Antibióticos para la rotura prematura de membranas
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised trial. No mention of method of randomisation. Not placebo‐controlled or blinded. | |
| Participants | 82 women treatment 43 control 39. Inclusions: 20‐34 weeks' pregnant. PPROM confirmed by sterile speculum. Singleton pregnancy only. | |
| Interventions | Treatment group: ampicillin 1 g IV every 6 hours for 24 hours. Maintained on oral 500 mg ampicillin 6‐hourly until delivery. In labour they were recommenced on 1 g IV ampicillin. | |
| Outcomes | Death only included. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given. |
| Allocation concealment (selection bias) | Unclear risk | No information given. |
| Blinding (performance bias and detection bias) | High risk | Not blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Data appear complete. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. |
| Other bias | Unclear risk | No information available. |
| Methods | Randomised trial ‐ no mention of the method of randomisation. | |
| Participants | 31 women with premature rupture of the membranes between 28‐34 weeks gestation. PPROM confirmed by speculum. Exclusions: women who go into active labour within 24 hours or who need induction of labour. Multiple pregnancy and fetal malformations. Women with serious medical conditions or who need antibiotic treatment for a known infection. Women who have received antibiotics in the last 10 days or who are allergic to penicillin. | |
| Interventions | Treatment group oral ampicillin 1 g 4 x daily. | |
| Outcomes | Death only included. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given. |
| Allocation concealment (selection bias) | Unclear risk | No information given. |
| Blinding (performance bias and detection bias) | High risk | Not blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Data appear complete. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. |
| Other bias | Unclear risk | No information available. |
| Methods | Randomised trial. Using sequentially numbered sealed envelopes. Not placebo‐controlled or blinded. The control group received IV fluids without antibiotics for first 24 hours. | |
| Participants | 94 women randomised 48 treatment, 46 control. Inclusions: singleton pregnancies 20‐34 weeks with PPROM confirmed by sterile speculum. | |
| Interventions | Treatment: 24 hours IV ampicillin 2 g every 6 hours for 4 doses; gentamycin 90 mg loading dose 60 mg every 8 hours for 3 doses. Then oral amoxicillin + clavulanic acid. 500 mg 3 x day for 7 days. Control IV fluids without antibiotics for 24 hours. | |
| Outcomes | Death only included. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given. |
| Allocation concealment (selection bias) | Low risk | Using sequentially numbered sealed envelopes. |
| Blinding (performance bias and detection bias) | High risk | Not blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Data appear complete. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. |
| Other bias | Unclear risk | No information given. |
| Methods | Randomised controlled trial. | |
| Participants | 62 women PPROM between 24 and 29 weeks' pregnant. Not stated whether multiple pregnancy included. | |
| Interventions | Co‐amoxiclav 3 g 6‐hourly for 4 doses then co‐amoxiclav 500 mg 6‐hourly for 5 days or matching placebo. | |
| Outcomes | Prolongation of pregnancy. | |
| Notes | Data extracted from abstract only. Further data requested from Dr Cox but not made available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given. |
| Allocation concealment (selection bias) | Unclear risk | No information given. |
| Blinding (performance bias and detection bias) | Low risk | Stated as double blind study. |
| Incomplete outcome data (attrition bias) | Low risk | Data appear complete. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. |
| Other bias | Unclear risk | No information given. |
| Methods | Randomised double‐blind, placebo‐controlled trial. A table of random numbers was used. Drugs and placebo were prepared by research nurses. The authors specify that participants and caregivers were blinded as to group. | |
| Participants | 148 women at 21‐37 weeks with premature rupture of the membranes preterm confirmed with positive nitrazine test and 'ferning' of amniotic fluid or by seeing vaginal pool of amniotic fluid from os. No tocolytics or steroids given. Multiple pregnancies included. | |
| Interventions | 4‐hourly IV 1 million units benzylpenicillin for 12‐24 hours ‐ oral 250 mg penicillin twice daily before delivery or a matched placebo. | |
| Outcomes | Latency period, infection complications and neonatal | |
| Notes | Study conducted from March 2 1989 to May 29 1991, in a single site (North Carolina, USA). 148 women. Information on neonatal death not given. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers. |
| Allocation concealment (selection bias) | Low risk | Stated that nurses were not involved in the preparation or release of either antibiotic or placebo. |
| Blinding (performance bias and detection bias) | Low risk | Patients and staff blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Data excluded for 4 women who were treated with antibiotics outside the protocol. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. |
| Other bias | Unclear risk | No information given. |
| Methods | Randomised double‐blind, placebo‐controlled trial ‐ multicentre. | |
| Participants | 105 pregnant women with PROM between 24+0 and 32+6 weeks. Exclusion criteria not clearly stated nor whether multiple pregnancy included. | |
| Interventions | Metzlocillin 2 g given 3 x day for 7 days or placebo. All women given corticosteroids and tocolytics IV. | |
| Outcomes | Prolongation of pregnancy and neonatal mortality and morbidity. | |
| Notes | 5 centres in Germany ‐ dates not given. 47 women in treatment arm and 58 in control. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given. |
| Allocation concealment (selection bias) | Unclear risk | No information given. |
| Blinding (performance bias and detection bias) | Low risk | Stated as double‐blind trial. |
| Incomplete outcome data (attrition bias) | Low risk | Data appear complete. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. |
| Other bias | Unclear risk | No information given. |
| Methods | Randomised double‐blind, placebo‐controlled trial. | |
| Participants | 60 singleton pregnancy women. Preterm PROM under 36 weeks' pregnant. Ruptured membranes confirmed by sterile speculum examination, ferning test and nitrazine test. Exclusions: > 37/40. | |
| Interventions | Erythromycin 500 mg 6‐hourly orally until delivery. Matched placebo given until delivery. | |
| Outcomes | Maternal morbidity. Neonatal mortality and morbidity. | |
| Notes | 60 women recruited during 1992 from single centre in Madrid, Spain. Paper in Spanish and data extracted with help from Dr Pigem. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | No information given. |
| Blinding (performance bias and detection bias) | Low risk | Stated as double‐blind trial. |
| Incomplete outcome data (attrition bias) | Low risk | Data appear complete. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. |
| Other bias | Unclear risk | No information given. |
| Methods | 60 women randomised to double blind placebo controlled trial. Randomisation based on random numbers tables with blocks providing 1:1 ratio and balancing every 6 women. Randomisation conducted in pharmacy. | |
| Participants | 60 women randomised. Inclusions <= 35 weeks with documented PPROM. | |
| Interventions | IV ampicillin 2 g every 6 hours for 24 hours followed by 500 mg oral ampicillin until delivery or discharge. Matched placebos. | |
| Outcomes | Maternal morbidity. Neonatal mortality and morbidity. | |
| Notes | Study divided into GBS positive and negative patients. Unclear whether clinician knew of positive culture. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation based on random numbers tables with blocks providing 1:1 ratio and balancing every 6 women. |
| Allocation concealment (selection bias) | Low risk | Randomisation and preparation of drugs conducted in pharmacy. |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind trial. |
| Incomplete outcome data (attrition bias) | Low risk | Data appear complete. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. |
| Other bias | Unclear risk | No information given. |
| Methods | Randomised double‐blind, placebo‐controlled trial. Randomisation table generated by consecutive coin toss, the randomisation schedule kept in pharmacy. | |
| Participants | 85 women randomised. Inclusions: mothers with singleton gestations between 20‐34 weeks with PPROM confirmed by sterile speculum for pooling, ferning and nitrazine paper testing. | |
| Interventions | IV mezlocillin for 48 hours followed by oral ampicillin until delivery or matched (IV + oral) placebo. | |
| Outcomes | Not clearly defined other than maternal or perinatal morbidity and mortality. | |
| Notes | Single centre ‐ University Medical Centre ‐ Jacksonville Florida. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation table generated by consecutive coin toss. |
| Allocation concealment (selection bias) | Low risk | The randomisation schedule kept in pharmacy. |
| Blinding (performance bias and detection bias) | Low risk | Randomisation schedule stated as not being available to clinicians. |
| Incomplete outcome data (attrition bias) | Low risk | Data appear complete. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. |
| Other bias | Unclear risk | No information given. |
| Methods | Randomised double‐blind, placebo‐controlled trial. | |
| Participants | 4826 women under 37 weeks' pregnant with PROM. Multiple pregnancies included. UK follow‐up at 7 years of age of the 4378 children of the 4148 eligible women who joined the ORACLE trial using a parental questionnaire. Exclusions 661 women (246 due to perinatal death, 376 randomised outside UK and 39 women withdrew). | |
| Interventions | Co‐amoxiclav 375 mg QDS, erythromycin 250 mg QDS orally for 10 days or until delivery matched placebo (2 x 2 factorial design). | |
| Outcomes | Primary outcome: neonatal death or abnormal brain scans on discharge from hospital or oxygenation at 36 weeks' postconceptual age. Functional impairment was assessed using the Mark III Multi‐Attribute Health Status classification system. Primary outcome was defined as any level of functional impairment (severe, moderate or mild). Other outcomes included death, behaviour (using the Strengths and Difficulties questionnaire) prespecified questions on respiratory symptoms, hospital admissions, convulsions, other prespecified medical conditions and demographic data. Educational attainment was evaluated for children in England using data from National Cirriculum Tests at 7 years of age (Key Stage 1). | |
| Notes | Multicentre trial (161 centres, 135 in the UK). Randomised 4826 women. 2 women lost to follow‐up and 15 women were excluded due to protocol violations. 4809 women analysed. For twin pregnancies adverse outcomes were considered present if one twin affected. Consumers involved in drawing up of protocol and information for women. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By computer using randomly generated blocks of 4. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered drug boxes of identical appearance. |
| Blinding (performance bias and detection bias) | Low risk | Stated that clinicians remained blind to treatment allocation in all but 9 cases and that all staff and participants remained blind to treatment allocation. For the follow‐up study all participants bar 1 women and all Study staff remained blind to treatment allocation. |
| Incomplete outcome data (attrition bias) | Low risk | 2 women lost to follow‐up and 15 protocol violations. In the follow‐up study outcome data were determined for 75% of eligible children. |
| Selective reporting (reporting bias) | Low risk | No selective reporting. Protocol published for follow‐up study. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Randomised double‐blind, placebo‐controlled trial. | |
| Participants | 101 women randomised between 23‐36 weeks' pregnant with visible leakage of amniotic fluid who did not go into labour within 12 hours of admission. Sterile speculum, digital examination and infection screening was performed on admission. Multiple pregnancies included. | |
| Interventions | 2 doses of IV penicillin (5 mu) or matched placebo. | |
| Outcomes | Prolongation of pregnancy. Infection, neonatal morbidity and mortality. Long‐term development at 2 years. | |
| Notes | Department of Obstetrics and Gynaecology, Helsinki, Finland. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given. |
| Allocation concealment (selection bias) | Low risk | Stated as being by sealed envelope. |
| Blinding (performance bias and detection bias) | Low risk | Stated as double‐blind trial. |
| Incomplete outcome data (attrition bias) | Low risk | Data appear complete. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. |
| Other bias | Unclear risk | No information given. |
| Methods | Randomised trial looking at 3 or 7 days antibiotic therapy. Randomised using table of arbitrary numbers in blocks of 10. Indicator cards placed in sealed envelopes which were sequentially numbered and stored on an area away from the enrolment site. | |
| Participants | 84 singleton pregnancies were randomised between 24‐34 weeks' gestation. Exclusions included known infection, absence of cervical cerclage, not penicillin allergic. Corticosteroids given to all participants. | |
| Interventions | Ampicillin‐sulbactam 3 g intravenously every six hours for either 3 or 7 days. | |
| Outcomes | Primary outcome was latency period between membrane rupture and delivery. Infection and neonatal morbidity and mortality. | |
| Notes | 3 study sites in Tennessee USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using table of arbitrary numbers in blocks of 10. |
| Allocation concealment (selection bias) | Low risk | Indicator cards placed in sealed envelopes which were sequentially numbered and stored on an area away from the enrolment site |
| Blinding (performance bias and detection bias) | Low risk | Stated that all carers were unaware of the randomisation process. |
| Incomplete outcome data (attrition bias) | Low risk | Data appear complete. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. |
| Other bias | Unclear risk | No information given. |
| Methods | Randomised double‐blind placebo‐controlled trial. | |
| Participants | 75 women randomised with a single fetus at 24‐34 completed weeks (accurate gestational age), admitted with PROM. No digital examination unless active labour. Women had infection screening. | |
| Interventions | Piperacillin 3 g IV 6‐hourly 72 hours or placebo. | |
| Outcomes | Prolongation of pregnancy. | |
| Notes | Recruitment in 3 centres (USA) from January 1987 to January 1992. 75 women were randomised (treatment 38, placebo 37). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Same deposited in pharmacy. |
| Blinding (performance bias and detection bias) | Low risk | Stated all healthcare providers were blinded to allocation. |
| Incomplete outcome data (attrition bias) | Low risk | Data appear complete. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. |
| Other bias | Unclear risk | No information given. |
| Methods | Randomised trial not placebo‐controlled. Randomisation by opening sealed consecutive opaque envelopes in admission room. | |
| Participants | 171 women randomised 84 in treatment group 87 in no treatment group. Inclusion PROM 26‐36 weeks' gestation drainage of liquor confirmed by sterile speculum. Exclusions: clinical signs of chorioamnionitis, multiple pregnancy, those with any contraindication to continuing the pregnancy and those who had just completed a course of antibiotics for another reason. | |
| Interventions | Co‐amoxiclav for 5 days. No mention of daily frequency or mg of drugs. | |
| Outcomes | Death only included. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given. |
| Allocation concealment (selection bias) | Low risk | Stated as being by sealed envelope. |
| Blinding (performance bias and detection bias) | High risk | Not blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Minimal loss to follow‐up ‐ 2 in the treatment and 1 in the no treatment group. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. |
| Other bias | Unclear risk | No information available. |
| Methods | Randomised double‐blind placebo‐controlled trial. | |
| Participants | 65 women with singleton pregnancies. | |
| Interventions | Erythromycin 333 mg 3 x daily or placebo 7 days or until active labour started. | |
| Outcomes | Prolongation of pregnancy. Maternal and neonatal infectious morbidity. | |
| Notes | July 1986‐June 1988 University Hospital Denver. No replies received to letter requesting breakdown between stillbirths and neonatal deaths and asking if Blanco's paper has ever been published. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number list. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered bottles. |
| Blinding (performance bias and detection bias) | Low risk | Stated as double‐blind trial. |
| Incomplete outcome data (attrition bias) | Low risk | Data appear complete after 10 exclusions. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. |
| Other bias | Unclear risk | No information available. |
| Methods | Randomised double‐blind, placebo‐controlled trial. | |
| Participants | Inclusions: 220 women 20‐34/6 weeks pregnant with PPROM ‐ sterile speculum and evaluation of cervix. Amniocentesis done for infection screen. Multiple pregnancies included. | |
| Interventions | Oral 333 mg erythromycin. 8‐hourly from randomisation to delivery with matched placebo. | |
| Outcomes | Not clearly stated. | |
| Notes | Single centre (Memphis, Tennessee, USA). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random number tables. |
| Allocation concealment (selection bias) | Low risk | Administered by the pharmacy. |
| Blinding (performance bias and detection bias) | Low risk | States that all involved remained blind to treatment allocation. |
| Incomplete outcome data (attrition bias) | Low risk | Data appear complete. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. |
| Other bias | Unclear risk | No information given. |
| Methods | Randomised double‐blind, placebo‐controlled trial. Urn randomisation scheme (a procedure to increase the likelihood of obtaining an equal number of subjects in each arm), stratified by centre. | |
| Participants | 614 women with PPROM at 24‐32 weeks' gestation. Inclusion criteria: membrane rupture within 36 hours of randomisation; cervical dilatation 3 cm or less on usual examination; < 5 contractions in 6 minutes. | |
| Interventions | Ampicillin 2 g 6‐hourly and erythromycin 250 mg 6‐hourly IV for 48 hours, then oral amoxacillin 250 mg every 8 hours and erythromycin 333 mg 8‐hourly for 5 days and a matching placebo regimen. | |
| Outcomes | Composite primary outcome included pregnancies complicated by at least 1 of the following: fetal or infant death, respiratory distress, severe intraventricular haemorrhage, stage 2 or 3 NEC, or sepsis within 72 hours of birth. These perinatal morbidities were also assessed separately and pregnancy prolongation assessed. | |
| Notes | 11 centres ‐ USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Urn randomisation scheme (a procedure to increase the likelihood of obtaining an equal number of subjects in each arm), stratified by centre. |
| Allocation concealment (selection bias) | Low risk | Treatment given by pharmacy. |
| Blinding (performance bias and detection bias) | Low risk | States all involved remained blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) | Low risk | Only 3 withdrawals (2 in placebo and 1 in treatment arm). |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Unclear risk | No information given. |
| Methods | Randomised trial not placebo controlled. RCT of antenatal steroids + ampicillin. 4 groups ‐ GP1 ‐ neither, GP2 steroids only, GP3 antibiotic only, GP4 both. Randomised by using sealed envelopes into 1 of groups. | |
| Participants | Randomised: 41 = GP1, 43 = GP2, 37 = GP3, 44 = GP4. | |
| Interventions | 2 g IV ampicillin every 6 hours until results of cervical cultures negative. | |
| Outcomes | Death only included. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given. |
| Allocation concealment (selection bias) | Low risk | States as sealed envelopes into 1 of 4 groups. |
| Blinding (performance bias and detection bias) | High risk | Not blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Data appear complete. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. |
| Other bias | Unclear risk | No information given. |
| Methods | Randomised double‐blind, placebo‐controlled trial. No comment as to method of randomisation. | |
| Participants | 88 women. | |
| Interventions | Clindamycin 600 mg IV every 6 hours for 48 hours + 4 mg/kg/day gentamycin IV for 48 hours followed by Clindamycin 300 mg orally every 6 hours for 5 days + gentamycin 2 mg/kg/day IM every 12 hours for 5 days. | |
| Outcomes | Prolongation of pregnancy, maternal infection related morbidity, birthweight, neonatal morbidity and admission to neonatal intensive care unit. | |
| Notes | November 1990‐September 1994. 3 sites: 2 Chile, 1 USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given. |
| Allocation concealment (selection bias) | Unclear risk | No information given. |
| Blinding (performance bias and detection bias) | Low risk | Stated as double‐blind trial. |
| Incomplete outcome data (attrition bias) | Low risk | Data appear complete with 1 loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. |
| Other bias | High risk | Trial stopped after intermediate evaluation showed treatment group had better outcome. |
| Methods | Randomised not placebo‐controlled. Randomised using sealed opaque envelopes determined by computer algorithm. | |
| Participants | 118 randomised 1 lost to follow‐up. 59 treatment 58 controls. Inclusions 24 to 34 weeks' gestation. PPROM confirmed by speculum. Exclusions in labour, clinical evidence of infection suspected fetal compromise, membrane rupture over 2 days, fetal abnormality, antibiotics in last 7 days, multiple pregnancy, cervical cerclage, prompt delivery required. | |
| Interventions | IV 1 g ampicillin 6‐hourly for 24 hours then 500 mg ampicillin orally every 6 hours. If allergic to penicillin 500 mg erythromycin used 6‐hourly. Treatment continued with delivery or diagnosis of chorioamnionitis. | |
| Outcomes | Death only included. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By computer algorithm. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelope. |
| Blinding (performance bias and detection bias) | High risk | Not blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Data appear complete ‐ 1 woman lost to follow‐up in control group. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. |
| Other bias | Unclear risk | No information given. |
| Methods | Randomised double‐blind, placebo‐controlled trial of 3 or 7 days treatment. Pharmacy provided randomisation with a computer‐generated random number table in blocks of 4. | |
| Participants | 48 women randomised: 24 in each arm‐analysis on 23 in each arm. Women 24‐33 weeks with clinically confirmed ruptured membranes. Exclusions included penicillin allergy, active labour, suspected infection, multiple pregnancy, known medical maternal or fetal problems and exposure to antibiotics within 1 week before admission. | |
| Interventions | For first 48 hours all women received parenteral ampicillin 2 g every 6 hours. Women were then randomly selected to receive either 3 or 7 days oral ampicillin. Women allocated the 3‐day course received a matching placebo. | |
| Outcomes | Primary outcome of prolongation of pregnancy for at least 7 days. Secondary outcomes included rated of chorioamnionitis, postpartum endometritis and neonatal morbidity and mortality. | |
| Notes | Study took place between September 1999 ‐ December 2001, Pennsylvania USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table in blocks of 4. |
| Allocation concealment (selection bias) | Low risk | Study medicine given by pharmacy in identical packs. |
| Blinding (performance bias and detection bias) | Low risk | Stated as double‐blind trial. |
| Incomplete outcome data (attrition bias) | Low risk | Data appear complete. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. |
| Other bias | Unclear risk | No information given. |
| Methods | Randomised double‐blind, placebo‐controlled trial. Block randomisation done using computer‐generated numbers. | |
| Participants | 67 women randomised. 26 + 0 ‐ 33 + 6 rupture of membranes, leakage of amniotic fluid at vaginal speculum examination. Preceding onset of uterine contractions. Singleton pregnancies. | |
| Interventions | Ampicillin 2 g IV 6‐hourly. 24 hours ‐ pivampicillin 500 g orally 8‐hourly for 7 days plus IV metronidazole 500 mg every 8 hours for 24 hours, followed by metronidazole 400 mg orally every 8 hours for 7 days or identical placebo. | |
| Outcomes | Latency period from admission ‐ delivery. Gestational age at delivery. Preterm delivery less than 37/40 maternal ‐ neonatal infection birthweight. | |
| Notes | October 1991‐April 1994. 6 centres around Copenhagen. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation done using computer‐generated numbers. |
| Allocation concealment (selection bias) | Unclear risk | No information available. |
| Blinding (performance bias and detection bias) | Low risk | Stated as double‐blind trial. |
| Incomplete outcome data (attrition bias) | Low risk | Data appear complete. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. |
| Other bias | Unclear risk | No information available |
cx: cervix
EDD: expected date of delivery
GBS: group B Streptococcus
GP: group
IM: intramuscular
IUCD: intrauterine contraceptive device
IUGR: intrauterine growth retardation
IV: intravenous
IVH: intraventricular haemorrhage
L/S: lecithin/sphingomyelin
NEC: necrotising enterocolitis
NICU: neonatal intensive care unit
PIH: pregnancy induced hypertension
PPROM: preterm prelabour rupture of membranes
PROM: premature rupture of membranes
QDS: four times per day
RCT: randomised controlled trial
RDS: respiratory distress syndrome
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Joint venture between Mozambique (where women were recruited), Sweden and Norway. | |
| Random allocation to 2 management protocols (conservative versus induction). | |
| Abstract only ‐ data requested. | |
| Abstract only ‐ study randomised but no mention of whether blinded. Comparison of ampicillin versus ceftriaxone (doses not given). Minimal data expressed as P values. | |
| Abstract only containing no usable data (P values only). | |
| Randomised trial of antibiotic treatment (mezlocillin) for women with PPROM. Not placebo‐controlled and no clinical outcomes reported. Mortality data requested from author. | |
| Study of 48 women with PPROM 26 to 34 weeks of pregnancy, given either oral ritodrine or cephalexin or both or neither (factorial design) ‐ not placebo‐controlled. No concealment of allocation for some participants (Latin Square method). | |
| Study that investigated active versus passive management of women with PPROM. 55 women were recruited when admitted and given antibiotics. The control group were women who presented with PPROM. 1985‐1987 before use of active protocol. Excluded as not double‐blinded, randomised or controlled. | |
| Participants allocated to treatment or no treatment group on the arbitrary basis of the last digit of the admission number (unsatisfactory concealment of allocation). No mention of blinding. | |
| This was a trial registration. The trial did not take place and no results are available. | |
| Abstract containing no usable data. GBS prophylaxis also given for carriers. | |
| Study comparing two macrolide antibiotics: i.e. comparison of similar antibiotics ‐ so excluded as this antibiotic comparison was not included in this review. | |
| Study compared antibiotic versus placebo only after 48 hour intravenous antibiotic treatment to all. | |
| Study was neither randomised nor placebo‐controlled. | |
| Study comparing a beta‐lactam antibiotic with the same antibiotic plus macrolide. This antibiotic comparison was not included in this review. | |
| Double‐blind randomised controlled trial of 1912 women but no mention of gestation at recruitment. | |
| Study comparing treatment of women with PROM at 25 to 35 weeks' gestation in a randomised blinded trial comparing ampicillin‐sulbactam with ampicillin: i.e. comparison of similar antibiotics ‐ so excluded as this antibiotic comparison was not included in this review. | |
| This is a randomised trial of corticosteroids in women with PPROM after a minimum of 12 hours ampicillin sulbactam. 77 women were enrolled. No statistically significant difference in latency period was noted. Neonatal and maternal infectious morbidity were similar. A significant reduction in the incidence of RDS, 18.4% versus 43.6%, was observed in the steroid group. | |
| Double‐blind, placebo‐controlled trial of 112 women with PPROM 23 to 25 weeks' gestation to receive ampicillin/sulbactam or ampicillin or placebo. Excluded because of a high rate of exclusions (52/164: 32%). Further information has been requested from the authors. | |
| Comparative study; not placebo‐controlled. | |
| Prospective study, not randomised, of conservative versus aggressive management of women with PPROM. Aggressive management: IV antibiotics + tocolytics. Conservative management consisted of bedrest only. | |
| Allocation by alternation. | |
| Double‐blind, placebo‐controlled trial of 84 women with PPROM (19 to 34 weeks' pregnant) who received ampicillin or placebo. 112 randomised ‐ 12 non‐compliant so excluded and 26 removed from study (does not add up). Letter sent to Mr McCaul to get excluded women's data; in the meantime, excluded. | |
| Abstract only ‐ does not say whether study was placebo‐controlled nor could any publication be found. | |
| Abstract only ‐ data in further publication. | |
| Randomised prospective study of 51 women with either PROM or SPL. Not placebo‐controlled and all women were given IV ampicillin 2 g every 6 hours until GBS status known. | |
| Randomised, double‐blind, placebo‐controlled trial of 60 women between 22 and 34 weeks pregnant with either PROM or SPL. All women were given IV ampicillin 2 g every 6 hours until GBS status known | |
| Randomised placebo‐controlled study looking at chorioamnionitis. No clear details of method of randomisation. 100 women recruited ‐71 analysed‐excluded as large number lost to follow‐up. | |
| Comparison of neonatal infection rates in 2 groups of women, with PPROM. Both groups were treated with tocolytic and steroid therapy. The first group was given antibiotic therapy continuously from onset of PPROM until delivery. The second group received antibiotic therapy for the first 3 days after PPROM and for a 3‐day period around each successive dose of corticosteroids. The study was neither randomised, nor placebo‐controlled or blinded. |
GBS: group B Streptococcus
IV: intravenous
PPROM: preterm prelabour rupture of membranes
PROM: premature rupture of membranes
RDS: respiratory distress syndrome
SPL: spontaneous preterm labour
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal death Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Any antibiotic versus placebo, Outcome 1 Maternal death. | ||||
| 1.1 Any antibiotic versus placebo | 3 | 763 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 All penicillin (excluding co‐amoxiclav) versus placebo | 1 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Beta lactum (including co‐amoxiclav) versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Macrolide (including erythromycin) versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 Other antibiotic versus placebo | 2 | 678 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Serious maternal morbidity | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Any antibiotic versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 All penicillin (excluding co‐amoxiclav) versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Beta lactum (including co‐amoxiclav) versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Macrolide (including erythromycin) versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 Other antibiotic versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Perinatal death/death before discharge Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Any antibiotic versus placebo, Outcome 3 Perinatal death/death before discharge. | ||||
| 3.1 Any antibiotic versus placebo | 12 | 6301 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.76, 1.14] |
| 3.2 All penicillin (excluding co‐amoxiclav) versus placebo | 4 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.31, 1.97] |
| 3.3 Beta lactum (including co‐amoxiclav) versus placebo | 2 | 1880 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.15, 2.56] |
| 3.4 Macrolide (including erythromycin) versus placebo | 4 | 2138 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.43, 1.60] |
| 3.5 Other antibiotic versus placebo | 3 | 762 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.68, 1.88] |
| 4 Neonatal infection including pneumonia Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.4 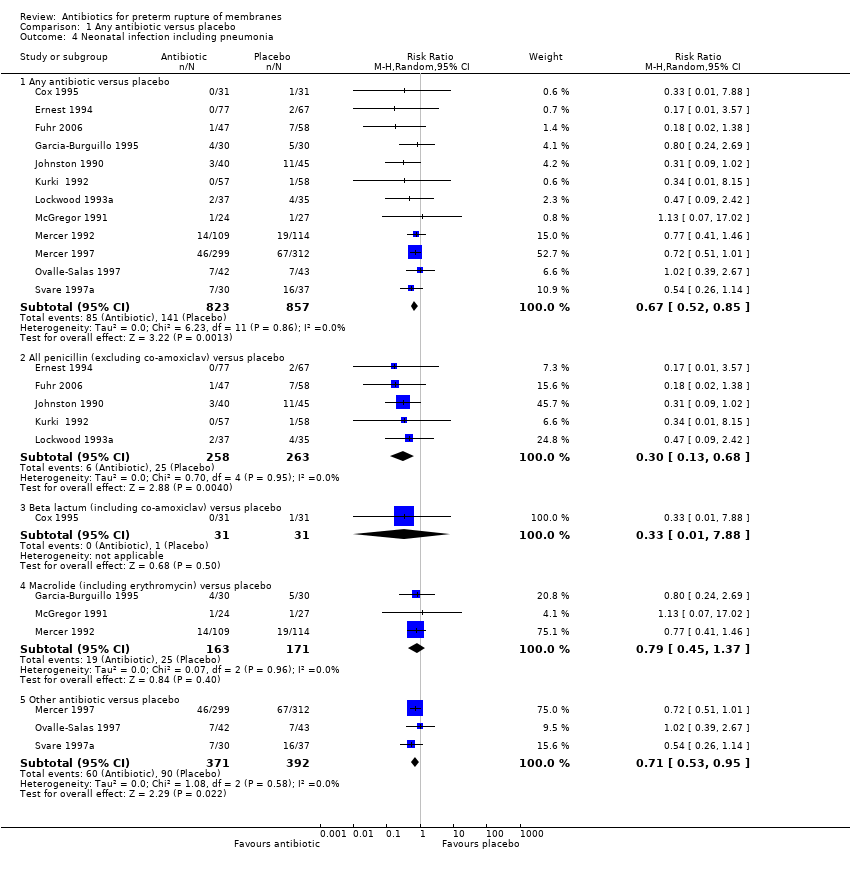 Comparison 1 Any antibiotic versus placebo, Outcome 4 Neonatal infection including pneumonia. | ||||
| 4.1 Any antibiotic versus placebo | 12 | 1680 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.52, 0.85] |
| 4.2 All penicillin (excluding co‐amoxiclav) versus placebo | 5 | 521 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.13, 0.68] |
| 4.3 Beta lactum (including co‐amoxiclav) versus placebo | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.88] |
| 4.4 Macrolide (including erythromycin) versus placebo | 3 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.45, 1.37] |
| 4.5 Other antibiotic versus placebo | 3 | 763 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.53, 0.95] |
| 5 Neonatal necrotising enterocolitis Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.5 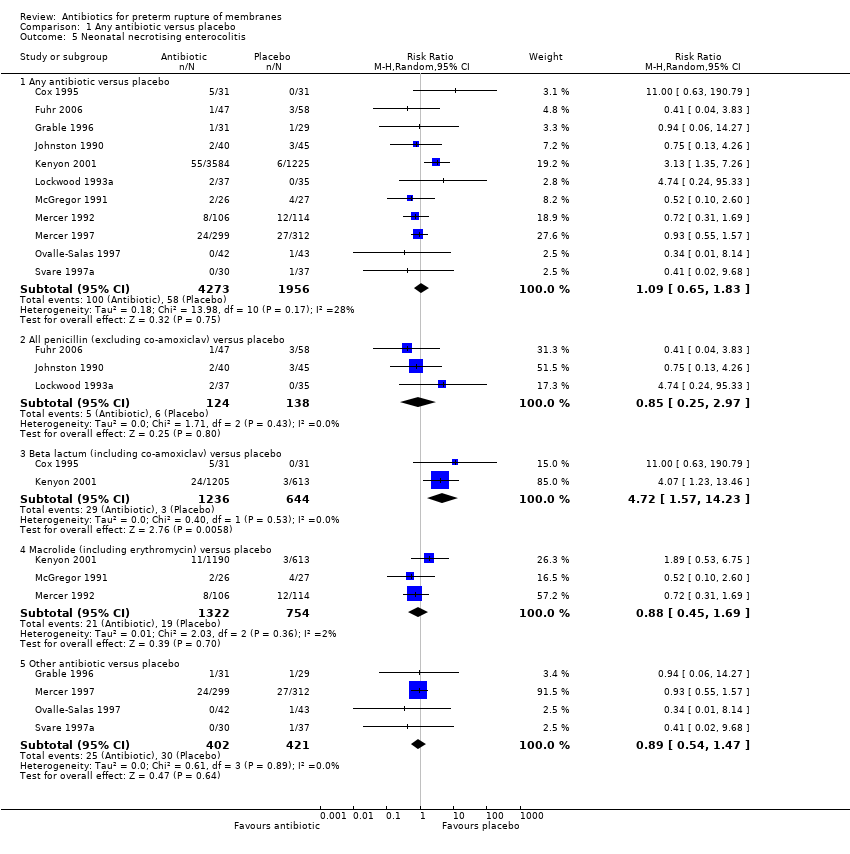 Comparison 1 Any antibiotic versus placebo, Outcome 5 Neonatal necrotising enterocolitis. | ||||
| 5.1 Any antibiotic versus placebo | 11 | 6229 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.65, 1.83] |
| 5.2 All penicillin (excluding co‐amoxiclav) versus placebo | 3 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.25, 2.97] |
| 5.3 Beta lactum (including co‐amoxiclav) versus placebo | 2 | 1880 | Risk Ratio (M‐H, Random, 95% CI) | 4.72 [1.57, 14.23] |
| 5.4 Macrolide (including erythromycin) versus placebo | 3 | 2076 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.45, 1.69] |
| 5.5 Other antibiotic versus placebo | 4 | 823 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.54, 1.47] |
| 6 Oxygen treatment > 36 weeks' postconceptual age Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Any antibiotic versus placebo, Outcome 6 Oxygen treatment > 36 weeks' postconceptual age. | ||||
| 6.1 Any antibiotic versus placebo | 1 | 4809 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.70, 1.17] |
| 6.2 All penicillin (excluding co‐amoxiclav) versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Beta lactum (including co‐amoxiclav) versus placebo | 1 | 1818 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.63, 1.36] |
| 6.4 Macrolide (including erythromycin) versus placebo | 1 | 1803 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.61, 1.32] |
| 6.5 Other antibiotic versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Major cerebral abnormality on ultrasound before discharge Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 Any antibiotic versus placebo, Outcome 7 Major cerebral abnormality on ultrasound before discharge. | ||||
| 7.1 Any antibiotic versus placebo | 12 | 6289 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.68, 0.98] |
| 7.2 All penicillin (excluding co‐amoxiclav) versus placebo | 3 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.25, 0.96] |
| 7.3 Beta lactum (including co‐amoxiclav) versus placebo | 2 | 1880 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.52, 1.16] |
| 7.4 Macrolide (including erythromycin) versus placebo | 4 | 2136 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.60, 1.44] |
| 7.5 Other antibiotic versus placebo | 4 | 823 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.45, 1.64] |
| 8 Birth before 37 weeks' gestation Show forest plot | 3 | 4931 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.98, 1.03] |
| Analysis 1.8  Comparison 1 Any antibiotic versus placebo, Outcome 8 Birth before 37 weeks' gestation. | ||||
| 9 Major adverse drug reaction Show forest plot | 3 | 5487 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 1.9  Comparison 1 Any antibiotic versus placebo, Outcome 9 Major adverse drug reaction. | ||||
| 10 Maternal infection after delivery prior to discharge Show forest plot | 4 | 5547 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.80, 1.02] |
| Analysis 1.10  Comparison 1 Any antibiotic versus placebo, Outcome 10 Maternal infection after delivery prior to discharge. | ||||
| 11 Chorioamnionitis Show forest plot | 11 | 1559 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.46, 0.96] |
| Analysis 1.11 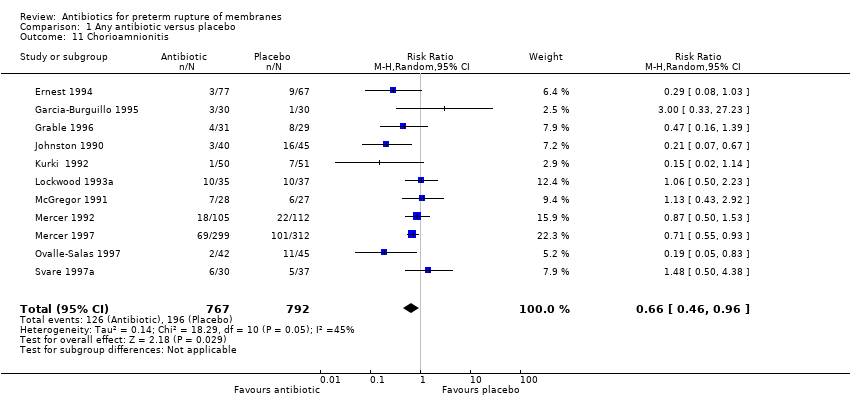 Comparison 1 Any antibiotic versus placebo, Outcome 11 Chorioamnionitis. | ||||
| 12 Caesarean section Show forest plot | 11 | 6317 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.05] |
| Analysis 1.12  Comparison 1 Any antibiotic versus placebo, Outcome 12 Caesarean section. | ||||
| 13 Days from birth till discharge of mother | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Days from randomisation to birth | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Birth within 48 hours of randomisation Show forest plot | 7 | 5927 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.58, 0.87] |
| Analysis 1.15 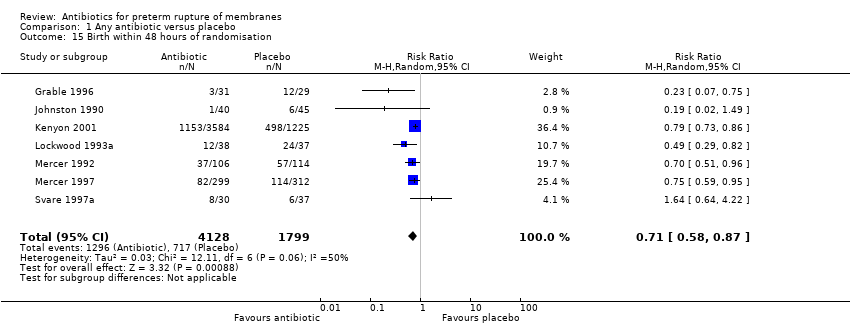 Comparison 1 Any antibiotic versus placebo, Outcome 15 Birth within 48 hours of randomisation. | ||||
| 16 Birth within 7 days of randomisation Show forest plot | 7 | 5965 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.71, 0.89] |
| Analysis 1.16  Comparison 1 Any antibiotic versus placebo, Outcome 16 Birth within 7 days of randomisation. | ||||
| 17 Birthweight Show forest plot | 12 | 6374 | Mean Difference (IV, Random, 95% CI) | 53.83 [7.06, 100.60] |
| Analysis 1.17 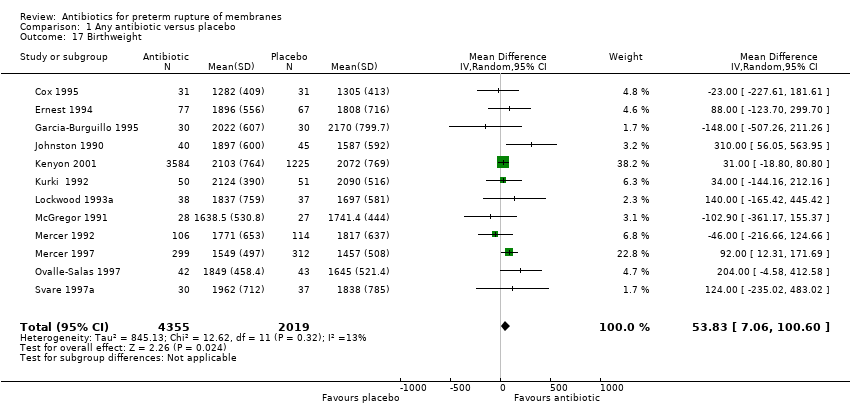 Comparison 1 Any antibiotic versus placebo, Outcome 17 Birthweight. | ||||
| 18 Birthweight < 2500 g Show forest plot | 2 | 4876 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.96, 1.04] |
| Analysis 1.18  Comparison 1 Any antibiotic versus placebo, Outcome 18 Birthweight < 2500 g. | ||||
| 19 Neonatal intensive care Show forest plot | 4 | 5023 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.84, 1.13] |
| Analysis 1.19  Comparison 1 Any antibiotic versus placebo, Outcome 19 Neonatal intensive care. | ||||
| 20 Days in neonatal intensive care unit Show forest plot | 3 | 225 | Mean Difference (IV, Random, 95% CI) | ‐5.05 [‐9.77, ‐0.33] |
| Analysis 1.20 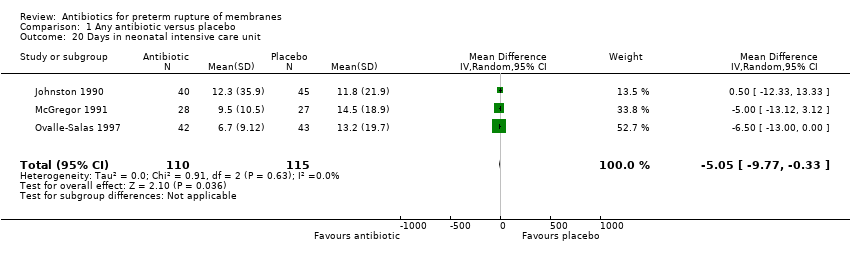 Comparison 1 Any antibiotic versus placebo, Outcome 20 Days in neonatal intensive care unit. | ||||
| 21 Positive neonatal blood culture Show forest plot | 3 | 4961 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.63, 0.99] |
| Analysis 1.21  Comparison 1 Any antibiotic versus placebo, Outcome 21 Positive neonatal blood culture. | ||||
| 22 Neonatal respiratory distress syndrome Show forest plot | 12 | 6287 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.83, 1.09] |
| Analysis 1.22  Comparison 1 Any antibiotic versus placebo, Outcome 22 Neonatal respiratory distress syndrome. | ||||
| 23 Treatment with surfactant Show forest plot | 1 | 4809 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.72, 0.96] |
| Analysis 1.23  Comparison 1 Any antibiotic versus placebo, Outcome 23 Treatment with surfactant. | ||||
| 24 Number of babies requiring ventilation Show forest plot | 2 | 4924 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.80, 1.02] |
| Analysis 1.24 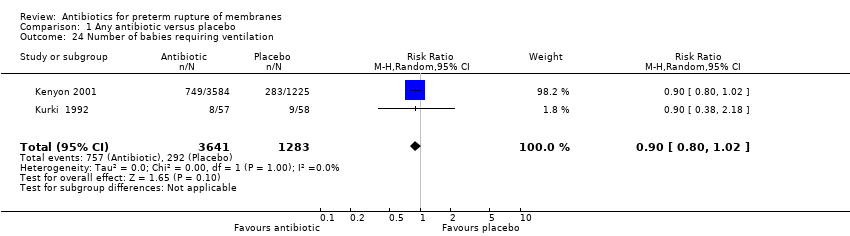 Comparison 1 Any antibiotic versus placebo, Outcome 24 Number of babies requiring ventilation. | ||||
| 25 Number of babies requiring oxygen therapy Show forest plot | 1 | 4809 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.81, 0.96] |
| Analysis 1.25  Comparison 1 Any antibiotic versus placebo, Outcome 25 Number of babies requiring oxygen therapy. | ||||
| 26 Neonatal oxygenation > 28 days Show forest plot | 3 | 5487 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.61, 1.03] |
| Analysis 1.26 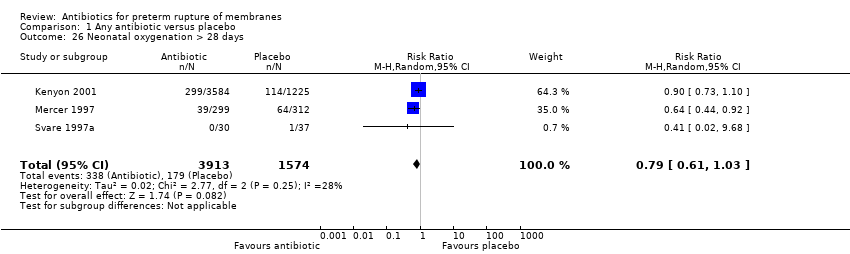 Comparison 1 Any antibiotic versus placebo, Outcome 26 Neonatal oxygenation > 28 days. | ||||
| 27 Neonatal encephalopathy Show forest plot | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 1.27  Comparison 1 Any antibiotic versus placebo, Outcome 27 Neonatal encephalopathy. | ||||
| 28 Serious childhood disability at 7 years Show forest plot | 1 | 3171 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.91, 1.12] |
| Analysis 1.28 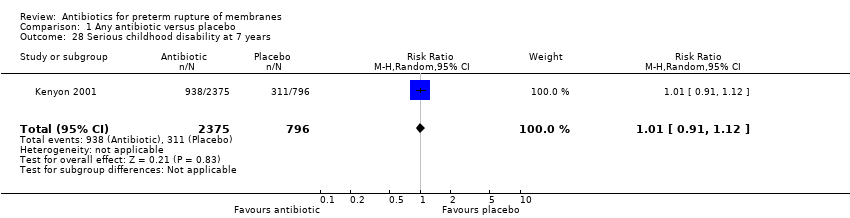 Comparison 1 Any antibiotic versus placebo, Outcome 28 Serious childhood disability at 7 years. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal death | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Serious maternal morbidity | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Major adverse drug reaction Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 2.3  Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 3 Major adverse drug reaction. | ||||
| 4 Maternal infection after delivery prior to discharge Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.87, 1.20] |
| Analysis 2.4 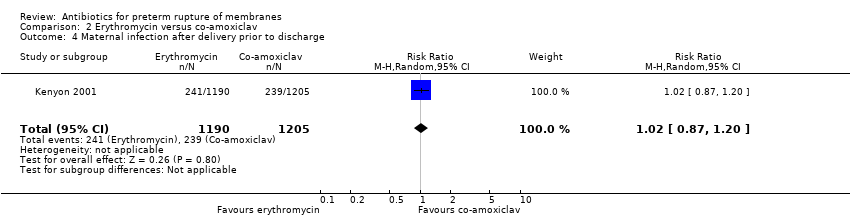 Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 4 Maternal infection after delivery prior to discharge. | ||||
| 5 Chorioamnionitis | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Caesarean section Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.90, 1.16] |
| Analysis 2.6 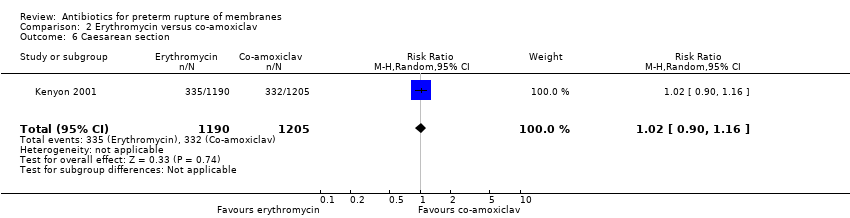 Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 6 Caesarean section. | ||||
| 7 Days from randomisation to birth | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Days from birth till discharge of mother | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Birth within 48 hours of randomisation Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [1.02, 1.28] |
| Analysis 2.9 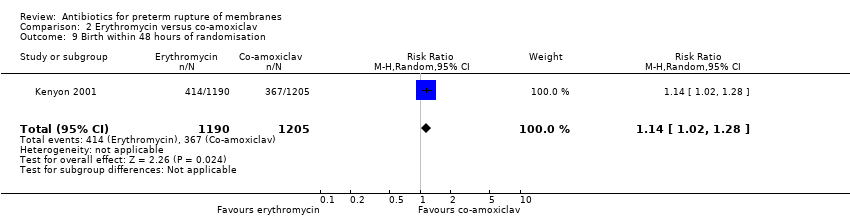 Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 9 Birth within 48 hours of randomisation. | ||||
| 10 Birth within 7 days of randomisation Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.99, 1.13] |
| Analysis 2.10 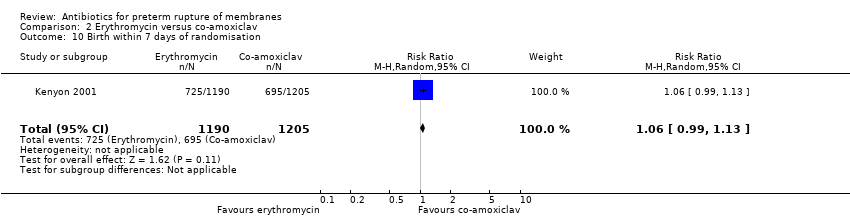 Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 10 Birth within 7 days of randomisation. | ||||
| 11 Birth before 37 weeks' gestation Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.96, 1.03] |
| Analysis 2.11 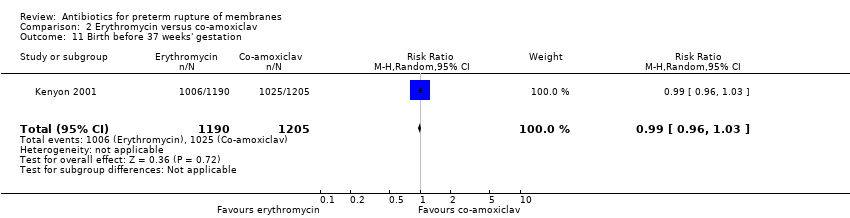 Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 11 Birth before 37 weeks' gestation. | ||||
| 12 Birthweight Show forest plot | 1 | 2395 | Mean Difference (IV, Random, 95% CI) | 19.0 [‐41.92, 79.92] |
| Analysis 2.12  Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 12 Birthweight. | ||||
| 13 Birthweight < 2500 g Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.95, 1.05] |
| Analysis 2.13  Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 13 Birthweight < 2500 g. | ||||
| 14 Neonatal intensive care Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.95, 1.05] |
| Analysis 2.14 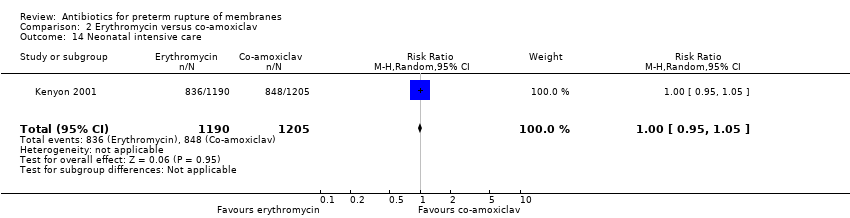 Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 14 Neonatal intensive care. | ||||
| 15 Days in neonatal intensive care unit | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Neonatal infection including pneumonia | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Positive neonatal blood culture Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.62, 1.15] |
| Analysis 2.17  Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 17 Positive neonatal blood culture. | ||||
| 18 Neonatal necrotising enterocolitis Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.23, 0.94] |
| Analysis 2.18 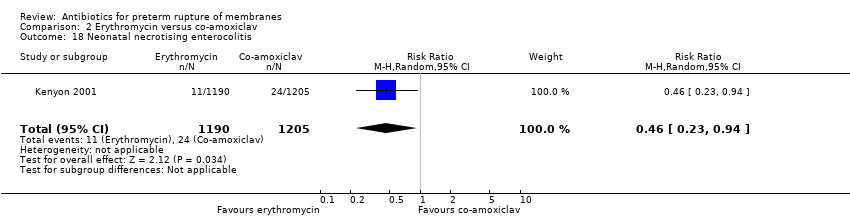 Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 18 Neonatal necrotising enterocolitis. | ||||
| 19 Neonatal respiratory distress syndrome Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.84, 1.16] |
| Analysis 2.19  Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 19 Neonatal respiratory distress syndrome. | ||||
| 20 Treatment with surfactant Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.81, 1.19] |
| Analysis 2.20 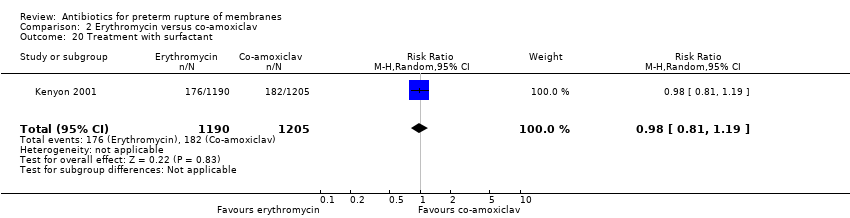 Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 20 Treatment with surfactant. | ||||
| 21 Number of babies requiring ventilation Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.86, 1.17] |
| Analysis 2.21  Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 21 Number of babies requiring ventilation. | ||||
| 22 Number of babies requiring oxygen therapy Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.87, 1.10] |
| Analysis 2.22  Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 22 Number of babies requiring oxygen therapy. | ||||
| 23 Neonatal oxygenation > 28 days Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.66, 1.12] |
| Analysis 2.23 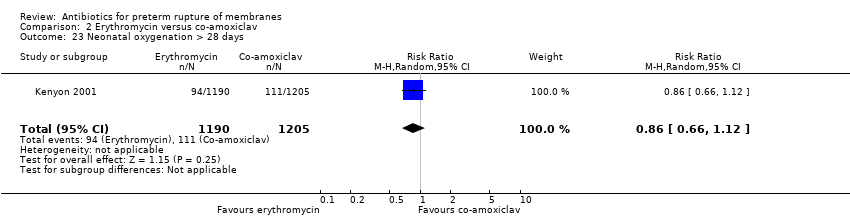 Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 23 Neonatal oxygenation > 28 days. | ||||
| 24 Oxygen treatment > 36 weeks' postconceptual age Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.70, 1.34] |
| Analysis 2.24  Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 24 Oxygen treatment > 36 weeks' postconceptual age. | ||||
| 25 Neonatal encephalopathy | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Major cerebral abnormality on ultrasound before discharge Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.74, 1.63] |
| Analysis 2.26 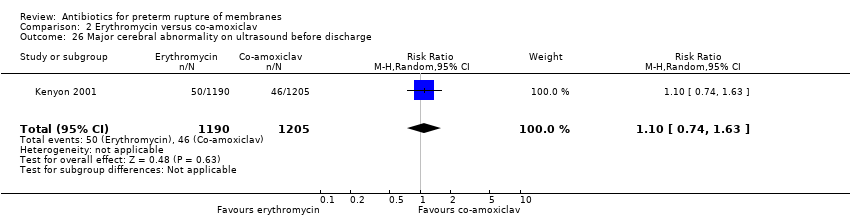 Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 26 Major cerebral abnormality on ultrasound before discharge. | ||||
| 27 Perinatal death/death before discharge Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.66, 1.23] |
| Analysis 2.27  Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 27 Perinatal death/death before discharge. | ||||
| 28 Serious childhood disability at 7 years Show forest plot | 1 | 1612 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.79, 1.01] |
| Analysis 2.28 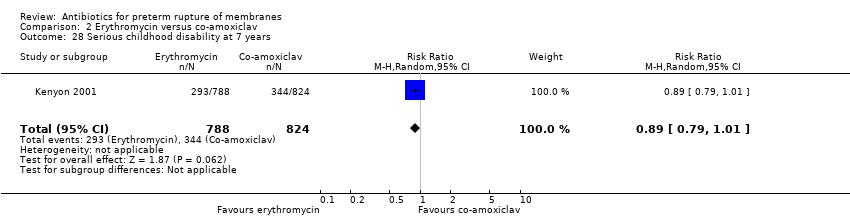 Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 28 Serious childhood disability at 7 years. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Perinatal death/death before discharge Show forest plot | 18 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.1  Comparison 4 Antibiotics versus no antibiotic, Outcome 1 Perinatal death/death before discharge. | ||||
| 1.1 Antibiotics versus no antibiotics (all studies) | 18 | 6872 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.74, 1.08] |
| 1.2 Antibiotics versus no treatment (no placebo) | 6 | 571 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.41, 1.14] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal death | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Serious maternal morbidity | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Major adverse drug reaction | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Maternal infection after delivery prior to discharge Show forest plot | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.36, 4.33] |
| Analysis 5.4 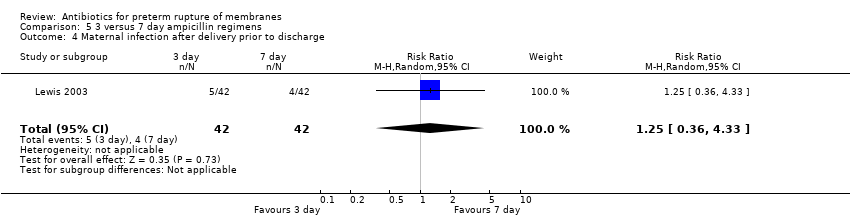 Comparison 5 3 versus 7 day ampicillin regimens, Outcome 4 Maternal infection after delivery prior to discharge. | ||||
| 5 Chorioamnionitis Show forest plot | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.33, 1.63] |
| Analysis 5.5  Comparison 5 3 versus 7 day ampicillin regimens, Outcome 5 Chorioamnionitis. | ||||
| 6 Caesarean section Show forest plot | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.72, 1.91] |
| Analysis 5.6  Comparison 5 3 versus 7 day ampicillin regimens, Outcome 6 Caesarean section. | ||||
| 7 Days from randomisation to birth | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Days from birth till discharge of mother | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Birth within 48 hours of randomisation Show forest plot | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.46, 2.87] |
| Analysis 5.9 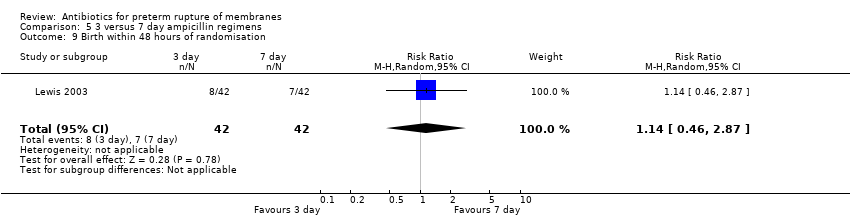 Comparison 5 3 versus 7 day ampicillin regimens, Outcome 9 Birth within 48 hours of randomisation. | ||||
| 10 Birth within 7 days of randomisation Show forest plot | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.70, 1.42] |
| Analysis 5.10  Comparison 5 3 versus 7 day ampicillin regimens, Outcome 10 Birth within 7 days of randomisation. | ||||
| 11 Birth before 37 weeks' gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Birthweight | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Birthweight < 2500 g | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Neonatal intensive care Show forest plot | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.84, 1.19] |
| Analysis 5.14 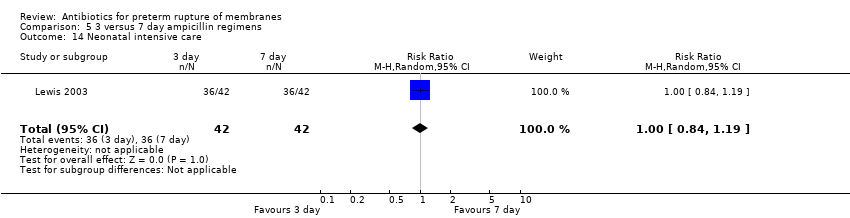 Comparison 5 3 versus 7 day ampicillin regimens, Outcome 14 Neonatal intensive care. | ||||
| 15 Days in neonatal intensive care unit | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Neonatal infection including pneumonia | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Positive neonatal blood culture | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Neonatal necrotising enterocolitis Show forest plot | 2 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.07, 2.86] |
| Analysis 5.18  Comparison 5 3 versus 7 day ampicillin regimens, Outcome 18 Neonatal necrotising enterocolitis. | ||||
| 19 Neonatal respiratory distress syndrome Show forest plot | 2 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.62, 1.49] |
| Analysis 5.19  Comparison 5 3 versus 7 day ampicillin regimens, Outcome 19 Neonatal respiratory distress syndrome. | ||||
| 20 Treatment with surfactant | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Number of babies requiring ventilation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Number of babies requiring oxygen therapy | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Neonatal oxygenation > 28 days | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Oxygen treatment > 36 weeks' postconceptual age | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Neonatal encephalopathy | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Neonatal intraventricular haemorrhage Show forest plot | 2 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 3.12] |
| Analysis 5.26  Comparison 5 3 versus 7 day ampicillin regimens, Outcome 26 Neonatal intraventricular haemorrhage. | ||||
| 27 Perinatal death/death before discharge Show forest plot | 2 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.05, 2.94] |
| Analysis 5.27 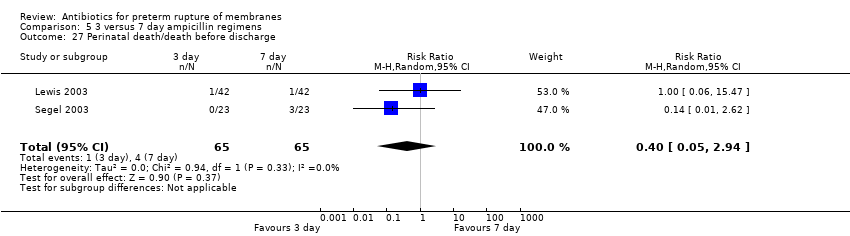 Comparison 5 3 versus 7 day ampicillin regimens, Outcome 27 Perinatal death/death before discharge. | ||||
| 28 Serious childhood disability at 7 years | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
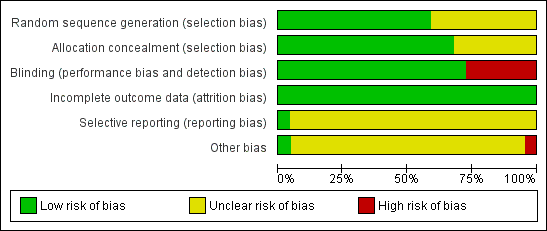
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Funnel plot of comparison: 1 Any antibiotic versus placebo, outcome: 1.3 Perinatal death/death before discharge.

Funnel plot of comparison: 1 Any antibiotic versus placebo, outcome: 1.4 Neonatal infection including pneumonia.
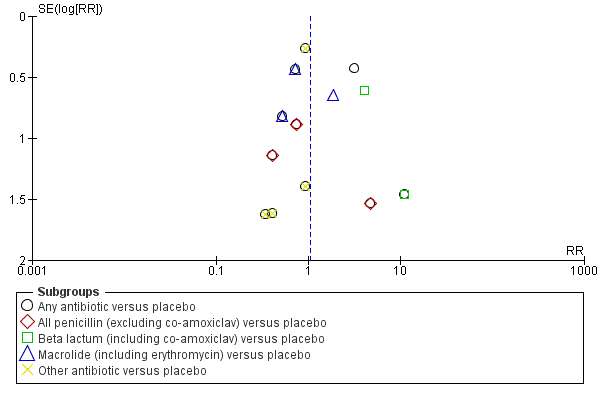
Funnel plot of comparison: 1 Any antibiotic versus placebo, outcome: 1.5 Neonatal necrotising enterocolitis.

Funnel plot of comparison: 1 Any antibiotic versus placebo, outcome: 1.7 Major cerebral abnormality on ultrasound before discharge.

Comparison 1 Any antibiotic versus placebo, Outcome 1 Maternal death.

Comparison 1 Any antibiotic versus placebo, Outcome 3 Perinatal death/death before discharge.

Comparison 1 Any antibiotic versus placebo, Outcome 4 Neonatal infection including pneumonia.

Comparison 1 Any antibiotic versus placebo, Outcome 5 Neonatal necrotising enterocolitis.

Comparison 1 Any antibiotic versus placebo, Outcome 6 Oxygen treatment > 36 weeks' postconceptual age.

Comparison 1 Any antibiotic versus placebo, Outcome 7 Major cerebral abnormality on ultrasound before discharge.

Comparison 1 Any antibiotic versus placebo, Outcome 8 Birth before 37 weeks' gestation.
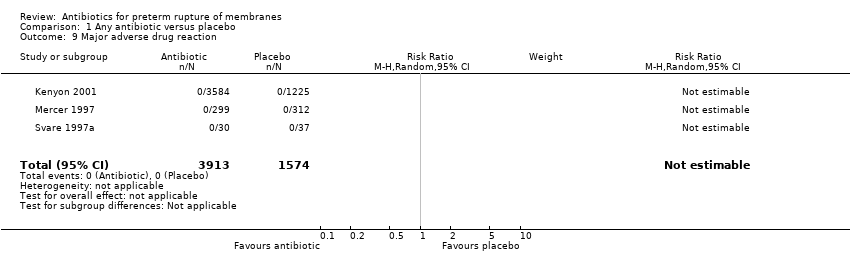
Comparison 1 Any antibiotic versus placebo, Outcome 9 Major adverse drug reaction.

Comparison 1 Any antibiotic versus placebo, Outcome 10 Maternal infection after delivery prior to discharge.

Comparison 1 Any antibiotic versus placebo, Outcome 11 Chorioamnionitis.

Comparison 1 Any antibiotic versus placebo, Outcome 12 Caesarean section.

Comparison 1 Any antibiotic versus placebo, Outcome 15 Birth within 48 hours of randomisation.
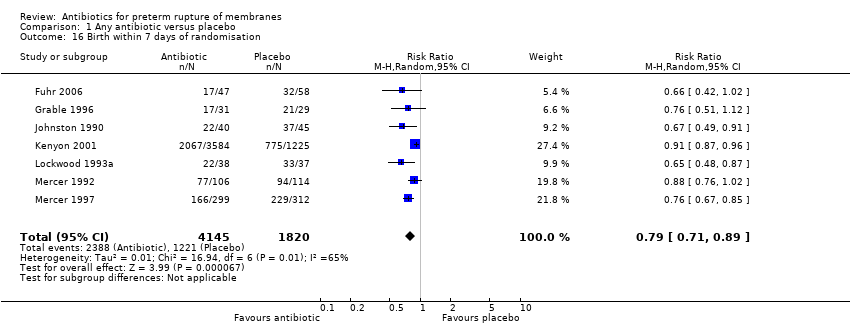
Comparison 1 Any antibiotic versus placebo, Outcome 16 Birth within 7 days of randomisation.
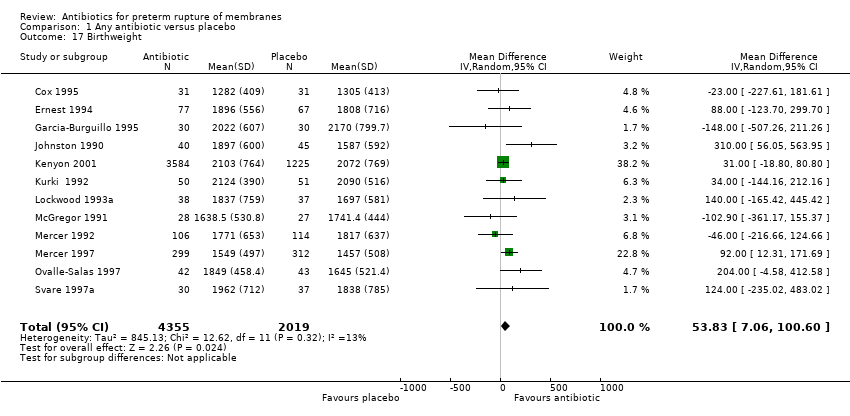
Comparison 1 Any antibiotic versus placebo, Outcome 17 Birthweight.

Comparison 1 Any antibiotic versus placebo, Outcome 18 Birthweight < 2500 g.

Comparison 1 Any antibiotic versus placebo, Outcome 19 Neonatal intensive care.

Comparison 1 Any antibiotic versus placebo, Outcome 20 Days in neonatal intensive care unit.
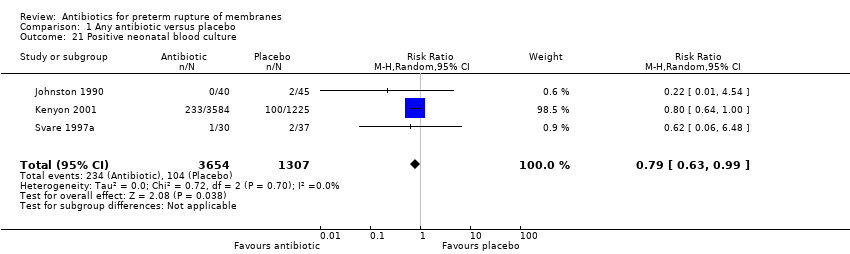
Comparison 1 Any antibiotic versus placebo, Outcome 21 Positive neonatal blood culture.

Comparison 1 Any antibiotic versus placebo, Outcome 22 Neonatal respiratory distress syndrome.

Comparison 1 Any antibiotic versus placebo, Outcome 23 Treatment with surfactant.
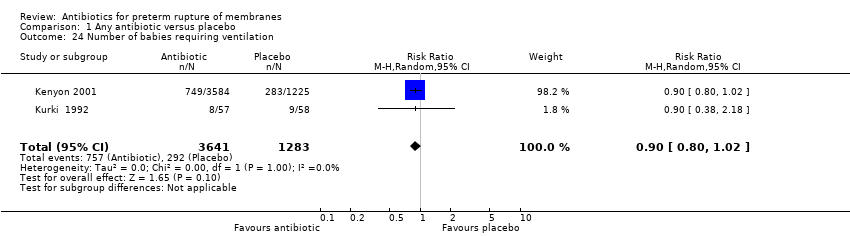
Comparison 1 Any antibiotic versus placebo, Outcome 24 Number of babies requiring ventilation.
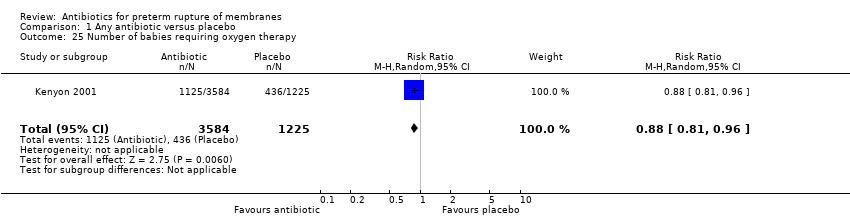
Comparison 1 Any antibiotic versus placebo, Outcome 25 Number of babies requiring oxygen therapy.

Comparison 1 Any antibiotic versus placebo, Outcome 26 Neonatal oxygenation > 28 days.
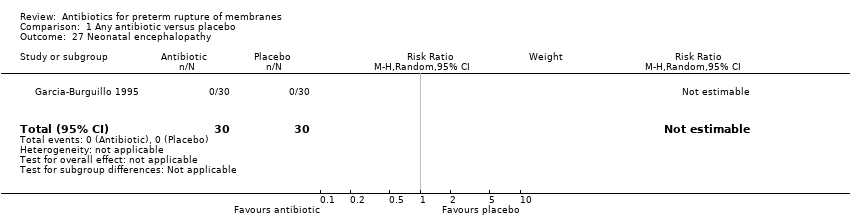
Comparison 1 Any antibiotic versus placebo, Outcome 27 Neonatal encephalopathy.

Comparison 1 Any antibiotic versus placebo, Outcome 28 Serious childhood disability at 7 years.

Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 3 Major adverse drug reaction.

Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 4 Maternal infection after delivery prior to discharge.

Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 6 Caesarean section.
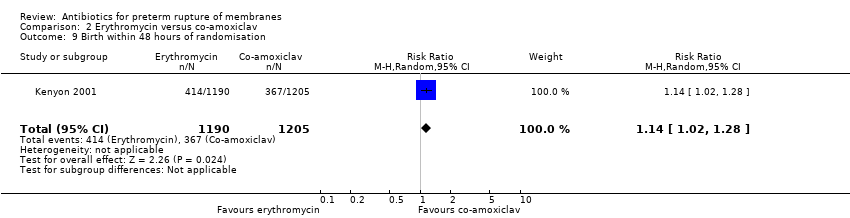
Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 9 Birth within 48 hours of randomisation.

Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 10 Birth within 7 days of randomisation.
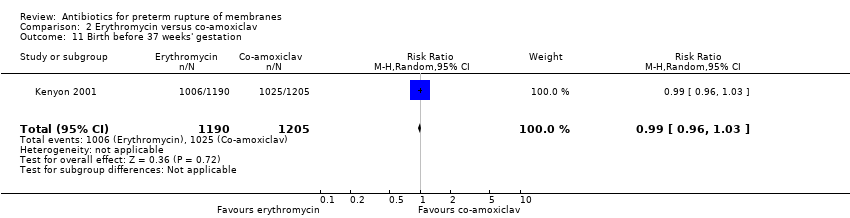
Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 11 Birth before 37 weeks' gestation.

Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 12 Birthweight.
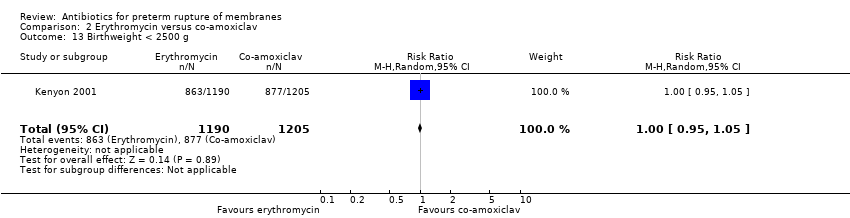
Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 13 Birthweight < 2500 g.

Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 14 Neonatal intensive care.

Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 17 Positive neonatal blood culture.

Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 18 Neonatal necrotising enterocolitis.
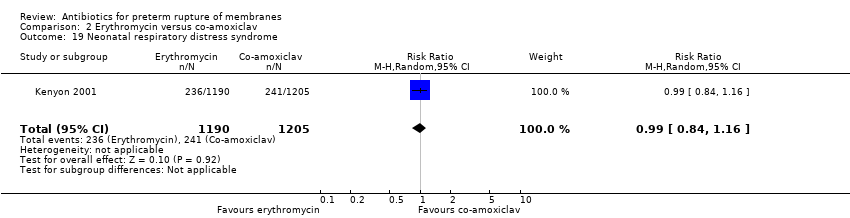
Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 19 Neonatal respiratory distress syndrome.
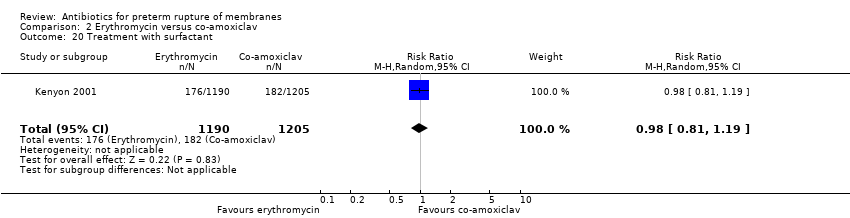
Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 20 Treatment with surfactant.
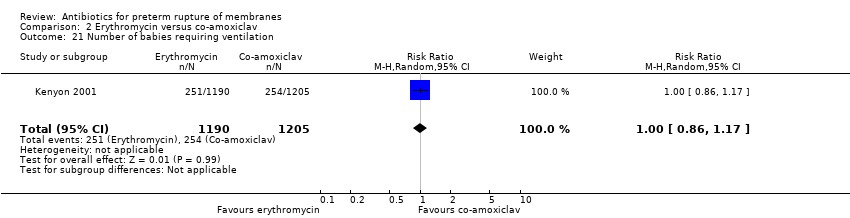
Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 21 Number of babies requiring ventilation.
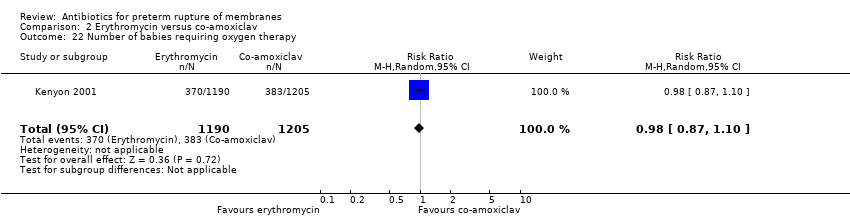
Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 22 Number of babies requiring oxygen therapy.
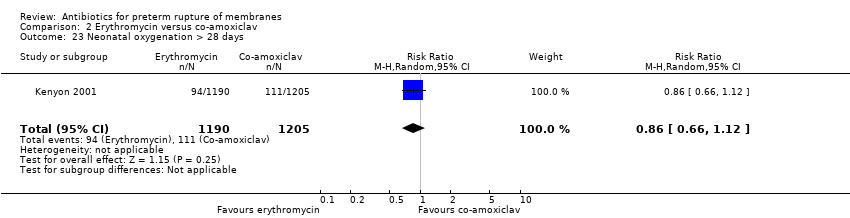
Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 23 Neonatal oxygenation > 28 days.
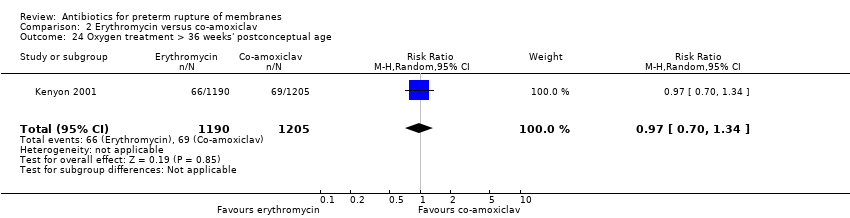
Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 24 Oxygen treatment > 36 weeks' postconceptual age.

Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 26 Major cerebral abnormality on ultrasound before discharge.
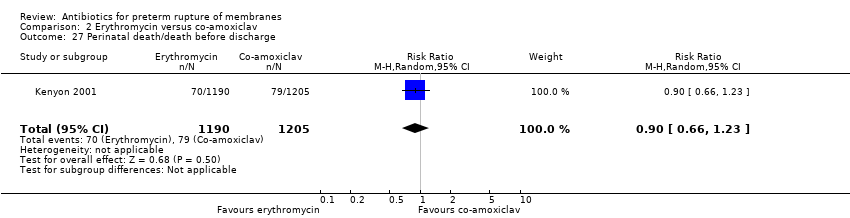
Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 27 Perinatal death/death before discharge.

Comparison 2 Erythromycin versus co‐amoxiclav, Outcome 28 Serious childhood disability at 7 years.

Comparison 4 Antibiotics versus no antibiotic, Outcome 1 Perinatal death/death before discharge.
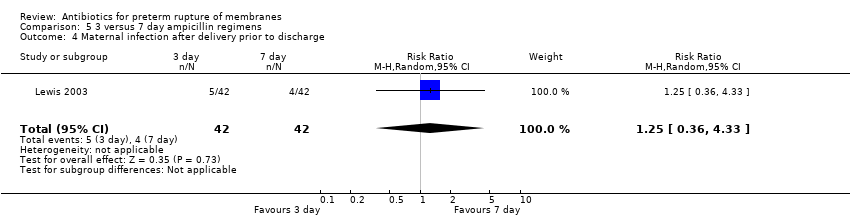
Comparison 5 3 versus 7 day ampicillin regimens, Outcome 4 Maternal infection after delivery prior to discharge.
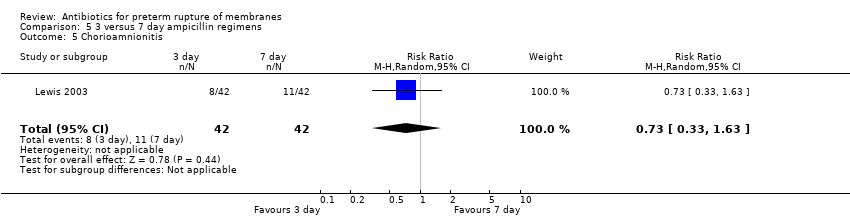
Comparison 5 3 versus 7 day ampicillin regimens, Outcome 5 Chorioamnionitis.
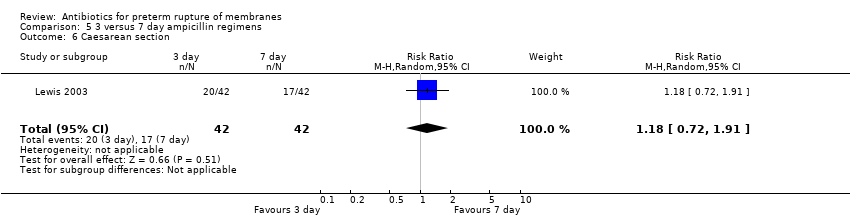
Comparison 5 3 versus 7 day ampicillin regimens, Outcome 6 Caesarean section.

Comparison 5 3 versus 7 day ampicillin regimens, Outcome 9 Birth within 48 hours of randomisation.

Comparison 5 3 versus 7 day ampicillin regimens, Outcome 10 Birth within 7 days of randomisation.

Comparison 5 3 versus 7 day ampicillin regimens, Outcome 14 Neonatal intensive care.
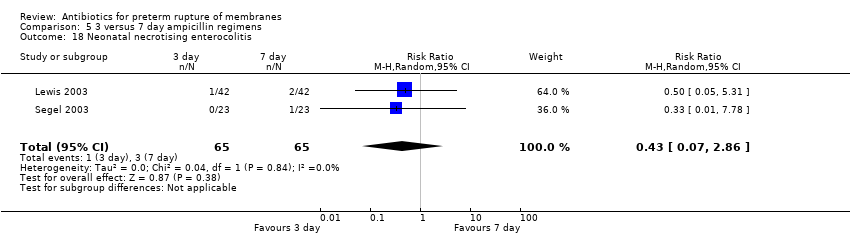
Comparison 5 3 versus 7 day ampicillin regimens, Outcome 18 Neonatal necrotising enterocolitis.

Comparison 5 3 versus 7 day ampicillin regimens, Outcome 19 Neonatal respiratory distress syndrome.
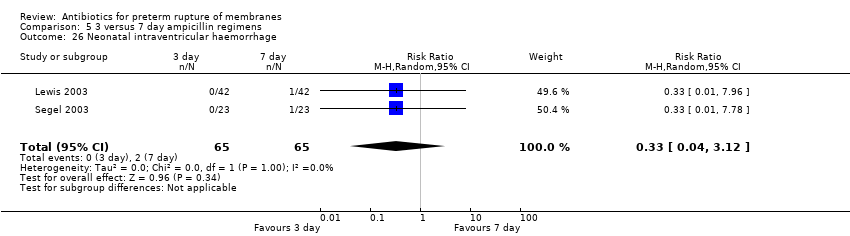
Comparison 5 3 versus 7 day ampicillin regimens, Outcome 26 Neonatal intraventricular haemorrhage.

Comparison 5 3 versus 7 day ampicillin regimens, Outcome 27 Perinatal death/death before discharge.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal death Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Any antibiotic versus placebo | 3 | 763 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 All penicillin (excluding co‐amoxiclav) versus placebo | 1 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Beta lactum (including co‐amoxiclav) versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Macrolide (including erythromycin) versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 Other antibiotic versus placebo | 2 | 678 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Serious maternal morbidity | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Any antibiotic versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 All penicillin (excluding co‐amoxiclav) versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Beta lactum (including co‐amoxiclav) versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Macrolide (including erythromycin) versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 Other antibiotic versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Perinatal death/death before discharge Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Any antibiotic versus placebo | 12 | 6301 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.76, 1.14] |
| 3.2 All penicillin (excluding co‐amoxiclav) versus placebo | 4 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.31, 1.97] |
| 3.3 Beta lactum (including co‐amoxiclav) versus placebo | 2 | 1880 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.15, 2.56] |
| 3.4 Macrolide (including erythromycin) versus placebo | 4 | 2138 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.43, 1.60] |
| 3.5 Other antibiotic versus placebo | 3 | 762 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.68, 1.88] |
| 4 Neonatal infection including pneumonia Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Any antibiotic versus placebo | 12 | 1680 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.52, 0.85] |
| 4.2 All penicillin (excluding co‐amoxiclav) versus placebo | 5 | 521 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.13, 0.68] |
| 4.3 Beta lactum (including co‐amoxiclav) versus placebo | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.88] |
| 4.4 Macrolide (including erythromycin) versus placebo | 3 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.45, 1.37] |
| 4.5 Other antibiotic versus placebo | 3 | 763 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.53, 0.95] |
| 5 Neonatal necrotising enterocolitis Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Any antibiotic versus placebo | 11 | 6229 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.65, 1.83] |
| 5.2 All penicillin (excluding co‐amoxiclav) versus placebo | 3 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.25, 2.97] |
| 5.3 Beta lactum (including co‐amoxiclav) versus placebo | 2 | 1880 | Risk Ratio (M‐H, Random, 95% CI) | 4.72 [1.57, 14.23] |
| 5.4 Macrolide (including erythromycin) versus placebo | 3 | 2076 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.45, 1.69] |
| 5.5 Other antibiotic versus placebo | 4 | 823 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.54, 1.47] |
| 6 Oxygen treatment > 36 weeks' postconceptual age Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Any antibiotic versus placebo | 1 | 4809 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.70, 1.17] |
| 6.2 All penicillin (excluding co‐amoxiclav) versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Beta lactum (including co‐amoxiclav) versus placebo | 1 | 1818 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.63, 1.36] |
| 6.4 Macrolide (including erythromycin) versus placebo | 1 | 1803 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.61, 1.32] |
| 6.5 Other antibiotic versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Major cerebral abnormality on ultrasound before discharge Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Any antibiotic versus placebo | 12 | 6289 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.68, 0.98] |
| 7.2 All penicillin (excluding co‐amoxiclav) versus placebo | 3 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.25, 0.96] |
| 7.3 Beta lactum (including co‐amoxiclav) versus placebo | 2 | 1880 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.52, 1.16] |
| 7.4 Macrolide (including erythromycin) versus placebo | 4 | 2136 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.60, 1.44] |
| 7.5 Other antibiotic versus placebo | 4 | 823 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.45, 1.64] |
| 8 Birth before 37 weeks' gestation Show forest plot | 3 | 4931 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.98, 1.03] |
| 9 Major adverse drug reaction Show forest plot | 3 | 5487 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Maternal infection after delivery prior to discharge Show forest plot | 4 | 5547 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.80, 1.02] |
| 11 Chorioamnionitis Show forest plot | 11 | 1559 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.46, 0.96] |
| 12 Caesarean section Show forest plot | 11 | 6317 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.05] |
| 13 Days from birth till discharge of mother | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Days from randomisation to birth | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Birth within 48 hours of randomisation Show forest plot | 7 | 5927 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.58, 0.87] |
| 16 Birth within 7 days of randomisation Show forest plot | 7 | 5965 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.71, 0.89] |
| 17 Birthweight Show forest plot | 12 | 6374 | Mean Difference (IV, Random, 95% CI) | 53.83 [7.06, 100.60] |
| 18 Birthweight < 2500 g Show forest plot | 2 | 4876 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.96, 1.04] |
| 19 Neonatal intensive care Show forest plot | 4 | 5023 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.84, 1.13] |
| 20 Days in neonatal intensive care unit Show forest plot | 3 | 225 | Mean Difference (IV, Random, 95% CI) | ‐5.05 [‐9.77, ‐0.33] |
| 21 Positive neonatal blood culture Show forest plot | 3 | 4961 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.63, 0.99] |
| 22 Neonatal respiratory distress syndrome Show forest plot | 12 | 6287 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.83, 1.09] |
| 23 Treatment with surfactant Show forest plot | 1 | 4809 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.72, 0.96] |
| 24 Number of babies requiring ventilation Show forest plot | 2 | 4924 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.80, 1.02] |
| 25 Number of babies requiring oxygen therapy Show forest plot | 1 | 4809 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.81, 0.96] |
| 26 Neonatal oxygenation > 28 days Show forest plot | 3 | 5487 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.61, 1.03] |
| 27 Neonatal encephalopathy Show forest plot | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Serious childhood disability at 7 years Show forest plot | 1 | 3171 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.91, 1.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal death | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Serious maternal morbidity | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Major adverse drug reaction Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Maternal infection after delivery prior to discharge Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.87, 1.20] |
| 5 Chorioamnionitis | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Caesarean section Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.90, 1.16] |
| 7 Days from randomisation to birth | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Days from birth till discharge of mother | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Birth within 48 hours of randomisation Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [1.02, 1.28] |
| 10 Birth within 7 days of randomisation Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.99, 1.13] |
| 11 Birth before 37 weeks' gestation Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.96, 1.03] |
| 12 Birthweight Show forest plot | 1 | 2395 | Mean Difference (IV, Random, 95% CI) | 19.0 [‐41.92, 79.92] |
| 13 Birthweight < 2500 g Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.95, 1.05] |
| 14 Neonatal intensive care Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.95, 1.05] |
| 15 Days in neonatal intensive care unit | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Neonatal infection including pneumonia | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Positive neonatal blood culture Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.62, 1.15] |
| 18 Neonatal necrotising enterocolitis Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.23, 0.94] |
| 19 Neonatal respiratory distress syndrome Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.84, 1.16] |
| 20 Treatment with surfactant Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.81, 1.19] |
| 21 Number of babies requiring ventilation Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.86, 1.17] |
| 22 Number of babies requiring oxygen therapy Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.87, 1.10] |
| 23 Neonatal oxygenation > 28 days Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.66, 1.12] |
| 24 Oxygen treatment > 36 weeks' postconceptual age Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.70, 1.34] |
| 25 Neonatal encephalopathy | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Major cerebral abnormality on ultrasound before discharge Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.74, 1.63] |
| 27 Perinatal death/death before discharge Show forest plot | 1 | 2395 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.66, 1.23] |
| 28 Serious childhood disability at 7 years Show forest plot | 1 | 1612 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.79, 1.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Perinatal death/death before discharge Show forest plot | 18 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Antibiotics versus no antibiotics (all studies) | 18 | 6872 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.74, 1.08] |
| 1.2 Antibiotics versus no treatment (no placebo) | 6 | 571 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.41, 1.14] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal death | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Serious maternal morbidity | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Major adverse drug reaction | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Maternal infection after delivery prior to discharge Show forest plot | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.36, 4.33] |
| 5 Chorioamnionitis Show forest plot | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.33, 1.63] |
| 6 Caesarean section Show forest plot | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.72, 1.91] |
| 7 Days from randomisation to birth | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Days from birth till discharge of mother | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Birth within 48 hours of randomisation Show forest plot | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.46, 2.87] |
| 10 Birth within 7 days of randomisation Show forest plot | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.70, 1.42] |
| 11 Birth before 37 weeks' gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Birthweight | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Birthweight < 2500 g | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Neonatal intensive care Show forest plot | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.84, 1.19] |
| 15 Days in neonatal intensive care unit | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Neonatal infection including pneumonia | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Positive neonatal blood culture | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Neonatal necrotising enterocolitis Show forest plot | 2 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.07, 2.86] |
| 19 Neonatal respiratory distress syndrome Show forest plot | 2 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.62, 1.49] |
| 20 Treatment with surfactant | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Number of babies requiring ventilation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Number of babies requiring oxygen therapy | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Neonatal oxygenation > 28 days | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Oxygen treatment > 36 weeks' postconceptual age | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Neonatal encephalopathy | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Neonatal intraventricular haemorrhage Show forest plot | 2 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 3.12] |
| 27 Perinatal death/death before discharge Show forest plot | 2 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.05, 2.94] |
| 28 Serious childhood disability at 7 years | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |

