無排卵性多嚢胞性卵巣症候群の女性における排卵誘発のための腹腔鏡下卵巣開孔術
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised trial conducted in Eygpt Timing: July 2007 to February 2010 | |
| Participants | 156 women assessed for eligibility in fertility clinics and 147 randomised Mean age of women in the letrozole group was 23.9 ± 3.2 years and in the LOD group was 23.6 ± 3.2 years Inclusion: Women with clomiphene‐resistant PCOS, primary or secondary infertility because of anovulation and clomiphene resistance for at least 1 year, normal sperm analysis from partner, patent tubes as seen by hysterosalpingography or diagnostic laparoscopy Exclusion: Age < 20 or > 35 years, hormonal treatment within 3 months prior to study, hyperprolactinaemia, any other endocrine, hepatic or renal disorder, presence of an organic pelvic mass, history of abdominal surgery that might have caused pelvic factor infertility | |
| Interventions | Letrozole 5 mg/day for 5 days starting on day 3 of menses for a maximum of 6 cycles (n = 74), versus LOD ‐ each ovary was punctured 4 to 6 times depending on the size of the ovary (n = 73) Follow‐up for 6 months | |
| Outcomes | Endometrial thickness, biochemical pregnancy, clinical pregnancy, spontaneous abortion, ovulation rate | |
| Notes | No conflict of interest Clinical trial registration number: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated random numbers table" |
| Allocation concealment (selection bias) | Low risk | Quote: "achieved using serially numbered opaque envelopes that were only opened once the interventions were assigned" |
| Blinding of participants and personnel (performance bias) | High risk | There are no details of blinding in the paper. Blinding was unlikely to have occurred as the interventions were oral medication versus surgery. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There are no details of outcome assessors being blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | 147 randomised; 4 in the letrozole group and 3 in the LOD dropped out of the trial, all for non‐compliance. Intention‐to‐treat analysis was not conducted |
| Selective reporting (reporting bias) | High risk | We could not retrieve the original protocol. Live birth rate was reported in the Results section and was not listed as an outcome in the Methods section of the paper. Adverse effects on the mother and congenital malformations were also addressed in the Discussion section of the paper but had not been reported in the results section |
| Other bias | Low risk | No evidence of other risk of bias |
| Methods | Randomised controlled trial conducted in UK Timing: not stated. | |
| Participants | 21 women randomised (this may be a typographical error in the abstract). Mean age 27 and 28 years; mean duration of infertility was 5.0 versus 4.8 years and the mean BMI was 19 versus 17 kg/m2 Included: women with clomiphene‐resistant PCOS (150 mg clomiphene) with chronic anovulation, and 5 were resistant to FSH ovulation induction | |
| Interventions | Bilateral ovarian surgery by diathermy (n = 10), versus 12 months follow‐up | |
| Outcomes | Pregnancy rate (by participant) | |
| Notes | Conflict of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "allocated randomly"; no other details in conference abstract |
| Allocation concealment (selection bias) | Unclear risk | No details in conference abstract. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No evidence of blinding of researchers, participants |
| Blinding of outcome assessment (detection bias) | Unclear risk | No evidence of blinding of outcome assessors |
| Incomplete outcome data (attrition bias) | Low risk | All participants appear to have been followed through the study and all those randomised were analysed |
| Selective reporting (reporting bias) | High risk | No live birth data |
| Other bias | High risk | Conference abstract only |
| Methods | Randomised trial conducted in UK fertility clinic Timing: March 2002 to March 2006 | |
| Participants | 72 anovulatory women with PCOS. Mean age of women in LOD group 28.1 ± 4.3 years and in CC group 29.1 ± 4.8 years Inclusion: Women with anovulatory infertility with PCOS. Aged 18 to 39 years, BMI ≤ 32 kg/m2, duration of infertility ≥ 1 year. At least 1 patent fallopian tube on hysterosalpingogram and normal semen analysis Exclusion: Inability to give informed consent, contra‐indication to clomiphene citrate or general anaesthetic. Any ovarian induction therapy in previous 6 months | |
| Interventions | Laparoscopic ovarian diathermy: 4 punctures per ovary in both ovaries. CC was also given if there was no ovulation 6 ‐ 8 weeks after surgery (n = 36), versus CC daily dose increasing from 50 mg to 150 mg on days 2 to 6 of a menstrual period or after a progestogen withdrawal bleed using medroxyprogesterone acetate. Treatment for 6 cycles and then offered LOD (n = 36) | |
| Outcomes | Ovulation, pregnancy (biochemical, cumulative), multiple pregnancies, live birth rate | |
| Notes | Conflict of interest: not stated Supported by a grant from the University of Sheffield Clinical trial registration number: NCT00220545 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...block randomisation method using a random number table .." |
| Allocation concealment (selection bias) | Low risk | Quote: "held centrally by a trial administrator" Comment: Appears to be central allocation |
| Blinding of participants and personnel (performance bias) | High risk | There was no blinding; once randomised the allocation was revealed to the investigator and the participant |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | LOD: 3 conceived before LOD, 1 discontinued and 1 postponed. 33 /36 were analysed CC: 3 conceived before CC and 1 postponed treatment. 32 were analysed |
| Selective reporting (reporting bias) | Low risk | We found the registered protocol on ClinicalTrials.gov (NCT00220545). All the outcomes mentioned in the protocol were presented in the published report |
| Other bias | Low risk | No evidence of other risk of bias |
| Methods | Prospective randomised trial conducted in Iran from March 2006 to February 2008 | |
| Participants | 126 women attending a fertility clinic aged 15 to 45 years with a history of infertility for at least 1 year and 3 treatment cycles of clomiphene citrate with no response. Mean age of women in LOD group was 26.54 ± 4.72 years and in the metformin group was 25.13 ± 3.47 years Inclusion: Irregular menstruation, clinical and biochemical signs of hyperandrogenism, polycystic ovaries Exclusion: Diseases that would disturb clinical and hormonal responses, pregnancy during follow‐up, BMI > 30 or < 17 | |
| Interventions | LOD performed 4 times in each ovary (n = 63), versus Metformin 1500 g daily (n = 63) Follow‐up for 6 months | |
| Outcomes | Menstrual regularity, hormonal levels, Ferriman‐Gallwey score | |
| Notes | No conflict of interest We have contacted authors for obstetric outcomes Clinical trial registration number: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details in paper |
| Allocation concealment (selection bias) | Low risk | Quote: "serially numbered opaque envelopes" |
| Blinding of participants and personnel (performance bias) | High risk | There was no evidence that participants or researchers were blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | All participants appear to have been followed through the study and all those randomised were analysed |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. All outcomes mentioned in the Method section are presented in the Results. There are no reproductive outcomes. Authors have been contacted. |
| Other bias | Low risk | No evidence of other risk of bias |
| Methods | Prospective randomised controlled trial conducted in UK (Middlesex Hospital, London) Timing: not stated | |
| Participants | 10 women randomised. Refractory PCO. Mean age (range) of the women was 29.5 (27 to 33) years and mean duration (range) of infertility was 5.6 years (4 to 8). Infertility work‐up consisted of tubal patency testing by laparoscopy, semen analysis, endocrinology. In one case the tubes were blocked, 2 had pelvic adhesions, 3 had severe oligospermia or azoospermia and underwent donor insemination. Mean BMI 23 kg/m2 | |
| Interventions | Bilateral ovarian surgery by diathermy (N=6), versus Follow‐up for 3 months | |
| Outcomes | Pregnancy rate (by participant) | |
| Notes | Conflict of interest: not stated Definitions: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details in paper |
| Allocation concealment (selection bias) | Unclear risk | No details in paper |
| Blinding of participants and personnel (performance bias) | High risk | No evidence of blinding of researchers or participants |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | Data reported from all 10 women |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. The outcomes mentioned in the Method section are presented in the Results section of the abstract. No live birth |
| Other bias | Low risk | No evidence of other risk of bias |
| Methods | Parallel randomised controlled trial. Multicentre (n = 25 centres) in The Netherlands Timing: February 1998 to October 2001 | |
| Participants | 168 women randomised Time of randomisation: during diagnostic laparoscopy, after determining eligibility. Mean age 29 years, mean duration of infertility was 2.8 years and the mean BMI was 27 kg/m2. Infertility was primary in 76% of women | |
| Interventions | Laparoscopic electrocautery of the ovaries strategy: each ovary was punctured 5 to 10 times depending on its size. If the woman ovulated in 6 subsequent cycles, no further treatment was given. If ovulatory cycles were not established 8 weeks after surgery or the woman became anovulatory again then clomiphene citrate was given in increasing doses. If the woman still remained anovulatory, rFSH was given in increasing, doses starting at 75 IU daily (n = 83) | |
| Outcomes | Primary: ongoing pregnancy rate within 12 months, defined as a viable pregnancy of at least 12 weeks Followed up to 1 year | |
| Notes | Analyses on an intention‐to‐treat basis No conflict of interest Funding: Serono Benelux provided financial support for rFSH during the first eight months of the study when this drug was not funded by the health services. FvdV was supported by a grant from the Health Insurance Funds Council (OG 97/007), Amstelveen, Netherlands. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation, stratified by centre |
| Allocation concealment (selection bias) | Low risk | Telephone call to central office |
| Blinding of participants and personnel (performance bias) | High risk | There was no evidence of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | All women randomised were analysed in the primary study |
| Selective reporting (reporting bias) | Low risk | The original protocol was supplied by the authors. All the outcomes mentioned in the protocol were presented in the published report |
| Other bias | Low risk | No evidence of other risk of bias |
| Methods | Parallel randomised controlled trial conducted in Womens Health University Hospital, Eygpt Timing: June 2013 to November 2014 | |
| Participants | 88 women randomised. 80 women analysed. Mean age of women in monopolar group was 25 ± 4.7 years and for the bipolar group was 24.8 ± 4.4 years Inclusion criteria: Clomiphene‐resistant PCOS (Rotterdam 2003) Exclusion criteria: Male‐factor infertility, tubal or peritoneal factor infertility and endometriosis. One or both tubes blocked. Pelvic adhesions | |
| Interventions | Monopolar LOD: monopolar needle. 4 seconds with 40 W, 4 punctures to each ovary. Energy for each ovary 640 J (n = 45), versus Bipolar LOD: bipolar needle. 4 seconds with 40 W, 4 punctures to each ovary. Energy for each ovary 640 J (n = 43) Follow‐up for 6 months | |
| Outcomes | Regularity of menstrual cycle, ovulation rate, pregnancy rate | |
| Notes | No conflict of interest No funding Clinical trial registration number: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned" "computerized random table" |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) | Low risk | 45 women allocated to monopolar group; 5 cases lost to follow‐up due to difficulty in travelling and follow‐up by own doctor 43 women allocated to bipolar group; 3 cases lost to follow‐up due to difficulty in travelling and follow‐up by own doctor |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. The outcomes mentioned in the Methods section are presented in the Results section |
| Other bias | Low risk | No evidence of other risk of bias |
| Methods | Parallel arm "randomized clinical study" conducted in Zagazig University Hospital, Egypt Timing: November 2015 to January 2017 | |
| Participants | 100 women randomised (50 per group), 95 women analysed (48 in group 1 and 47 in group 2). Mean age: Group 1: 27.5 ± 4.25; Group 2: 28.03 ± 4.32 Inclusion criteria: Infertile women with clomiphene citrate‐resistant PCOS (150 mg/day for 5 days), aged between 25 and 35 years, infertility duration of ≤ 3 years, BMI < 30 kg/m2 luteinising hormone ≥ 10 IU/ml or LH/FSH ratio ≥ 2, Free androgen index ≥ 4, normal semen analysis in the husband, normal oral glucose tolerance test Exclusion criteria: Hyper‐androgenic disorders such as late onset congenital adrenal hyperplasia, hyperprolactinaemia, thyroid diseases, Cushing's syndrome, androgen‐secreting tumours | |
| Interventions | Unilateral laparoscopic ovarian surgery on the right side, using thermal dose adjusted according to ovarian volume (n = 50), versus Bilateral laparoscopic ovarian surgery using thermal dose adjusted to ovarian volume on both sides (n = 50) Follow‐up for 6 months | |
| Outcomes | Menstrual cycle resumption, ovulation rate, cumulative pregnancy rate | |
| Notes | Further information confirming methods requested from authors 2 August 2017 No conflict of interest Clinical trial registration number: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done using a computer |
| Allocation concealment (selection bias) | Unclear risk | No details in the paper |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details in the paper but unlikely to have occurred |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details in the paper |
| Incomplete outcome data (attrition bias) | Low risk | Unilateral LOD group: 2 participants excluded; 1 had a tubal disease which was identified during laparoscopy and 1 missed the follow‐up Bilateral LOD group: 3 participants excluded; 1 was excluded due to endometriosis which was diagnosed during laparoscopy, and 2 participants missed follow‐up |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. The outcomes mentioned in the Methods section are presented in the Results section |
| Other bias | Low risk | No evidence of other risk of bias |
| Methods | Parallel randomised controlled trial conducted in Zagazig University Hospital infertility clinic, Egypt Timing: not stated | |
| Participants | 146 women randomised. Mean age of women in LOD group 25.1 ± 2.1 years; mean age for metformin + letrozole group 24.7 ± 1.8 years Inclusion criteria: Women with PCOS (Rotterdam 2003 criteria) and clomiphene resistance (failure to achieve adequate follicular maturation after 3 consecutive induction cycles with clomiphene citrate 150 mg/day for 5 days) Exclusion criteria: Women with other causes of infertility, endocrine disorders, women who had received hormonal treatment or ovulation induction drugs in the previous 3 months | |
| Interventions | Bilateral LOD: 4 punctures to ovary then the ovary cooled by irrigating with normal saline and 500 ml of this solution was left in the pelvis at the end of the procedure (n = 73), versus Metformin + letrozole: Metformin started from the first day with a dose of 850 mg/day and increased after 1 week up to 1700 mg/day. Letrozole 5 mg was added for 5 days from day 3 of spontaneous or induced bleeding. Metformin was stopped only when pregnancy was documented (n = 73) Follow‐up for 6 months | |
| Outcomes | Serum LH and FSH, fasting glucose concentration, testosterone concentration, menstrual calender, ovulation, biochemical pregnancy, clinical pregnancy, spontaneous abortion | |
| Notes | No evidence of sample size calculation No conflict of interest Clinical trial registration number: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated random numeric table" |
| Allocation concealment (selection bias) | Low risk | Quote: "The random allocation sequence was concealed in sealed dark envelopes..." |
| Blinding of participants and personnel (performance bias) | High risk | No evidence of blinding. Blinding unlikely as 1 intervention is a surgical procedure, versus oral medication. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details of blinding of outcome assessors |
| Incomplete outcome data (attrition bias) | Low risk | All women randomised appear to be analysed |
| Selective reporting (reporting bias) | Unclear risk | We found the registered protocol on ClinicalTrials.gov (NCT01693289), but it was first posted retrospective. All the outcomes mentioned in the protocol were presented in the published report |
| Other bias | Low risk | Baseline data of groups appeared balanced |
| Methods | Randomised trial conducted in Fertility Plus, National Women's Hospital, New Zealand Timing: mid 1996 to late 1999 | |
| Participants | 50 women randomised,3 cycles/participant, mean age 30 years, mean BMI 28 kg/m2, mean length of infertility: 36 months in the LOD group and 29 months in the gonadotrophin group Included: women aged 20 to 38 years with clomiphene‐resistant PCOS (150 mg clomiphene for 5 days), BMI < 32 (for European women) and < 34 (for Polynesian women) | |
| Interventions | Bilateral ovarian drilling by diathermy, versus Follow‐up for 6 months | |
| Outcomes | Pregnancy rate 6 months after drilling or after 3 cycles of gonadotrophins (per participant), live birth, ovulation rate (per participant), costs | |
| Notes | Analyses on an intention‐to‐treat basis. Conflict of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated sequences.." |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed numbered opaque envelopes" |
| Blinding of participants and personnel (performance bias) | High risk | There was no evidence that researchers or participants were blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details of blinding of outcome assessors |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. All outcomes listed in Methods were reported in the Results |
| Other bias | Low risk | No evidence of other risk of bias |
| Methods | Parallel randomised controlled trial conducted in France Timing: June 2009 to June 2012 | |
| Participants | 40 of 252 women randomised, as trial stopped early. Mean age in LOD group was 28 ± 3 years and in the metformin + FSH group was 27 ± 3 years Inclusion criteria: Clomiphene‐resistant, polycystic ovaries Exclusion criteria: Other causes of infertility including tubal factors, male factor, > 36 years of age, thyroid dysfunction | |
| Interventions | LOD: Bipolar needle, 10 punctures at 100 to 130 W 8 mm depth and 2 mm diameter. (n = 19), versus Recombinant FSH plus metformin: 3 months treatment by metformin (start dose 500 g up to a max 1500 g a day) followed by 3 hyperstimulation by FSH + insemination Follow‐up for 6 months | |
| Outcomes | Pregnancy, BMI, hormone levels, follicle count, changing strategy during the study follow‐up | |
| Notes | No conflict of interest Clinical trial registration number: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Central randomisation through a website |
| Allocation concealment (selection bias) | Unclear risk | Centralised randomisation |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Unclear risk | Planned to recruit 126 women but only recruited 40 before trial stopped. 4 women failed to consent and 2 women were lost to follow‐up. Not stated which group they were allocated to. States that all women were analysed regardless of the group they were randomised to |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. All outcomes prespecified in the paper appear to have been reported |
| Other bias | Unclear risk | Trial stopped early due to "difficulty in the inclusion criteria with absence of final agreement by team included". |
| Methods | Randomised trial conducted in Iran Timing: not stated | |
| Participants | 100 infertile, clomiphene‐resistant women with PCOS | |
| Interventions | Gonadotrophin (n = 50), versus Laparoscopic ovarian electrocautery (n = 50) Follow‐up for 4 months | |
| Outcomes | Pregnancy, live birth | |
| Notes | Conflict of interest: not stated Clinical trial registration number: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised". Awaiting further details in translation but numbers are equal in both groups so probably satisfactory |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | All women accounted for at trial end and intention‐to‐treat data reported |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol |
| Other bias | High risk | Only abstract available |
| Methods | Parallel randomised controlled trial, conducted in Department of Obstetrics and Gynaecology, University of Naples, Italy Timing: December 2009 to July 2015 | |
| Participants | 246 women randomised, 201 analysed. Mean age of women in LOD group was 30.1 ± 7.5 years and in the THL group was 27.5 ± 6.8 years Inclusion criteria: Age 18 to 40 years, PCOS (Rotterdam 2003 criteria), clomiphene resistant Exclusion criteria: endocrine anomalies other than PCOS, any disease potentially responsible for ovarian adhesions, previous abdominal or pelvic surgery, presence of adhesions, fixed retroverted uterus, lateral displacement of the cervix, suspected pelvic tumour, vaginal infection, abnormalities at vaginal examination and transvaginal ultrasound, psychiatric disorder preventing ability to participate, obliteration of the Pouch of Douglas or inability to perform vaginal examination or any other contraindication to THL or laparoscopy | |
| Interventions | Laparoscopic ovarian drilling: Unipolar needle electrode with a power setting of 40 W for 4 to 5 seconds set at 30 W per ovary. 3 ‐ 6 punctures per ovary (n = 123), versus Transvaginal hydrolaparoscopy ovarian drilling: Bipolar electrosurgical probe and 3 ‐ 6 points per ovary drilled at a power setting of 110 ‐ 130 W (n = 123) . At 6 months, all women offered follow‐up with THL and asked to monitor menstrual cycles for next 12 months for spontaneous pregnancy | |
| Outcomes | Presence and type of adhesions, peri‐ and post‐operative complications, cumulative pregnancy rate, multiple pregnancy rate | |
| Notes | Only overall cumulative pregnancy rate reported in the paper. We contacted the authors 25 October 2016 for additional data on pregnancy rate by group No conflict of interest Clinical trial registration number: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated" |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation sequence was concealed from the researchers' 'sequentially numbered opaque, sealed and stapled envelope" |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of surgeons or participants |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors of participants were blinded to allocation |
| Incomplete outcome data (attrition bias) | High risk | 246 women were randomised. 19 women in the LOD group refused follow‐up with THL and therefore follow‐up was completed on 104 women. 26 women in the THL group refused follow‐up with THL and therefore follow‐up was completed on 97 women. Unclear if cumulative pregnancy rate is for all 246 women or only for those who had follow‐up with THL |
| Selective reporting (reporting bias) | High risk | We could not retrieve the original protocol. Data for cumulative pregnancy are given as an overall value and not by group. Pregnancy rate and multiple pregnancy rate are not prespecified as outcomes in the Methods |
| Other bias | Low risk | Groups were balanced at baseline |
| Methods | Randomised trial conducted in Turkey at the University of Hecettepi, Ankara, Turkey. | |
| Participants | 40 women randomised, clomiphene‐resistant PCOS patients (see definitions). Mean age (range) of the participants was 25.2 years (21 to 31) and mean duration of infertility was 4.4 years. 33 participants had primary and 7 had secondary infertility. Infertility work‐up consisted of semen analysis (normal in 36 participants and mildly oligo/asthenospermia in 4) and normal HSG. All women were anovulatory There were no clear inclusion or exclusion criteria specified | |
| Interventions | 2nd look laparoscopic adhesiolysis following ovarian laser drilling, versus | |
| Outcomes | Pregnancy rate (by participant), ovulation rate (by participant), miscarriage rate (by pregnancy), multiple pregnancy rate (by pregnancy) Follow‐up for 6 months | |
| Notes | Conflict of interest: not stated Definitions: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | No details in paper |
| Blinding of participants and personnel (performance bias) | High risk | No details in paper but blinding unlikely to have occurred |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | 40 women randomised, 1 refused second‐look laparoscopy |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. A priori outcomes in Methods section of paper were reported in Results section |
| Other bias | Low risk | No evidence of other risk of bias |
| Methods | Randomised trial conducted in Egypt Timing: May 2007 to September 2008 | |
| Participants | 110 participants. The mean age of the women in the metformin group was 23.6 ± 2.6 years and in the LOD group was 24.3 ± 4.5 years Inclusion: Women with diagnosis of PCOS attending infertility clinic. Clomiphene resistance. Age 20 to 35 years. Patent fallopian tubes shown by hysterosalpingography, insulin resistance, normal semen analysis Exclusion: women < 20 years and > 35 years, received gonadotrophins or hormonal contraception in previous 3 months, having hyperprolactinaemia, or other endocrine, hepatic, or renal disorders, having organic pelvic mass, or previous abdominal surgery suggesting pelvic factor infertility | |
| Interventions | 850 mg metformin orally twice daily (n = 55), versus LOD using 4 to 8 punctures (n = 55) Follow‐up for 6 cycles/30 weeks | |
| Outcomes | BMI, ovulation, pregnancy (biochemical, clinical), miscarriage, resuming regular cycles, glucose/insulin ratio | |
| Notes | No conflict of interest Clinical trial registration number: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "..computer generated random numbers tables" Comment: Satisfactory method. |
| Allocation concealment (selection bias) | Low risk | Quote: '..using serially numbered opaque envelopes" Comment: Satisfactory method |
| Blinding of participants and personnel (performance bias) | Unclear risk | There were no details in the paper on blinding, but blinding unlikely due to differences in interventions |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | There were 55 women allocated to each group and there were no losses to follow‐up or discontinuation of medication. All women were analysed |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. Report on adverse effects of treatment that were not prespecified as outcomes in the Methods section of the paper |
| Other bias | Low risk | No evidence of other risk of bias |
| Methods | Randomised controlled trial conducted in Minia University Hospital, El‐Minia, Egypt Timing: August 2015 to March 2016 | |
| Participants | 80 women randomised and analysed (40 per group); Mean age: Group A: 28.8 ± 3.13; Group B: 29.7 ± 3.65 Inclusion criteria: Between 20 and 35 years of age, diagnosis of PCOS based on the Revised 2003 Consensus Diagnostic Criteria for PCOS (must meet 2 of the 3 following criteria: ultrasound diagnosis of polycystic ovaries, oligo‐ or anovulation clinically diagnosed as oligo‐ or amenorrhoea, and clinical and biochemical hyperandrogenism), normal hysterosalpingogram, partner has normal semen analysis Exclusion criteria: Age < 20 or > 35 years, non‐PCOS, hyperprolactinaemia, hypo‐ and hyperthyroidism, diabetes, Cushing's syndrome, current or previous (within last 6 months) non‐classical congenital adrenal hyperplasia, use of oral contraceptives, glucocorticoids, antiandrogens, antidiabetic or anti‐obesity drugs or any other hormonal drugs, any neoplastic, metabolic, hepatic or cardiovascular disorder or other concurrent medical illness, pelvic diseases, previous pelvic surgery, suspected peritoneal factor infertility, tubal infertility, male‐factor infertility | |
| Interventions | Laparoscopic ovarian drilling (n = 40), versus Letrozole 2.5 mg orally twice daily for 5 days from the 3rd day of menses, repeated for up to 6 cycles if ovulation failed (n = 40) Follow‐up for 6 months | |
| Outcomes | Ovulation rate, pregnancy rate | |
| Notes | No conflict of interest No funding Clinical trial registration number: not stated We requested further information on methods from the authors on 03 August 2017 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved by the use of a randomisation number allocated prior to dosing |
| Allocation concealment (selection bias) | Low risk | Randomisation schedule was produced by an interactive voice response system vendor |
| Blinding of participants and personnel (performance bias) | High risk | Once the participants had been allocated to 1 of the 2 groups, the treatment was revealed to the investigator |
| Blinding of outcome assessment (detection bias) | Low risk | The doctor responsible for performing the transvaginal ultrasound follow‐up assessment was blinded to the treatment groups |
| Incomplete outcome data (attrition bias) | Low risk | In the Results there was no loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. Outcomes in the Method section were reported in the Results section |
| Other bias | Low risk | No evidence of other risk of bias |
| Methods | Parallel randomised controlled trial conducted in University Hospital, Jeddah, Saudi Arabia Timing: 1995 to 1998 | |
| Participants | 35 women randomised Inclusion criteria: not clearly specified but included women with refractory anovulatory infertility with polycystic ovaries with unsuccessful medical treatment Exclusion criteria: no details | |
| Interventions | Unilateral laparoscopic ovarian drilling of 5 points in each ovary for 5 seconds, versus Bilateral laparoscopic ovarian drilling of 5 points in each ovary for 5 seconds Follow‐up for 3 months | |
| Outcomes | Ovulaton rate and endocrine changes (no details) | |
| Notes | Conference abstract only Conflict of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) | High risk | States that 35 women randomised but no details on the numbers in each group, no details for any losses. Conference abstract only |
| Selective reporting (reporting bias) | High risk | Conference abstract only including no data that could be included in an analysis |
| Other bias | High risk | Conference abstract only. Unable to judge if groups were balanced at baseline |
| Methods | Randomised prospective trial conducted in Turkey Timing: January 2000 to January 2004 | |
| Participants | Clomiphene‐resistant PCOS participants (see definitions). Mean age of LOMNT group was 26.3 ± 4.3 years and for gonadotrophin group 25.6 ± 4.08 years. All women had anovulatory infertility for > 1 year Exclusions: History of abdominopelvic surgery, systemic disease, proven or suspected pelvic inflammatory disease or ectopic pregnancy | |
| Interventions | Bilateral ovarian drilling by diathermy (n = 17), versus Laparoscopic ovarian drilling was performed with a specially‐designed instrument which was then applied across the ovary and then squeezed Follow‐up for 6 months | |
| Outcomes | Pregnancy rate by participant, multiple pregnancy rate and ovarian hyperstimulation rate, costs by treatment 8/17 who underwent ovarian drilling had second‐look laparoscopy for adhesion formation All women followed up for 6 months | |
| Notes | Conflict of interest: not stated Definitions: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated random sequence" |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed opaque envelopes" |
| Blinding of participants and personnel (performance bias) | High risk | No details of blinding, which is unlikely to have occurred |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) | Low risk | 1 woman in the LOD group and 2 women in the gonadotrophin group were lost to follow‐up, but their data were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. A priori outcomes stated in the Methods section of the paper were reported in the Results section |
| Other bias | Low risk | No evidence of other risk of bias |
| Methods | Randomised trial, cross‐over design, data available prior to cross‐over. Study conducted in Institute for Obstetrics and Gynaecology, University of Belgrade, Belgrade, Yugoslavia Timing: not stated. | |
| Participants | 56 participants randomised, 6 cycles/patient. Clomiphene‐resistant PCOS participants (high LH). Mean age, duration of infertility, infertility work‐up, mean BMI not stated | |
| Interventions | Ovarian drilling with diathermy or laser vaporisation with CO2 (n = 28), versus Gonadotrophins (FSH or hMG) for ovulation induction for 6 cycles. Number of drill holes per ovary is not stated. (n = 28) Follow‐up for 6 months | |
| Outcomes | Pregnancy rate (by participant), miscarriage rate (by pregnancy), multiple pregnancy rate (by pregnancy) | |
| Notes | Conflict of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details in paper |
| Allocation concealment (selection bias) | Unclear risk | No details in paper |
| Blinding of participants and personnel (performance bias) | High risk | No details of blinding, but unlikely to have occurred. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | All participants appear to be included in the analysis |
| Selective reporting (reporting bias) | High risk | This is a conference abstract only. No full paper was identified |
| Other bias | High risk | Conference abstract |
| Methods | Parallel randomised controlled trial conducted in Centre for Reproductive Medicine, Tongji University, China Timing: Not stated | |
| Participants | 141 women randomised. Mean age of women in the LOD group 28.1 ± 3.6 years and in the letrozole group was 29.5 ± 3.3 years Inclusion criteria: Diagnosed with PCOS (Revised 2003 Consensus Diagnostic Criteria for PCOS); clomiphene resistance, patent fallopian tubes, normal semen analysis for partner, normal serum prolactin, TSH and 17‐OH progesterone; no systemic disease; no gonadotropin or other hormonal drug treatment during preceding 3 months, normal blood count and blood chemistry; normal glucose and urinalysis Exclusion criteria: Infertility for other reasons than PCOS; uterine cavity lesions or ovarian cyst; > 40 years of age; BMI > 26 kg/m2; contraindications to general anaesthesia; history of pelvic surgery; other endocrine diseases; or a history of liver or renal disease | |
| Interventions | LOD: Both ovaries cauterised at 4 to 6 points, each for 4 seconds at 40 W at a depth of 7 to 8 mm and a diameter of 3 to 5 mm using a monopolar electrosurgical needle (n = 70), versus Letrozole 2.5 mg orally administered on the 5th day of menses and then every day for 5 days. Treatment was repeated for up to 6 cycles (n = 71). Follow‐up for 6 months. Natural intercourse advised | |
| Outcomes | Ovulation, biochemical pregnancy, clinical pregnancy | |
| Notes | Conflict of interest: not stated Clinical trial registration number: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated" Comment: no other details. |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | High risk | Once the allocation had been made the intervention was revealed to the investigator |
| Blinding of outcome assessment (detection bias) | Low risk | The doctor responsible for performing the transvaginal ultrasound follow‐up assessment was blinded to the treatment groups |
| Incomplete outcome data (attrition bias) | Low risk | 141 women randomised and 141 women analysed |
| Selective reporting (reporting bias) | High risk | Live birth and spontaneous abortion were reported as outcomes, but not prespecified in the Methods |
| Other bias | Low risk | Groups balanced at baseline. No other bias identified |
| Methods | Randomised controlled trial conducted in King Hussein Medical Centre, Amman, Jordan Timing: January 2000 to December 2001 | |
| Participants | 161 women were randomised, 64 assigned to receive metformin and 97 to undergo LOD. Mean age: Metformin group = 27.4 ± 3.0; LOD group = 27.1 ± 4.4 Inclusion criteria: Clomiphene citrate‐resistant PCOS, normal uterine cavity and tubal patency on hysterosalpingography, normal semen parameters in male partner Exclusion criteria: Congenital adrenal hyperplasia, Cushing's syndrome, hyperprolactinaemia and thyroid disease | |
| Interventions | Metformin 850 mg twice daily throughout the cycle (n = 64), versus LOD (n = 97) Follow‐up: not stated | |
| Outcomes | Ovulation rate, pregnancy rate, multiple pregnancies, miscarriage rate, ectopic pregnancy rate, OHSS, hormonal profile | |
| Notes | Conflict of interest: not stated Clinical trial registration number: not stated We contacted the authors in August 2017 to provide confirmation of randomisation and allocation concealment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was by random‐number table |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | High risk | No details of blinding, but unlikely due to nature of intervention and comparison |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details of blinding of outcome assessors |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | High risk | We could not retrieve the original protocol. Ovulation rate and pregnancy rate were prespecified in the study report, but ovarian hyperstimulation, menstrual cycle regularity, and hormone profile were not prespecified outcomes |
| Other bias | Low risk | Appears free of other bias |
| Methods | Prospective randomised trial conducted in the Ukraine Timing: not stated | |
| Participants | 128 women with clomiphene‐resistant PCOS. 84% were obese | |
| Interventions | Metrodin High Purity for up to 6 cycles (n = 62), versus Laparoscopic electrocoagulation of the ovarian surface (n = 66). Follow‐up for 1½ years | |
| Outcomes | Pregnancy, miscarriage | |
| Notes | Conflict of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "..were randomized.." Comment: no other details in abstract |
| Allocation concealment (selection bias) | Unclear risk | No details in abstract |
| Blinding of participants and personnel (performance bias) | High risk | No evidence of blinding of researchers or participants |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear details |
| Selective reporting (reporting bias) | High risk | No outcomes were listed in the Methods section. Study only available in abstract form |
| Other bias | High risk | Conference abstract only |
| Methods | Parallel randomised controlled trial conducted in Obstetrics and Gynaecology clinic, Isfahan, Iran Timing: not stated | |
| Participants | 104 women randomised. Mean age of women in LOD group was 29.2 ± 5.5 years and in gonadotropin group was 28.5 ± 5.5 years Inclusion criteria: Nuliparous, aged < 40 years, clomiphene‐resistant, PCOS Exclusion criteria: Male‐factor or tubal‐factor infertility | |
| Interventions | LOD: 10 to 15 punctures per ovary depending on size (n = 52), versus Gonadotropin: HMG given after the bleeding withdrawal and from day 3 of the cycle with 10 mg medroxyprogesterone (n = 52) Follow‐up: not stated | |
| Outcomes | Pregnancy, miscarriage, ectopic pregnancy, OHSS, multiple pregnancy | |
| Notes | Conflict of interest: not stated Clinical trial registration number: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer ‐generated random numbers" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | High risk | No details, but unlikely due to different interventions |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | All women randomised were analysed |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. Prespecified outcomes appear to be reported |
| Other bias | Low risk | Groups balanced at baseline |
| Methods | Parallel randomised controlled trial in university‐affiliated tertiary centre, Egypt Timing: Not stated | |
| Participants | 80 women randomised Mean age of unilateral drilling group was 28.4 ± 2.2 years and in bilateral group was 29.2 ± 1.9 years Inclusion criteria: Clomiphene‐resistant PCOS Exclusion criteria: No details | |
| Interventions | Unilateral drilling (n = 40), versus Bilateral drilling (n = 40) 40 normally‐ovulating women were included as controls but not included in this review and were not randomised Follow‐up for 6 months | |
| Outcomes | Serum anti‐Mullerian hormone at 6 months follow‐up | |
| Notes | Conflict of interest: not stated Clinical trial registration number: not stated Conference abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized" Comment: no other details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Unclear risk | 80 women randomised but not clear if all women were analysed at 6 months |
| Selective reporting (reporting bias) | High risk | Conference abstract that only reported on anti‐Mullerian hormone, which was not a prespecified outcome for this review |
| Other bias | High risk | States that groups were balanced at baseline but conference abstract only. No tables or P values identified |
| Methods | Parallel randomised controlled trial conducted in Women's Health Centre, Assiut University. Egypt Timing: Not stated | |
| Participants | 80 women randomised. Mean age of women in adjusted group was 27.7 ± 2.1 years and in the fixed group was 28.5 ± 1.9 years Inclusion criteria: Clomiphene‐resistant PCOS Exclusion criteria: No details | |
| Interventions | Adjusted thermal dose based on ovarian volume (n = 40), versus Fixed thermal dose 600 J per ovary through 4 punctures regardless of size (n = 40) A third group of normally‐ovulating women acted as controls but are not included in these analyses Follow‐up for 6 months | |
| Outcomes | AMH levels, ovulation, conception (no details), early abortion rates | |
| Notes | Conflict of interest: not stated Clinical trial registration number: not stated Conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details are provided of method used to generate the random sequence |
| Allocation concealment (selection bias) | Unclear risk | No details are provided of the method used to conceal allocation to treatment groups |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details provided of blinding of participants or trial personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details provided of blinding of outcome assessors |
| Incomplete outcome data (attrition bias) | High risk | No details provided of levels of attrition |
| Selective reporting (reporting bias) | High risk | Only available as a conference abstract, no full publication available |
| Other bias | High risk | Conference abstract only |
| Methods | Randomised double‐blind study conducted in Italy Timing: October 2001 to December 2002 | |
| Participants | 120 women; mean age of metformin group was 26.8 ± 2.2 and in LOD group 27.5 ± 2.4 years Inclusion: Overweight (BMI 25 ‐ 30 kg/m2) women with PCOS, clomiphene‐resistant Exclusion: Age < 22 or > 34 years; hypothyroidism, hyperprolactinaemia, Cushings syndrome, nonclassical congenital adrenal hyperplasia, and current or previous (within 6 months) use of oral contraceptives, glucocorticoids, antiandrogens, ovulation induction agents, antidiabetic or anti‐obesity drugs, or other hormonal drugs; neoplasms, metabolic, hepatic, or cardiovascular disorder or other concurrent medical illness; women who were intending to start a diet or a specific programme of physical activity; having organic pelvic disease, previous pelvic surgery, suspected peritoneal factor infertility , and tubal or male infertility | |
| Interventions | Diagnostic laparoscopy followed by metformin cloridrate 850 mg twice daily. If anovulatory at 6 months clomiphene citrate 150 mg daily from Day 3 ‐ 7 (n = 60), versus LOD (3 to 6 punctures in each ovary depending on size of ovary) followed by multivitamins twice daily. If anovulatory at 6 months clomiphene citrate 150 mg daily from day 3 ‐7 (n = 60) Follow‐up for 6 months | |
| Outcomes | Live birth, adverse events, menstrual cycle characteristics, ovulation rate, pregnancy, miscarriage, costs | |
| Notes | Conflict of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation was carried out using online software to generate a random allocation sequence in double block as method of restriction" |
| Allocation concealment (selection bias) | Unclear risk | Quote: 'The random allocation sequence was concealed until the interventions were assigned" Comment: there were no further details in the paper |
| Blinding of participants and personnel (performance bias) | Low risk | Participants were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors were blinded |
| Incomplete outcome data (attrition bias) | Low risk | 6 women in metformin group and 5 in the LOD group. Reasons given were evidence of minimal endometriosis by laparoscopy (4 in Group A and 2 from Group B) and non‐compliance (1 from each group). 1 woman from Group A and 2 from group B were excluded for weight loss observed in the first 3 months of the study |
| Selective reporting (reporting bias) | Unclear risk | The original protocol could not be retrieved. All outcomes cited in the Methods section were reported |
| Other bias | Low risk | No evidence of other risk of bias |
| Methods | Randomised trial conducted in Italy Timing: February 2003 to May 2004 | |
| Participants | 50 participants Inclusion: Anovulatory, clomiphene‐resistant, with PCOS, seeking pregnancy Exclusion: < 18 or > 35 years, BMI > 35 kg/m2, neoplastic, metabolic, endocrine, hepatic, renal , and cardiovascular disorders, or other concurrent medical illnesses; and current or previous use of any drug that affected hormone levels, metabolism or appetite. Organic or pelvic diseases, previous pelvic surgery, suspected peritoneal factor infertility/ subfertility, and tubal or male‐factor infertility or subfertility that was excluded by hysterosalpingogram and semen analysis. Wanting to start a diet or a specific programme of physical activity, cigarette smokers or alcoholic beverage abusers | |
| Interventions | LOD followed by 6 cycles of observation (n = 25), versus Clomiphene citrate (incremental dose) plus metformin (850 mg increasing to 1700g daily) for 6 cycles (n = 25) Follow‐up for 6 months | |
| Outcomes | Live birth, pregnancy rates, multiple pregnancy, miscarriage, ovulation rate, adverse events, compliance, cost | |
| Notes | Conflict of interest: not stated Clinical trial registration number: NCT00558077 No funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "achieved using online software (www.randomization.it)" |
| Allocation concealment (selection bias) | Low risk | Concealed in sealed dark envelopes until the interventions were assigned |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details but blinding unlikely due to differences in the interventions |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) | Low risk | 3 women (1 in the LOD group and 2 in the CC + metformin group) were lost to follow‐up because they missed a follow‐up visit |
| Selective reporting (reporting bias) | Low risk | We found the registered protocol on ClinicalTrials.gov (NCT00558077). All the outcomes mentioned in the protocol were presented in the published report |
| Other bias | Low risk | No evidence of other risk of bias |
| Methods | Parallel randomised controlled trial. Single centre, Department of Obstetrics and Gynaecology, Menoufia University Hospital, Egypt Timing: October 2014 to July 2015 | |
| Participants | 108 women randomised. Mean age of women in unilateral ovarian drilling group was 29.7 ± 1.5 years and in bilateral ovarian drilling group was 29.8 ± 1.4 years Inclusion criteria: Clomiphene‐resistant PCOS (revised Rotterdam criteria); normal semen analysis for partner, normal uterine cavity, bilateral tubal patency Exclusion criteria: FSH > 15 IU/ml, medical disorders such as diabetes and hypertension, contraindications for laparoscopy, endocrine disorders, hyperprolactinaemia, thyroid disorder, Cushing syndrome, acromegaly, pelvic organ disease, abnormal semen analysis from partner | |
| Interventions | Unilateral ovarian drilling of the larger ovary. Number of punctures was calculated as Np = 60 J/cm3/ 30 W x 4 seconds (n = 52), versus Bilateral ovarian drilling: 5 punctures per ovary at 30 W for 4 seconds. Each ovary received 600 J (n = 53) Follow‐up for 6 months | |
| Outcomes | Ovulation rate, clinical pregnancy, ovarian reserve measures. | |
| Notes | Clinical trial registration number: PACTR201405000757313 No conflict of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned into two groups" "computer generated simple random tables" |
| Allocation concealment (selection bias) | Unclear risk | No details provided . |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details provided . |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details provided . |
| Incomplete outcome data (attrition bias) | Low risk | 108 women randomised.105 women analysed (2 lost in Unilateral group and 1 lost in Bilateral group ‐ reasons were loss to follow‐up) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | Groups balanced at baseline. No other bias identified |
| Methods | Randomised prospective study conducted in a Fertility clinic in Wales, UK Timing: not stated | |
| Participants | 50 women, mean age in IVF group was 31 (95% CI 29.8 to 32.2) and for LOE + IVF the mean age was 31.8 (95% CI 30.3 to 33.2) Inclusion: Diagnosis of PCOS, requiring IVF for reasons other than anovulation, at least 1 previous unsuccessful ovarian stimulation cycle with gonadotrophins Exclusion: Aged > 40 years, history of > 2 miscarriages, severe male‐factor infertility | |
| Interventions | IVF (n = 25), versus Ovarian electrocautery and IVF (grid of holes 10 mm apart) ovarian stimulation started 1 week after LOE (n = 25). Follow‐up for 1 cycle | |
| Outcomes | Number of abandoned cycles, OHSS, pregnancy, miscarriage | |
| Notes | Conflict of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Blocked method of randomisation.." |
| Allocation concealment (selection bias) | Unclear risk | No details in paper |
| Blinding of participants and personnel (performance bias) | High risk | There was no evidence of blinding of researchers or participants |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) | Low risk | All women randomised appear to be analysed |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. All outcomes listed in the Methods section were reported in the Results |
| Other bias | Low risk | No evidence of other risk of bias |
| Methods | Prospective randomised trial conducted in India Timing: June 2005 to June 2007 | |
| Participants | 44 women with PCOS, normal hysterosalpingography, normal semen parameters in partners; women were also clomiphene‐resistant. Mean age of women in unilateral group was 28.2 ± 12.7 and in the bilateral group was 28.8 ± 2.9 years Exclusion: Other causes of infertility like hypothalamic amenorrhoea, Cushing syndrome, premature ovarian failure, congenital adrenal hyperplasia, androgenic ovarian tumours, endometrial tuberculosis, abnormal TSH and prolactin; had already received other regimens of ovulation induction; tubal obstruction, extensive adhesions of the ovaries or fallopian tubes and endometriosis | |
| Interventions | Unilateral laparoscopic drilling (n = 22), versus Bilateral laparoscopic drilling (n = 22) 5 drills performed per ovary. If there was no ovulation evident within 3 months, the women were started on clomiphene citrate 50 mg daily for 5 days increasing up to a maximum of 150 mg daily for 5 days for a maximum of 6 cycles Follow‐up for 1 year | |
| Outcomes | Clinical and biochemical response, ovulation rate and pregnancy rate | |
| Notes | No conflict of interest Clinical trial registration number: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "..randomly allocated.." Comment: No other details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) | High risk | No evidence of blinding of researchers or participant |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) | Low risk | All women randomised appear to have been analysed |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. The outcomes listed in the Methods section were reported in the Results |
| Other bias | Low risk | No evidence of other risk of bias |
| Methods | Prospective randomised trial conducted in India Timing: January 2006 to January 2009 | |
| Participants | Women from a gynaecological clinic. Mean age of rosiglitazone group was 27.32 ± 4.25 and for LOD group was 28.42 ± 3.65 years Inclusion: Age between 20 and 40 years, having primary infertility with clomiphene‐resistant PCOS, documented patent tubes on hysterosalpingography and no other infertility factor, normal semen parameters in partner Exclusion: Other PCOS‐like syndromes such as Cushings syndrome, congenital adrenal hyperplasia, androgen producing tumours, hyperprolactinaemia and hypothyroidism | |
| Interventions | All participants had laparoscopy Unilateral LOD using 5 punctures + multivitamins twice daily + CC (n = 25), versus Rosiglitazone 4 mg twice daily + CC (n = 25). Treatment continued for 6 months after laparoscopy | |
| Outcomes | Ovulation, pregnancy, number of follicles, serum E2, endocrine parameters | |
| Notes | No conflict of interest Clinical trial registration number: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using online software to generate a random number table" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "opening sealed envelopes containing numbers from the computer generated random table" Comment: Method looks okay but unclear if envelopes were opaque and if they were opened sequentially |
| Blinding of participants and personnel (performance bias) | Low risk | Participants were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessor was blinded to allocation group |
| Incomplete outcome data (attrition bias) | High risk | 5 women were lost to follow‐up, an additional 2 women refused to participate before randomisation and therefore 43 were analysed. The reasons for loss to follow‐up are not described |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. The outcomes listed in the Methods section were reported in the Results |
| Other bias | Low risk | No evidence of other risk of bias |
| Methods | Randomised prospective pilot study, conducted in India Timing: not stated | |
| Participants | 20 women with clomiphene‐resistant PCOS, patent tubes on hysterosalpingography and normal partner semen. Average age of unipolar group was 27.3 (range 21 to 32), and for the bipolar group was 25.5 (range 23 to 30) years No exclusion criteria detailed. | |
| Interventions | Unipolar (n = 10), versus Bipolar ovarian drilling (n = 10) The average number of punctures across both groups was 14.85 per ovary Follow‐up for 3 months and if no evidence of ovulation then started on clomiphene citrate | |
| Outcomes | Ovulation and pregnancy rate, androgen and biochemical measurements | |
| Notes | Conflict of interest: not stated Clinical trial registration number: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned by using computerized random table" |
| Allocation concealment (selection bias) | Unclear risk | No details in paper |
| Blinding of participants and personnel (performance bias) | High risk | No evidence of blinding of researchers or participants |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) | Low risk | Although not stated it appears as though all women randomised were analysed |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. The outcomes listed in the Methods section were reported in the Results |
| Other bias | Low risk | No evidence of other risk of bias |
| Methods | Parallel randomised controlled trial. Single centre in Fertility clinic, Al‐Zahara Hospital, Iran Timing: June 2011 to July 2012. | |
| Participants | 100 women randomised. Mean age of women in the unilateral group was 27.6 ± 4.3 years and in bilateral group was 28.0 ± 4.3 years Inclusion criteria: Women with PCOS (Rotterdam 2003 criteria) and clomiphene resistance Exclusion criteria: Tubal disease, peritoneal adhesions to tubes or ovaries, endometriosis, endocrine abnormality, concomitant male infertility. | |
| Interventions | Unilateral ovarian drilling (right ovary) (n = 50), versus Bilateral ovarian drilling (n = 50) Unipolar diathermy needle, 8 mm, 60 W and 5 points per ovary Follow‐up for 6 months | |
| Outcomes | Menstrual calender, serum LH and FSH, ovulation, clinical pregnancy | |
| Notes | No conflict of interest Funding from Guilan University of Medical Sciences Clinical trial registration number: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Blocked sample randomisation, no other details |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | Surgeons were blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) | Unclear risk | 45 women in each group were analysed. In the unilateral group 2 women were excluded with tubal disease found during laparoscopy and 3 missed follow‐up visit. In the bilateral group, 1 woman was excluded because of endometriosis found during laparoscopy and 4 were excluded as they missed follow‐up visits |
| Selective reporting (reporting bias) | Low risk | We found the registered protocol on irct.ir (IRCT138903291306N2). All the outcomes mentioned in the protocol were presented in the published report |
| Other bias | Low risk | Groups balanced at baseline |
| Methods | Randomised trial, no method stated. Conducted at First Department of Obstetrics and Gynaecology, University of Milan and Gynaecology Unit, University of Pavia, Varese, Italy | |
| Participants | 29 participants randomised, 6 cycles/participant. Clomiphene‐resistant PCO women (high LH). Mean age not stated | |
| Interventions | Ovarian drilling with diathermy (at least 20 drill holes per ovary), (N = 16) versus Follow‐up for 6 months | |
| Outcomes | Pregnancy rate (per participant), miscarriage rate (per pregnancy), multiple pregnancy rate (per pregnancy) | |
| Notes | Interim results only ‐ further patients will be randomised and a later publication is expected Conflict of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants or study personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol |
| Other bias | Unclear risk | Interim details only |
| Methods | Randomized controlled prospective trial conducted in India Timing: January 2012 to May 2015 | |
| Participants | 109 women randomised. The mean age of women was 26.23 ± 2.9 years in gonadotropin group and 26.11 ± 2.7 years in ovarian drilling group Inclusion criteria: chronic anovulation, polycystic ovaries diagnosed by transvaginal ultrasonography, clomiphene citrate‐resistant, shown by anovulation after taking 150 mg clomiphene citrate daily for 5 days for at least 3 cycles. Aged between 21 and 35 years. Exclusion criteria: severe male‐factor subfertility, other causes of infertility like tubal obstruction and extensive adhesion (endometriosis) stages III and IV according to the classification of the American Fertility Society Secondary exclusion criteria identified during diagnostic laparoscopy: tubal obstruction, extensive adhesion of the ovaries or fallopian tubes, and endometriosis stage III or IV | |
| Interventions | Gonadotrophins (N = 44), versus LOD with CC or gonadotrophins (N = 45) (4 to 5 puncture sites, 40 W, monopolar needle) Follow‐up for 6 months | |
| Outcomes | Ovulation rate, pregnancy rate, live birth, abortion, ectopic, multiple pregnancies | |
| Notes | No conflict of interest No funding Clinical trial registration number: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly allocated2 Comment: no other details in the paper |
| Allocation concealment (selection bias) | Unclear risk | No details in the paper |
| Blinding of participants and personnel (performance bias) | High risk | There was no evidence that participants or researchers were blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details in the paper |
| Incomplete outcome data (attrition bias) | Low risk | Out of 109 women, 8 were excluded after diagnostic laparoscopy because of the presence of endometriosis and adhesions. 12 women did not complete the study protocol |
| Selective reporting (reporting bias) | High risk | We could not retrieve the original protocol. The primary outcome in the Method section was ongoing pregnancy within 12 months. The primary outcome in the Result section was a positive urine pregnancy test after 3 and 6 cycles |
| Other bias | Unclear risk | Women with LOD received CC or gonadotrophins |
| Methods | Randomised trial conducted in Egypt Timing: January 2003 to December 20 | |
| Participants | 87 women with PCOS. Mean age of unilateral group was 31.1 ± 4.2, and for the bilateral group was 29.8 ± 3.7 years Inclusion: infertility secondary to anovulation, unsuccessful treatment with clomiphene citrate and gonadotrophins | |
| Interventions | Weight reduction and insulin sensitising drugs were tried first for 3 months Clomiphene citrate 50 mg daily for 5 days from day 3 to 7. If no response then increased up to 150 mg daily for 5 days. If still no response HMG used to stimulate ovulation Unilateral LOD: If both ovaries equal size the right one was drilled, if of unequal size then the larger one was treated (n = 43), versus Bilateral LOD (n = 44). Ovaries were cauterised at 4 points Follow‐up for 1 year | |
| Outcomes | Postoperative pain, postoperative nausea, ovulation, pregnancy, miscarriage | |
| Notes | Conflict of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided in paper |
| Allocation concealment (selection bias) | Low risk | Quote: "randomly allocated by an independent investigator blinded to the treatment group...using the closed envelope method" |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details provided |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors were blinded |
| Incomplete outcome data (attrition bias) | Low risk | All women appear to have been followed up and analysed |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. All outcomes listed in the Methods section were reported in the Results |
| Other bias | Low risk | No evidence of other risk of bias |
| Methods | Randomised trial conducted in Egypt Timing: January 2007 to February 2009 | |
| Participants | 150 women with clomiphene‐resistant PCOS attending an infertility clinic. Mean age for CC + tamoxifen group 25.6 ± 3.5 years, LOD group 25.6 ± 4.1 years Inclusion: Age between 18 and 38 years, at least 2 years of primary or secondary infertility due to anovulation, patent fallopian tubes on hysterosalpingography or diagnostic laparoscopy, no hormonal treatment in previous 3 months and normal semen values | |
| Interventions | CC (150 mg) + tamoxifen (40 mg) from day 3 to day 7 for a maximum of 6 consecutive cycles (n = 75), versus LOD performed through triple‐puncture laparoscopy (4 to 6 puncture points were made through the ovarian capsule of each ovary) (n = 75) Follow‐up for 6 months | |
| Outcomes | Pregnancy (biochemical, clinical, live birth), miscarriage, endometrial thickness, ovulation rate (follicles ≥ 18 mm) | |
| Notes | No conflicts of interest Clinical trial registration number: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a computer generated random number table.." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "sealed envelopes" Comment: Not clear if opaque and serially numbered |
| Blinding of participants and personnel (performance bias) | High risk | No details provided but unlikely that there was blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) | Low risk | There was no loss to follow‐up and all 150 women were analysed |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. All a priori outcomes in paper were reported |
| Other bias | Low risk | No evidence of other risk of bias |
| Methods | Parallel randomised controlled trial conducted in Women's Health Centre and Physiology Department, Assiut University, Egypt Timing: January 2007 to December 2009 | |
| Participants | 120 women randomised. Mean age of women in adjusted thermal dose group was 25.7 ± 5.9 years and in the fixed‐dose group was 25.4 ± 5.7 years Inclusion criteria: PCOS (Rotterdam 2003); aged 18 to 38 years, clomiphene‐resistant, anovulatory infertility for 2 years or more, confirmed patent tubes, normal semen analysis from male partner Exclusion criteria: Endocrine abnormalities or pelvic pathology | |
| Interventions | Adjusted thermal dose thermal dose based on ovarian volume (4 to 9 holes delivering a thermal dose of 480 to 1080 J per ovary) (n = 60), versus Fixed 4‐puncture thermal dose 600 J per ovary regardless of size (n = 60) Follow‐up for 6 months. If no pregnancy after 6 months then evaluated using second‐look laparoscopy for presence of adhesions | |
| Outcomes | Ovulation rate, menstrual cycle regularity, pregnancy. Serum AMH, FSH, AFC and ovarian volume | |
| Notes | Conflict of interest: not stated Clinical trial registration number: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "assigned randomly" "computer generated random number table" |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) | Low risk | 60 women allocated to each group. Adjusted thermal dose group lost 2 women to follow‐up (no reasons provided) analysed 58 women. The fixed‐dose group lost 3 women to follow‐up (no reasons provided), analysed 57 women |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. Miscarriage rate reported but not prespecified in Methods |
| Other bias | Low risk | Groups balanced at baseline |
AMH: anti‐Müllerian hormone; AFC: antral follicle count; BBT: basal body temperature; BMI: body mass index; CC: clomiphene citrate; FSH: follicle stimulating hormone; hMG: human menopausal (urinary) gonadotrophins; IUI: intra uterine insemination; J: joules; LH: luteinizing hormone; LOD: laparoscopic ovarian drilling; LOE: laparoscopic ovarian electrocautery; OCP: oral contraceptive pill; OHSS: ovarian hyperstimulation syndrome; PCOS: polycystic ovary syndrome; rFSH: recombinant follicle stimulating hormone; THL: transvaginal hydrolaparoscopy; TSH: thyroid stimulating hormone
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Serial randomisation | |
| Participants had CC failure (defined as failure to achieve pregnancy despite successful CC‐induced ovulation for 6 cycles) as opposed to CC resistance | |
| Randomisation was by cards numbered 1 to 20; even numbers allocated to one group and odd numbers to another group | |
| Trial compared methods of drilling only | |
| Compared 5 to 10 punctures in each ovary | |
| Ineligible intervention: transabdominal versus transvaginal laparoscopic ovarian drilling | |
| Serial method of randomisation | |
| RCT comparing drilling by diathermy + Interceed to 1 ovary versus drilling only to the other ovary 1. Unit of randomisation: ovaries, not participants | |
| Use of concurrent controls | |
| Use of concurrent controls | |
| Compared re‐electrocautery with FSH | |
| Compares transvaginal ovarian needle drilling with LOD | |
| Non‐randomised controlled trial comparing Nd:YAG laser drilling versus CO2 laser drilling Different duration of follow‐up between the 2 groups (8 versus 18 to 30 months) | |
| Ineligible comparisons. LOD was compared with LOD + metformin | |
| Conference abstract only; lack of usable data; we were not able to obtain data after multiple attempts to contact the authors. | |
| Not an RCT | |
| Randomisation used an 'alternate' allocation method | |
| Both groups underwent LOD | |
| Quasi‐RCT | |
| Ineligible intervention: LOD by harmonic scalpel versus monopolar drilling needle | |
| Ineligible intervention: RCT comparing LOD under local anaesthetic versus general anaesthetic | |
| RCT comparing LOD + interceed to 1 ovary versus drilling only to the other ovary Outcome is adhesion formation at second‐look laparoscopy | |
| Not an RCT; prospective controlled study | |
| Not an RCT; quasi‐random allocation | |
| RCT comparing 5 versus 10 versus 15 points electrocautery of the ovary | |
| No interventions of interest | |
| Excluded due to article being retracted | |
| Ineligible intervention: trial comparing needle puncture drainage with unipolar electrocoagulation drilling | |
| This trial compared different numbers of coagulation points |
CC: clomiphene citrate; FSH: follicle stimulating hormone; LOD: laparoscopic ovarian drilling; RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Prospective randomised trial conducted in Egypt |
| Participants | 260 women attending fertility clinics. Mean age of women in letrozole group was 27.3 ± 2.6 years and in the LOD group was 26.4 ± 2.4 years Inclusion: Clomiphene‐resistant PCOS, patent fallopian tubes assessed by hysterosalpingography, normal semen analysis from partner, normal serum prolactin, thyroid stimulating hormone and 17‐hydroyprogesterone Exclusion: Other causes of infertility, age > 40 years, BMI > 35, contraindications to anaesthesia, previous history of LOD, and having received metformin, gonadotrophin, other hormonal drugs or OCP in preceding 6 months. Women intending to start a diet or a specific programme of physical activity were also excluded |
| Interventions | Letrozole 2.5 mg orally daily from day 3 of the menses for 5 days for 6 cycles (n = 128), versus LOD ‐ each ovary was cauterised at 4 points and women were followed up for 6 months (n = 132) |
| Outcomes | Biochemical pregnancy, clinical pregnancy, ovulation, miscarriage, live birth rates, endometrial thickness |
| Notes |
| Methods | Randomised prospective trial conducted in Egypt |
| Participants | 282 women attending fertility clinics in Egypt. Mean age of women in the metformin group was 27.2 ± 2.5 years and in the LOD group was 26.5 ± 2.3 years Inclusion: Clomiphene‐resistant PCOS, patent fallopian tubes assessed by hysterosalpingography, normal semen analysis from partner, normal serum prolactin, thyroid stimulating hormone and 17‐hydroyprogesterone Exclusion: Other causes of infertility, age > 40 years, contraindications to anaesthesia and having received metformin, gonadotrophin or OCP in preceding 6 months |
| Interventions | Metformin 500 mg 3 times a day for 6 to 8 weeks, followed by 100 mg of clomiphene citrate for 5 days starting on day 3 of spontaneous or induced menstruation. Dosage increased by 50 mg at next cycle if still anovulatory; treated for 6 cycles (n = 138), versus LOD: each ovary was cauterised at 4 points and women were followed up for 6 months (n = 144). |
| Outcomes | Pregnancy, miscarriage, ovulation rate, endometrial thickness |
| Notes | Author contacted in September 2011 for details on pregnancy rates by woman rather than by cycle |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Comparison of ovulation rate after laparoscopic electrocautery in infertile women with clomiphene‐resistant PCOS |
| Methods | Randomised, double‐blinded, parallel‐assignment |
| Participants | Inclusion criteria: Age: 20 ‐ 38, BMI < 32, infertile women with polycystic ovarian syndrome and infertility duration > 1 year caused by disorder of ovulation, normal semen analysis in partner, normal hysterosalpingography, resistance to CC, absence of any major disease Exclusion criteria: Other cause of infertility, use of other indicated ovulation diets like metformin, any abnormalities of fallopian tubes and endometriosis during laparoscopy Age minimum 20 years, maximum 38 years |
| Interventions | Laparoscopic bilateral ovarian cauterisation, versus Laparoscopic unilateral ovarian cauterisation |
| Outcomes | Primary outcome: ovulation rate Secondary outcomes: CC dose for induction ovulation (If ovulation spontaneously does not return), pregnancy rate, serum hormonal level (FSH, LH, testosterone) |
| Starting date | 23 July 2010 |
| Contact information | Dr Ziba Zahiri, Iran |
| Notes |
| Trial name or title | N‐acetyl cysteine for ovulation induction in clomiphene citrate‐resistant polycystic ovary syndrome |
| Methods | Randomised controlled trial, parallel‐assignment |
| Participants | Inclusion criteria: 18 to 39 years; PCOS women according to Rotterdam criteria who failed to respond to 6 months ovulation induction therapy with clomiphene citrate; normal semen analysis of partner; normal tubo‐peritoneal anatomy as assessed by laparoscopy Exclusion Criteria: Other causes of infertility; receiving gonadotrophin ovulation induction |
| Interventions | LOD versus LOD plus N‐Acetyl cysteine 1200 mg daily in 2 divided doses starting on cycle day 2 for 6 months |
| Outcomes | Primary outcome measures: ovulation rate |
| Starting date | January 2012 |
| Contact information | Esraa Yousef Badran, Egypt |
| Notes |
| Trial name or title | Comparison between letrozole and LOD in women with clomiphene‐resistant PCOS |
| Methods | Randomised controlled trial, parallel‐assignment |
| Participants | Inclusion criteria: 20 to 40 years, clomiphene‐resistant PCOS women Exclusion Criteria: Other causes of infertility, hyperprolactinaemia, BMI > 35, previous letrozole or LOD |
| Interventions | LOD versus Letrozole 2.5 mg |
| Outcomes | Primary outcome measures: ovulation |
| Starting date | November 2014 |
| Contact information | AbdelGany MA Hassan, Cairo University, Egypt |
| Notes |
| Trial name or title | Extended CC regimen versus LOD for ovulation Induction in clomiphene‐resistant women With PCOS |
| Methods | Randomised controlled trial, parallel‐assignment. |
| Participants | Inclusion criteria: Aged 18 ‐ 35 years,> 2 years infertility, serum level of FSH < 10 U/L in the early follicular phase, CC‐resistant PCOS, as they failed to ovulate with a dose of CC of 150 mg/day for 5 days per cycle for at least 3 consecutive cycles. All women had patent fallopian tubes proved by hysterosalpingography or laparoscopy and their partners satisfied the normal parameters of semen analysis according to the modified WHO criteria Exclusion Criteria: Infertility due to causes other than CC‐ resistant PCOS or due to combined factors, BMI ≥ 35 Kg/m2, use of metformin, gonadotropins, hormonal contraception or diet regimen within the last 6 months; women with congenital adrenal hyperplasia, hyperprolactinaemia or abnormal thyroid function, hypersensitivity or contraindications to letrozole or clomiphene treatment; previous LOD |
| Interventions | CC 100 mg daily for 10 days starting on day 3 of cycle, versus LOD |
| Outcomes | Primary outcome measures: ovulation rate |
| Starting date | June 2014 |
| Contact information | Khalid Abd Aziz Mohamed, Benha University, Egypt |
| Notes |
| Trial name or title | N‐acetyl‐cysteine in clomiphene‐resistant PCOS after LOD: a randomised controlled trial |
| Methods | Randomised controlled trial, parallel‐assignment |
| Participants | Inclusion criteria: Aged 18 to 35 years; BMI between 25 and 30 Kg/m2; CC‐resistant PCOS Exclusion criteria: BMI < 25 or > 30 Kg/m2, hyper‐ or hypothyroidism, or hyperprolactinaemia; current or previous (within the last 6 months) use of oral contraceptives, glucocorticoids, antiandrogens, antidiabetic and anti‐obesity drugs or other hormonal drugs; intention to start a diet or a specific programme of physical activity; organic pelvic diseases; tubal or male‐factor infertility; interval of earlier treatment with any of the fertility drugs of < 6 months; contraindication to clomiphene citrate; liver disease, undiagnosed abnormal uterine bleeding, uterine fibroids, endometrial cancer, ovarian enlargement or OHSS or HCG injection: ovarian enlargement or hyper stimulation |
| Interventions | LOD + N‐acetyl‐cysteine + CC, versus LOD + CC |
| Outcomes | Primary outcome measures: Biochemical pregnancy rate |
| Starting date | May 2016 |
| Contact information | Mohamed S Sweed, Ain Shams University, Cairo, Egypt |
| Notes |
| Trial name or title | Letrozole versus LOD in PCOS |
| Methods | Randomised controlled trial, parallel‐assignment |
| Participants | Inclusion criteria: Aged 20 to 35 years; history of at least 1 year of infertility either primary or secondary; BMI 25 ‐ 35; normal fallopian tubes; normal semen analysis of the husband; women who will agree to participate in the study Exclusion criteria: BMI > 35; contraindication to general anaesthesia; previous laparoscopic drilling; presence of other causes of infertility; had received metformin, gonadotrophin, oral contraceptives or other hormonal drugs during the preceding 6 months; intended to start a diet programme; refuse to participate in the study |
| Interventions | LOD versus Letrozole 2.5 mg oral tablets |
| Outcomes | Primary outcome: ovulation rate |
| Starting date | January 2017 |
| Contact information | Ahmed Mohamed Abbas, Assiut Univeristy, Egypt |
| Notes |
| Trial name or title | LESS surgery versus conventional multiport laparoscopy in ovarian drilling |
| Methods | Randomised controlled trial, parallel‐assignment |
| Participants | Inclusion criteria: Aged 16 to 50 years; PCOS according to Rotterdam Criteria (2 out of 3): polycystic ovaries (12 or more follicles in each ovary and/or increased ovarian volume > 10 cm3), oligo‐ or an‐ovulation, clinical and/or biochemical hyperandrogenism after exclusion of other aetiologies for irregular cycles. Indications of laparoscopic ovarian drilling: clomiphene citrate‐resistance or failure: failure to conceive after 6 to 9 cycles, other indications for laparoscopy; before gonadotropin administration to decrease risk of OHSS and multiple pregnancy; before ART to decrease risk of severe OHSS in women who previously had cancelled IVF cycles due to OHSS risk or who suffered from OHSS in a previous treatment. Exclusion criteria: Previous 2 or more laparotomies; chronic pelvic pain, endometriosis or pelvic inflammatory diseases to avoid pelvic adhesions and bias in the quantification of postoperative pain; high BMI (> 35kg/m2); do not possess a native umbilicus; advanced gynaecological surgeries or malignant disorders (total laparoscopic hysterectomy, laparoscopically assisted vaginal hysterectomy , laparoscopic myomectomy); contraindication to any laparoscopy‐like medical condition worsened by pneumoperitoneum or Trendelnburg position |
| Interventions | Laparoscopic ovarian drilling for PCOS in infertile women using laparo‐endoscopic single‐site surgery (LESS surgery: single incision through the umbilicus using modified Hasson technique), versus Conventional multi‐port laparoscopy LOD for PCOS |
| Outcomes | Primary outcome: successful surgical procedure |
| Starting date | August 2017 |
| Contact information | Ahmed Mohamed Bahaa Eldin Ahmed, Ain Shams Maternity Hospital, Egypt |
| Notes |
| Trial name or title | LOD versus letrozole In clomiphene‐resistant polycystic ovary |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: diagnosed as PCOS according to Roterdam (2003) criteria; clomiphene‐resistance, i.e. failure to ovulate following 100 mg CC for 5 days for at least 3 cycles; patent fallopian tubes, confirmed by hysterosalpingography or hysteroscopic diagnosis; normal semen analysis parameters of the spouse according to the modified criteria of the World Health Organization; normal serum prolactin, thyroid stimulating hormone and 17‐OH progesterone; no systemic disease; no gonadotropin or other hormonal drug treatment during the preceding 3 months Exclusion criteria: Infertility induced by reasons other than PCOS; uterine cavity lesions or ovarian cyst; > 40 years old; BMI > 26 kg/m2; contraindications to general anaesthesia; history of pelvic surgery; other endocrine diseases; a history of liver or kidney disease |
| Interventions | 2.5 mg letrozole oral tablets on the 2nd ‐ 3rd day of menses and then every day for 5 days. Treatment to be repeated for up to 3 cycles if the participant failed to conceive, versus Bilateral LOD: each ovary will be cauterised at 4 points, each for 4 sec at 40 W, at a depth of 7 ‐ 8 mm and a diameter of 3 ‐ 5 mm, using a monopolar electrosurgical needle according to the size of each ovary |
| Outcomes | Primary outcome: ovulation rate Secondary outcomes: biochemical pregnancy rate, clinical pregnancy rate |
| Starting date | September 2018 |
| Contact information | Ahmed Abdelshafy, Ain shams university maternity hospital, Cairo, Egypt |
| Notes |
| Trial name or title | Impact of unilateral versus bilateral LOD on ovarian reserve and pregnancy rate: A randomised clinical trial |
| Methods | Parallel randomised |
| Participants | Inclusion criteria: women with PCOS who were resistant to CC; Age minimum 20 years, maximum 32 years Exclusion criteria: women with adrenal hyperplasia, thyroid disease, Cushings syndrome, hyperprolactinaemia and a tumour‐related excess of androgen |
| Interventions | Bilateral LOD versus Unilateral LOD |
| Outcomes | Clinical pregnancy rate, ovarian reserve measures, ovulation rate |
| Starting date | 10 January 2014 |
| Contact information | Hytham Hamza, Egypt |
| Notes |
ART: assisted reproductive technology; BMI: body mass index; CC: clomiphene citrate; FSH: follicle stimulating hormone; HCG: human chorionic gonadotropin; LOD: laparoscopic ovarian drilling; OHSS: ovarian hyperstimulation syndrome; PCOS: polycystic ovary syndrome; WHO: World Health Organization
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth Show forest plot | 9 | 1015 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.54, 0.92] |
| Analysis 1.1  Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 1 Live birth. | ||||
| 1.1 LOD versus CC + metformin | 2 | 170 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.32, 1.09] |
| 1.2 LOD versus CC + tamoxifen | 1 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.42, 1.53] |
| 1.3 LOD versus gonadotrophins | 4 | 407 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.56, 1.36] |
| 1.4 LOD versus letrozole | 2 | 288 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.32, 0.92] |
| 2 Multiple pregnancy Show forest plot | 14 | 1161 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.34 [0.18, 0.66] |
| Analysis 1.2  Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 2 Multiple pregnancy. | ||||
| 2.1 LOD versus clomiphene citrate | 1 | 72 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 LOD versus CC + metformin | 2 | 170 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 LOD versus CC + rosiglitazone | 1 | 43 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.12 [0.21, 21.52] |
| 2.4 LOD versus gonadotrophins | 7 | 532 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.22 [0.10, 0.46] |
| 2.5 LOD versus gonadotrophins (rFSH) + metformin | 1 | 36 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 LOD versus letrozole | 1 | 147 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.7 LOD versus metformin | 1 | 161 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.32 [0.25, 6.94] |
| 3 Clinical pregnancy Show forest plot | 21 | 2016 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.72, 1.03] |
| Analysis 1.3  Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 3 Clinical pregnancy. | ||||
| 3.1 LOD versus clomiphene citrate | 1 | 72 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.19, 1.44] |
| 3.2 LOD versus CC + metformin | 2 | 170 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.39, 1.31] |
| 3.3 LOD versus CC + tamoxifen | 1 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.47, 1.71] |
| 3.4 LOD versus CC + rosiglitazone | 1 | 43 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.23, 2.50] |
| 3.5 LOD versus gonadotrophins | 9 | 760 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.74, 1.36] |
| 3.6 LOD versus gonadotrophins (rFSH) + metformin | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.04, 1.00] |
| 3.7 LOD versus letrozole | 3 | 368 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.42, 1.01] |
| 3.8 LOD versus letrozole + metformin | 1 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.42, 1.65] |
| 3.9 LOD versus metformin | 2 | 271 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.75, 2.08] |
| 4 Miscarriage Show forest plot | 19 | 1909 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.78, 1.59] |
| Analysis 1.4  Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 4 Miscarriage. | ||||
| 4.1 LOD versus CC + metformin | 2 | 170 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.69, 5.54] |
| 4.2 LOD versus CC + tamoxifen | 1 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.39, 7.45] |
| 4.3 LOD versus CC + rosiglitazone | 1 | 43 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.06, 17.95] |
| 4.4 LOD versus gonadotrophins | 8 | 725 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.49, 1.33] |
| 4.5 LOD versus gonadotrophins (rFSH) + metformin | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 LOD versus letrozole | 3 | 368 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.86 [0.61, 5.67] |
| 4.7 LOD versus letrozole + metformin | 1 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.16, 3.43] |
| 4.8 LOD versus metformin | 2 | 271 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.53, 4.82] |
| 5 OHSS Show forest plot | 8 | 722 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.25 [0.07, 0.91] |
| Analysis 1.5  Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 5 OHSS. | ||||
| 5.1 LOD versus clomiphene citrate | 1 | 72 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 6.82] |
| 5.2 LOD versus CC + metformin | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 LOD versus CC + rosiglitazone | 1 | 43 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 LOD versus gonadotrophins | 5 | 446 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.02, 0.64] |
| 5.5 LOD versus letrozole | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.6 LOD versus metformin | 1 | 161 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.31 [0.13, 13.44] |
| 6 Ovulation Show forest plot | 10 | 951 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.73, 1.28] |
| Analysis 1.6  Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 6 Ovulation. | ||||
| 6.1 LOD versus clomiphene citrate | 1 | 72 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.7 [0.27, 1.83] |
| 6.2 LOD versus CC + metformin | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.32, 3.10] |
| 6.3 LOD versus CC + tamoxifen | 1 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.56, 3.17] |
| 6.4 LOD versus CC + rosiglitazone | 1 | 43 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.13, 3.44] |
| 6.5 LOD versus gonadotrophins | 2 | 139 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.32, 1.36] |
| 6.6 LOD versus letrozole | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.23, 1.46] |
| 6.7 LOD versus letrozole + metformin | 1 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.49, 1.81] |
| 6.8 LOD versus metformin | 2 | 271 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.86, 2.68] |
| 7 Costs Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 7 Costs. | ||||
| 7.1 LOD versus CC + metformin | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 3711.3 [3585.17, 3837.43] |
| 7.2 LOD versus gonadotrophins only (short‐term) | 2 | 203 | Mean Difference (IV, Fixed, 95% CI) | ‐1115.75 [‐1309.72, ‐921.77] |
| 7.3 LOD versus gonadotrophins only (long‐term) | 1 | 168 | Mean Difference (IV, Fixed, 95% CI) | ‐2235.0 [‐4433.16, ‐36.84] |
| 8 Quality of Life (Health related quality of life: SF‐36) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.8  Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 8 Quality of Life (Health related quality of life: SF‐36). | ||||
| 8.1 Physical functioning at 24 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Social functioning at 24 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Role limitations (physical) at 24 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.4 Role limitations (emotional) at 24 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.5 Mental health at 24 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.6 Vitality at 24 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.7 Pain at 24 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.8 General health at 24 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Quality of life (Rotterdam Symptom Checklist at 24 weeks) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.9  Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 9 Quality of life (Rotterdam Symptom Checklist at 24 weeks). | ||||
| 9.1 Physical symptoms | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Psychological distress | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Activity level | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.4 Overall quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Quality of life (Depression scales (CES‐D) at 24 weeks) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.10  Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 10 Quality of life (Depression scales (CES‐D) at 24 weeks). | ||||
| 10.1 Gonadotrophins | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Multiple pregnancy per pregnancy Show forest plot | 14 | 577 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.34 [0.17, 0.66] |
| Analysis 1.11  Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 11 Multiple pregnancy per pregnancy. | ||||
| 11.1 LOD versus clomiphene citrate | 1 | 23 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 LOD versus CC + metformin | 2 | 99 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 LOD versus CC + rosiglitazone | 1 | 20 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.66 [0.24, 29.46] |
| 11.4 LOD versus gonadotrophins | 7 | 280 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.20 [0.09, 0.43] |
| 11.5 LOD versus gonadotrophins (rFSH) + metformin | 1 | 11 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.6 LOD versus letrozole | 1 | 45 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.7 LOD versus metformin | 1 | 99 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.42 [0.27, 7.53] |
| 12 Miscarriage per pregnancy Show forest plot | 19 | 900 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.88, 1.88] |
| Analysis 1.12  Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 12 Miscarriage per pregnancy. | ||||
| 12.1 LOD versus CC + metformin | 2 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.49 [0.86, 7.24] |
| 12.2 LOD versus CC + tamoxifen | 1 | 78 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.87 [0.41, 8.43] |
| 12.3 LOD versus CC + rosiglitazone | 1 | 20 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.07, 23.26] |
| 12.4 LOD versus gonadotrophins | 8 | 373 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.53, 1.56] |
| 12.5 LOD versus gonadotrophins (rFSH) + metformin | 1 | 11 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.6 LOD versus letrozole | 3 | 118 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.75 [0.86, 8.79] |
| 12.7 LOD versus letrozole + metformin | 1 | 49 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.16, 4.15] |
| 12.8 LOD versus metformin | 2 | 131 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.41, 4.08] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 LOD + IVF versus IVF, Outcome 1 Live birth. | ||||
| 2 Multiple pregnancy Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 LOD + IVF versus IVF, Outcome 2 Multiple pregnancy. | ||||
| 3 Clinical pregnancy Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 LOD + IVF versus IVF, Outcome 3 Clinical pregnancy. | ||||
| 4 Miscarriage Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.4  Comparison 2 LOD + IVF versus IVF, Outcome 4 Miscarriage. | ||||
| 5 OHSS Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.5  Comparison 2 LOD + IVF versus IVF, Outcome 5 OHSS. | ||||
| 6 Multiple pregnancy per pregnancy Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.6  Comparison 2 LOD + IVF versus IVF, Outcome 6 Multiple pregnancy per pregnancy. | ||||
| 7 Miscarriage per pregnancy Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.7  Comparison 2 LOD + IVF versus IVF, Outcome 7 Miscarriage per pregnancy. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical pregnancy Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.1  Comparison 3 LOD + second‐look laparoscopy versus LOD + expectant management, Outcome 1 Clinical pregnancy. | ||||
| 2 Miscarriage Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.2  Comparison 3 LOD + second‐look laparoscopy versus LOD + expectant management, Outcome 2 Miscarriage. | ||||
| 3 Ovulation Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.3  Comparison 3 LOD + second‐look laparoscopy versus LOD + expectant management, Outcome 3 Ovulation. | ||||
| 4 Miscarriage per pregnancy Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.4  Comparison 3 LOD + second‐look laparoscopy versus LOD + expectant management, Outcome 4 Miscarriage per pregnancy. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.1  Comparison 4 Unilateral versus bilateral, Outcome 1 Live birth. | ||||
| 2 Clinical pregnancy Show forest plot | 7 | 470 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.39, 0.84] |
| Analysis 4.2  Comparison 4 Unilateral versus bilateral, Outcome 2 Clinical pregnancy. | ||||
| 3 Miscarriage Show forest plot | 2 | 131 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.31, 3.33] |
| Analysis 4.3  Comparison 4 Unilateral versus bilateral, Outcome 3 Miscarriage. | ||||
| 4 Ovulation Show forest plot | 6 | 449 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.40, 0.90] |
| Analysis 4.4  Comparison 4 Unilateral versus bilateral, Outcome 4 Ovulation. | ||||
| 5 Miscarriage per pregnancy Show forest plot | 2 | 71 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.28, 3.36] |
| Analysis 4.5  Comparison 4 Unilateral versus bilateral, Outcome 5 Miscarriage per pregnancy. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical pregnancy Show forest plot | 3 | 354 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.62, 1.44] |
| Analysis 5.1  Comparison 5 Monopolar versus bipolar, Outcome 1 Clinical pregnancy. | ||||
| 2 Ovulation Show forest plot | 2 | 108 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.14, 0.76] |
| Analysis 5.2  Comparison 5 Monopolar versus bipolar, Outcome 2 Ovulation. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical pregnancy Show forest plot | 2 | 195 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.84 [1.04, 3.26] |
| Analysis 6.1  Comparison 6 Adjusted thermal dose versus fixed thermal dose, Outcome 1 Clinical pregnancy. | ||||
| 2 Miscarriage Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.2  Comparison 6 Adjusted thermal dose versus fixed thermal dose, Outcome 2 Miscarriage. | ||||
| 3 Ovulation Show forest plot | 2 | 195 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.01, 3.33] |
| Analysis 6.3  Comparison 6 Adjusted thermal dose versus fixed thermal dose, Outcome 3 Ovulation. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth Show forest plot | 4 | 415 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.59, 1.36] |
| Analysis 7.1  Comparison 7 Sensitivity analysis low risk of bias: LOD with and without medical ovulation versus medical ovulation alone, Outcome 1 Live birth. | ||||
| 1.1 LOD versus gonadotrophins | 2 | 218 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.59, 1.85] |
| 1.2 LOD versus CC + metformin | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.39, 3.56] |
| 1.3 LOD versus letrozole | 1 | 147 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.30, 1.31] |
| 2 Multiple pregnancy Show forest plot | 6 | 522 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.18 [0.06, 0.57] |
| Analysis 7.2  Comparison 7 Sensitivity analysis low risk of bias: LOD with and without medical ovulation versus medical ovulation alone, Outcome 2 Multiple pregnancy. | ||||
| 2.1 LOD versus CC + metformin | 1 | 50 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 LOD versus gonadotrophins | 3 | 253 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.18 [0.06, 0.57] |
| 2.3 LOD versus letrozole | 1 | 147 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 LOD versus clomiphene citrate | 1 | 72 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
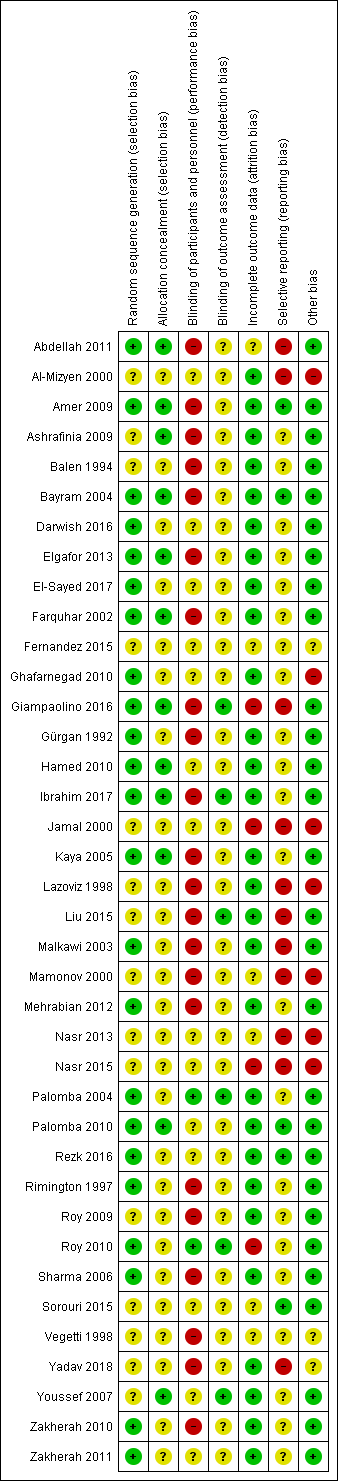
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 LOD with and without medical ovulation versus medical ovulation alone, outcome: 1.1 Live birth.
MOI: Medical ovulation induction alone
LOD: laparoscopic ovarian drilling with or without medical ovulation induction
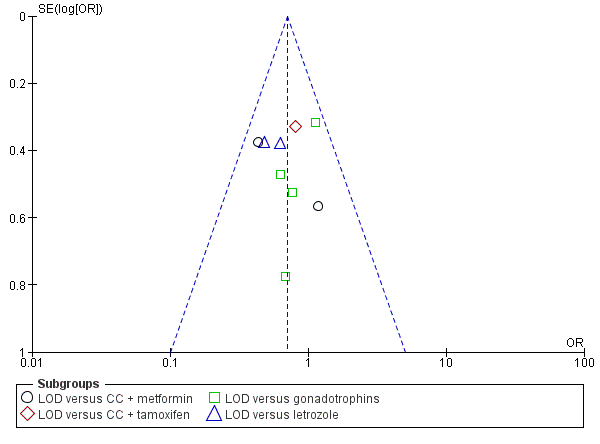
Funnel plot of comparison: 1 LOD with and without medical ovulation versus medical ovulation alone, outcome: 1.1 Live birth.
LOD: laparoscopic ovarian drilling with or without medical ovulation induction
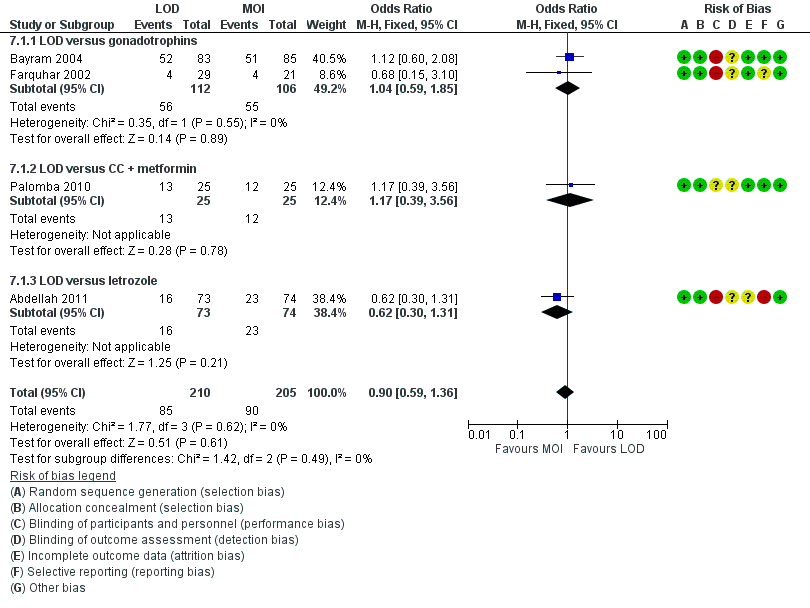
Forest plot of comparison: 5 Sensitivity analysis low risk of bias: LOD with and without medical ovulation versus medical ovulation alone, outcome: 5.1 Live birth.
MOI: Medical ovulation induction alone
LOD: laparoscopic ovarian drilling with or without medical ovulation induction
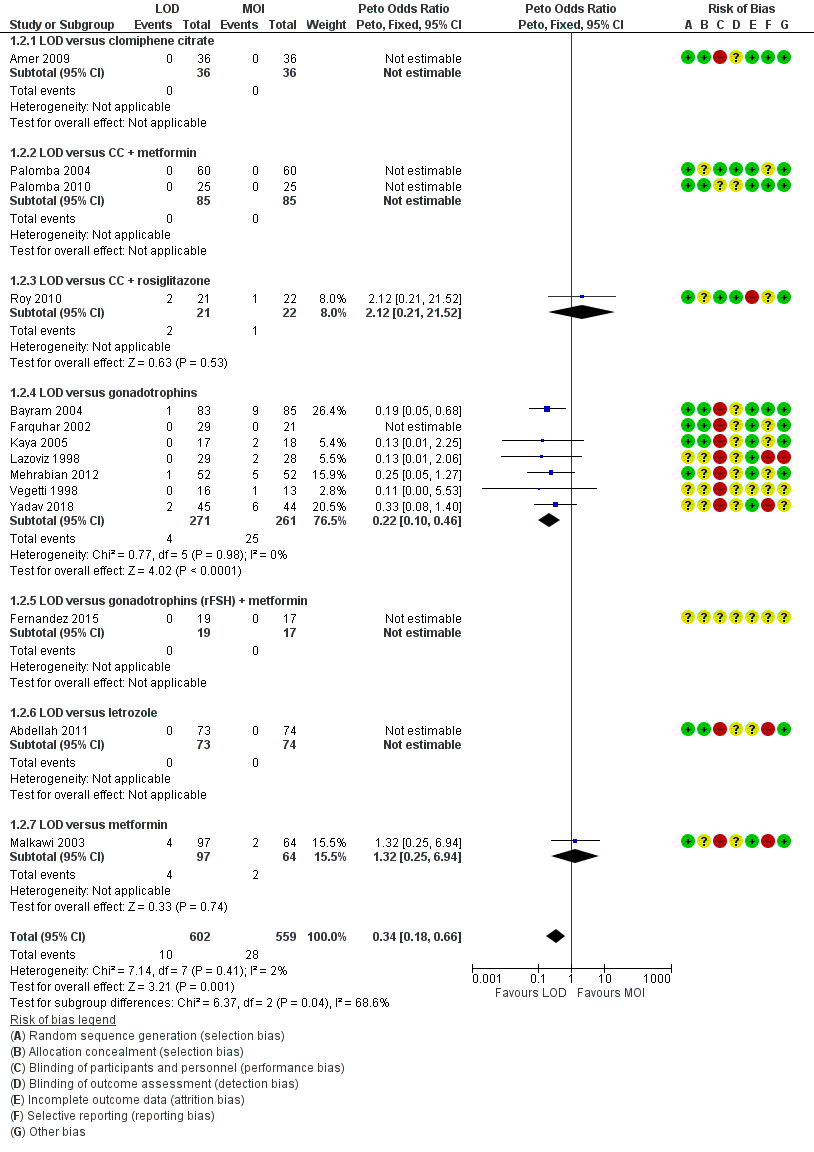
Forest plot of comparison: 1 LOD with and without medical ovulation versus medical ovulation alone, outcome: 1.4 Multiple pregnancy rate (per ongoing pregnancy).
MOI: Medical ovulation induction alone
LOD: laparoscopic ovarian drilling with or without medical ovulation induction
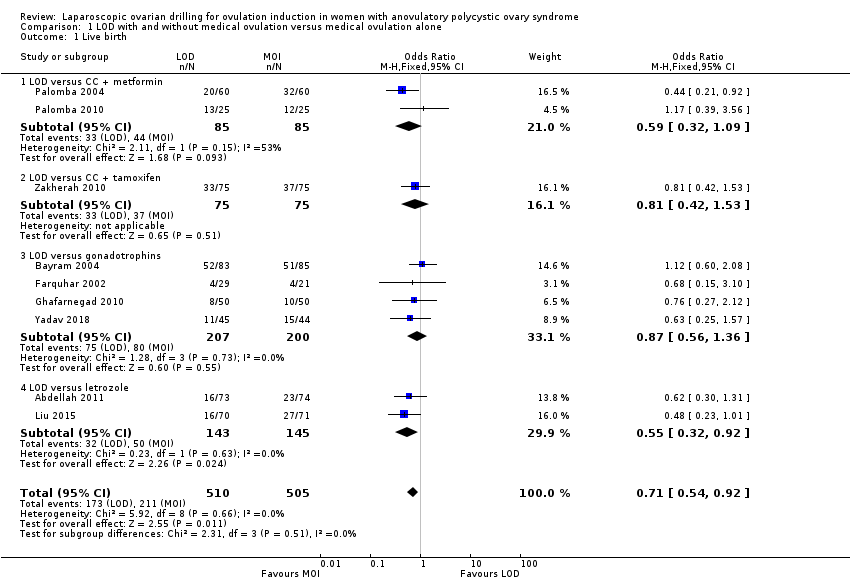
Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 1 Live birth.

Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 2 Multiple pregnancy.
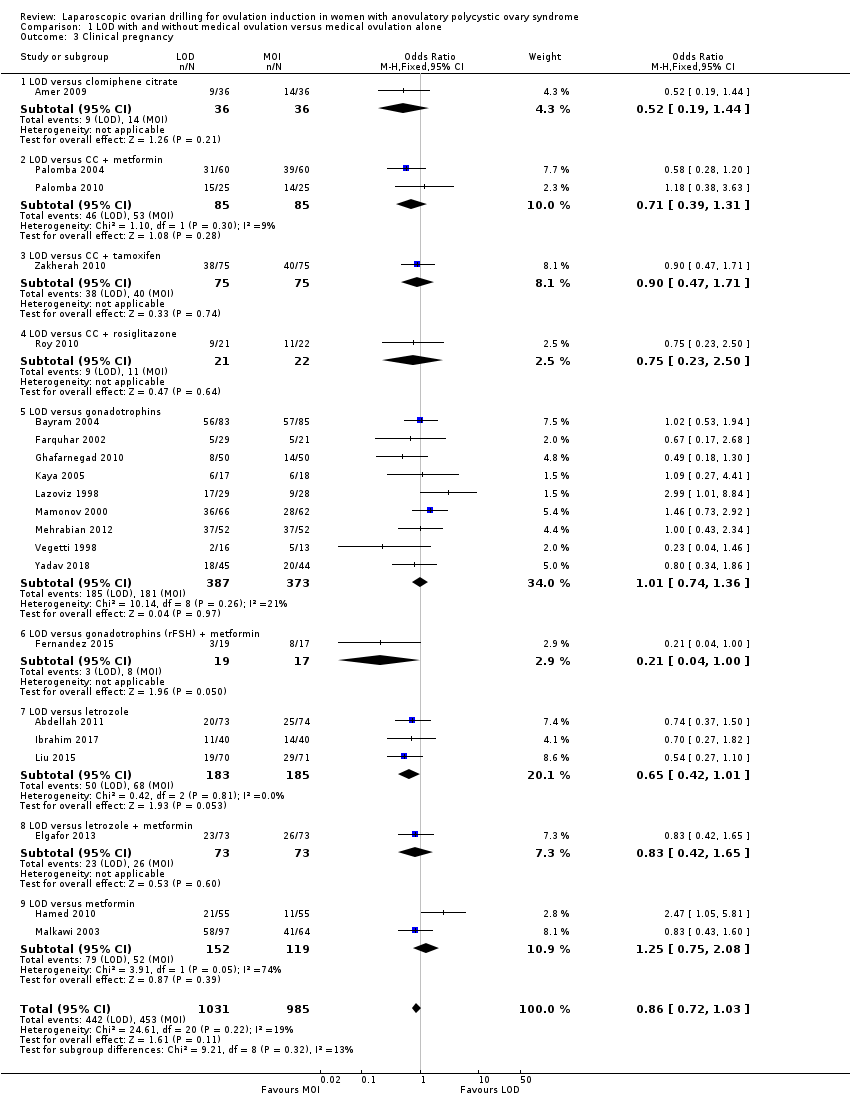
Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 3 Clinical pregnancy.

Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 4 Miscarriage.

Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 5 OHSS.
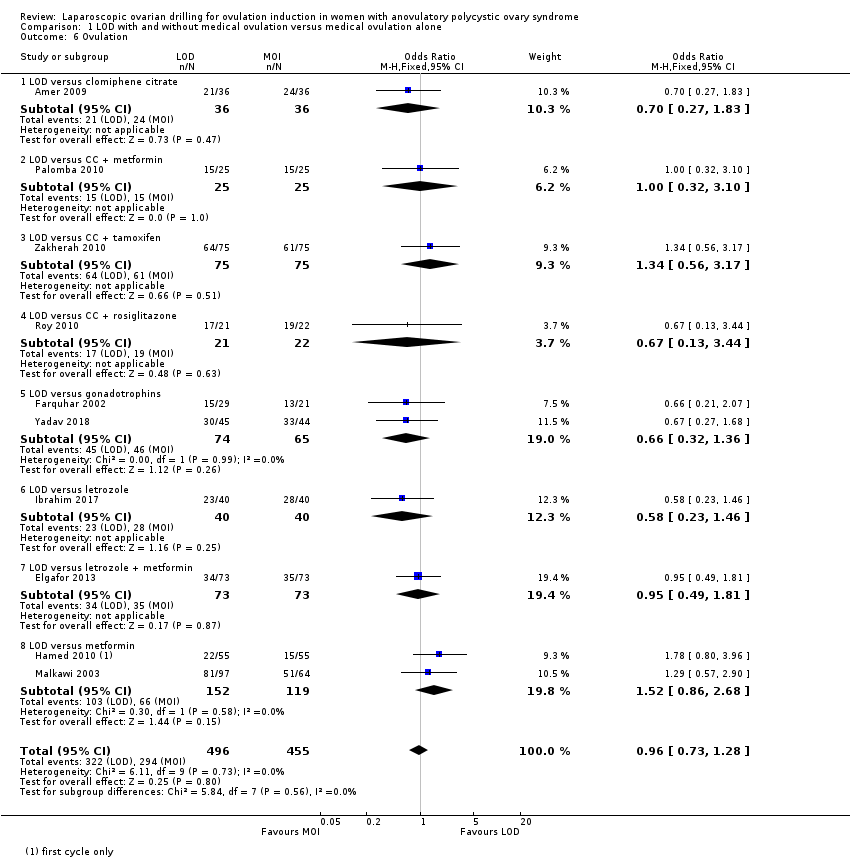
Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 6 Ovulation.

Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 7 Costs.

Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 8 Quality of Life (Health related quality of life: SF‐36).
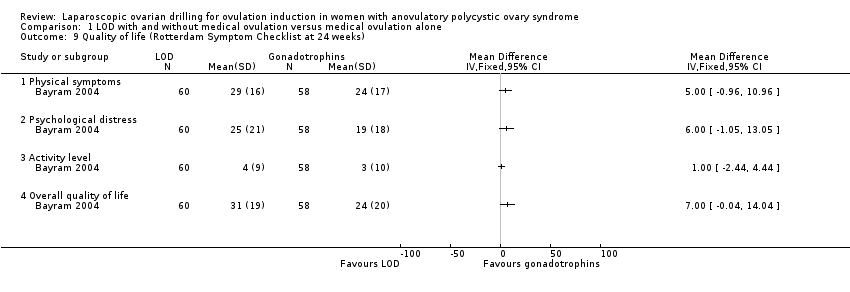
Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 9 Quality of life (Rotterdam Symptom Checklist at 24 weeks).
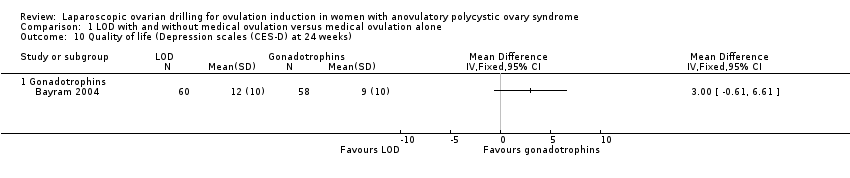
Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 10 Quality of life (Depression scales (CES‐D) at 24 weeks).

Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 11 Multiple pregnancy per pregnancy.
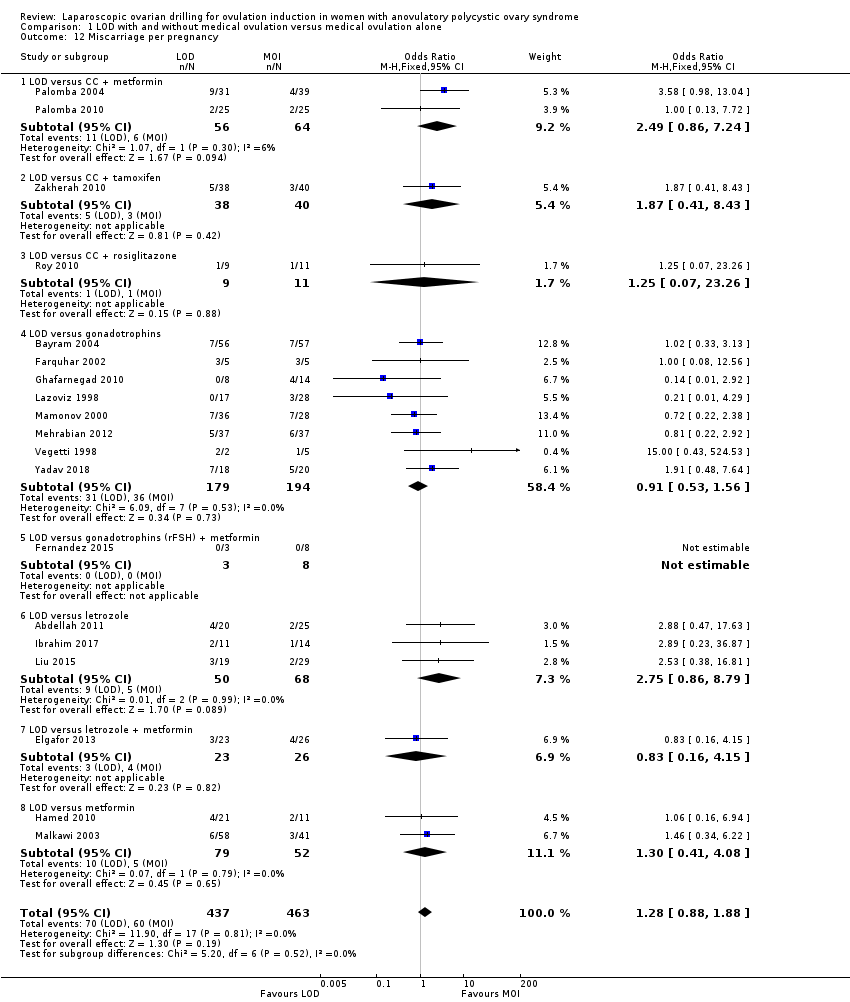
Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 12 Miscarriage per pregnancy.
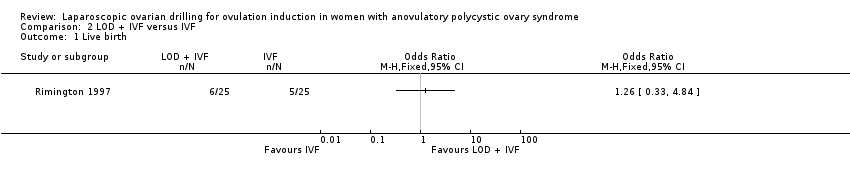
Comparison 2 LOD + IVF versus IVF, Outcome 1 Live birth.

Comparison 2 LOD + IVF versus IVF, Outcome 2 Multiple pregnancy.

Comparison 2 LOD + IVF versus IVF, Outcome 3 Clinical pregnancy.

Comparison 2 LOD + IVF versus IVF, Outcome 4 Miscarriage.
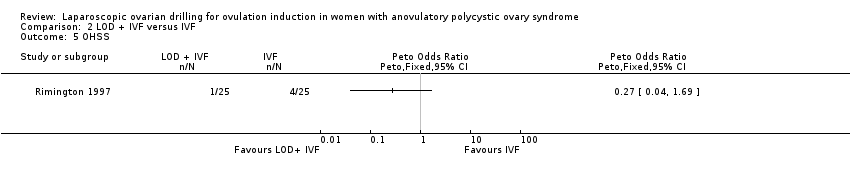
Comparison 2 LOD + IVF versus IVF, Outcome 5 OHSS.

Comparison 2 LOD + IVF versus IVF, Outcome 6 Multiple pregnancy per pregnancy.
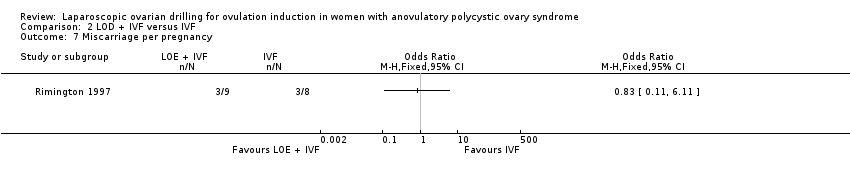
Comparison 2 LOD + IVF versus IVF, Outcome 7 Miscarriage per pregnancy.

Comparison 3 LOD + second‐look laparoscopy versus LOD + expectant management, Outcome 1 Clinical pregnancy.
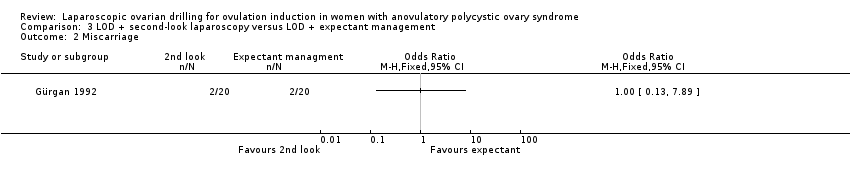
Comparison 3 LOD + second‐look laparoscopy versus LOD + expectant management, Outcome 2 Miscarriage.

Comparison 3 LOD + second‐look laparoscopy versus LOD + expectant management, Outcome 3 Ovulation.

Comparison 3 LOD + second‐look laparoscopy versus LOD + expectant management, Outcome 4 Miscarriage per pregnancy.

Comparison 4 Unilateral versus bilateral, Outcome 1 Live birth.

Comparison 4 Unilateral versus bilateral, Outcome 2 Clinical pregnancy.
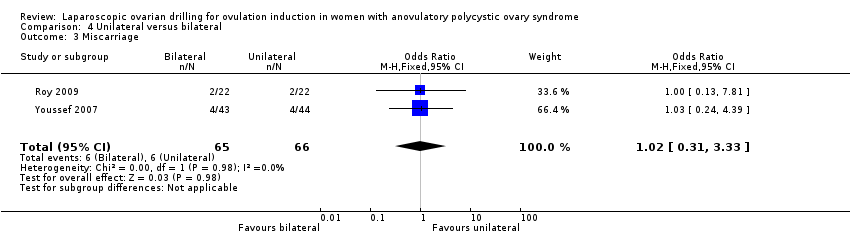
Comparison 4 Unilateral versus bilateral, Outcome 3 Miscarriage.
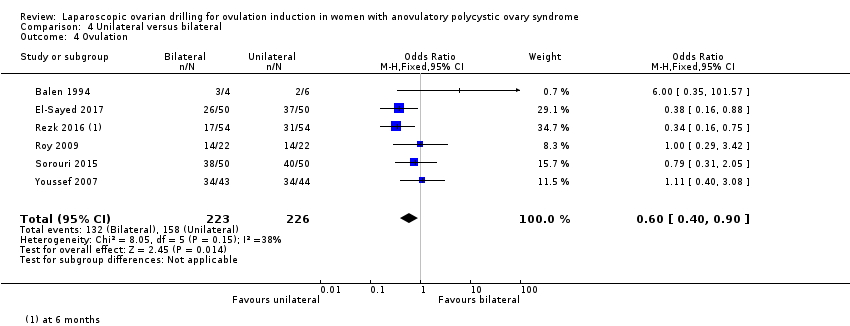
Comparison 4 Unilateral versus bilateral, Outcome 4 Ovulation.
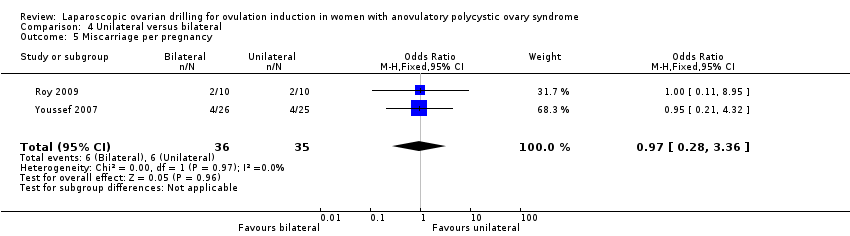
Comparison 4 Unilateral versus bilateral, Outcome 5 Miscarriage per pregnancy.

Comparison 5 Monopolar versus bipolar, Outcome 1 Clinical pregnancy.

Comparison 5 Monopolar versus bipolar, Outcome 2 Ovulation.

Comparison 6 Adjusted thermal dose versus fixed thermal dose, Outcome 1 Clinical pregnancy.

Comparison 6 Adjusted thermal dose versus fixed thermal dose, Outcome 2 Miscarriage.

Comparison 6 Adjusted thermal dose versus fixed thermal dose, Outcome 3 Ovulation.
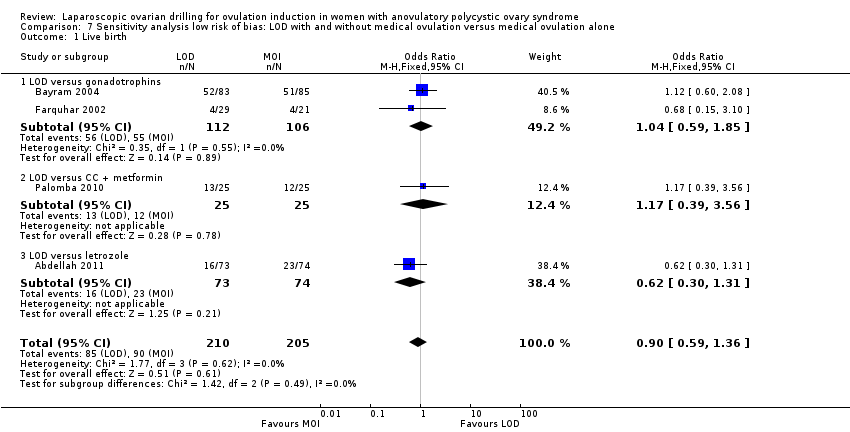
Comparison 7 Sensitivity analysis low risk of bias: LOD with and without medical ovulation versus medical ovulation alone, Outcome 1 Live birth.

Comparison 7 Sensitivity analysis low risk of bias: LOD with and without medical ovulation versus medical ovulation alone, Outcome 2 Multiple pregnancy.
| Laparoscopic ovarian drilling with and without medical ovulation compared to medical ovulation induction alone | ||||||
| Patient or population: women with anovulatory PCOS and CC resistance | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with medical ovulation induction alone | Risk with LOD ±medical ovulation | |||||
| Live birth | 418 per 1000 | 338 per 1000 | OR 0.71 | 1015 | ⊕⊕⊝⊝ | |
| Live birth (sensitivity analysis) | 439 per 1000 | 413 per 1000 (316 to 516) | OR 0.90 (0.59 to 1.36) | 415 (4 RCTs) | ⊕⊕⊝⊝ | |
| Multiple pregnancy | 50 per 1000 | 18 per 1000 | Peto OR 0.34 | 1161 | ⊕⊕⊕⊝ | |
| Clincial pregnancy | 460 per 1000 | 423 per 1000 | OR 0.86 | 2016 | ⊕⊕⊝⊝ | |
| Miscarriage | 64 per 1000 | 71 per 1000 | Peto OR 1.11 | 1909 | ⊕⊕⊝⊝ | |
| OHSS | 23 per 1000 | 6 per 1000 | Peto OR 0.25 | 722 | ⊕⊕⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded by two levels for very serious risk of bias; inadequate randomisation or allocation concealment and no evidence of blinding. bDowngraded by one level for serious risk of bias; no evidence of blinding. cDowngraded by one level for serious imprecision. | ||||||
| LOD of one ovary (unilateral) versus LOD of both ovaries (bilateral) | ||||||
| Patient or population: women with anovulatory PCOS and CC resistance | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with Bilateral | Risk with Unilateral | |||||
| Live birth | 409 per 1000 | 365 per 1000 | OR 0.83 | 44 | ⊕⊝⊝⊝ | ‐ |
| Multiple pregnancy | ‐ | ‐ | ‐ | ‐ | ‐ | No data were reported for this outcome. |
| Clinical pregnancy | 464 per 1000 | 331 per 1000 | OR 0.57 | 470 | ⊕⊕⊝⊝ | ‐ |
| Miscarriage | 91 per 1000 | 93 per 1000 | Peto OR 1.02 | 131 | ⊕⊝⊝⊝ | ‐ |
| OHSS | ‐ | ‐ | ‐ | ‐ | ‐ | No data were reported for this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded by two levels for very serious risk of bias; inadequate randomisation or allocation concealment and no evidence of blinding. bDowngraded by one level for serious imprecision. | ||||||
| Study | LOD ± CC | Other treatment | P value |
| EUR 1050 | Metformin ± CC EUR 50 | < 0.05 | |
| Total cost per patient NZD 2953 Chance of pregnancy 28% Cost per pregnancy NZD 10,938 Chance of live birth 14% Cost per live birth NZD 21,095 | Gonadotrophin Total cost per woman NZD 5461 Chance of pregnancy 33% Cost per pregnancy NZD 16,549 Chance of live birth 19% Cost per live birth NZD 28,744 | NS NS |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth Show forest plot | 9 | 1015 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.54, 0.92] |
| 1.1 LOD versus CC + metformin | 2 | 170 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.32, 1.09] |
| 1.2 LOD versus CC + tamoxifen | 1 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.42, 1.53] |
| 1.3 LOD versus gonadotrophins | 4 | 407 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.56, 1.36] |
| 1.4 LOD versus letrozole | 2 | 288 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.32, 0.92] |
| 2 Multiple pregnancy Show forest plot | 14 | 1161 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.34 [0.18, 0.66] |
| 2.1 LOD versus clomiphene citrate | 1 | 72 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 LOD versus CC + metformin | 2 | 170 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 LOD versus CC + rosiglitazone | 1 | 43 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.12 [0.21, 21.52] |
| 2.4 LOD versus gonadotrophins | 7 | 532 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.22 [0.10, 0.46] |
| 2.5 LOD versus gonadotrophins (rFSH) + metformin | 1 | 36 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 LOD versus letrozole | 1 | 147 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.7 LOD versus metformin | 1 | 161 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.32 [0.25, 6.94] |
| 3 Clinical pregnancy Show forest plot | 21 | 2016 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.72, 1.03] |
| 3.1 LOD versus clomiphene citrate | 1 | 72 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.19, 1.44] |
| 3.2 LOD versus CC + metformin | 2 | 170 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.39, 1.31] |
| 3.3 LOD versus CC + tamoxifen | 1 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.47, 1.71] |
| 3.4 LOD versus CC + rosiglitazone | 1 | 43 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.23, 2.50] |
| 3.5 LOD versus gonadotrophins | 9 | 760 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.74, 1.36] |
| 3.6 LOD versus gonadotrophins (rFSH) + metformin | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.04, 1.00] |
| 3.7 LOD versus letrozole | 3 | 368 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.42, 1.01] |
| 3.8 LOD versus letrozole + metformin | 1 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.42, 1.65] |
| 3.9 LOD versus metformin | 2 | 271 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.75, 2.08] |
| 4 Miscarriage Show forest plot | 19 | 1909 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.78, 1.59] |
| 4.1 LOD versus CC + metformin | 2 | 170 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.69, 5.54] |
| 4.2 LOD versus CC + tamoxifen | 1 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.39, 7.45] |
| 4.3 LOD versus CC + rosiglitazone | 1 | 43 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.06, 17.95] |
| 4.4 LOD versus gonadotrophins | 8 | 725 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.49, 1.33] |
| 4.5 LOD versus gonadotrophins (rFSH) + metformin | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 LOD versus letrozole | 3 | 368 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.86 [0.61, 5.67] |
| 4.7 LOD versus letrozole + metformin | 1 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.16, 3.43] |
| 4.8 LOD versus metformin | 2 | 271 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.53, 4.82] |
| 5 OHSS Show forest plot | 8 | 722 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.25 [0.07, 0.91] |
| 5.1 LOD versus clomiphene citrate | 1 | 72 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 6.82] |
| 5.2 LOD versus CC + metformin | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 LOD versus CC + rosiglitazone | 1 | 43 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 LOD versus gonadotrophins | 5 | 446 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.02, 0.64] |
| 5.5 LOD versus letrozole | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.6 LOD versus metformin | 1 | 161 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.31 [0.13, 13.44] |
| 6 Ovulation Show forest plot | 10 | 951 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.73, 1.28] |
| 6.1 LOD versus clomiphene citrate | 1 | 72 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.7 [0.27, 1.83] |
| 6.2 LOD versus CC + metformin | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.32, 3.10] |
| 6.3 LOD versus CC + tamoxifen | 1 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.56, 3.17] |
| 6.4 LOD versus CC + rosiglitazone | 1 | 43 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.13, 3.44] |
| 6.5 LOD versus gonadotrophins | 2 | 139 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.32, 1.36] |
| 6.6 LOD versus letrozole | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.23, 1.46] |
| 6.7 LOD versus letrozole + metformin | 1 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.49, 1.81] |
| 6.8 LOD versus metformin | 2 | 271 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.86, 2.68] |
| 7 Costs Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 LOD versus CC + metformin | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 3711.3 [3585.17, 3837.43] |
| 7.2 LOD versus gonadotrophins only (short‐term) | 2 | 203 | Mean Difference (IV, Fixed, 95% CI) | ‐1115.75 [‐1309.72, ‐921.77] |
| 7.3 LOD versus gonadotrophins only (long‐term) | 1 | 168 | Mean Difference (IV, Fixed, 95% CI) | ‐2235.0 [‐4433.16, ‐36.84] |
| 8 Quality of Life (Health related quality of life: SF‐36) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Physical functioning at 24 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Social functioning at 24 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Role limitations (physical) at 24 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.4 Role limitations (emotional) at 24 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.5 Mental health at 24 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.6 Vitality at 24 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.7 Pain at 24 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.8 General health at 24 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Quality of life (Rotterdam Symptom Checklist at 24 weeks) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Physical symptoms | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Psychological distress | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Activity level | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.4 Overall quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Quality of life (Depression scales (CES‐D) at 24 weeks) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 Gonadotrophins | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Multiple pregnancy per pregnancy Show forest plot | 14 | 577 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.34 [0.17, 0.66] |
| 11.1 LOD versus clomiphene citrate | 1 | 23 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 LOD versus CC + metformin | 2 | 99 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 LOD versus CC + rosiglitazone | 1 | 20 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.66 [0.24, 29.46] |
| 11.4 LOD versus gonadotrophins | 7 | 280 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.20 [0.09, 0.43] |
| 11.5 LOD versus gonadotrophins (rFSH) + metformin | 1 | 11 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.6 LOD versus letrozole | 1 | 45 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.7 LOD versus metformin | 1 | 99 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.42 [0.27, 7.53] |
| 12 Miscarriage per pregnancy Show forest plot | 19 | 900 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.88, 1.88] |
| 12.1 LOD versus CC + metformin | 2 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.49 [0.86, 7.24] |
| 12.2 LOD versus CC + tamoxifen | 1 | 78 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.87 [0.41, 8.43] |
| 12.3 LOD versus CC + rosiglitazone | 1 | 20 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.07, 23.26] |
| 12.4 LOD versus gonadotrophins | 8 | 373 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.53, 1.56] |
| 12.5 LOD versus gonadotrophins (rFSH) + metformin | 1 | 11 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.6 LOD versus letrozole | 3 | 118 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.75 [0.86, 8.79] |
| 12.7 LOD versus letrozole + metformin | 1 | 49 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.16, 4.15] |
| 12.8 LOD versus metformin | 2 | 131 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.41, 4.08] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Multiple pregnancy Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 3 Clinical pregnancy Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Miscarriage Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 OHSS Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 6 Multiple pregnancy per pregnancy Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 7 Miscarriage per pregnancy Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical pregnancy Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Miscarriage Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Ovulation Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Miscarriage per pregnancy Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Clinical pregnancy Show forest plot | 7 | 470 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.39, 0.84] |
| 3 Miscarriage Show forest plot | 2 | 131 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.31, 3.33] |
| 4 Ovulation Show forest plot | 6 | 449 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.40, 0.90] |
| 5 Miscarriage per pregnancy Show forest plot | 2 | 71 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.28, 3.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical pregnancy Show forest plot | 3 | 354 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.62, 1.44] |
| 2 Ovulation Show forest plot | 2 | 108 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.14, 0.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical pregnancy Show forest plot | 2 | 195 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.84 [1.04, 3.26] |
| 2 Miscarriage Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Ovulation Show forest plot | 2 | 195 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.01, 3.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth Show forest plot | 4 | 415 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.59, 1.36] |
| 1.1 LOD versus gonadotrophins | 2 | 218 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.59, 1.85] |
| 1.2 LOD versus CC + metformin | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.39, 3.56] |
| 1.3 LOD versus letrozole | 1 | 147 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.30, 1.31] |
| 2 Multiple pregnancy Show forest plot | 6 | 522 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.18 [0.06, 0.57] |
| 2.1 LOD versus CC + metformin | 1 | 50 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 LOD versus gonadotrophins | 3 | 253 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.18 [0.06, 0.57] |
| 2.3 LOD versus letrozole | 1 | 147 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 LOD versus clomiphene citrate | 1 | 72 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |

