Anticonceptivos orales para el dolor asociado con la endometriosis
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Parallel randomised controlled trial. | |
| Participants | 150 women randomised. Inclusion criteria: no details. Exclusion criteria: no details. Setting: Egypt. Timing: no details. | |
| Interventions | Monophasic oral contraceptive norethisterone 1 mg + ethinylestradiol 35 μg (PO). Leuprolide 3.75 mg (IM). 3 months' treatment. | |
| Outcomes | Biberoglu and Behram pain scores. | |
| Notes | Conference abstract only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized;" no other details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Stated double blind but no details. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Stated double blind but no details. |
| Incomplete outcome data (attrition bias) | High risk | No details on allocation to groups for final number of women analysed. |
| Selective reporting (reporting bias) | High risk | Protocol not available. Conference abstract with only 1 outcome reported. |
| Other bias | Unclear risk | Unable to judge due to lack of information. |
| Methods | Parallel randomised controlled trial. | |
| Participants | 47 women with endometriosis‐associated pain. Inclusion criteria: > 18 years of age, premenopausal, pelvic pain ≥ 3 months' duration, diagnosis of endometriosis by laparoscopy or laparotomy within 3 years of study entry, moderate‐to‐severe pelvic pain associated with endometriosis, willingness to comply with study protocol. Exclusion criteria: use of OCPs within 1 month of trial enrolment, use of leuprolide within previous 3 months if given monthly or 5 months if given 3 monthly, any contraindication to the use of OCPs or GnRH analogues, history of hysterectomy or bilateral salpingo‐oophrectomy, pregnant, breastfeeding, serious mental or chronic condition that would prevent the completion of the study. Setting: medical centre, USA. Timing: 2005‐2008. | |
| Interventions | Monophasic oral contraceptive (norethisterone 1 mg + ethinylestradiol 35 μg) given daily + placebo Leuprolide, 11.25 mg depot IM every 12 weeks with hormonal add‐back using norethisterone acetate 5 mg PO, daily + placebo; 48 weeks' treatment. | |
| Outcomes | Biberoglu and Behrman pain scores, NRS, BDI and ISS. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized;" no other details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants were blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details. |
| Incomplete outcome data (attrition bias) | High risk | Sample size calculation suggested 188 women required. Only 47 women randomised and 7 dropped out immediately after screening (3 from COCP group and 4 from leuprolide group). Only 24 women completed the trial but unclear which groups they were in. Reasons given included lack of improvement in pain symptoms, adverse effects and a decision to proceed with alternative therapies such as surgery. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. Adverse effects reported that were not prespecified in the study methods section. |
| Other bias | Low risk | Groups were balanced at baseline. |
| Methods | Multicentre (18 sites), parallel randomised controlled trial. | |
| Participants | 100 women randomised. Mean age: 31.7 (SD 5.6) years in OCP group; 31.5 (SD 6.3) years in placebo group. Inclusion criteria: aged ≥ 18 years; regular menstrual cycles (28 ± 2 days); symptomatic endometriosis (diagnosed by laparoscopy or laparotomy) or ovarian Exclusion criteria: no details. Setting: Japan. Timing: no details. | |
| Interventions | 4 cycles of treatment. Monophasic OCP (ethinylestradiol 0.035 mg + norethisterone 1 mg) for 21 days + placebo for 7 days. Placebo for 28 days. Women could continue to use analgesia of their choice during study. | |
| Outcomes | VRS and VAS to measure the severity of disability because of dysmenorrhoea in daily life and non‐menstrual pain, and the women's use of analgesics. Clinical evaluation of pelvic induration and size of ovarian endometrioma. | |
| Notes | All authors received consulting fees from Nobelpharma Co., Ltd. Tokyo, Japan, the pharmaceutical company provided the randomisation service. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Randomly assigned." "Randomization was done by the pharmaceutical company...using the permuted block method." The pharmaceutical company appeared to have been responsible for randomisation of a trial of their own drug. |
| Allocation concealment (selection bias) | High risk | "Randomization was done by the pharmaceutical company." "Allocation concealment was accomplished centrally by the company, not broken until after all data were collected." The pharmaceutical company appeared to have been responsible for allocation concealment. There were no details as to whether sequentially number opaque envelopes were used. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Both the patients and the doctors were blinded regarding the medication." As the trial was funded by the pharmaceutical company that was responsible for randomisation and allocation concealment, it is likely that it may also have communicated with the doctors who were involved in conducting the trial. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details provided on blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) | Low risk | 51 women randomised to OCP; 1 became pregnant and 1 was lost to follow‐up. 49 randomised to placebo; 2 lost to follow‐up. 14 women (7 in each group) discontinued study but had data included in the analysis. 4 women in OCP group were discontinued because of adverse effects (1 rupture of ovarian cyst; 1 nausea and headache; 1 ovarian haemorrhagic cyst; 1 oedema), 2 women were lost to follow‐up, and 1 woman took a prohibited drug. 7 women in placebo group terminated: 3 had adverse effects (1 oedema and headache; 1 ovarian haemorrhagic cyst; 1 worsened dysmenorrhoea), 3 were lost to follow‐up, and 1 used a prohibited drug. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. All prespecified outcomes appeared to be reported. |
| Other bias | High risk | Some women had radiological diagnosis of endometriosis rather than surgical diagnosis as most women had endometrioma. Groups were balanced at baseline. All authors received consulting fees from Nobelpharma Co., Ltd. Tokyo, Japan, the pharmaceutical company that provided the randomisation service. |
| Methods | Parallel, multicentre (32 sites), randomised controlled trial. 3 treatment arms (1 not used in this review). | |
| Participants | Inclusion criteria: aged ≥ 20 years with a clinical diagnosis of endometriosis who had pelvic tenderness, induration in the cul de sac or uterine immobility; diagnosed as having endometriosis by laparotomy/laparoscopy or by identification of endometriomas. Pelvic pain (defined as a VAS score ≥ 40 mm) and regular menstrual cycles (25‐38 days) during the baseline observation phase and a normal or clinically insignificant cervical smear not requiring further follow‐up collected during screening or documented within the previous 6 months. Women willing to use a barrier method of contraception for duration of study. Exclusion criteria: surgical treatment for endometriosis by laparotomy or laparoscopy within 2 months of start of study; antiphospholipid antibody syndrome; requirement for analgesics for medical reasons other than relief of endometrial pain during study (occasional use was permitted; prophylaxis was not permitted); aged ≥ 40 years with endometriomas for which the largest diameter was > 10 cm; endometriomas containing solid components; any disease or condition that was likely to worsen under hormonal treatment; receipt of hormone preparations containing progestin, oestrogen, GnRH analogues, testosterone derivatives, oestrogen antagonists, aromatase inhibitors, medications or their derivatives that were presumed to affect the secretion of sex hormones, or St John's Wort within 2 months prior to the baseline observation phase; pregnant or breastfeeding; undiagnosed abnormal vaginal bleeding; known hypersensitivity to any ingredient in study drug; and aged > 35 years who smoked or those of any age who smoked ≥ 15 cigarettes/day. Setting: Japan. Timing: October 2012 to December 2014. | |
| Interventions | Ethinylestradiol 20 μg + drospirenone 3 mg (FlexibleMIB) (130 women), 1 tablet per day. Treatment began between the first and fifth day of menstruation. Tablets administered continuously for 120 days, followed by a 4‐day tablet‐free interval. In the event of ≥3 consecutive days of spotting or bleeding (or both) on days 25‐120 of the cycle, women began and completed the 4‐day tablet‐free interval, then started next cycle of treatment. Placebo (129 women), 1 tablet daily following the same instructions as the FlexibleMIB group for 24 weeks, after which these women were changed to FlexibleMIB for the open‐label extension phase. There was also an unblinded reference arm that received dienogest (53 women). This arm was used as the reference for the extension phase and was, therefore, not included in this analysis. Randomisation was 2.5:2.5:1. | |
| Outcomes | Primary outcome: change in most severe endometriosis associated pelvic pain (VAS scale 0‐100 mm). Secondary outcomes: pelvic pain, dyspareunia, defecation pain, rated 0‐10 or by VAS) induration in the cul de sac, limitation of uterine mobility, pelvic tenderness, size and number of endometriomas, endometrial thickness, serum oestrogen and progesterone levels. Clinical Global Improvement/Change subscale of the Clinical Global Impression rating scale. Treatment satisfaction. Adverse events. | |
| Notes | At the end of the blinded 24‐week treatment phase, the arms were unblinded and the women who had been allocated to the placebo group were given the OCP for an extension phase of the trial. Trial registration: NCT01697111. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Randomization was via an Interactive Web Response system, with numbers generated by a Randomization Management Group from Bayer and was stratified by baseline visual analog scale (VAS) score <60mm vs 60mm or more." Randomisation is conducted by the pharmaceutical company sponsoring and running the trial. No details on how random numbers were generated. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding of participants and personnel (performance bias) | Unclear risk | States that placebo tablets were the same as FlexibleMIB to maintain masking but paper does not state who was blinded. Blinding was unmasked at the end of the treatment phase and women in the placebo group changed to FlexibleMIB in the extension phase. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | High risk | 20% in each group discontinued the study by 24 weeks of treatment. OCP: 130 women. 26 withdrawals: adverse event (12), withdrawal of consent (11), protocol deviation (2), wish for pregnancy (1). Placebo: 1 women did not receive treatment. 128 women. 17 withdrawals: adverse event (2), withdrawal of consent (9), lost to follow‐up (2), protocol deviation (1), wish for pregnancy (0), other (3). |
| Selective reporting (reporting bias) | Low risk | Outcomes appeared matched those of manuscript and those registered in the clinical trials registry. |
| Other bias | High risk | Declaration in paper stated that the trial was supported by Bayer Yakuhin Ltd., which participate in trial design and managed all operational aspects of the study, including monitoring data collection, statistical analysis and writing the report. The first author reported that they received advisory fees from Bayer, 1 author received speakers bureau fees from multiple pharmaceutical companies including Bayer. The other authors reported that they had nothing to disclose. |
| Methods | Parallel randomised controlled trial. | |
| Participants | 57 women with laparoscopically diagnosed endometriosis and ≥ 1 moderate or severe pain symptom as judged by both a VRS and VAS. Exclusion criteria: any treatment for endometriosis other than non‐steroidal anti‐inflammatory drugs in the preceding 3 months; ≥ 1 moderate or severe symptom on 2 separate pain scales. Setting: University Hospital, Milan, Italy. Timing: not stated. | |
| Interventions | Goserelin 3.6 mg subcutaneous depot formulation monthly for 6 months. | |
| Outcomes | Change in pain scores (VAS and VRS) after 6 months' treatment and after 6 months' follow‐up. | |
| Notes | Power calculation completed. Funded by a grant from the Italian National Research Council Applied Project "Prevention and Control of Disease Factors" subproject 5 (Fertility Control), grant no 91.00131.PF41.115.05532, Milan Italy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated that "a randomization list" was used to allocate participants to treatment group but no further details were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details were provided of method used to conceal sequence of allocation to treatment groups. |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the treatment administration (injection vs oral tablet) blinding of study participants or investigators was not undertaken. |
| Blinding of outcome assessment (detection bias) | High risk | No details provided of blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) | Low risk | 29 women were randomised to the goserelin group and data were presented for 26 women. There was 1 loss to follow‐up and 2 women did not complete the follow‐up ‐ 1 due to pregnancy and 1 requested additional hormonal therapy at end of treatment period. 28 women were randomised to OCP group and data were presented on 24 women. There was 1 loss to follow‐up. 1 woman withdrew because of headaches, and 2 women did not complete follow‐up due to requesting additional hormonal therapy at end of treatment period. |
| Selective reporting (reporting bias) | Low risk | Protocol did not appear to be available; however, all outcomes prespecified in the materials and methods section of the article were reported on. |
| Other bias | Low risk | Groups appeared balanced for age and parity. |
BDI: Beck Depression Inventory; GnRH: gonadotrophin‐releasing hormone; IM: intramuscular; ISS: Index of Sexual Satisfaction; NRS: numerical rating score; OCP: oral contraceptive pill; PO: orally; SD: standard deviation; VAS: visual analogue scale; VRS: verbal rating scale.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Ineligible study population. Participants were healthy women without endometriosis. | |
| Ineligible study design. Not a randomised controlled trial. | |
| Hormonal therapy used as an adjunct to surgery, a criterion for exclusion. | |
| Hormonal therapy used as an adjunct to surgery, a criterion for exclusion. | |
| Dose of cyproterone acetate used was 27 mg. Dose in modern contraceptive (Dianette) is 2 mg. Therefore, therapeutic effect of trial medication unlikely to be extendable to modern low‐dose contraceptives. | |
| Ineligible study design. Secondary report detailing cytoscopic changes in a small subgroup of women with bladder endometriosis participating in a larger non‐randomised trial. | |
| Hormonal therapy used as an adjunct to surgery, a criterion for exclusion. | |
| Ineligible study design. Following correspondence with authors, it was established that participants were allocated to treatment group based on chart number, not a randomised control trial. | |
| Hormonal therapy used as an adjunct to other medical treatment, a criterion for exclusion. | |
| Hormonal therapy used as an adjunct to surgery, a criterion for exclusion. | |
| Hormonal therapy used as an adjunct to surgery, a criterion for exclusion. | |
| Ineligible study population. Participants were women with menstrual‐related pelvic pain without any identifiable pelvic disease. | |
| Hormonal therapy used as an adjunct to surgery, a criterion for exclusion. | |
| Hormonal therapy used as an adjunct to surgery, a criterion for exclusion. | |
| Trial published in Bulgarian. English abstract stated that the included oral contraceptive was Ovostat, which contained ethinylestradiol 0.05 mg; therefore, did not meet inclusion criteria of a 'modern' oral contraceptive. | |
| Ineligible study population. Participants were women with primary dysmenorrhoea without any identifiable pelvic disease. | |
| Ineligible study design. Not a randomised controlled trial. | |
| Ineligible study design. Not a randomised controlled trial. | |
| Medical treatment used as an adjunct to surgery, a criterion for exclusion. | |
| Hormonal therapy used as an adjunct to surgery, a criterion for exclusion. | |
| Hormonal therapy used as an adjunct to surgery, a criterion for exclusion. | |
| Hormonal therapy used as an adjunct to surgery, a criterion for exclusion. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Self‐reported pain (dysmenorrhoea) at end of treatment Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Combined oral contractive pill (COCP) versus placebo/no treatment, Outcome 1 Self‐reported pain (dysmenorrhoea) at end of treatment. | ||||
| 1.1 Dysmenorrhoea at end of treatment: verbal rating scale (VRS) | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐1.84, ‐0.76] |
| 1.2 Dysmenorrhoea at end of treatment: visual analogue scale (VAS) | 2 | 327 | Mean Difference (IV, Fixed, 95% CI) | ‐23.68 [‐28.75, ‐18.62] |
| 1.3 Menstrual pain reduction from baseline to end of treatment: VAS) | 1 | 169 | Mean Difference (IV, Fixed, 95% CI) | 2.1 [1.38, 2.82] |
| 2 Cyclical pain (non‐menstrual) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Combined oral contractive pill (COCP) versus placebo/no treatment, Outcome 2 Cyclical pain (non‐menstrual). | ||||
| 2.1 Non‐menstrual pain at end of treatment: VRS | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.48, 0.68] |
| 2.2 Non‐menstrual pain at end of treatment: VAS | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐11.72, 7.92] |
| 2.3 Non‐menstrual pain reduction from baseline to end of treatment: VAS | 1 | 212 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [0.30, 1.70] |
| 3 Dyspareunia Show forest plot | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 1.4 [0.46, 2.34] |
| Analysis 1.3 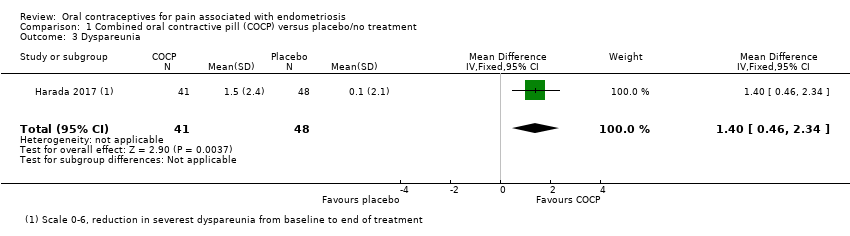 Comparison 1 Combined oral contractive pill (COCP) versus placebo/no treatment, Outcome 3 Dyspareunia. | ||||
| 4 Dyschezia (pain on defecation) Show forest plot | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | 1.2 [0.56, 1.84] |
| Analysis 1.4  Comparison 1 Combined oral contractive pill (COCP) versus placebo/no treatment, Outcome 4 Dyschezia (pain on defecation). | ||||
| 5 Satisfaction (very highly/highly satisfied) Show forest plot | 1 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.24 [2.44, 7.37] |
| Analysis 1.5  Comparison 1 Combined oral contractive pill (COCP) versus placebo/no treatment, Outcome 5 Satisfaction (very highly/highly satisfied). | ||||
| 6 Withdrawal from treatment Show forest plot | 2 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.83, 2.18] |
| Analysis 1.6  Comparison 1 Combined oral contractive pill (COCP) versus placebo/no treatment, Outcome 6 Withdrawal from treatment. | ||||
| 7 Adverse effects occurring during treatment Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 Combined oral contractive pill (COCP) versus placebo/no treatment, Outcome 7 Adverse effects occurring during treatment. | ||||
| 7.1 Pregnancy | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.88 [0.12, 68.98] |
| 7.2 Spotting/irregular bleeding/menorrhagia | 2 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.44 [1.44, 4.15] |
| 7.3 Nausea | 2 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.14 [1.79, 9.54] |
| 7.4 Any treatment‐associated adverse effect | 1 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.00, 1.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Self‐reported pain (dysmenorrhoea) at end of treatment (continuous data) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 1 Self‐reported pain (dysmenorrhoea) at end of treatment (continuous data). | ||||
| 1.1 Dysmenorrhoea at 6 months' follow‐up: visual analogue scale (VAS) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐1.28, 1.08] |
| 1.2 Dysmenorrhoea at 6 months' follow‐up: verbal rating scale (VRS) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.99, 0.79] |
| 2 Self‐reported pain (dysmenorrhoea): VAS (dichotomous data) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.2 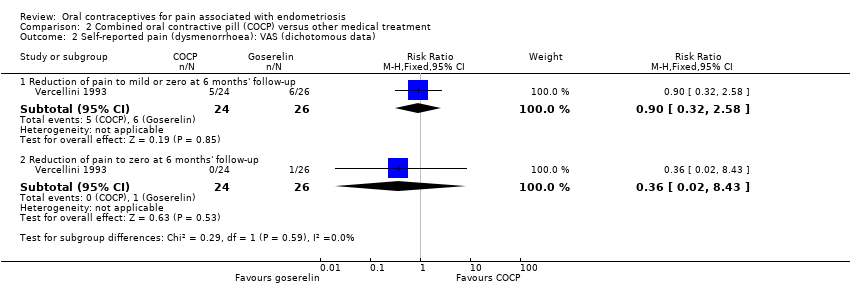 Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 2 Self‐reported pain (dysmenorrhoea): VAS (dichotomous data). | ||||
| 2.1 Reduction of pain to mild or zero at 6 months' follow‐up | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.32, 2.58] |
| 2.2 Reduction of pain to zero at 6 months' follow‐up | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.43] |
| 3 Self‐reported pain (dysmenorrhoea): VRS (dichotomous data) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 3 Self‐reported pain (dysmenorrhoea): VRS (dichotomous data). | ||||
| 3.1 Reduction of pain to mild or zero at 6 months' follow‐up | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.70, 1.28] |
| 3.2 Reduction of pain to zero at 6 months' follow‐up | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.93, 1.08] |
| 4 Cyclical pain (non‐menstrual pain) (continuous data) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.4  Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 4 Cyclical pain (non‐menstrual pain) (continuous data). | ||||
| 4.1 Non‐menstrual pain at end of treatment: VAS | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.51, 1.11] |
| 4.2 Non‐menstrual pain at end of treatment: VRS | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.51, 1.31] |
| 4.3 Non‐menstrual pain at 6 months' follow‐up: VAS | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.85, 1.25] |
| 4.4 Non‐menstrual pain at 6 months' follow‐up: VRS | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.08, 1.08] |
| 5 Cyclical pain (non‐menstrual pain) (dichotomous data) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.5  Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 5 Cyclical pain (non‐menstrual pain) (dichotomous data). | ||||
| 5.1 Reduction of pain to mild or zero at end of treatment: VAS | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.81, 1.21] |
| 5.2 Reduction of pain to zero at end of treatment: VAS | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.51, 1.89] |
| 5.3 Reduction of pain to mild or zero at end of treatment: VRS | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.84, 1.17] |
| 5.4 Reduction to zero at end of treatment: VRS | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.51, 1.89] |
| 5.5 Reduction of pain to mild or zero at 6 months' follow‐up: VAS | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.85, 1.53] |
| 5.6 Reduction of pain to zero at 6 months' follow‐up: VAS | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.26, 2.85] |
| 5.7 Reduction of pain to mild or zero at 6 months' follow‐up: VRS | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.63, 1.32] |
| 5.8 Reduction of pain to zero at 6 months' follow‐up: VRS | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.26, 2.85] |
| 6 Dyspareunia (continuous data) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.6  Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 6 Dyspareunia (continuous data). | ||||
| 6.1 Dyspareunia at end of treatment: VAS | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 1.80 [0.18, 3.42] |
| 6.2 Dyspareunia at end of treatment: VRS | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.41, 0.61] |
| 6.3 Dyspareunia at 6 months' follow‐up: VAS | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐1.30, 2.10] |
| 6.4 Dyspareunia at 6 months' follow‐up: VRS | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.47, 0.67] |
| 7 Dyspareunia (dichotomous data) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.7  Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 7 Dyspareunia (dichotomous data). | ||||
| 7.1 Reduction of pain to mild or zero at end of treatment: VAS | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.53, 1.02] |
| 7.2 Reduction of pain to zero at end of treatment: VAS | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.19, 1.48] |
| 7.3 Reduction of pain to mild or zero at end of treatment: VRS | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.56, 1.65] |
| 7.4 Reduction of pain to zero at end of treatment: VRS | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.99, 1.84] |
| 7.5 Reduction of pain to mild or zero at 6 months' follow‐up: VAS | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.62, 1.78] |
| 7.6 Reduction of pain to zero at 6 months' follow‐up: VAS | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.13, 3.77] |
| 7.7 Reduction of pain to mild or zero at 6 months' follow‐up: VRS | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.55, 1.68] |
| 7.8 Reduction of pain to zero at 6 months' follow‐up: VRS | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.13, 3.77] |
| 8 Withdrawal from treatment Show forest plot | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.34, 5.62] |
| Analysis 2.8  Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 8 Withdrawal from treatment. | ||||
| 9 Adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.9 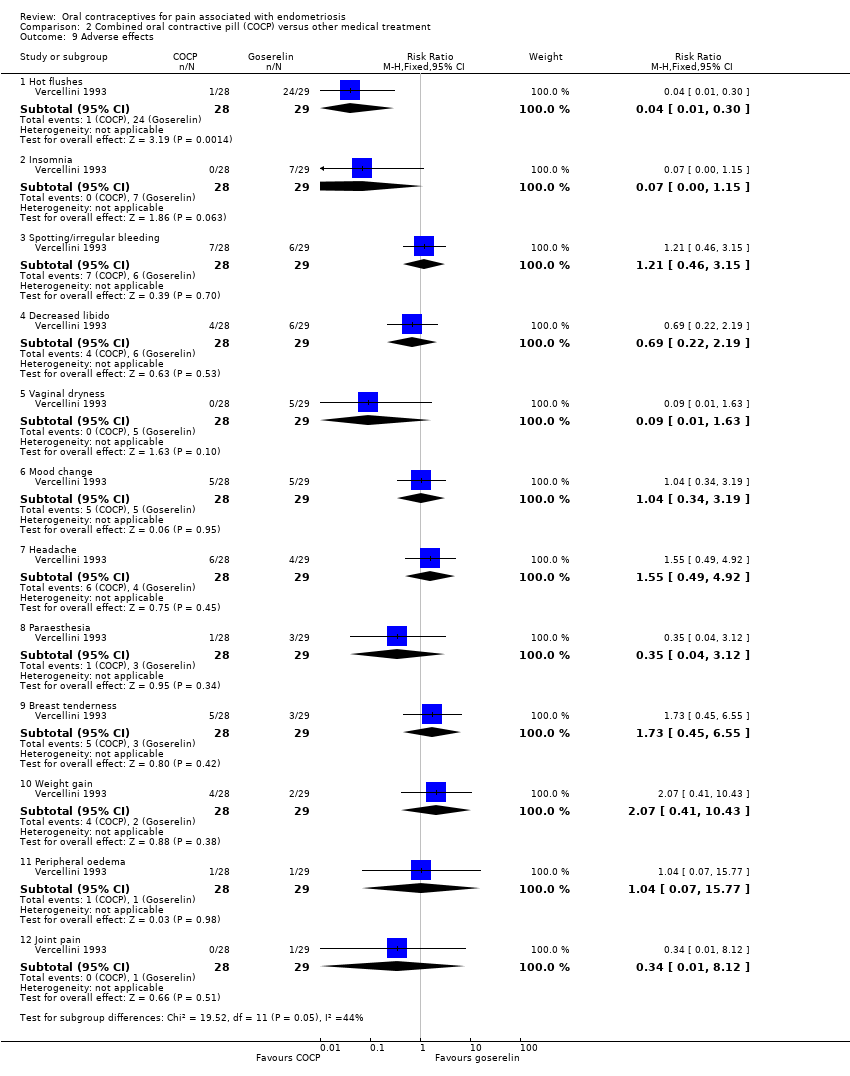 Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 9 Adverse effects. | ||||
| 9.1 Hot flushes | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.01, 0.30] |
| 9.2 Insomnia | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.00, 1.15] |
| 9.3 Spotting/irregular bleeding | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.46, 3.15] |
| 9.4 Decreased libido | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.22, 2.19] |
| 9.5 Vaginal dryness | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.63] |
| 9.6 Mood change | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.34, 3.19] |
| 9.7 Headache | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.49, 4.92] |
| 9.8 Paraesthesia | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.04, 3.12] |
| 9.9 Breast tenderness | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.45, 6.55] |
| 9.10 Weight gain | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.41, 10.43] |
| 9.11 Peripheral oedema | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.07, 15.77] |
| 9.12 Joint pain | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.12] |

Study flow diagram.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
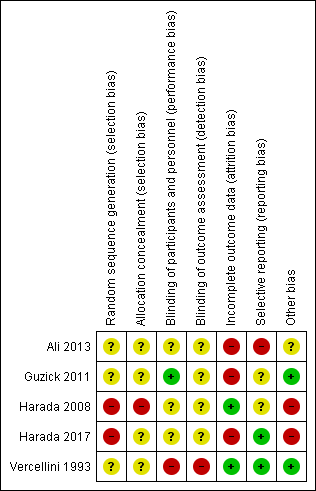
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Forest plot of comparison: 1 Combined oral contractive pill (COCP) versus placebo/no treatment, outcome: 1.1 Self‐reported pain (dysmenorrhoea) at end of treatment.

Forest plot of comparison: 2 Combined oral contractive pill (COCP) versus other medical treatment, outcome: 2.1 Self‐reported pain (dysmenorrhoea) at end of treatment (continuous data).

Forest plot of comparison: 2 Combined oral contractive pill (COCP) versus other medical treatment, outcome: 2.2 Self‐reported pain (dysmenorrhoea): VAS (dichotomous data).

Forest plot of comparison: 2 Combined oral contractive pill (COCP) versus other medical treatment, outcome: 2.3 Self‐reported pain (dysmenorrhoea): VRS (dichotomous data).
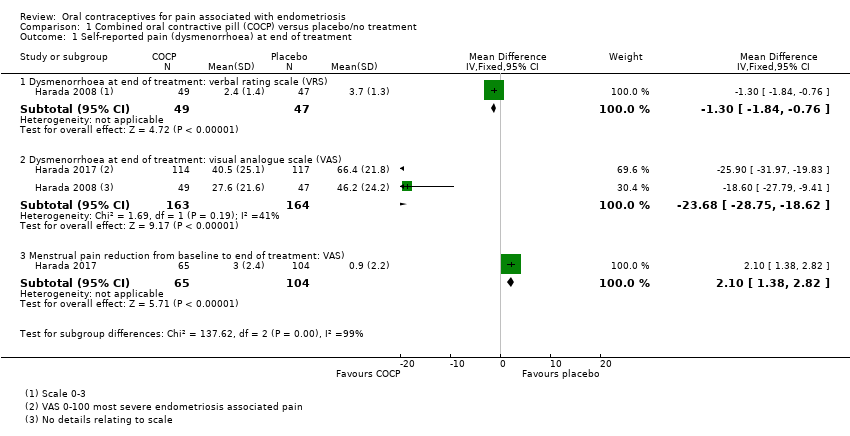
Comparison 1 Combined oral contractive pill (COCP) versus placebo/no treatment, Outcome 1 Self‐reported pain (dysmenorrhoea) at end of treatment.

Comparison 1 Combined oral contractive pill (COCP) versus placebo/no treatment, Outcome 2 Cyclical pain (non‐menstrual).

Comparison 1 Combined oral contractive pill (COCP) versus placebo/no treatment, Outcome 3 Dyspareunia.

Comparison 1 Combined oral contractive pill (COCP) versus placebo/no treatment, Outcome 4 Dyschezia (pain on defecation).

Comparison 1 Combined oral contractive pill (COCP) versus placebo/no treatment, Outcome 5 Satisfaction (very highly/highly satisfied).

Comparison 1 Combined oral contractive pill (COCP) versus placebo/no treatment, Outcome 6 Withdrawal from treatment.

Comparison 1 Combined oral contractive pill (COCP) versus placebo/no treatment, Outcome 7 Adverse effects occurring during treatment.

Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 1 Self‐reported pain (dysmenorrhoea) at end of treatment (continuous data).
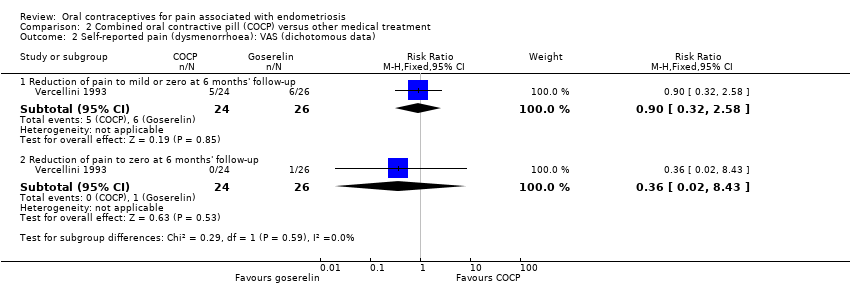
Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 2 Self‐reported pain (dysmenorrhoea): VAS (dichotomous data).

Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 3 Self‐reported pain (dysmenorrhoea): VRS (dichotomous data).
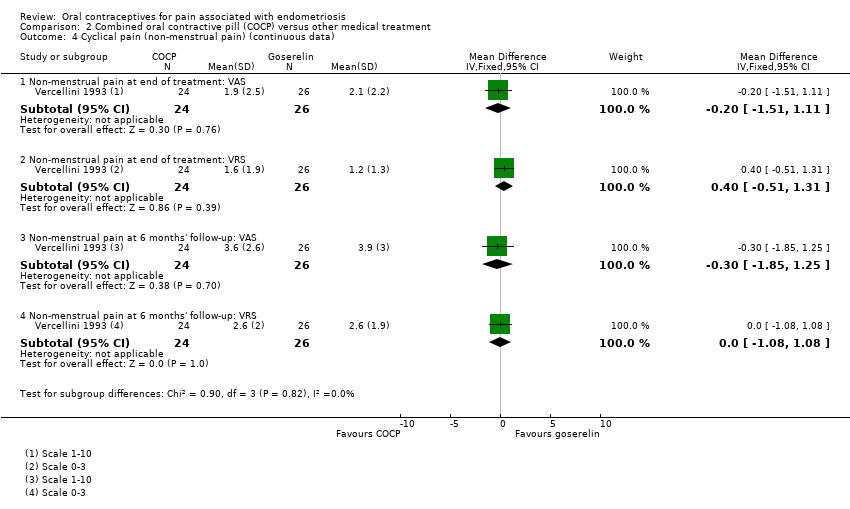
Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 4 Cyclical pain (non‐menstrual pain) (continuous data).

Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 5 Cyclical pain (non‐menstrual pain) (dichotomous data).

Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 6 Dyspareunia (continuous data).

Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 7 Dyspareunia (dichotomous data).

Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 8 Withdrawal from treatment.

Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 9 Adverse effects.
| Combined oral contraceptive pill (COCP) compared to placebo/no treatment for pain associated with endometriosis | ||||||
| Patient or population: women with endometriosis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with placebo/no treatment | Risk with COCP | |||||
| Self‐reported pain (dysmenorrhoea) at the end of treatment: dysmenorrhoea VRS | The mean self‐reported pain (dysmenorrhoea) at the end of treatment: Dysmenorrhoea VRS was 3.7 | MD 1.3 lower | ‐ | 96 | ⊕⊝⊝⊝ | VRS ranged from 0 to 3. |
| Self‐reported pain (dysmenorrhoea) at the end of treatment: dysmenorrhoea VAS | The mean self‐reported pain (dysmenorrhoea) at the end of treatment: Dysmenorrhoea VAS was 46.2 | MD 23.68 lower | ‐ | 327 | ⊕⊝⊝⊝ | No details provided for the VAS. |
| Self‐reported pain: menstrual pain reduction from baseline to end of treatment | The mean menstrual pain (reduction from baseline to end of treatment) was 3.00 | MD 2.10 lower (1.38 lower to 2.82 lower) | ‐ | 169 (1 RCT) | ⊕⊝⊝⊝ | Used a VAS from 0 to 10 where 10 was extreme pain. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; COCP: combined oral contraceptive pill; MD: mean difference; RCT: randomised controlled trial; VAS: visual analogue scale; VRS: verbal rating scale. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Imprecision: evidence was based on a single small trial; downgraded one level. 2Risk of bias: trial judged to be at high risk of bias; downgraded two levels. 3Imprecision: evidence based on a single trial including 96 women; wide confidence intervals; downgraded two levels. | ||||||
| Combined oral contraceptive pill (COCP) compared to other medical treatment for pain associated with endometriosis | ||||||
| Patient or population: women with endometriosis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with other medical treatment | Risk with COCP | |||||
| Self‐reported pain (dysmenorrhoea) at the end of treatment (continuous data): dysmenorrhoea at 6 months' follow‐up: VAS | The mean self‐reported pain (dysmenorrhoea) at the end of treatment (continuous data): dysmenorrhoea at 6 months' follow‐up was 7.5 | MD 0.1 lower | ‐ | 50 | ⊕⊝⊝⊝ | VAS ranged from 1 to 10. |
| Self‐reported pain (dysmenorrhoea) at the end of treatment (continuous data): dysmenorrhoea at 6 months' follow‐up: VRS | The mean self‐reported pain (dysmenorrhoea) at the end of treatment (continuous data): dysmenorrhoea at 6 months' follow‐up was 4.8 | MD 0.1 lower | ‐ | 50 | ⊕⊝⊝⊝ | VRS ranged from 0 to 3. |
| Self‐reported pain (dysmenorrhoea) (dichotomous data): reduction of pain to zero at 6 months' follow‐up: VAS | 38 per 1000 | 14 per 1000 | RR 0.36 | 50 | ⊕⊝⊝⊝ | VAS ranged from 1 to 10. |
| Self‐reported pain (dysmenorrhoea) (dichotomous data) ‐ reduction of pain to zero at 6 months' follow‐up: VRS | 1000 per 1000 | 1000 per 1000 | RR 1.00 | 49 | ⊕⊕⊝⊝ | VRS ranged from 0 to 3. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; COCP: combined oral contraceptive pill; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; VAS: visual analogue scale; VRS: verbal rating scale. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias: no blinding and randomisation and allocation concealment unclear; downgraded one level. 2Imprecision: evidence from a single small trial including 50 women; wide confidence intervals crossing the line of no effect; downgraded two levels. 3Imprecision: evidence from a single small trial reporting data on 49 women; downgraded one level. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Self‐reported pain (dysmenorrhoea) at end of treatment Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Dysmenorrhoea at end of treatment: verbal rating scale (VRS) | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐1.84, ‐0.76] |
| 1.2 Dysmenorrhoea at end of treatment: visual analogue scale (VAS) | 2 | 327 | Mean Difference (IV, Fixed, 95% CI) | ‐23.68 [‐28.75, ‐18.62] |
| 1.3 Menstrual pain reduction from baseline to end of treatment: VAS) | 1 | 169 | Mean Difference (IV, Fixed, 95% CI) | 2.1 [1.38, 2.82] |
| 2 Cyclical pain (non‐menstrual) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Non‐menstrual pain at end of treatment: VRS | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.48, 0.68] |
| 2.2 Non‐menstrual pain at end of treatment: VAS | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐11.72, 7.92] |
| 2.3 Non‐menstrual pain reduction from baseline to end of treatment: VAS | 1 | 212 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [0.30, 1.70] |
| 3 Dyspareunia Show forest plot | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 1.4 [0.46, 2.34] |
| 4 Dyschezia (pain on defecation) Show forest plot | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | 1.2 [0.56, 1.84] |
| 5 Satisfaction (very highly/highly satisfied) Show forest plot | 1 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.24 [2.44, 7.37] |
| 6 Withdrawal from treatment Show forest plot | 2 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.83, 2.18] |
| 7 Adverse effects occurring during treatment Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Pregnancy | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.88 [0.12, 68.98] |
| 7.2 Spotting/irregular bleeding/menorrhagia | 2 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.44 [1.44, 4.15] |
| 7.3 Nausea | 2 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.14 [1.79, 9.54] |
| 7.4 Any treatment‐associated adverse effect | 1 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.00, 1.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Self‐reported pain (dysmenorrhoea) at end of treatment (continuous data) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Dysmenorrhoea at 6 months' follow‐up: visual analogue scale (VAS) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐1.28, 1.08] |
| 1.2 Dysmenorrhoea at 6 months' follow‐up: verbal rating scale (VRS) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.99, 0.79] |
| 2 Self‐reported pain (dysmenorrhoea): VAS (dichotomous data) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Reduction of pain to mild or zero at 6 months' follow‐up | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.32, 2.58] |
| 2.2 Reduction of pain to zero at 6 months' follow‐up | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.43] |
| 3 Self‐reported pain (dysmenorrhoea): VRS (dichotomous data) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Reduction of pain to mild or zero at 6 months' follow‐up | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.70, 1.28] |
| 3.2 Reduction of pain to zero at 6 months' follow‐up | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.93, 1.08] |
| 4 Cyclical pain (non‐menstrual pain) (continuous data) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Non‐menstrual pain at end of treatment: VAS | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.51, 1.11] |
| 4.2 Non‐menstrual pain at end of treatment: VRS | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.51, 1.31] |
| 4.3 Non‐menstrual pain at 6 months' follow‐up: VAS | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.85, 1.25] |
| 4.4 Non‐menstrual pain at 6 months' follow‐up: VRS | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.08, 1.08] |
| 5 Cyclical pain (non‐menstrual pain) (dichotomous data) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Reduction of pain to mild or zero at end of treatment: VAS | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.81, 1.21] |
| 5.2 Reduction of pain to zero at end of treatment: VAS | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.51, 1.89] |
| 5.3 Reduction of pain to mild or zero at end of treatment: VRS | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.84, 1.17] |
| 5.4 Reduction to zero at end of treatment: VRS | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.51, 1.89] |
| 5.5 Reduction of pain to mild or zero at 6 months' follow‐up: VAS | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.85, 1.53] |
| 5.6 Reduction of pain to zero at 6 months' follow‐up: VAS | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.26, 2.85] |
| 5.7 Reduction of pain to mild or zero at 6 months' follow‐up: VRS | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.63, 1.32] |
| 5.8 Reduction of pain to zero at 6 months' follow‐up: VRS | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.26, 2.85] |
| 6 Dyspareunia (continuous data) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Dyspareunia at end of treatment: VAS | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 1.80 [0.18, 3.42] |
| 6.2 Dyspareunia at end of treatment: VRS | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.41, 0.61] |
| 6.3 Dyspareunia at 6 months' follow‐up: VAS | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐1.30, 2.10] |
| 6.4 Dyspareunia at 6 months' follow‐up: VRS | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.47, 0.67] |
| 7 Dyspareunia (dichotomous data) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Reduction of pain to mild or zero at end of treatment: VAS | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.53, 1.02] |
| 7.2 Reduction of pain to zero at end of treatment: VAS | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.19, 1.48] |
| 7.3 Reduction of pain to mild or zero at end of treatment: VRS | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.56, 1.65] |
| 7.4 Reduction of pain to zero at end of treatment: VRS | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.99, 1.84] |
| 7.5 Reduction of pain to mild or zero at 6 months' follow‐up: VAS | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.62, 1.78] |
| 7.6 Reduction of pain to zero at 6 months' follow‐up: VAS | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.13, 3.77] |
| 7.7 Reduction of pain to mild or zero at 6 months' follow‐up: VRS | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.55, 1.68] |
| 7.8 Reduction of pain to zero at 6 months' follow‐up: VRS | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.13, 3.77] |
| 8 Withdrawal from treatment Show forest plot | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.34, 5.62] |
| 9 Adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Hot flushes | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.01, 0.30] |
| 9.2 Insomnia | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.00, 1.15] |
| 9.3 Spotting/irregular bleeding | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.46, 3.15] |
| 9.4 Decreased libido | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.22, 2.19] |
| 9.5 Vaginal dryness | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.63] |
| 9.6 Mood change | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.34, 3.19] |
| 9.7 Headache | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.49, 4.92] |
| 9.8 Paraesthesia | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.04, 3.12] |
| 9.9 Breast tenderness | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.45, 6.55] |
| 9.10 Weight gain | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.41, 10.43] |
| 9.11 Peripheral oedema | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.07, 15.77] |
| 9.12 Joint pain | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.12] |

