4 and 6 months. | |
| Notes | Control group received home visit from public health nurse during the first month after birth followed by other contacts (face‐to‐face or by telephone) as determined by the mother. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation by "drawing numbered papers". |
| Allocation concealment (selection bias) | Unclear risk | Not clear. |
| Blinding (performance bias and detection bias) | Unclear risk | There was an attempt to blind outcome assessors. |
| Incomplete outcome data (attrition bias) | Unclear risk | Reasons for drop‐out recorded. 200 randomise, 3 babies died and 3 other women lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Not clear over what time period women were recruited or whether controls and intervention women were recruited at the same time. "Subjects were recruited during various periods of time, depending on the availability of volunteers". |
| Methods | RCT. 2‐arm trial, individual randomisation.Single‐site study recruiting over 14 months, n = 632. | |
| Participants | Urban UK. Background rates of breastfeeding initiation: Intermediate. National baseline prevalence 66% breastfeeding at birth and 42% at 4 months. Exclusive breastfeeding 21% at 4 months. All English‐speaking women 17 years or over giving birth at the study hospital unless their baby spent more than 48 hours on the SCBU. | |
| Interventions | Control: standard UK care (includes postnatal home visits from midwives and health visitors). Intervention: community postnatal support worker. 8 week training. Home‐based support of up to 10 visits in the first 28 days. Maximum 3 hours per visit. | |
| Outcomes | Exclusive or any breastfeeding at 6 weeks and 6 months. | |
| Notes | Study population not limited to those intending to breastfeed. Women consenting to participation more likely to be white and have had a CS. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed opaque envelopes. |
| Blinding (performance bias and detection bias) | High risk | Not stated whether there was any attempt to blind outcome assessors. |
| Incomplete outcome data (attrition bias) | Unclear risk | 623 women randomised. Stated that analysis was by ITT; 30 women who declined visits were included in the analysis. 78% follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | There was some baseline imbalance between groups. Women in the intervention group were more likely to have twins (9 vs 1), to have another adult resident in their household and to have used TENS in labour. |
| Methods | Community‐based cluster‐randomised study. Recruitment over 18 months, n = 130. | |
| Participants | Peri‐urban Mexican community. Background rates of breastfeeding initiation: High. All pregnant or postnatal women in 39 geographical clusters. Perinatal death only clinical exclusion criterion. Baseline breastfeeding prevalence: 92% initiation; 4% exclusivity at 2 weeks and 3 months; 50% cessation by 6 months. | |
| Interventions | Control: not specified. Intervention: home visits by peer‐counsellors trained by La Leche League. | |
| Outcomes | Breastfeeding at 3 and 6 months. Incidence of diarrhoea in infants 0‐3 months. | |
| Notes | Subgroup analysis: antenatal and postpartum support; proactive intervention with scheduled contacts at home; initial face‐to‐face contact; intervention delivered by trained counsellors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster‐randomisation. Clusters stratified by area. Randomisation schedule generated by computer. |
| Allocation concealment (selection bias) | Low risk | Clusters randomised by computer. |
| Blinding (performance bias and detection bias) | High risk | Clusters randomised to avoid contamination but women and counsellors would be aware of intervention; outcome measurement was by staff who were aware of group allocation. |
| Incomplete outcome data (attrition bias) | Unclear risk | 130 women from 31 cluster areas randomised. 125 remaining to follow‐up at 3 months and 104 at 6 months (20% attrition at 6 months). |
| Selective reporting (reporting bias) | Unclear risk | The way that cluster design was taken into account was not clear. It was stated that ICC values were "negligible" and "these results show that the cluster‐randomisation design achieved the equivalent of individual randomisation". |
| Other bias | Unclear risk | No baseline imbalance apparent although group size was uneven (this may have been due to chance). |
| Methods | RCT with 2 arms. Individual randomisation (n = 225). | |
| Participants | Setting: General practice in Ayrshire, Scotland. Background rates of breastfeeding initiation: Low. Participant characteristics: mean age of intervention group: 28.5 years; SD 5.2; range 17‐43. Mean age of control group: 27.8 years; SD 5.5; range 16‐40. Parity: 53% primiparous. | |
| Interventions | Control group (n = 113): standard care, including visits from community midwife for the first 10 days, health visitor after 10 days, breastfeeding support groups and breastfeeding workshops available. Intervention group (n = 112): women were assigned 2 peer supporters (women with previous breastfeeding experience) who contacted them at least once in the antenatal period and provided further antenatal support on request. In the postnatal period after hospital discharge peer supporters contacted women who were still breastfeeding at least every 2 days by phone or by home visit up until 28 days, and further support was available up to 16 weeks postpartum. | |
| Outcomes | Initiation of breastfeeding, any and exclusive breastfeeding at 6 weeks and 6 months, median breastfeeding duration and reasons for giving up breastfeeding. | |
| Notes | The researchers note that "health professionals varied in their commitment to breastfeeding and also in their acceptance of lay assistance, such as peer support". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation sequences for each stratum (primagravidae, previous formula feeding, previously breastfed < 6 weeks, previously breastfed > 6 weeks) were generated at the start of the trial by computer in blocks of 10 (that is, 5 random allocations to each of the peer support and control groups in each different block of 10) to give approximate numerical balance between groups. |
| Allocation concealment (selection bias) | Low risk | Allocation to control or peer support group was by post‐recruitment concealed allocation. Telephone call for the next allocation on the list |
| Blinding (performance bias and detection bias) | High risk | "There was no post‐allocation concealment as once a woman was allocated to the peer support or control group this was known to the peer supporters and others associated with the trial.” It was stated that the trial team were not involved in outcome assessment. |
| Incomplete outcome data (attrition bias) | Low risk | Low loss to follow‐up. Peer support group (intervention group) (112): followed up for 16 weeks, n = 110. Control group (113): followed up for 16 weeks, n = 110. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Planned recruitment was for 320 women but ended after 225 women recruited, therefore the study had reduced power to detect differences between groups. Little demographic data were reported so it was not clear whether or not there was baseline imbalance although recruitment was balanced for parity by stratification. |
| Methods | RCT, 2 arms with individual randomisation (n = 104). | |
| Participants | Setting: maternal and paediatric clinic for low‐income inner city population (New Jersey, USA). Background rates of breastfeeding initiation in this population: High. Inclusion criteria: WIC program‐qualified pregnant women in the third trimester of a singleton pregnancy without HIV, cancer, or illegal drug use. Participant characteristics: 87.5% of the women were of Hispanic origin, 89% spoke Spanish at home, 30% were single, approximately 70% were educated at less than high school level. 37% of the intervention group, compared with 42% of controls, were expecting their first child. | |
| Interventions | Control group (n = 52): routine breastfeeding education and support during the pregnancy and postpartum. Lactation consultant services were available for all postpartum women if any breastfeeding problems arose during the hospital stay. Intervention group (n = 52): in addition to routine care, allocated to 2 individual educational/support sessions with a lactation consultant in the third trimester of pregnancy lasting 15‐20 minutes. After birth the lactation consultant provided support at the hospital or by phone soon after discharge, with further phone support after the first or second week then after 1 and 2 months. The participants were asked to contact the lactation consultant if they experienced any breastfeeding problems. | |
| Outcomes | Exclusive and any breastfeeding at 7 days and 1, 2 and 3 months postpartum. | |
| Notes | Among multiparous participants, 27/29 (93%) in the intervention group had previously breastfed, compared with 17/25 (68%) in the control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “ We used computer generated random numbers to assign women to the control and intervention groups. Each random number was related to an ordinal number that was assigned to the woman once she assigned the informed consent." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) | High risk | Not described. |
| Incomplete outcome data (attrition bias) | High risk | 104 women randomised. 82% available to follow‐up at 1 month follow‐up (data included in the review) 70% of women followed up for 3 months. (35 out of 52 in intervention group completed the 3‐month follow‐up (loss of 17). 38 out of 52 in the control group completed the 3‐month follow‐up (loss of 14).
High attrition but reasons for loss given and balanced across groups (e.g. phone disconnected; women did not answer phone; some women did not notify the research team about their delivery.) |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | There was some baseline imbalance between groups that means that differences between groups are difficult to interpret. Of the multiparous women 93% in the intervention group had previous breastfeeding experience compared with 68% in the control group. More women in the control group had CS (40% vs 14%). Both of these differences possibly relate to breastfeeding outcomes. |
| Methods | RCT, 2‐arm trial with individual randomisation. Single‐site study recruiting over 3 months, n = 52. | |
| Participants | Urban Canada. Background rates of breastfeeding initiation: Intermediate. Baseline breastfeeding prevalence at 4 months: approximately 33%. Inclusion criteria: singleton pregnancy, healthy mother and child, vaginal delivery, self‐identified on breastfeeding questionnaire as unsupported. | |
| Interventions | Control: hospital care may have been from any member of the mother‐child nursing team. Intervention: breastfeeding support from the researcher, a community midwife, consisting of daily visits in hospital, telephone call within 72 hours of discharge and weekly through the 4th week postpartum, and at least 1 home visit (in the first week), with further home visits as required. Home visits lasted 60‐90 minutes. | |
| Outcomes | Exclusive and partial breastfeeding at 4 weeks. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised block randomisation procedure (stratified by planned length of breastfeeding, parity and education). |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) | High risk | The intervention was provided by the investigator so outcome assessment not blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | 52 randomised, 51 appeared to complete the study. Follow‐up 98%. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Recruitment limited by availability of investigator. No baseline imbalance apparent. |
| Methods | RCT, 2‐arm trial with individual randomisation. | |
| Participants | Women were recruited at a US community hospital and had diverse socio‐economic status. Background rates of breastfeeding initiation: Intermediate. Inclusion: women who had experienced vaginal deliveries after full‐term pregnancies. Exclusion: not stated. Mean age: 24.4 years; married, n = 47 (78%); White n = 55 (93%); completed high school, n = 58 (97%); income of $20,000 or less: n = 13 (22%). | |
| Interventions | Standard care included routine breastfeeding support in hospital following delivery. Control group: home visit on day 3 or 4 by a hospital nurse (the visit was not specifically about breastfeeding). Intervention group: 2 home visits by a professional community health nurse and phone call from a qualified lactation consultant. The professional nurse provided a structured teaching and support protocol. The focus of the first visit was to enhance breastfeeding. For the second visit, of up to 2 hours duration, mothers could choose the content from options including help with dishes or laundry. Most chose education or infant assessment; 2 asked for child care help so they could rest and/or spend time with a partner. | |
| Outcomes | Primary outcome: duration of breastfeeding. Secondary outcomes: fatigue, symptoms of anxiety and depression. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to the treatment (n = 30) or control group (n = 30). |
| Allocation concealment (selection bias) | Unclear risk | No information. |
| Blinding (performance bias and detection bias) | High risk | Participants and caregivers were not blinded. For outcome assessors, outcome data were collected by a research assistant (by telephone). It was not clear whether blinding was achieved. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not clear. Loss to follow‐up was not mentioned and denominators were not provided for the results.
|
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Little information was provided on study methods. No information on how many women were followed up, blinding, how randomisation occurred. |
| Methods | Single‐site study. 2‐arm RCT. Recruitment April 1999‐February 2000, n = 41; 21 assigned to intervention and 20 to control group. | |
| Participants | Community intervention in urban USA. Background rates of breastfeeding initiation: Low. Inclusion criteria: low‐income women receiving financial medical assistance. Exclusion criteria not stated. Ethnic composition: 95.2% African American. | |
| Interventions | Control: usual breastfeeding support consisted of support from hospital nurses, assistance by means of a telephone "warm line" and if mothers gave birth on a weekday, 1 hospital visit from a lactation consultant. Intervention: breastfeeding support visits by community health nurse/peer counsellor team. Support offered daily when in hospital, and at home during weeks 1, 2 and 4 and at the team's discretion. Telephone support from peer counsellor twice weekly through week 8 and monthly through month 6. | |
| Outcomes | Duration of breastfeeding to 6 months; healthcare services use by infants; costs. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "assigned randomly". |
| Allocation concealment (selection bias) | Low risk | Described as "a sealed envelope technique". |
| Blinding (performance bias and detection bias) | High risk | Outcome assessment by person or by phone; not blinded. |
| Incomplete outcome data (attrition bias) | Low risk | 41 women randomised, all appeared to have been followed up. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Low risk | Groups similar at baseline. |
| Methods | RCT, 2‐arm trial with individual randomisation (n = 328). | |
| Participants | Setting: 2 hospitals (1 university and 1 community hospital) serving urban areas in Baltimore, Maryland, USA. Background rates of breastfeeding initiation: Intermediate. Inclusion criteria: mother English speaking, with phone access and living within 25 miles of the hospital, intending to breastfeed, family eligible for WIC program. Singleton term infant (> 37 weeks’ gestation) Exclusion criteria: Infants or mothers with positive drug screen, infants with craniofacial abnormalities, infants admitted to NICU. Participant characteristics: All enrolled in WIC program; mean age 23.1 years; 87% African Americans; 26.5% with less than high school education; 79.6% single; 17.4% not employed or in school; 26.6% caesarean births; 50.6% first time mothers; 32.3% with previous breastfeeding experience; | |
| Interventions | Control group (n = 160): usual care included access to a lactation consultant in hospital and phone access after discharge home. Intervention group (n = 168): In addition to usual care, a structured programme of education and support. This comprised postnatal visits by a breastfeeding team (community nurse and peer counsellor) daily in hospital, 2 home visits in the first week after discharge, a third visit at approximately 4 weeks, then scheduled phone calls by the peer counsellor at least fortnightly until 24 weeks and phone access to the community nurse (24 hr) for 24 weeks. Home visits lasted approximately 45‐60 minutes and the average length of phone calls was approximately 20 minutes. | |
| Outcomes | Any breastfeeding (breastfed at least once during the previous 24 hours) at 6, 12, and 24 weeks postpartum. | |
| Notes | Baseline variables were measured using established valid instruments and were used as co‐variates to adjust for differences between randomisation groups in some of the analyses in the paper. In our analyses we have reported unadjusted figures. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence. Block randomisation (block size 10). |
| Allocation concealment (selection bias) | Low risk | “sealed envelope technique” ... not entirely clear, not described in detail but probably adequate. |
| Blinding (performance bias and detection bias) | High risk | Baseline data were collected before randomisation therefore this was collected in a blind fashion, however following randomisation women and staff would be aware of group assignment. There was a serious risk of bias associated with the lack of blinding of outcome assessors. In the intervention group outcome data were collected by the staff carrying out the intervention whereas in the control group outcome data were collected by a research interviewer who the women will not have met. |
| Incomplete outcome data (attrition bias) | Low risk | 70% of those approached randomised. 328 randomised and followed up, 29% lost to follow‐up by 24 weeks but all women included in the analyses. Women who withdrew from the study early in the project were assumed not to be breastfeeding and those who were lost subsequently were assumed not to be breastfeeding since their last contact. Both I and C groups were treated in the same way and loss was similar in the 2 groups. The numbers recorded as still breastfeeding therefore represent a conservative estimate. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | There was no apparent baseline imbalance although baseline characteristics were used in regression analysis to determine adjusted treatment effect. In our results we have reported the unadjusted data. |
| Methods | RCT. 2‐arm trial. Single‐site study. Recruitment July 1998‐December 2000, n = 136. | |
| Participants | Urban Australia. Background rates of breastfeeding initiation: High. Participants were recruited at a teenage pregnancy clinic serving mostly disadvantaged young women. The intervention was offered regardless of feeding intention or practice. Inclusion criteria: teenagers aged less than 18 years attending first antenatal appointment at public‐care teenage pregnancy clinic for first time mothers; English speaking; intending to continue with the pregnancy and not relinquish the infant. Exclusion criteria: residence > 150 km from the study hospital; known fetal abnormality. Ethnic composition of sample: 24% indigenous Australian. Socio‐economic status: 86.5% of sample scored low or destitute on score derived from educational level of participant and her parents, and family income. Baseline prevalence of breastfeeding in Australia = 83% at hospital discharge. | |
| Interventions | Control: routine postnatal support, counselling and information services provided by the hospital included access to routine hospital domiciliary home‐visiting services. Intervention: structured home visits in weeks 1 and 2 by certified nurse‐midwives to teach feeding and maternal‐infant bonding skills. Further visits at months 1, 2, 3 and 4 to provide advice and support. | |
| Outcomes | Adverse neonatal outcomes (infant death, severe non‐accidental injury and non‐voluntary foster care); knowledge and practice of contraception, vaccination schedules and breastfeeding. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By computer‐generated randomised allocation schedule. |
| Allocation concealment (selection bias) | Low risk | Concealed in numbered, sealed opaque envelopes. |
| Blinding (performance bias and detection bias) | High risk | Women, staff and outcome assessors all aware of intervention group. |
| Incomplete outcome data (attrition bias) | Unclear risk | 65 assigned to the intervention and 71 to the control group. Reasons for drop‐out recorded. 124 completed trial (91%). |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | It was not clear how the intervention related to some of the outcomes (e.g. early infant death). No baseline imbalance apparent with similar numbers of women in the 2 groups initiating breastfeeding. |
| Methods | Quasi‐RCT. 2‐arm trial. | |
| Participants | Setting: study in a hospital in Zambia. Recruitment between 1989‐1992. 408 women recruited 1 hour following delivery at the study hospital. Inclusion criteria: normal birth, term, singleton, Apgar score > 7 at 1 min, no visible malformation and mother and baby assessed as healthy. | |
| Interventions | Control (n = 200): home visit by a midwife at 42 days only. Intervention (n = 208): home visits by a midwife at 3, 7, 28 and 42 days. Home visits lasted about 1 hour. Midwives examined women and infants and asked about their health; any health problems and related actions; breastfeeding patterns; social support (if any). If indicated, midwives referred women for medical help. | |
| Outcomes | Maternal and infant health problems. | |
| Notes | We have not included data from this study in the review as data were not reported in a way that allowed us to enter them in to RevMan 2011 for meta‐analysis. Numbers of breastfeeding women were not reported by randomisation group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 2‐stage randomisation process with recruitment on certain days, when women were randomly selected to be randomised to treatment groups. |
| Allocation concealment (selection bias) | Unclear risk | It was not clear whether the person carrying out the randomisation had any control over the sample selection and the randomisation process. |
| Blinding (performance bias and detection bias) | Unclear risk | Some outcome data were collected by staff blind to group allocation. |
| Incomplete outcome data (attrition bias) | Unclear risk | Patients seen at follow‐up for Group A ‐ 98.5% at day 3, 97.5% at day 7, 87% at day 28 and 89% at day 42. Patients seen at follow‐up for Group B ‐ 87% at day 42. Loss to follow‐less than 20% at each follow‐up visit. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Baseline characteristics similar. |
| Methods | RCT with 2 arms. | |
| Participants | Setting: 235 eligible and consenting women booked for delivery at an Australian hospital in 1989. Inclusion criteria: primiparous women who expressed a wish to breastfeed, who booked for delivery before 20 weeks’ gestation, aged between 18 and 35 and lived within 20 km of the hospital. Women who received care in addition from independent midwives were excluded. | |
| Interventions | Control: usual breastfeeding care and advice along with routine antenatal classes. Intervention: programme of care based on health belief model and cognitive‐behavioural principles, including a 3‐hour group teaching session in the antenatal period and a visit by a lactation consultant shortly after hospital birth, phone support 2‐3 weeks later and at 3 months, with a home visit if needed. The lactation consultant was available to provide telephone support at other times. | |
| Outcomes | Breastfeeding at 6 weeks and 4 months post delivery and reasons for stopping breastfeeding. Satisfaction with the intervention. | |
| Notes | We have not included data from this study in the review due to very high attrition rates which means results are difficult to interpret. In this study women were recruited in the antenatal period. 235 women were randomised; 30% were lost to follow‐up by 6 weeks postpartum (and full interview data were available for only 56% of the sample). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternate. Odd or even numbered consent forms. It was stated that forms were given out sequentially. |
| Allocation concealment (selection bias) | Unclear risk | Odd or evenly numbered consent forms. It was stated that those carrying out recruitment and women were not aware of the code for allocation. |
| Blinding (performance bias and detection bias) | High risk | Not blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | High loss to follow‐up with interview data at 6 weeks for only 56% of the sample randomised. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Baseline characteristics similar ‐ no significant differences between control and intervention groups on any of these variables. |
| Methods | Single‐site study. Recruitment: August 2000‐July 2002, n = 101; RCT 3‐arm trial with individual randomisation. | |
| Participants | Urban setting in Minas Gerais, Brazil. Background rates of breastfeeding initiation: High. Inclusion criteria: mother breastfeeding her well, term baby when appointment for paediatric clinic made; first clinic consultation took place, at 30 days or less. Exclusion criteria: mothers who expressed a preference to see a particular paediatrician; babies no longer breastfed at the first appointment. Ethnic composition: 62% of babies white. Baseline prevalence of breastfeeding in Brazil in the first 30 days = 88%. | |
| Interventions | Control group: babies were monitored by a paediatrician who did not have formal training to promote exclusive breastfeeding. Intervention group 1: babies were monitored by a paediatrician working with a multidisciplinary breastfeeding team. The paediatrician and team had all received training to promote exclusive breastfeeding (PNIAM: Programa de Incentivo ao Aleitamento Materno, Brazil). Intervention group 2: babies were monitored by the same paediatrician, in individual consultations. | |
| Outcomes | Exclusive breastfeeding to 4 months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | (Risk of bias assessment from translation notes.) Random assignment by drawing lots. |
| Allocation concealment (selection bias) | Unclear risk | Random assignment by drawing lots. Described as simple randomisation in translation notes. |
| Blinding (performance bias and detection bias) | High risk | It was stated that staff were aware of group assignment. |
| Incomplete outcome data (attrition bias) | Unclear risk | It was not clear at what time randomisation took place or the number randomised to each group "the exclusion percentages were similar in the three groups". 190 were eligible and 101 completed the study. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | No baseline imbalance apparent. It was not clear how many women were randomised. |
| Methods | RCT, 2 arms with individual randomisation (n = 52). | |
| Participants | Volunteers attending prenatal clinics in Massachusetts USA who intended to breastfeed their babies for 2 months or longer (recruitment 1986‐1987). Background rates of breastfeeding initiation: Low. Inclusion: Breastfeeding for the first time, or unsuccessful previous attempts. English speaking. All women received prenatal breastfeeding information. | |
| Interventions | Control group: usual care. Women were given contact details for the clinic nutritionist if problems arose. Interventions group: home visits and telephone contacts up to 2 months postpartum from an experienced breastfeeding counsellor (who also recruited women to the study). Women received 5‐8 visits lasting 30‐60 minutes. | |
| Outcomes | Breastfeeding at 2 months postpartum and 6 months postpartum. | |
| Notes | Only 1 result was reported that we were able to include in the review: numbers breastfeeding (any) at 8 weeks postpartum 61.% of 26 in the intervention group and 34.6% of 26 in the control group. The remaining data were in a graph and are not easy to interpret or data were not reported by randomisation group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Method not described “randomised the clients”. |
| Blinding (performance bias and detection bias) | High risk | No blinding.
|
| Incomplete outcome data (attrition bias) | High risk | 52 women were recruited. It appeared that all women were followed up at 8 weeks postpartum but that approximately half of the comparison group were lost to follow‐up by 6 months. We have not included any data in the review relating to the outcomes measured at 6 months. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Very low recruitment rate “it took 14 months to enrol just 52 participants from 4 clinics serving in total approximately 1000 pregnant women per year”. Results may not be generalisable. |
| Methods | Single‐centre, single blind trial. RCT, 2 arms. Recruitment 2005‐2006. | |
| Participants | Setting: maternity unit in Northern Ireland with Baby‐Friendly accreditation. Background rates of breastfeeding initiation: Intermediate. One‐fifth of women reported to stop breastfeeding before hospital discharge. Participants randomised n = 182. Exclusion criteria: Women < 20 years old who had commenced the ‘young mums’ parentcraft programme prior to the 20 weeks visit. Vulnerable women, e.g. women who neither spoke nor understood English. Mothers separated from their babies, for example when a baby was admitted to the neonatal unit, who did not receive routine instruction (post‐randomisation exclusion). Sample characteristics for n = 144 who completed the research (not reported by randomised group) Age 21‐30 years 79/144 (55%); 31‐40 years 53/144 (37%) SES 38 (26%) professionals; 20 (14%) not working | |
| Interventions | Standard care at the study hospital, received by all study participants, met Baby‐Friendly standards and complied with National Institute for Clinical Excellence (NICE) guidelines. Women in the control group (n = 89) received a 2‐hour antenatal infant feeding class, a breastfeeding book and midwife support for the first 3 postnatal weeks. The intervention was staff training. Women in the intervention group (n = 93) received a “motivationally enhanced” version of control group care from staff who had been trained in a programme called ‘Designer Breastfeeding’. | |
| Outcomes | Primary outcome: using 7‐point Likert scales women’s motivational profile was measured in relation to 3 motivational factors: total value placed on breastfeeding, perceived midwife support and expectancy for successful breastfeeding. Secondary outcomes: breastfeeding behaviour was measured as a secondary outcome on discharge from hospital and at 3 weeks postnatal. Breastfeeding initiation was defined according to the Department of Health as giving 1 breastfeed or 1 episode of expressed breastmilk. Duration of breastfeeding was categorised in accordance with the Index of Breastfeeding Status, which classified breastfeeding on a scale in accordance with the amount of breast milk the infant is receiving. | |
| Notes | Only 53 of the 89 women randomised to the control group were known to have initiated breastfeeding. In the intervention group 57 of 93 randomised initiated breastfeeding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Prior to recruitment a randomised table was created." |
| Allocation concealment (selection bias) | Low risk | The authors state: "Neither the researcher, nor the research participants could predict their allocated treatment". |
| Blinding (performance bias and detection bias) | Unclear risk | Described as single blind. Women were said to be not aware of groups, but there were stickers on the notes so care providers would be aware of group assignment. |
| Incomplete outcome data (attrition bias) | Unclear risk | 234 assessed for eligibility, 182 consented and 144 completed. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | There were some baseline differences between groups. Women in the control group were more likely to be discharged from hospital early and were less likely to attend antenatal infant feeding classes. It is not clear what impact these differences had on the results. |
| Methods | Single‐site quasi‐randomised trial (n = 146). Duration 12 months. | |
| Participants | Urban Sweden ‐ maternity ward of University Hospital. Background rates of breastfeeding initiation: High. Inclusion criteria: resident in Uppsala; normal birth; healthy babies weighing > 3 kg. Ethnic composition not stated. 28% of mothers had completed college or university education. Baseline prevalence (1972): 4% breastfeeding at 24 weeks. | |
| Interventions | 'Interview' with paediatrician in hospital on days 1 and 4 and at home at 2 and 6 weeks and 3 months; telephone contact weekly while breastfeeding followed by home visit if problem noted. | |
| Outcomes | Partial and exclusive breastfeeding at 2, 4, 8, 12, 16, 20 and 24 weeks. | |
| Notes | Primarily designed as a study of the reasons for breastfeeding difficulties and the cessation of breastfeeding. Recruitment halted during holidays. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomisation depending on time of day of birth. |
| Allocation concealment (selection bias) | High risk | Quasi‐randomisation. |
| Blinding (performance bias and detection bias) | High risk | Women in the control group were not told about the study until the 6 months follow‐up interview. All interviews were carried out by the same investigator who was aware of group allocation. |
| Incomplete outcome data (attrition bias) | Unclear risk | Interviews took place while women continued to breastfeed and it was not clear how many women remained to follow‐up at different points although no drop‐out was reported for the final data collection interview at 6 months. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Women in the intervention group reported outcomes at scheduled interviews whereas the control group were interviewed at 6 months postpartum only. Recall and response bias may have been different in the 2 groups. |
| Methods | RCT, 3‐arm trial with individual randomisation (n = 450). | |
| Participants | National University Hospital, Singapore. Background rates of breastfeeding initiation: High. Inclusion criteria: healthy pregnant women attending antenatal clinics at the study hospital, with no illness that would contraindicate breastfeeding or severely compromise its success; intending to breastfeed; birth at 34 weeks gestation or later. Exclusion criteria: women with high risk and multiple pregnancies. Participant characteristics: 40% primiparous, mixed ethnicity (Chinese 31‐44%, Malay 46‐54%, Indian and other), approximately a third educated beyond secondary school, approximately half employed outside the home, 56% had previously breastfed. | |
| Interventions | Control: women received routine antenatal, intrapartum and postnatal care, including optional antenatal classes and postnatal visits by a lactation consultant should any problems with breastfeeding arise. Intervention 1 ‐ Antenatal education: in addition to routine care, women received 1 session of antenatal breastfeeding education and printed guides on breastfeeding. Intervention 2 ‐ Postnatal lactation support: in addition to routine care, women received two postnatal sessions with a lactation consultant, 1 in hospital within the first 3 postnatal days (when they received the same printed guides on breastfeeding as the antenatal education group) and 1 during the first routine postnatal visit 1 to 2 weeks after the birth. Each session lasted about 30 minutes and covered latching on, proper positioning and other techniques to avoid common breastfeeding complications. | |
| Outcomes | Exclusive and any breastfeeding at hospital discharge and 2 weeks, 6 weeks, 3 and 6 months after the birth. Exclusive breastfeeding was defined as giving breast milk as the only food source, with no other foods or liquids, other than vitamins and minerals being given. | |
| Notes | The group receiving the antenatal intervention are not included in the analysis in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence by external clinical trials unit. |
| Allocation concealment (selection bias) | Low risk | Telephone allocation by external trials unit (with envelope back up used only on 4 occasions) |
| Blinding (performance bias and detection bias) | Unclear risk | Data collection was on standard forms and was entered by remote unit, therefore outcome assessment may have been partially blinded. For participants, blinding was not mentioned, but women would be aware of allocation. For caregivers, those who delivered the intervention, would be aware of the intervention. For outcome assessors, this was not clear but may have been partially blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | Low attrition in all arms. In total 450 randomised 347 completed follow‐up at 6 months (82%). In the data and analyses 2 arms included 299 randomised, 245 followed up at 6 months (82%).
|
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | There was an imbalance in the groups due to 4 women being randomised by using back up envelopes because of dysfunction in web randomisation, but groups appeared similar at baseline. Some of the data were based on assumptions. Sensitivity analyses were based on the assumption that none of the women lost to follow‐up were exclusively breastfeeding at any time point. |
| Methods | One of the 3 country sites that completed the cluster‐randomised trial ‐ Burkino Faso in French speaking West Africa (24 clusters, 12 intervention, 12 control). Mother‐infant pairs enrolled: 392 intervention and 402 control (794 total). Followed up at 24 weeks: 359/392 (92%) intervention and 372/402 (93%) control. | |
| Participants | Rural area. Main source of income farming. 60 primary care facilities and a regional hospital. Mean age of women 25 years. None of the women had any formal education and more than half had a previous child death. 99% of women had none or open toilets and < 1% had piped water in yard or home. Monthly income was approximately 3 euros. Baseline prevalence of breastfeeding initiation: High (98.4%). Exclusive BF for babies under 6 months estimated at 16%. Inclusion Criteria: Women living in trial area at least 7 months pregnant and intending to breastfeed. Singleton live birth, no serious congenital malformations. Exclusion Criteria: If mothers or infants died they were not included in the analysis | |
| Interventions | Control: Mothers and infants in control clusters in Burkina Faso were given standard healthcare only. 76% of pregnant women attend antenatal care and 51% of deliveries were assisted by trained staff. Under 5 mortality rate 169/1000 live births and infant mortality 92/1000 live births. In Burkina Faso, only the peer counsellors were supporting mothers for exclusive breastfeeding. Intervention: Peer counselling. Supporters received a modified version of WHO/UNICEF training (1 week training). Women were given information about breastfeeding and peers provided support and addressed problems or referred women for specialist help. The intervention involved a minimum of 5 home visits 1 in the third trimester and at least 4 in the postnatal period up to 6 months postpartum. The supporters were local residents, literate, able to travel to visit women in their homes and to have a good reputation in the community. Peer counsellors visited the same women each time to achieve continuity of care. The intervention varied in the 3 study areas and was adapted to local circumstances. | |
| Outcomes | Prevalence of exclusive breastfeeding and prevalence of diarrhoea, reported by mothers for infants aged 12 weeks and 24 weeks. | |
| Notes | The paper states that current breastfeeding was assessed at all scheduled postpartum visits using past 24‐hour and 7‐day recalls. Babies reported to have received no other food or liquids other than breast milk (they may have been administered drugs) were classified as exclusively breastfed. This may have been during the last 24 hours/7 days rather than since birth. Prevalence of diarrhoea was based on the mothers’ reports of the past 2 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear (different procedures in different areas and procedure in 1 of the 4 areas was not clear) . |
| Allocation concealment (selection bias) | Unclear risk | There was no allocation concealment within clusters and participants would be aware of assignment. |
| Blinding (performance bias and detection bias) | High risk | There was no participant or staff blinding. There was an attempt to mask/ blind outcome assessors to randomisation group although it is possible women would have revealed whether or not they received support. The success of attempted blinding was not formally evaluated. |
| Incomplete outcome data (attrition bias) | Low risk | There was flooding in 1 of the 4 original study area and no results are reported for this area. For the remaining 82 clusters in 3 countries for primary outcomes the authors carried out an ITT analysis (i.e. those that were missing were recorded as non‐events, i.e. NOT exclusive breastfeeding and no diarrhoea). 2579 women enrolled. Missing data and missed visits at various data collection points. |
| Selective reporting (reporting bias) | Unclear risk | Not apparent. |
| Other bias | Unclear risk | Recruitment procedures and intervention delivery was slightly different in each of the study countries which meant that results were difficult to interpret. It was reported that the ICC in each country for primary outcomes varied considerably and therefore results were reported separately for each country.
Authors state “The community‐based approach could possibly have resulted in socially desirable answers, and the results were based on self‐reports. A bias towards desirable answers and thereby an increased effect size cannot be ruled out. We also noted some questionnaire fatigue in the Ugandan site—i.e., reluctance to fully engage in answering similar questions after a few interviews". |
| Methods | Second of 3 country sites that completed the cluster‐randomised trial ‐ Mbale district in Eastern Uganda (24 clusters, 12 intervention, 12 control). Mother‐infant pairs enrolled: 396 intervention and 369 control (765 total). Followed up at 24 weeks: 368/396 (93%) intervention and 329/369 (89%) control. | |
| Participants | Urban and rural areas. Urban area included “large slum migrant settlements”. HIV prevalence for fertile women was 6.2%. 26% of women none or open toilets and 5% had piped water in yard or home. Mean age 25. Women had approximately 6 years of formal education and approximately a third had a previous child death. Monthly income was approximately 12 euros monthly. Background rates of breastfeeding initiation: High (> 95%). Inclusion Criteria: Women living in trial area at least 7 months pregnant and intending to breastfeed. Singleton live birth, no serious congenital malformations. Exclusion Criteria: If mothers or infants died they were not included in the analysis | |
| Interventions | Control: Mothers and infants in control clusters in Uganda were given standard healthcare only. In 2006, 95% of pregnant women attended antenatal care and 41% delivered in a healthcare facility. The under 5 mortality rate was 135/1000 live births and the infant mortality rate was 85/1000 live births in 2008. In Uganda, only the peer counsellors were supporting mothers for exclusive breastfeeding. Peer counselling intervention. As in Burkina Faso. Paper states the intervention varied in the 3 study areas and was adapted to local circumstances. | |
| Outcomes | Prevalence of exclusive breastfeeding and prevalence of diarrhoea, reported by mothers for infants aged 12 weeks and 24 weeks. | |
| Notes | The paper states that current breastfeeding was assessed at all scheduled postpartum visits using past 24‐hour and 7‐day recalls. Babies reported to have received no other food or liquids other than breast milk (they may have been administered drugs) were classified as exclusively breastfed. This may have been during the last 24 hours/ 7 days rather than since birth. Prevalence of diarrhoea was based on the mothers’ reports of the past 2 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear (different procedures in different areas and procedure in 1 of the 4 areas was not clear) . |
| Allocation concealment (selection bias) | Unclear risk | There was no allocation concealment within clusters and participants would be aware of assignment. |
| Blinding (performance bias and detection bias) | High risk | There was no participant or staff blinding. There was an attempt to mask/blind outcome assessors to randomisation group although it is possible women would have revealed whether or not they received support. The success of attempted blinding was not formally evaluated. |
| Incomplete outcome data (attrition bias) | Low risk | There was flooding in 1 of the 4 original study area and no results are reported for this area. For the remaining 82 clusters in 3 countries for primary outcomes the authors carried out an ITT analysis (i.e. those that were missing were recorded as non‐events, i.e. NOT exclusive breastfeeding and no diarrhoea). 2579 women enrolled. Missing data and missed visits at various data collection points. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Recruitment procedures and intervention delivery was slightly different in each of the study countries which meant that results were difficult to interpret. It was reported that the ICC in each country for primary outcomes varied considerably and therefore results were reported separately for each country.
Authors state “The community‐based approach could possibly have resulted in socially desirable answers, and the results were based on self‐reports. A bias towards desirable answers and thereby an increased effect size cannot be ruled out. We also noted some questionnaire fatigue in the Ugandan site—i.e., reluctance to fully engage in answering similar questions after a few interviews". |
| Methods | Third of 3 country sites that completed the cluster‐randomised trial ‐ 3 geographically separate sites in South Africa (Paarl, a town at the centre of a farming district near Cape Town; Umlazi, a large periurban township near Durban; and Rietvlei, one of the country's poorest rural districts). 34 clusters, 17 intervention, 17 control. Mother‐infant pairs enrolled: 535 intervention and 485 control (1020 total). Followed up at 24 weeks: 461/535 (86%) intervention and 410/485 (85%) control. | |
| Participants | South Africa (3 areas including 1 of the poorest rural area in South Africa). Under 5 mortality rate in South Africa 67/1000 and IMR 48/1000. Background rates of breastfeeding initiation: High (> 95%). Exclusive breastfeeding at 6 months was estimated at 8% in 2005‐9. 16% of women had none or open toilets and 66% had piped water in yard or home. Mean age 23 years. Women had approximately 10 years of formal education and approximately 7% had a previous child death. Monthly income was approximately 103 euros. Inclusion Criteria: Women living in trial area at least 7 months pregnant and intending to breastfeed. Singleton live birth, no serious congenital malformations. Exclusion Criteria: If mothers or infants died they were not included in the analysis | |
| Interventions | Control: In South Africa, antenatal attendance 94% and hospital delivery rates 84%. Control clusters were visited by peer counsellors, with the same schedule as the intervention clusters, but who assisted families in obtaining birth certificates and social welfare grants. The peer counsellors for the intervention and control clusters in South Africa were kept separate during the study. Peer counselling intervention: as in Burkina Faso and Uganda. Paper states the intervention varied in the 3 study areas and was adapted to local circumstances. | |
| Outcomes | Prevalence of exclusive breastfeeding and prevalence of diarrhoea, reported by mothers for infants aged 12 weeks and 24 weeks. | |
| Notes | The paper states that current breastfeeding was assessed at all scheduled postpartum visits using past 24‐hour and 7‐day recalls. Babies reported to have received no other food or liquids other than breast milk (they may have been administered drugs) were classified as exclusively breastfed. This may have been during the last 24 hours/ 7 days rather than since birth. Prevalence of diarrhoea was based on the mothers’ reports of the past 2 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear (different procedures in different areas and procedure in 1 of the 4 areas was not clear) . |
| Allocation concealment (selection bias) | Unclear risk | There was no allocation concealment within clusters and participants would be aware of assignment. |
| Blinding (performance bias and detection bias) | High risk | There was no participant or staff blinding. There was an attempt to mask/blind outcome assessors to randomisation group although it is possible women would have revealed whether or not they received support. The success of attempted blinding was not formally evaluated. |
| Incomplete outcome data (attrition bias) | Low risk | There was flooding in 1 of the 4 original study area and no results are reported for this area. For the remaining 82 clusters in 3 countries for primary outcomes the authors carried out an ITT analysis (i.e. those that were missing were recorded as non‐events, i.e. NOT exclusive breastfeeding and no diarrhoea). 2579 women enrolled. Missing data and missed visits at various data collection points. |
| Selective reporting (reporting bias) | Unclear risk | Not apparent. |
| Other bias | Unclear risk | Recruitment procedures and intervention delivery was slightly different in each of the study countries which meant that results were difficult to interpret. It was reported that the ICC in each country for primary outcomes varied considerably and therefore results were reported separately for each country.
Authors state “The community‐based approach could possibly have resulted in socially desirable answers, and the results were based on self‐reports. A bias towards desirable answers and thereby an increased effect size cannot be ruled out. We also noted some questionnaire fatigue in the Ugandan site—i.e., reluctance to fully engage in answering similar questions after a few interviews". |
| Methods | RCT 2 arms with individual randomisation (n = 500). | |
| Participants | Setting: urban, a low‐income area of the city of Sa˜o Leopoldo, Rio Grande do Sul, Brazil. Recruitment from maternity wards of the city's only publicly funded hospital, which mainly serves the low‐income population. Background rates of breastfeeding initiation: High. Inclusion: low‐income mothers with healthy, singleton, full‐term (> 37 week) babies with birthweight > 2500 g. Exclusion: impediments to breast‐feeding, HIV/AIDS, or congenital malformation. Demographics: 57% male children; 60% of intervention and 52% of control mothers had less than 8 years schooling; 73% of intervention and 67% of controls had low annual incomes (less than US $3000); 34% of mothers were not in paid work; 70% of children were living with mother and father; almost half of the mothers were overweight. | |
| Interventions | Both groups received routine assistance from paediatricians in the health service. Control (n = 300): standard care (not described). Intervention (n = 200): dietary advice about breastfeeding and the adequate introduction of complementary foods, given monthly to 6 months in home visits starting within 10 days of the child’s birth then at 8, 10, and 12 months by 12 trained field‐workers (undergraduate students in groups of 2) who counselled mothers on the Ten Steps for Healthy Feeding Children from Birth to Two Years of Age (Brazilian Ministry of Health). | |
| Outcomes | Exclusive breastfeeding at 4 and 6 months; any breastfeeding at 12 months; also diarrhoea, respiratory problems, dental caries, anaemia, hospitalisation and nutritional status at 12‐16 months. | |
| Notes | Although the paper called this intervention dietary counselling, we have included it as a breastfeeding support intervention because its main purpose was to promote exclusive breastfeeding for 6 months followed by healthy complementary foods, and it involved regular visits during the first year of life. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomisation in groups of 5. |
| Allocation concealment (selection bias) | Low risk | Randomisation was conducted by an investigator not involved in the eligibility and entry of participants into the study. Fieldworkers were informed of this allocation and then proceeded with the study. |
| Blinding (performance bias and detection bias) | Unclear risk | Women and care staff would be aware of group assignment. It was stated that fieldworkers collecting outcome data in interviews at 12‐16 months were not aware of group assignment. The impact of lack of blinding is unclear. |
| Incomplete outcome data (attrition bias) | Unclear risk | 500 women were randomised (200 to the intervention and 300 to the control group). By 12 months 163 intervention group (81%) and 234 control group (78%) remained available to follow‐up. Reasons for loss to follow‐up were give by group with reasons. However there were some discrepancies between publications and information provided by the author in the numbers followed up. |
| Selective reporting (reporting bias) | Low risk | Not apparent. |
| Other bias | Unclear risk | Method of randomisation led to imbalanced groups. Mothers and children appeared similar at baseline. There were some discrepancies between publications and information provided by the author in the numbers followed up and in the results. We have used information provided by the author. |
| Methods | RCT 3‐arm trial with individual randomisation (n = 390). | |
| Participants | The study was carried out in 7 prenatal clinics in the American Midwest. Clinics provided services to low‐income adolescent mothers. Baseline prevalence of breastfeeding initiation in country/setting: low. Inclusion criteria: Age 15‐18 years, in 2nd trimester of pregnancy, expecting first birth, planning to keep baby, able to read and speak English, with access to phone. Exclusion criteria: at birth, only mothers of singleton, term healthy babies were included. Women who had birth complications that prohibited or delayed breastfeeding beyond 48hrs were also excluded. Sample characteristics: mean age 17 years (SD 0.9); 61% African Americans; 75% low‐income; 74% single and living with their families and 71% in school. | |
| Interventions | Intervention (n = 128): 2 antenatal classes (1.5–2 hours) on benefits of breastfeeding and practical issues run by the lactation consultant and the peer counsellor, followed up by phone calls. After the birth, phone calls to those who had initiated breastfeeding, at 4, 7, 11, 18 days and 4 weeks to provide support. Control (group 1, n = 128): the same contact schedule of classes and phone calls as the intervention group, with content concentrating on more general pregnancy and health issues. Control (group 2, n = 134): usual care with no special intervention. | |
| Outcomes | Data on breastfeeding were available for women who initiated breastfeeding – this means results were difficult to interpret. | |
| Notes | We have not included outcome data from this study in the review due to very high levels of attrition. This was a study where women were recruited in the second trimester and interventions took place both prenatally and postnatally. For postnatal outcomes only those women who initiated breastfeeding were followed up. There was considerable loss to follow‐up. 390 were randomly assigned. Women who did not attend at least 1 of the study classes were dropped from the study. Follow‐up data on duration of breastfeeding were available for 201 women who initiated breastfeeding (51%). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “list of random codes generated by the study bio‐statistician." |
| Allocation concealment (selection bias) | Unclear risk | It was not clear how allocation was concealed at the point of randomisation. |
| Blinding (performance bias and detection bias) | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) | High risk | 390 women were randomised and those not attend at least 1 of the study classes were excluded. Follow‐up data on duration of breastfeeding was available for 201 who initiated breastfeeding (51%). |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Unclear ‐ data limited ‐ only reported in the form of an abstract. |
| Methods | Single‐site study. Duration of recruitment not reported, n = 72, 30 allocated to the intervention and 42 to the control group. | |
| Participants | Community study in North Trent, England, UK. Background rates of breastfeeding initiation: Intermediate. National baseline prevalence 66% breastfeeding at birth. Inclusion criteria: mothers attending for antenatal care on 1 area. Other details not reported. | |
| Interventions | The midwife asked mothers during their pregnancy to identify a close female confidante who could support them to breastfeed, and visited the mother and confidante together during the third trimester to discuss breastfeeding. | |
| Outcomes | Duration of breastfeeding to 3 months; women's satisfaction with the intervention; midwives' assessments of the intervention. | |
| Notes | Numerical outcome data provided by the researcher. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated". |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) | High risk | Not mentioned. |
| Incomplete outcome data (attrition bias) | Unclear risk | 72 randomised. It was not clear whether full data were available for all women at 3 months. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | No baseline characteristics. |
| Methods | RCT, 2 arms with individual randomisation. Few details of study methods reported. | |
| Participants | Partners of women attending for antenatal care at Baltimore hospital USA 2001‐2. (567 pregnant women were approached). | |
| Interventions | Intervention: 1 group session for fathers, lasting 2 hours, to encourage them to support their partners to breastfeed. Control: usual care. Fathers in the control group received classes on child safety and baby care. | |
| Outcomes | Breastfeeding at 4, 6 and 8 weeks and breastfeeding duration. | |
| Notes | We have not included data from this study in the review due to very high levels of attrition. 567 pregnant women approached, of the 431 that agreed to participate only 59 fathers completed the study (14%). It was not clear at what point randomisation occurred. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) | Unclear risk | Control group received alternative intervention. |
| Incomplete outcome data (attrition bias) | High risk | 431 women agreed to participate but only 14% were followed up. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | "The expectant mothers and fathers who were assigned randomly to the 2 study groups were demographically similar." Baseline characteristics tables were presented. |
| Methods | Single‐site, 2‐group quasi‐randomised study (even numbers to intervention and odd numbers to control group). Recruitment April 1999‐February 2000, n = 186, with 79 assigned to the intervention and 107 to the control group. | |
| Participants | Urban USA ‐ military hospital in Texas. Background rates of breastfeeding initiation: Intermediate. Baseline breastfeeding rate in Texas at hospital discharge = 67% in 1999. All participants were members of the armed forces or their dependents. Inclusion criteria: mothers on postpartum ward of study hospital; aged 18+; primiparous; uncomplicated delivery and postpartum; healthy baby; mother planned to breastfeed for at least 6 weeks. Exclusion criteria: hospitalisation of mother or baby for > 4 days; mothers who did not speak English. Ethnic composition of sample: 63% white, 11% black, 20% Hispanic, 2% Asian, 3% other. . | |
| Interventions | Control: standard care (not described). Intervention: breastfeeding support in hospital visit lasting approximately 30 minutes, home visit 2‐4 days after discharge lasting 45‐60 minutes, and phone call 10‐14 days after the home visit. | |
| Outcomes | Breastfeeding attrition to 6 weeks. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation by odd and even numbers in groups of 10. |
| Allocation concealment (selection bias) | High risk | Could be anticipated. |
| Blinding (performance bias and detection bias) | High risk | The person delivering the intervention seems to have collected outcome data. |
| Incomplete outcome data (attrition bias) | High risk | Information on drop‐outs incomplete and loss to follow‐up not balanced across groups. 79 in intervention group, 5 were lost to follow‐up, data at 6 weeks from 68. Outcome data were not obtained from 32 women in the control group at 6 weeks so more women were enrolled (107 enrolled to this group). Some breastfeeding duration data were obtained from drop‐outs by phone. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Replacing women in the control group lost to follow‐up means that this study is at high risk of bias. |
BFI: Baby Friendly Initiative (UNICEF)
EDD: expected date of delivery
h: hour(s)
HIV: Human Immunodeficiency Virus
ICC: intracluster correlation co‐efficient
ITT: intention‐to‐treat
LLLI: La Leche League International
MB training: maternal breastfeeding training
min: minute(s)
NICU: neonatal intensive care unit
pcm: per calendar month
RCT: randomised controlled trial
RG: Registrar General
SCBU: special care baby unit
SD: standard deviation
SPSS: Statistical Package for the Social Sciences
SSBC: supplementary structured breastfeeding counselling
VLBW: very low birthweight
WHO: World Health Organization
WIC: Special Supplemental Nutrition Programme for Women, Infants and Children (US Department of Agriculture, Food and Nutrition Service)
vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Low birthweight (LBW) infants. Under consideration for review of support for mothers breastfeeding infants who are not term and healthy. | |
| Premature infants. Under consideration for review of support for mothers breastfeeding infants who are not term and healthy. | |
| The intervention examined in this trial is not a breastfeeding support intervention. The trial examined the use of a baby cot that was clamped onto the side of the mother's bed so that the baby was within easy reach of the mother at all times. | |
| Excluded as the only breastfeeding outcome reported is "breastfeeding within one hour". | |
| Educational intervention not intended to facilitate continued breastfeeding. | |
| Intervention did not have the purpose of facilitating continued breastfeeding. | |
| Intervention did not have the purpose of facilitating continued breastfeeding. | |
| Evaluates an educational intervention. | |
| Low birthweight (LBW) infants. Under consideration for review of support for mothers breastfeeding infants who are not term and healthy. | |
| Intervention was staff training, and participants were hospitals. | |
| Not a randomised controlled trial (see Dyson et al). | |
| This study specifically focused on women with obesity. The study will be considered for inclusion in a proposed review on breastfeeding support for women at high risk of health problems that affect breastfeeding. | |
| Controlled study of breastfeeding counselling intervention without randomisation. | |
| Infants with diarrhoea. Under consideration for review of support for mothers breastfeeding infants who are not term and healthy. | |
| Not a randomised controlled trial. | |
| Participants were women with gestational diabetes. Under consideration for review of support for mothers with conditions affecting/ affected by breastfeeding. | |
| Evaluates an antenatal educational and marketing intervention. Both groups received postnatal breastfeeding support. Under consideration for the review Interventions for promoting the initiation of breastfeeding (Dyson et al). | |
| Evaluates an educational intervention. | |
| Antenatal intervention with no postnatal component. | |
| Intervention not relevant for this review. The intervention was an alternative to standard care. The intervention was not aimed at facilitating breastfeeding, rather the trial compared women who were randomised to early hospital discharge with telephone follow‐up (with home visits by nurses only for those women who left hospital within 36 hours of the birth “to encourage them to leave the hospital early”) versus usual care with later discharge from hospital. It was not clear that the intervention included any breastfeeding support. Although outcomes included breastfeeding the main focus was on “maternal competence” and infant outcomes.44% post‐randomisation exclusions. | |
| Educational intervention. Controls were matched, but not randomised. | |
| This study focused on families with a history of asthma. | |
| Paper is a review. | |
| Infants with diarrhoea. Under consideration for review of support for mothers breastfeeding infants who are not term and healthy. | |
| Not a randomised controlled trial (groups not concurrent). Sub‐study asking open‐ended questions. | |
| Intervention was a booklet and did not involve contact with an individual. | |
| Evaluates an educational intervention. | |
| Educational intervention which does not have the purpose of facilitating continued breastfeeding. | |
| Educational intervention. | |
| Not a randomised controlled trial (groups not concurrent). | |
| This study examined an intervention carried out in the antenatal period. | |
| Evaluates an education intervention. In this study women were offered specialist lactation advice by the researcher regarding returning to work and milk expression. This was a 1‐hour evidence based session and was reinforced with a written leaflet. Results were reported for those women still breastfeeding on their return to work. | |
| Very low birthweight (VLBW) infants. Under consideration for review of support for mothers breastfeeding infants who are not term and healthy. | |
| Non‐randomised observational study. | |
| Evaluates an educational intervention. | |
| Evaluates an educational intervention. | |
| Paper is a review. | |
| Support was not supplementary to standard care. | |
| Intervention was not breastfeeding support. No breastfeeding outcomes reported. | |
| Antenatal intervention with no postnatal component. | |
| Results from this study were not reported according to randomisation group. | |
| Evaluates an educational intervention. | |
| Geographical controls. | |
| This study specifically focused on smoking and the aim of the support intervention was to encourage women to quit or reduce smoking in pregnancy although breastfeeding outcomes were reported. The study will be considered for inclusion in a proposed review on breastfeeding support for women at high risk of health problems that affect breastfeeding. | |
| Infants in neonatal intensive care. Under consideration for review of support for mothers breastfeeding infants who are not term and healthy. | |
| Participants in this study may not have been healthy mothers. We excluded this study as it focused on parents with eczema or asthma and was examining the impact of a breastfeeding intervention on the occurrence of these diseases in babies. | |
| Correspondence with author established study was controlled but not randomised. | |
| It was not clear that this was a randomised trial. Only 66% follow‐up in intervention group. | |
| Antenatal intervention with no postnatal component. | |
| Not a randomised controlled trial. Qualitative study looking at women’s views of peer counselling. | |
| The intervention took place before the birth; there was no postnatal component. | |
| Paper is not about a trial. | |
| Study controlled but not randomised. | |
| Both groups received WIC breastfeeding education. The intervention group received social support for maternal diet, activity and weight loss outcomes. | |
| This study only recruited women whose babies were admitted to neonatal intensive care. Breastfeeding support for mothers of poorly babies will be considered in a separate review. | |
| Very low birthweight infants. Under consideration for review of support for mothers breastfeeding infants who are not term and healthy. | |
| This study did not examine a breastfeeding support intervention by professionals or peers. The intervention group completed daily feeding logs recording breastfeeding practices. | |
| This study specifically focused on women with obesity. The study will be considered for inclusion in a proposed review on breastfeeding support for women at high risk of health problems that affect breastfeeding. | |
| Intervention did not have the purpose of facilitating continued breastfeeding. | |
| Training intervention with no data on breastfeeding women. | |
| Evaluated an antenatal education intervention. | |
| Educational intervention. | |
| Abstract only available. No information on intervention used. | |
| Trial of hospital telephone help‐line. The intervention was an invitation to call a general telephone support line in the postnatal period; the help‐line was available to women in the control group but this new service development was not promoted with this group. The aim of the study was to examine the uptake of this service (i.e. reasons for and number of calls to the help‐line and to other hospital departments from control and intervention women). The intervention was general and was not specifically to encourage breastfeeding; breastfeeding and breastfeeding duration were not measured. | |
| All the participants had given birth by caesarean section. Both groups received an educational intervention. 1 group received a drug to promote lactation. | |
| Evaluates a purely educational intervention. | |
| Support intervention available to all women in the trial. | |
| Study controlled but not randomised. | |
| The intervention examined in this study is baby massage and not breastfeeding support. Breastfeeding was reported as a secondary outcome. | |
| Not a randomised controlled trial. | |
| Support was not supplementary to standard care. | |
| Participants were not randomised. | |
| Both groups received support as part of standard care. The intervention was not breastfeeding support. | |
| Educational intervention. Not a randomised controlled trial (assignment was 30 then another 30). | |
| Study controlled but not randomised. | |
| This study was excluded as it examined a brief educational intervention by midwives advising mothers on the correct positioning of the baby for breastfeeding. | |
| Intervention was training, and participants were hospitals. | |
| Evaluates a social support intervention. | |
| This study examined an intervention aimed at fathers which was offered as part of antenatal childbirth preparation classes. There was no postnatal component to the intervention. |
WIC: Special Supplemental Nutrition Programme for Women, Infants and Children (US Department of Agriculture, Food and Nutrition Service)
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | RCT with 4 groups: 1) mother and grandmother not cohabiting, without intervention; 2) mother and grandmother not cohabiting, with intervention directed only toward mother; 3) mother and grandmother cohabiting, without intervention; 4) mother and grandmother cohabiting, with intervention directed toward both. |
| Participants | Adolescent mothers and maternal grandmothers in Brazil. |
| Interventions | 6 breastfeeding counselling sessions; the first on the maternity ward and the others at home on days 7, 15, 30, 60 and 120. |
| Outcomes | The primary outcome was initiation of water and/or tea intake in the first 6 months of life. These were not reported in a way we could use for analyses in RevMan. We are awaiting further information on breastfeeding outcomes. |
| Notes |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | The Best Infant Nutrition for Good Outcomes (BINGO) trial. |
| Methods | A randomised, controlled, single centre, single blind, 2 x 2 factorial design. |
| Participants | Inclusion criteria include: 1. enrolled/plan to remain in care at the site throughout their pregnancy; 2. 1st or 2nd trimester (randomisation will include trimester as a blocking factor); 3. aged 18 or older; 4. can provide a reliable phone number and at least 2 alternative contacts; 5. are carrying a singleton pregnancy; 6. can communicate in English or Spanish.
Exclusion criteria include: 1. high risk of prematurity/NICU; 2. medical/obstetrical complications for which BF is or may be perceived to be contra‐indicated. |
| Interventions | Women will be randomised into 4 groups: PNC; LC; both (PNC and LC) and a control group. PNCs (certified nurse‐midwives and obstetrician/gynaecologist doctors) will use a brief, electronically prompted protocol with women in the PNC groups throughout pregnancy. A LC will arrange prenatal 1‐to‐1 meetings, daily hospital and home visits with women in the LC intervention groups. |
| Outcomes | Primary outcomes: breastfeeding intensity and exclusive breastfeeding at 1, 3 and 6 months. Secondary outcomes: infant health and participant and provider (PNC and LC) experiences of the interventions. |
| Starting date | February 2008 until February 2012. |
| Contact information | |
| Notes | Study (BINGO) is ongoing. Due to be completed Feb 2012. |
| Trial name or title | Part of the MINIMat study. |
| Methods | RCT. |
| Participants | Women in Bangladesh. |
| Interventions | Counselling to promote exclusive breastfeeding. Including 7 or 8 home visits. |
| Outcomes | Exclusive breastfeeding and infant health outcomes. |
| Starting date | Not clear. |
| Contact information | Uppsala University, Sweden. |
| Notes | Part of the MINIMat study. Unclear whether the participants of the MINIMat study would be classified as healthy or not for the purposes of our review. MINIMat is not yet published. |
| Trial name or title | Evaluation of the effectiveness of cell phone technology as community based intervention to improve exclusive breastfeeding and reduce infant morbidity rates. |
| Methods | Cluster‐randomised trial. |
| Participants | Staff training to promote breastfeeding. |
| Interventions | All the women in the trial will receive hospital maternity care at hospitals using BFHI (WHO/Unicef Baby Friendly Hospital Initiative) training for staff. Women in control group clusters: existing staff at the hospitals will be encouraged to set up their own systems to continue counselling during the antenatal period, at delivery and during immunisation visits. Women in intervention group clusters will receive, in addition to counselling in the hospitals during the scheduled antenatal visits, personalised lactation consultation and support via cell phone (handsets provided). Cell phone counselling will continue until 24 weeks after the birth. |
| Outcomes | Primary outcome: exclusive breastfeeding at 24 weeks Secondary outcomes: timely initiation of breastfeeding, timely initiation of complimentary feeding, duration of any breastfeeding, infant growth, hospital admissions/mortality for infants and mothers, maternal satisfaction, cost effectiveness. |
| Starting date | August 2010. |
| Contact information | |
| Notes | Clinical Trials.gov accessed 14 December 2011 showed "This study is currently recruiting participants" with the verification date June 2011. |
bf: breastfeeding
LC: lactation consultant
NICU: neonatal intensive care unit
PNC: prenatal care provider
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Stopping breastfeeding (any) before last study assessment up to 6 months Show forest plot | 40 | 14227 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.88, 0.96] |
| Analysis 1.1  Comparison 1 All forms of support versus usual care, Outcome 1 Stopping breastfeeding (any) before last study assessment up to 6 months. | ||||
| 2 Stopping exclusive breastfeeding before last study assessment Show forest plot | 33 | 11961 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.82, 0.91] |
| Analysis 1.2  Comparison 1 All forms of support versus usual care, Outcome 2 Stopping exclusive breastfeeding before last study assessment. | ||||
| 3 Stopping breastfeeding (any) at up to 4‐6 weeks Show forest plot | 25 | 8513 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.81, 0.96] |
| Analysis 1.3  Comparison 1 All forms of support versus usual care, Outcome 3 Stopping breastfeeding (any) at up to 4‐6 weeks. | ||||
| 4 Stopping exclusive breastfeeding at up to 4‐6 weeks Show forest plot | 24 | 7693 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.61, 0.89] |
| Analysis 1.4  Comparison 1 All forms of support versus usual care, Outcome 4 Stopping exclusive breastfeeding at up to 4‐6 weeks. | ||||
| 5 Stopping any breastfeeding at different times Show forest plot | 43 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 All forms of support versus usual care, Outcome 5 Stopping any breastfeeding at different times. | ||||
| 5.1 Before 4 to 6 weeks | 25 | 8513 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.81, 0.96] |
| 5.2 Before 2 months | 8 | 2520 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.71, 0.99] |
| 5.3 Before 3 months | 20 | 6859 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.75, 0.92] |
| 5.4 Before 4 months | 9 | 3780 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.77, 0.96] |
| 5.5 Before 6 months | 15 | 5346 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.79, 0.98] |
| 5.6 Before 9 months | 2 | 688 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.81, 0.99] |
| 5.7 Before 12 months | 3 | 2012 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.84, 1.07] |
| 6 Stopping exclusive breastfeeding at different times Show forest plot | 34 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 All forms of support versus usual care, Outcome 6 Stopping exclusive breastfeeding at different times. | ||||
| 6.1 Before 4 to 6 weeks | 24 | 7693 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.61, 0.89] |
| 6.2 Before 2 months | 7 | 1524 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.46, 0.89] |
| 6.3 Before 3 months | 12 | 2896 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.52, 0.82] |
| 6.4 Before 4 months | 9 | 3400 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.54, 0.88] |
| 6.5 Before 5 months | 1 | 590 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.40, 0.54] |
| 6.6 Before 6 months | 8 | 3149 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.82, 0.97] |
| 7 Duration of breastfeeding (mean number of days) Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 48.00 [‐10.98, 106.98] |
| Analysis 1.7  Comparison 1 All forms of support versus usual care, Outcome 7 Duration of breastfeeding (mean number of days). | ||||
| 8 Sensitivity analysis by risk of bias: stopping any breastfeeding at up to six months Show forest plot | 40 | 14227 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.88, 0.96] |
| Analysis 1.8  Comparison 1 All forms of support versus usual care, Outcome 8 Sensitivity analysis by risk of bias: stopping any breastfeeding at up to six months. | ||||
| 8.1 studies at low risk of bias | 22 | 8795 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.88, 0.98] |
| 8.2 Risk of bias unclear or high | 18 | 5432 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.83, 0.96] |
| 9 Sensitivity analysis by risk of bias: stopping exclusive breastfeeding at up to six months Show forest plot | 33 | 11961 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.82, 0.91] |
| Analysis 1.9  Comparison 1 All forms of support versus usual care, Outcome 9 Sensitivity analysis by risk of bias: stopping exclusive breastfeeding at up to six months. | ||||
| 9.1 Studies at low risk of bias | 19 | 7681 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.90, 0.98] |
| 9.2 Unclear or high risk of bias | 14 | 4280 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.45, 0.89] |
| 10 Sensitivity analysis by risk of bias: stopping any breastfeeding at 4‐6 weeks Show forest plot | 25 | 8513 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.81, 0.96] |
| Analysis 1.10  Comparison 1 All forms of support versus usual care, Outcome 10 Sensitivity analysis by risk of bias: stopping any breastfeeding at 4‐6 weeks. | ||||
| 10.1 Studies at low risk of bias | 13 | 4985 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.86, 1.00] |
| 10.2 Unclear or high risk of bias | 12 | 3528 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.68, 1.03] |
| 11 Sensitivity analysis by risk of bias: stopping exclusive breastfeeding by 4‐6 weeks Show forest plot | 24 | 7693 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.61, 0.89] |
| Analysis 1.11  Comparison 1 All forms of support versus usual care, Outcome 11 Sensitivity analysis by risk of bias: stopping exclusive breastfeeding by 4‐6 weeks. | ||||
| 11.1 Studies at low risk of bias | 14 | 5283 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.75, 0.98] |
| 11.2 Unclear of high risk of bias | 10 | 2410 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.43, 0.80] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Stopping any breastfeeding before last study assessment up to 6 months Show forest plot | 40 | 14227 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.88, 0.96] |
| Analysis 2.1  Comparison 2 All forms of support versus usual care: SUBGROUP ANALYSIS by who delivered the intervention, Outcome 1 Stopping any breastfeeding before last study assessment up to 6 months. | ||||
| 1.1 Professional support | 26 | 9644 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.88, 0.99] |
| 1.2 Lay support | 9 | 3109 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.77, 0.93] |
| 1.3 Both professional and lay support | 5 | 1474 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.91, 1.03] |
| 2 Stopping exclusive breastfeeding before last study assessment Show forest plot | 33 | 11961 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.82, 0.91] |
| Analysis 2.2  Comparison 2 All forms of support versus usual care: SUBGROUP ANALYSIS by who delivered the intervention, Outcome 2 Stopping exclusive breastfeeding before last study assessment. | ||||
| 2.1 Professional support | 18 | 6537 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.88, 0.98] |
| 2.2 Lay support | 12 | 4350 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.64, 0.87] |
| 2.3 Both professional and lay support | 3 | 1074 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.44, 1.32] |
| 3 Stopping any breastfeeding at up to 4‐6 weeks Show forest plot | 26 | 9148 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.78, 0.94] |
| Analysis 2.3  Comparison 2 All forms of support versus usual care: SUBGROUP ANALYSIS by who delivered the intervention, Outcome 3 Stopping any breastfeeding at up to 4‐6 weeks. | ||||
| 3.1 Professional support | 16 | 5685 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.73, 0.95] |
| 3.2 Lay support | 7 | 2541 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.73, 1.06] |
| 3.3 Both professional and lay support | 3 | 922 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.68, 1.11] |
| 4 Stopping exclusive breastfeeding at up to 4‐6 weeks Show forest plot | 24 | 7667 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.61, 0.89] |
| Analysis 2.4  Comparison 2 All forms of support versus usual care: SUBGROUP ANALYSIS by who delivered the intervention, Outcome 4 Stopping exclusive breastfeeding at up to 4‐6 weeks. | ||||
| 4.1 Professional support | 15 | 4408 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.57, 0.99] |
| 4.2 Lay support | 7 | 2114 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.45, 0.92] |
| 4.3 Both professional and lay support | 2 | 1145 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.89, 0.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Stopping any breastfeeding before last study assessment up to 6 months Show forest plot | 40 | 14227 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.88, 0.96] |
| Analysis 3.1  Comparison 3 All forms of support versus usual care: SUBGROUP ANALYSIS by type of support, Outcome 1 Stopping any breastfeeding before last study assessment up to 6 months. | ||||
| 1.1 Predominant telephone support | 3 | 677 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.65, 1.17] |
| 1.2 Predominant face‐to‐face contact | 16 | 7859 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.96] |
| 1.3 Balanced telephone and face‐to‐face support | 21 | 5691 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.87, 0.99] |
| 2 Stopping exclusive breastfeeding by last study assessment up to 6 months Show forest plot | 33 | 11475 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.87, 0.94] |
| Analysis 3.2  Comparison 3 All forms of support versus usual care: SUBGROUP ANALYSIS by type of support, Outcome 2 Stopping exclusive breastfeeding by last study assessment up to 6 months. | ||||
| 2.1 Predominant telephone support | 2 | 419 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.99, 1.01] |
| 2.2 Predominant face‐to‐face contact | 17 | 7113 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.75, 0.88] |
| 2.3 Balanced telephone and face‐to‐face | 14 | 3943 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.94, 1.02] |
| 3 Stopping any breastfeeding by 4‐6 weeks Show forest plot | 24 | 8409 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.81, 0.96] |
| Analysis 3.3  Comparison 3 All forms of support versus usual care: SUBGROUP ANALYSIS by type of support, Outcome 3 Stopping any breastfeeding by 4‐6 weeks. | ||||
| 3.1 Predominant telephone support | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.31, 1.58] |
| 3.2 Predominant face‐to‐face contact | 10 | 4178 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.70, 0.95] |
| 3.3 Balanced telephone and face‐to‐face | 12 | 3632 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.84, 1.06] |
| 4 Stopping exclusive breastfeeding by 4‐6 weeks Show forest plot | 23 | 7044 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.58, 0.90] |
| Analysis 3.4  Comparison 3 All forms of support versus usual care: SUBGROUP ANALYSIS by type of support, Outcome 4 Stopping exclusive breastfeeding by 4‐6 weeks. | ||||
| 4.1 Predominant telephone support | 2 | 419 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.68, 1.35] |
| 4.2 Predominant face‐to‐face contact | 12 | 3889 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.51, 0.77] |
| 4.3 Balanced telephone and face‐to‐face | 9 | 2736 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.88, 1.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Stopping any breastfeeding at last study assessment up to 6 months Show forest plot | 40 | 14227 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.88, 0.96] |
| Analysis 4.1  Comparison 4 All forms of support versus usual care: SUBGROUP ANALYSIS by timing of support, Outcome 1 Stopping any breastfeeding at last study assessment up to 6 months. | ||||
| 1.1 Postnatal support alone | 27 | 9527 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.86, 0.97] |
| 1.2 Antenatal component to support | 13 | 4700 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.86, 0.99] |
| 2 Stopping exclusive breastfeeding by last assessment up to 6 months Show forest plot | 32 | 11371 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.86, 0.94] |
| Analysis 4.2  Comparison 4 All forms of support versus usual care: SUBGROUP ANALYSIS by timing of support, Outcome 2 Stopping exclusive breastfeeding by last assessment up to 6 months. | ||||
| 2.1 Postnatal support alone | 21 | 7113 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.81, 0.94] |
| 2.2 Antenatal component to support | 11 | 4258 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.87, 0.98] |
| 3 Stopping any breastfeeding at 4‐6 weeks Show forest plot | 24 | 8595 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.81, 0.96] |
| Analysis 4.3  Comparison 4 All forms of support versus usual care: SUBGROUP ANALYSIS by timing of support, Outcome 3 Stopping any breastfeeding at 4‐6 weeks. | ||||
| 3.1 Postnatal support alone | 17 | 6062 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.75, 0.95] |
| 3.2 Antenatal component to support | 7 | 2533 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.84, 1.06] |
| 4 Stopping exclusive breastfeeding at up to 4‐6 weeks Show forest plot | 23 | 7044 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.58, 0.90] |
| Analysis 4.4  Comparison 4 All forms of support versus usual care: SUBGROUP ANALYSIS by timing of support, Outcome 4 Stopping exclusive breastfeeding at up to 4‐6 weeks. | ||||
| 4.1 Postnatal support alone | 18 | 5425 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.46, 1.02] |
| 4.2 Antenatal component to support | 5 | 1619 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.77, 1.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Stopping any breastfeeding by last assessment up to 6 months Show forest plot | 39 | 14071 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.88, 0.96] |
| Analysis 5.1  Comparison 5 All forms of support versus usual care: SUBGROUP ANALYSIS by breastfeeding initiation, Outcome 1 Stopping any breastfeeding by last assessment up to 6 months. | ||||
| 1.1 Settings with high breastfeeding initiation rates | 14 | 6549 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.82, 0.98] |
| 1.2 Settings with Intermediate initiation rates | 15 | 5396 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.88, 1.00] |
| 1.3 Settings with low initiation rates | 10 | 2126 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.84, 1.00] |
| 2 Stopping exclusive breastfeeding at last assessment up to 6 months Show forest plot | 31 | 11185 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.86, 0.94] |
| Analysis 5.2  Comparison 5 All forms of support versus usual care: SUBGROUP ANALYSIS by breastfeeding initiation, Outcome 2 Stopping exclusive breastfeeding at last assessment up to 6 months. | ||||
| 2.1 Settings with high breastfeeding initiation rates | 19 | 7606 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.78, 0.89] |
| 2.2 Settings with Intermediate initiation rates | 7 | 2210 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.79, 1.01] |
| 2.3 Settings with low initiation rates | 5 | 1369 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.99, 1.01] |
| 3 Stopping any breastfeeding at up to 4‐6 weeks Show forest plot | 23 | 7804 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.79, 0.96] |
| Analysis 5.3  Comparison 5 All forms of support versus usual care: SUBGROUP ANALYSIS by breastfeeding initiation, Outcome 3 Stopping any breastfeeding at up to 4‐6 weeks. | ||||
| 3.1 Settings with high breastfeeding initiation rates | 5 | 1897 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.55, 0.85] |
| 3.2 Settings with Intermediate initiation rates | 12 | 4369 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.83, 1.05] |
| 3.3 Settings with low initiation rates | 6 | 1538 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.79, 1.00] |
| 4 Stopping exclusive breastfeeding at up to 4‐6 weeks Show forest plot | 22 | 6858 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.56, 0.90] |
| Analysis 5.4  Comparison 5 All forms of support versus usual care: SUBGROUP ANALYSIS by breastfeeding initiation, Outcome 4 Stopping exclusive breastfeeding at up to 4‐6 weeks. | ||||
| 4.1 Settings with high breastfeeding initiation rates | 11 | 3568 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.47, 0.80] |
| 4.2 Settings with Intermediate initiation rates | 6 | 1921 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.68, 0.96] |
| 4.3 Settings with low initiation rates | 5 | 1369 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.86, 1.08] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Stopping any breastfeeding before last study assessment up to 6 months Show forest plot | 40 | 14227 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.88, 0.96] |
| Analysis 6.1  Comparison 6 All forms of support vs usual care: SUBGROUP ANALYSIS by number of postnatal contacts, Outcome 1 Stopping any breastfeeding before last study assessment up to 6 months. | ||||
| 1.1 Unspecified number of contacts | 5 | 2747 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.87, 1.02] |
| 1.2 Less than 4 postnatal contacts | 10 | 3667 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.82, 1.01] |
| 1.3 Between 4 and 8 postnatal contacts | 13 | 3183 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.75, 0.96] |
| 1.4 9 or more postnatal contacts | 12 | 4630 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.88, 1.01] |
| 2 Stopping exclusive breastfeeding before last study assessment Show forest plot | 33 | 11961 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.82, 0.91] |
| Analysis 6.2  Comparison 6 All forms of support vs usual care: SUBGROUP ANALYSIS by number of postnatal contacts, Outcome 2 Stopping exclusive breastfeeding before last study assessment. | ||||
| 2.1 Unspecified number of contacts | 2 | 1102 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.88, 1.11] |
| 2.2 Less than 4 postnatal contacts | 8 | 2550 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.84, 1.01] |
| 2.3 Between 4 and 8 postnatal contacts | 13 | 3943 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.60, 0.84] |
| 2.4 9 or more postnatal contacts | 10 | 4366 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.78, 0.99] |
| 3 Stopping any breastfeeding at up to 4‐6 weeks Show forest plot | 25 | 8513 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.81, 0.96] |
| Analysis 6.3  Comparison 6 All forms of support vs usual care: SUBGROUP ANALYSIS by number of postnatal contacts, Outcome 3 Stopping any breastfeeding at up to 4‐6 weeks. | ||||
| 3.1 Unspecified number of contacts | 4 | 1943 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.83, 1.06] |
| 3.2 Less than 4 postnatal contacts | 10 | 3949 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.70, 0.97] |
| 3.3 Between 4 and 8 postnatal contacts | 5 | 554 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.56, 1.15] |
| 3.4 9 or more postnatal contacts | 6 | 2067 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.77, 1.11] |
| 4 Stopping exclusive breastfeeding at up to 4‐6 weeks Show forest plot | 24 | 7693 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.61, 0.89] |
| Analysis 6.4  Comparison 6 All forms of support vs usual care: SUBGROUP ANALYSIS by number of postnatal contacts, Outcome 4 Stopping exclusive breastfeeding at up to 4‐6 weeks. | ||||
| 4.1 Unspecified number of contacts | 3 | 1284 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.78, 1.06] |
| 4.2 Less than 4 postnatal contacts | 9 | 2906 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.96] |
| 4.3 Between 4 and 8 postnatal contacts | 5 | 556 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.31, 0.87] |
| 4.4 9 or more postnatal contacts | 7 | 2947 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.28, 1.74] |
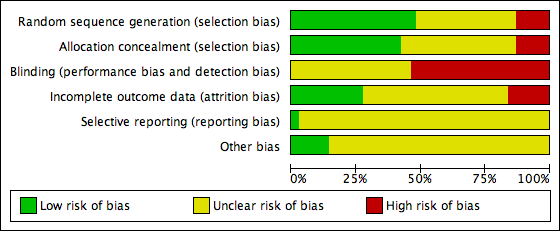
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
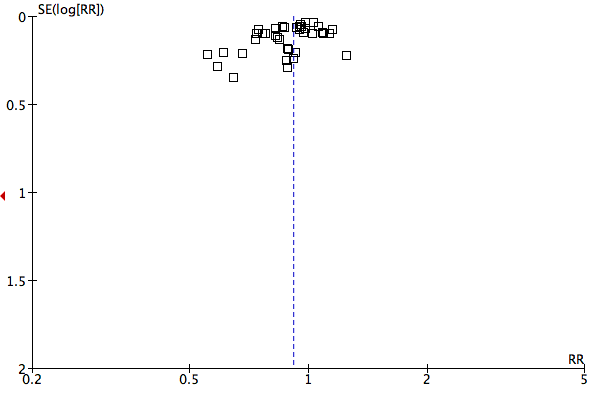
Funnel plot of comparison: 1 All forms of support versus usual care, outcome: 1.1 Stopping breastfeeding (any) before last study assessment up to 6 months.
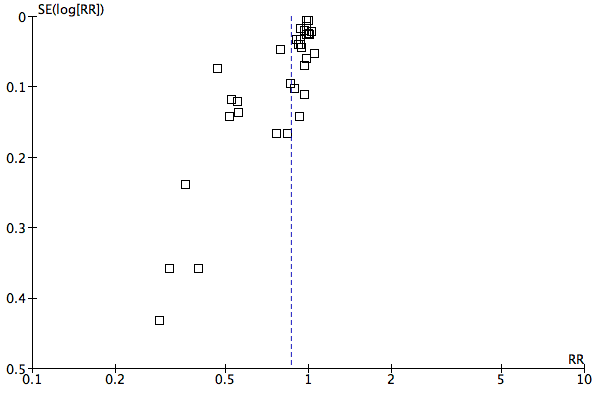
Funnel plot of comparison: 1 All forms of support versus usual care, outcome: 1.2 Stopping exclusive breastfeeding before last study assessment.

Funnel plot of comparison: 1 All forms of support versus usual care, outcome: 1.3 Stopping breastfeeding (any) at up to 4‐6 weeks.
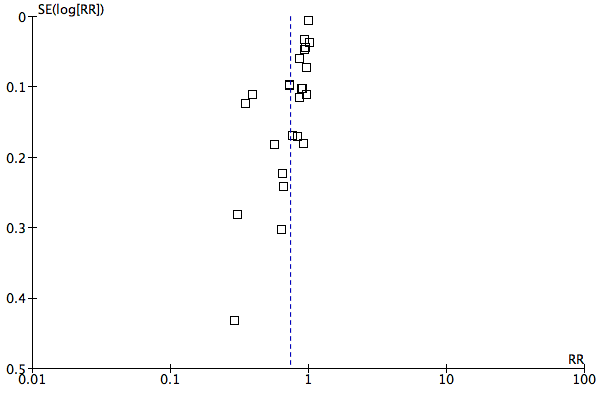
Funnel plot of comparison: 1 All forms of support versus usual care, outcome: 1.4 Stopping exclusive breastfeeding at up to 4‐6 weeks.
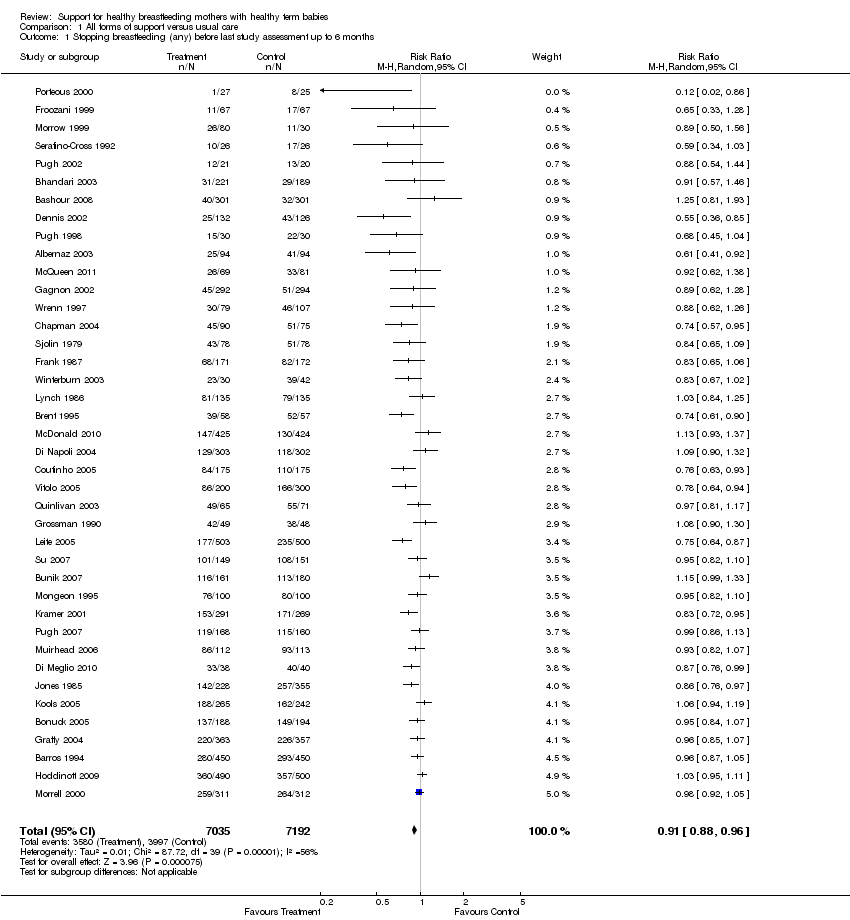
Comparison 1 All forms of support versus usual care, Outcome 1 Stopping breastfeeding (any) before last study assessment up to 6 months.

Comparison 1 All forms of support versus usual care, Outcome 2 Stopping exclusive breastfeeding before last study assessment.
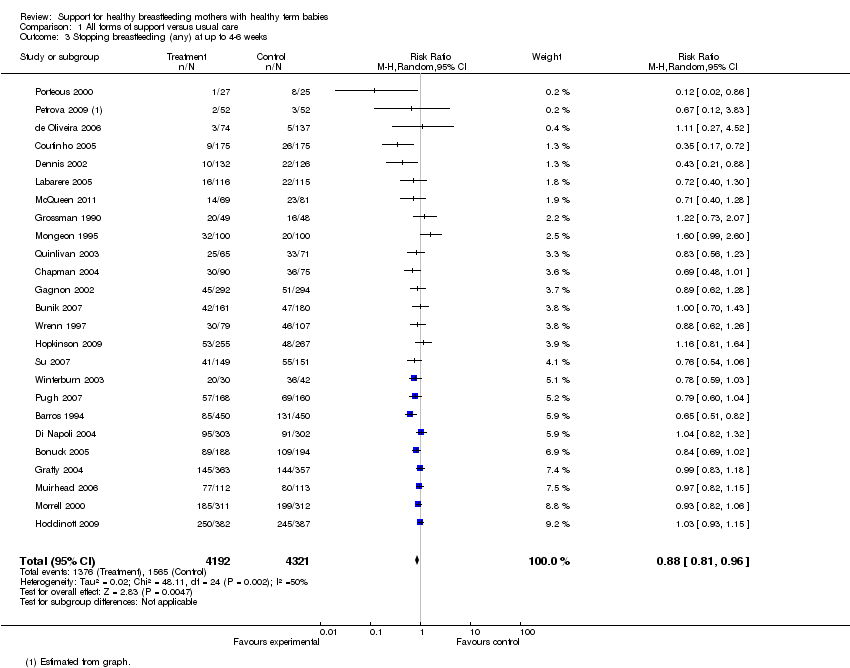
Comparison 1 All forms of support versus usual care, Outcome 3 Stopping breastfeeding (any) at up to 4‐6 weeks.

Comparison 1 All forms of support versus usual care, Outcome 4 Stopping exclusive breastfeeding at up to 4‐6 weeks.

Comparison 1 All forms of support versus usual care, Outcome 5 Stopping any breastfeeding at different times.

Comparison 1 All forms of support versus usual care, Outcome 6 Stopping exclusive breastfeeding at different times.

Comparison 1 All forms of support versus usual care, Outcome 7 Duration of breastfeeding (mean number of days).

Comparison 1 All forms of support versus usual care, Outcome 8 Sensitivity analysis by risk of bias: stopping any breastfeeding at up to six months.

Comparison 1 All forms of support versus usual care, Outcome 9 Sensitivity analysis by risk of bias: stopping exclusive breastfeeding at up to six months.

Comparison 1 All forms of support versus usual care, Outcome 10 Sensitivity analysis by risk of bias: stopping any breastfeeding at 4‐6 weeks.
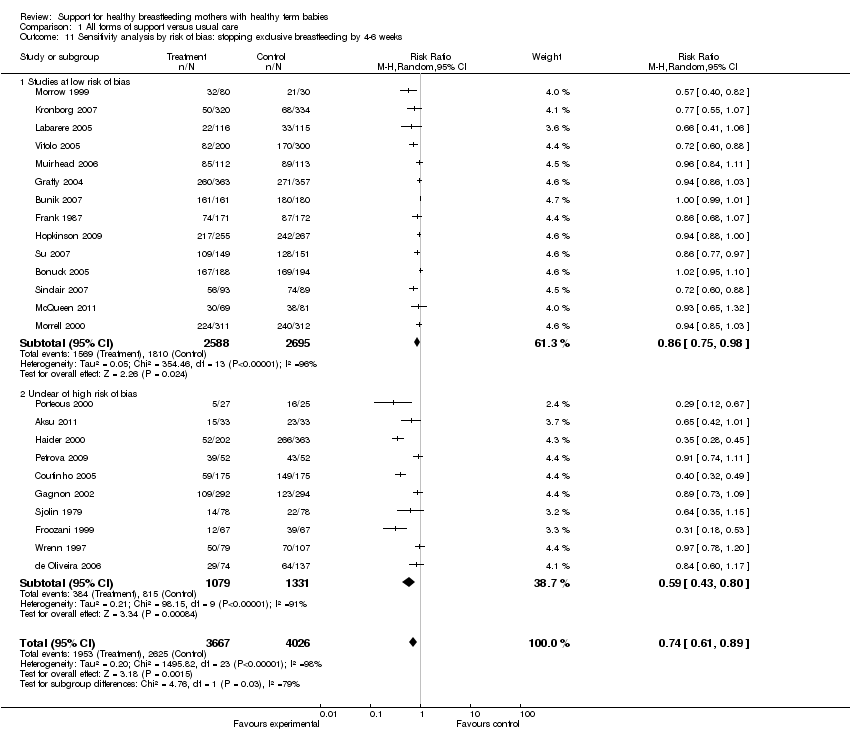
Comparison 1 All forms of support versus usual care, Outcome 11 Sensitivity analysis by risk of bias: stopping exclusive breastfeeding by 4‐6 weeks.
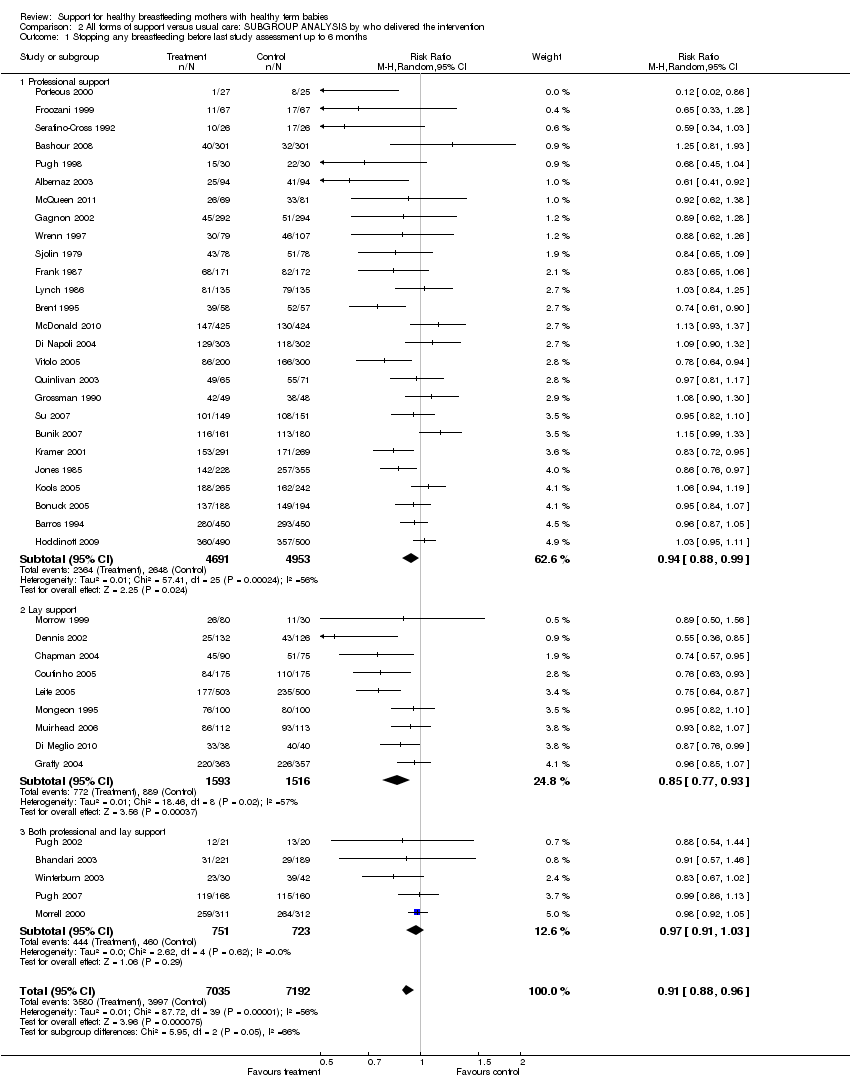
Comparison 2 All forms of support versus usual care: SUBGROUP ANALYSIS by who delivered the intervention, Outcome 1 Stopping any breastfeeding before last study assessment up to 6 months.

Comparison 2 All forms of support versus usual care: SUBGROUP ANALYSIS by who delivered the intervention, Outcome 2 Stopping exclusive breastfeeding before last study assessment.
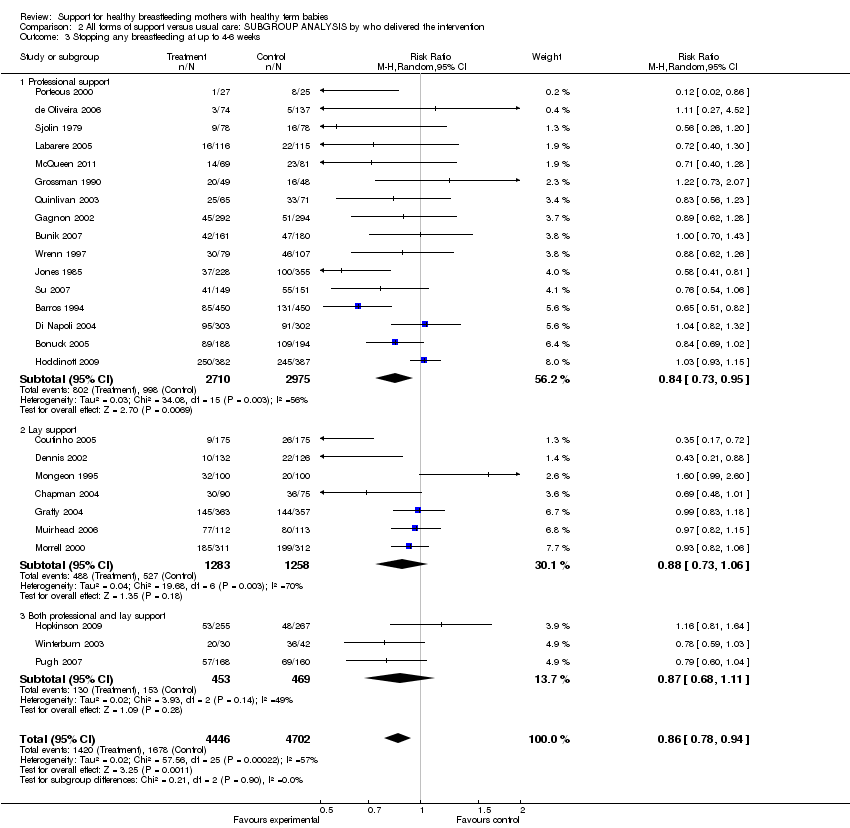
Comparison 2 All forms of support versus usual care: SUBGROUP ANALYSIS by who delivered the intervention, Outcome 3 Stopping any breastfeeding at up to 4‐6 weeks.
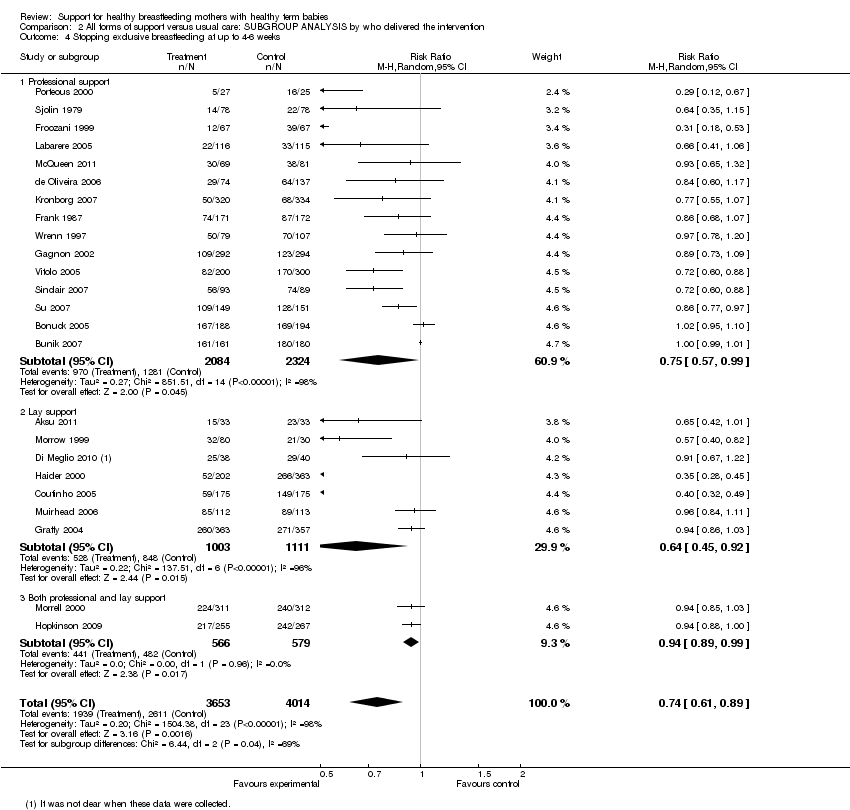
Comparison 2 All forms of support versus usual care: SUBGROUP ANALYSIS by who delivered the intervention, Outcome 4 Stopping exclusive breastfeeding at up to 4‐6 weeks.

Comparison 3 All forms of support versus usual care: SUBGROUP ANALYSIS by type of support, Outcome 1 Stopping any breastfeeding before last study assessment up to 6 months.

Comparison 3 All forms of support versus usual care: SUBGROUP ANALYSIS by type of support, Outcome 2 Stopping exclusive breastfeeding by last study assessment up to 6 months.

Comparison 3 All forms of support versus usual care: SUBGROUP ANALYSIS by type of support, Outcome 3 Stopping any breastfeeding by 4‐6 weeks.

Comparison 3 All forms of support versus usual care: SUBGROUP ANALYSIS by type of support, Outcome 4 Stopping exclusive breastfeeding by 4‐6 weeks.
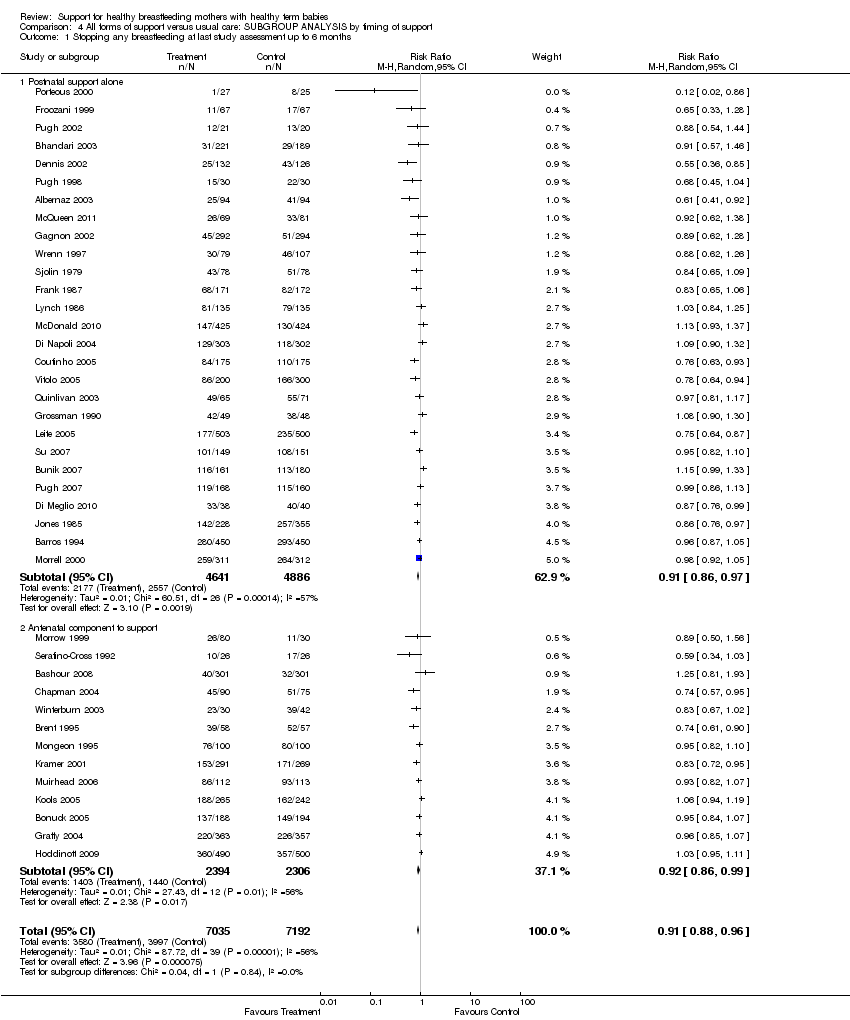
Comparison 4 All forms of support versus usual care: SUBGROUP ANALYSIS by timing of support, Outcome 1 Stopping any breastfeeding at last study assessment up to 6 months.
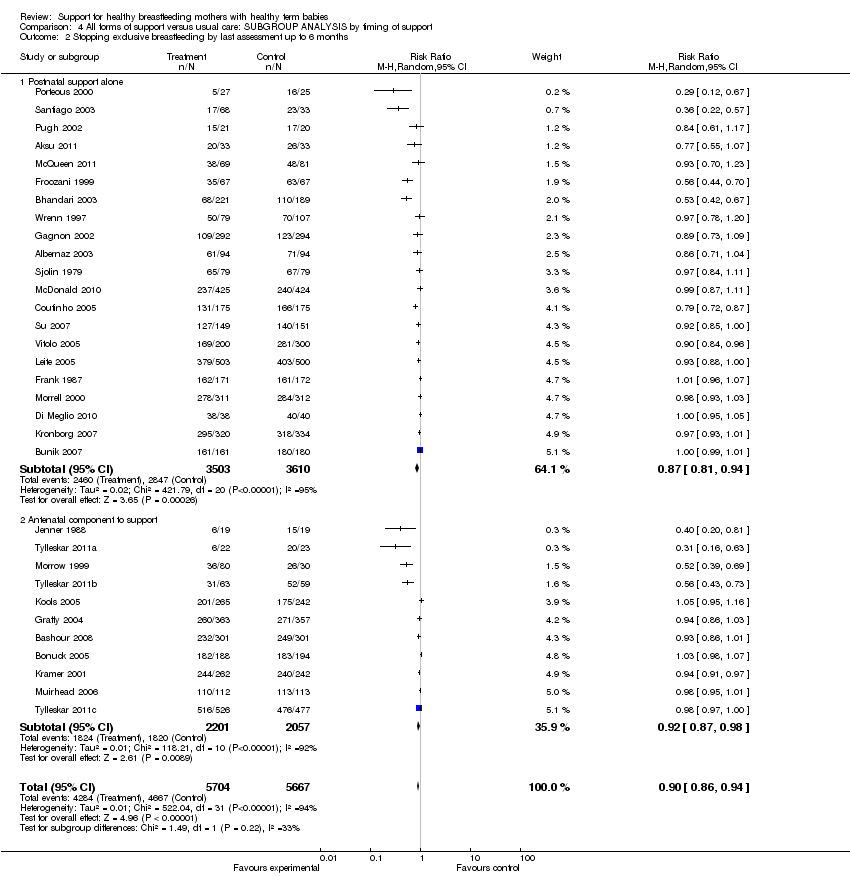
Comparison 4 All forms of support versus usual care: SUBGROUP ANALYSIS by timing of support, Outcome 2 Stopping exclusive breastfeeding by last assessment up to 6 months.

Comparison 4 All forms of support versus usual care: SUBGROUP ANALYSIS by timing of support, Outcome 3 Stopping any breastfeeding at 4‐6 weeks.

Comparison 4 All forms of support versus usual care: SUBGROUP ANALYSIS by timing of support, Outcome 4 Stopping exclusive breastfeeding at up to 4‐6 weeks.

Comparison 5 All forms of support versus usual care: SUBGROUP ANALYSIS by breastfeeding initiation, Outcome 1 Stopping any breastfeeding by last assessment up to 6 months.

Comparison 5 All forms of support versus usual care: SUBGROUP ANALYSIS by breastfeeding initiation, Outcome 2 Stopping exclusive breastfeeding at last assessment up to 6 months.

Comparison 5 All forms of support versus usual care: SUBGROUP ANALYSIS by breastfeeding initiation, Outcome 3 Stopping any breastfeeding at up to 4‐6 weeks.
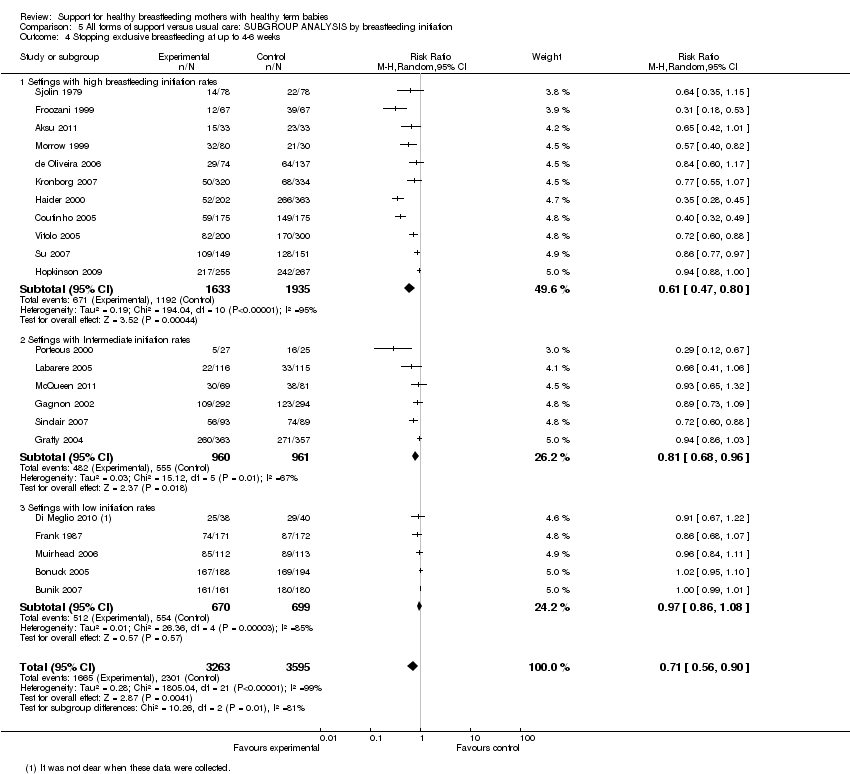
Comparison 5 All forms of support versus usual care: SUBGROUP ANALYSIS by breastfeeding initiation, Outcome 4 Stopping exclusive breastfeeding at up to 4‐6 weeks.
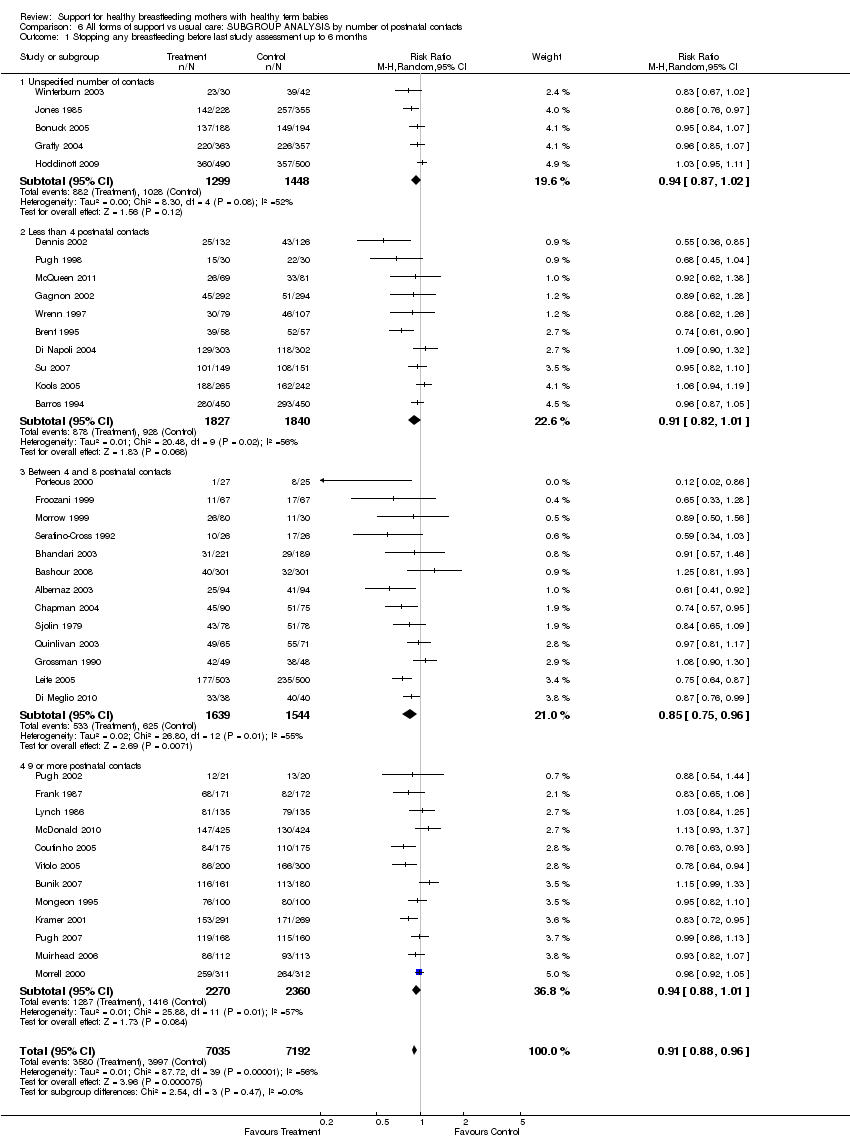
Comparison 6 All forms of support vs usual care: SUBGROUP ANALYSIS by number of postnatal contacts, Outcome 1 Stopping any breastfeeding before last study assessment up to 6 months.
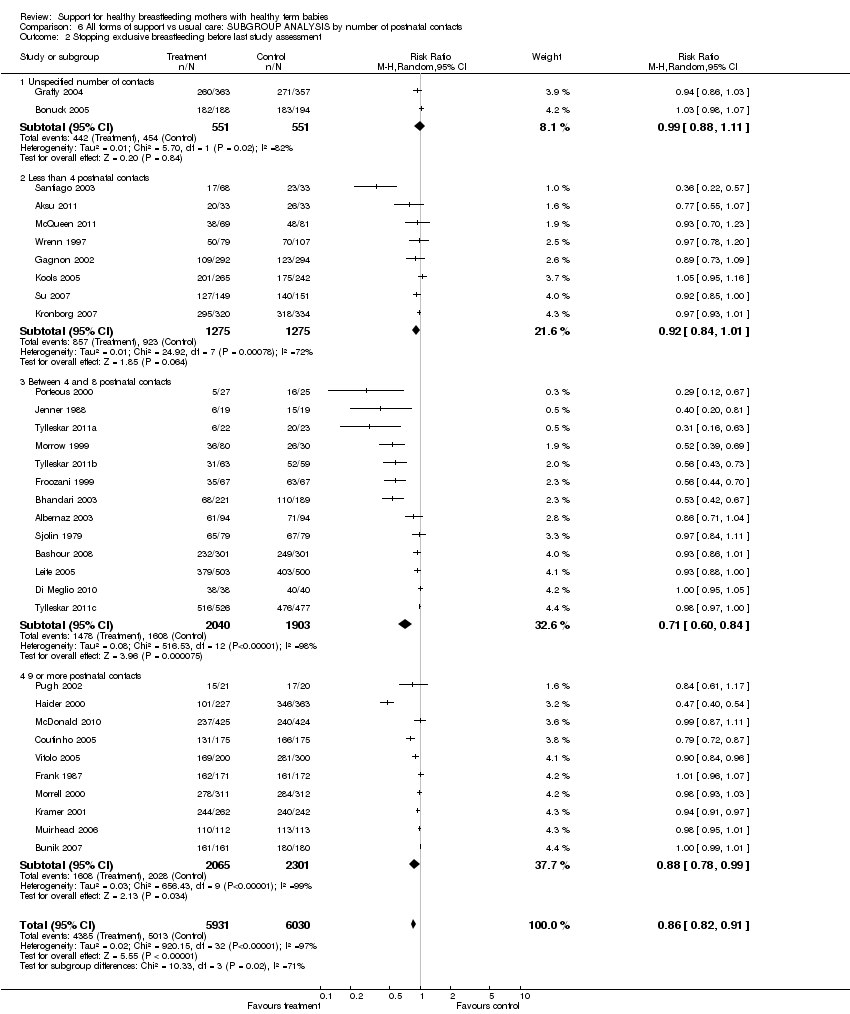
Comparison 6 All forms of support vs usual care: SUBGROUP ANALYSIS by number of postnatal contacts, Outcome 2 Stopping exclusive breastfeeding before last study assessment.

Comparison 6 All forms of support vs usual care: SUBGROUP ANALYSIS by number of postnatal contacts, Outcome 3 Stopping any breastfeeding at up to 4‐6 weeks.

Comparison 6 All forms of support vs usual care: SUBGROUP ANALYSIS by number of postnatal contacts, Outcome 4 Stopping exclusive breastfeeding at up to 4‐6 weeks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Stopping breastfeeding (any) before last study assessment up to 6 months Show forest plot | 40 | 14227 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.88, 0.96] |
| 2 Stopping exclusive breastfeeding before last study assessment Show forest plot | 33 | 11961 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.82, 0.91] |
| 3 Stopping breastfeeding (any) at up to 4‐6 weeks Show forest plot | 25 | 8513 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.81, 0.96] |
| 4 Stopping exclusive breastfeeding at up to 4‐6 weeks Show forest plot | 24 | 7693 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.61, 0.89] |
| 5 Stopping any breastfeeding at different times Show forest plot | 43 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Before 4 to 6 weeks | 25 | 8513 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.81, 0.96] |
| 5.2 Before 2 months | 8 | 2520 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.71, 0.99] |
| 5.3 Before 3 months | 20 | 6859 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.75, 0.92] |
| 5.4 Before 4 months | 9 | 3780 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.77, 0.96] |
| 5.5 Before 6 months | 15 | 5346 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.79, 0.98] |
| 5.6 Before 9 months | 2 | 688 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.81, 0.99] |
| 5.7 Before 12 months | 3 | 2012 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.84, 1.07] |
| 6 Stopping exclusive breastfeeding at different times Show forest plot | 34 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Before 4 to 6 weeks | 24 | 7693 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.61, 0.89] |
| 6.2 Before 2 months | 7 | 1524 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.46, 0.89] |
| 6.3 Before 3 months | 12 | 2896 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.52, 0.82] |
| 6.4 Before 4 months | 9 | 3400 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.54, 0.88] |
| 6.5 Before 5 months | 1 | 590 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.40, 0.54] |
| 6.6 Before 6 months | 8 | 3149 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.82, 0.97] |
| 7 Duration of breastfeeding (mean number of days) Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 48.00 [‐10.98, 106.98] |
| 8 Sensitivity analysis by risk of bias: stopping any breastfeeding at up to six months Show forest plot | 40 | 14227 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.88, 0.96] |
| 8.1 studies at low risk of bias | 22 | 8795 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.88, 0.98] |
| 8.2 Risk of bias unclear or high | 18 | 5432 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.83, 0.96] |
| 9 Sensitivity analysis by risk of bias: stopping exclusive breastfeeding at up to six months Show forest plot | 33 | 11961 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.82, 0.91] |
| 9.1 Studies at low risk of bias | 19 | 7681 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.90, 0.98] |
| 9.2 Unclear or high risk of bias | 14 | 4280 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.45, 0.89] |
| 10 Sensitivity analysis by risk of bias: stopping any breastfeeding at 4‐6 weeks Show forest plot | 25 | 8513 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.81, 0.96] |
| 10.1 Studies at low risk of bias | 13 | 4985 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.86, 1.00] |
| 10.2 Unclear or high risk of bias | 12 | 3528 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.68, 1.03] |
| 11 Sensitivity analysis by risk of bias: stopping exclusive breastfeeding by 4‐6 weeks Show forest plot | 24 | 7693 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.61, 0.89] |
| 11.1 Studies at low risk of bias | 14 | 5283 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.75, 0.98] |
| 11.2 Unclear of high risk of bias | 10 | 2410 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.43, 0.80] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Stopping any breastfeeding before last study assessment up to 6 months Show forest plot | 40 | 14227 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.88, 0.96] |
| 1.1 Professional support | 26 | 9644 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.88, 0.99] |
| 1.2 Lay support | 9 | 3109 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.77, 0.93] |
| 1.3 Both professional and lay support | 5 | 1474 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.91, 1.03] |
| 2 Stopping exclusive breastfeeding before last study assessment Show forest plot | 33 | 11961 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.82, 0.91] |
| 2.1 Professional support | 18 | 6537 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.88, 0.98] |
| 2.2 Lay support | 12 | 4350 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.64, 0.87] |
| 2.3 Both professional and lay support | 3 | 1074 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.44, 1.32] |
| 3 Stopping any breastfeeding at up to 4‐6 weeks Show forest plot | 26 | 9148 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.78, 0.94] |
| 3.1 Professional support | 16 | 5685 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.73, 0.95] |
| 3.2 Lay support | 7 | 2541 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.73, 1.06] |
| 3.3 Both professional and lay support | 3 | 922 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.68, 1.11] |
| 4 Stopping exclusive breastfeeding at up to 4‐6 weeks Show forest plot | 24 | 7667 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.61, 0.89] |
| 4.1 Professional support | 15 | 4408 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.57, 0.99] |
| 4.2 Lay support | 7 | 2114 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.45, 0.92] |
| 4.3 Both professional and lay support | 2 | 1145 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.89, 0.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Stopping any breastfeeding before last study assessment up to 6 months Show forest plot | 40 | 14227 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.88, 0.96] |
| 1.1 Predominant telephone support | 3 | 677 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.65, 1.17] |
| 1.2 Predominant face‐to‐face contact | 16 | 7859 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.96] |
| 1.3 Balanced telephone and face‐to‐face support | 21 | 5691 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.87, 0.99] |
| 2 Stopping exclusive breastfeeding by last study assessment up to 6 months Show forest plot | 33 | 11475 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.87, 0.94] |
| 2.1 Predominant telephone support | 2 | 419 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.99, 1.01] |
| 2.2 Predominant face‐to‐face contact | 17 | 7113 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.75, 0.88] |
| 2.3 Balanced telephone and face‐to‐face | 14 | 3943 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.94, 1.02] |
| 3 Stopping any breastfeeding by 4‐6 weeks Show forest plot | 24 | 8409 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.81, 0.96] |
| 3.1 Predominant telephone support | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.31, 1.58] |
| 3.2 Predominant face‐to‐face contact | 10 | 4178 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.70, 0.95] |
| 3.3 Balanced telephone and face‐to‐face | 12 | 3632 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.84, 1.06] |
| 4 Stopping exclusive breastfeeding by 4‐6 weeks Show forest plot | 23 | 7044 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.58, 0.90] |
| 4.1 Predominant telephone support | 2 | 419 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.68, 1.35] |
| 4.2 Predominant face‐to‐face contact | 12 | 3889 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.51, 0.77] |
| 4.3 Balanced telephone and face‐to‐face | 9 | 2736 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.88, 1.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Stopping any breastfeeding at last study assessment up to 6 months Show forest plot | 40 | 14227 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.88, 0.96] |
| 1.1 Postnatal support alone | 27 | 9527 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.86, 0.97] |
| 1.2 Antenatal component to support | 13 | 4700 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.86, 0.99] |
| 2 Stopping exclusive breastfeeding by last assessment up to 6 months Show forest plot | 32 | 11371 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.86, 0.94] |
| 2.1 Postnatal support alone | 21 | 7113 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.81, 0.94] |
| 2.2 Antenatal component to support | 11 | 4258 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.87, 0.98] |
| 3 Stopping any breastfeeding at 4‐6 weeks Show forest plot | 24 | 8595 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.81, 0.96] |
| 3.1 Postnatal support alone | 17 | 6062 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.75, 0.95] |
| 3.2 Antenatal component to support | 7 | 2533 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.84, 1.06] |
| 4 Stopping exclusive breastfeeding at up to 4‐6 weeks Show forest plot | 23 | 7044 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.58, 0.90] |
| 4.1 Postnatal support alone | 18 | 5425 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.46, 1.02] |
| 4.2 Antenatal component to support | 5 | 1619 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.77, 1.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Stopping any breastfeeding by last assessment up to 6 months Show forest plot | 39 | 14071 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.88, 0.96] |
| 1.1 Settings with high breastfeeding initiation rates | 14 | 6549 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.82, 0.98] |
| 1.2 Settings with Intermediate initiation rates | 15 | 5396 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.88, 1.00] |
| 1.3 Settings with low initiation rates | 10 | 2126 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.84, 1.00] |
| 2 Stopping exclusive breastfeeding at last assessment up to 6 months Show forest plot | 31 | 11185 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.86, 0.94] |
| 2.1 Settings with high breastfeeding initiation rates | 19 | 7606 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.78, 0.89] |
| 2.2 Settings with Intermediate initiation rates | 7 | 2210 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.79, 1.01] |
| 2.3 Settings with low initiation rates | 5 | 1369 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.99, 1.01] |
| 3 Stopping any breastfeeding at up to 4‐6 weeks Show forest plot | 23 | 7804 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.79, 0.96] |
| 3.1 Settings with high breastfeeding initiation rates | 5 | 1897 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.55, 0.85] |
| 3.2 Settings with Intermediate initiation rates | 12 | 4369 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.83, 1.05] |
| 3.3 Settings with low initiation rates | 6 | 1538 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.79, 1.00] |
| 4 Stopping exclusive breastfeeding at up to 4‐6 weeks Show forest plot | 22 | 6858 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.56, 0.90] |
| 4.1 Settings with high breastfeeding initiation rates | 11 | 3568 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.47, 0.80] |
| 4.2 Settings with Intermediate initiation rates | 6 | 1921 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.68, 0.96] |
| 4.3 Settings with low initiation rates | 5 | 1369 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.86, 1.08] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Stopping any breastfeeding before last study assessment up to 6 months Show forest plot | 40 | 14227 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.88, 0.96] |
| 1.1 Unspecified number of contacts | 5 | 2747 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.87, 1.02] |
| 1.2 Less than 4 postnatal contacts | 10 | 3667 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.82, 1.01] |
| 1.3 Between 4 and 8 postnatal contacts | 13 | 3183 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.75, 0.96] |
| 1.4 9 or more postnatal contacts | 12 | 4630 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.88, 1.01] |
| 2 Stopping exclusive breastfeeding before last study assessment Show forest plot | 33 | 11961 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.82, 0.91] |
| 2.1 Unspecified number of contacts | 2 | 1102 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.88, 1.11] |
| 2.2 Less than 4 postnatal contacts | 8 | 2550 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.84, 1.01] |
| 2.3 Between 4 and 8 postnatal contacts | 13 | 3943 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.60, 0.84] |
| 2.4 9 or more postnatal contacts | 10 | 4366 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.78, 0.99] |
| 3 Stopping any breastfeeding at up to 4‐6 weeks Show forest plot | 25 | 8513 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.81, 0.96] |
| 3.1 Unspecified number of contacts | 4 | 1943 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.83, 1.06] |
| 3.2 Less than 4 postnatal contacts | 10 | 3949 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.70, 0.97] |
| 3.3 Between 4 and 8 postnatal contacts | 5 | 554 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.56, 1.15] |
| 3.4 9 or more postnatal contacts | 6 | 2067 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.77, 1.11] |
| 4 Stopping exclusive breastfeeding at up to 4‐6 weeks Show forest plot | 24 | 7693 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.61, 0.89] |
| 4.1 Unspecified number of contacts | 3 | 1284 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.78, 1.06] |
| 4.2 Less than 4 postnatal contacts | 9 | 2906 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.96] |
| 4.3 Between 4 and 8 postnatal contacts | 5 | 556 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.31, 0.87] |
| 4.4 9 or more postnatal contacts | 7 | 2947 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.28, 1.74] |

