Inositol in preterm infants at risk for or having respiratory distress syndrome
Información
- DOI:
- https://doi.org/10.1002/14651858.CD000366.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 04 febrero 2015see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Neonatología
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
All review authors contributed to all stages of this update of the review in 2014.
Sources of support
Internal sources
-
Izaak Walton Killam Health Centre, Halifax, Nova Scotia, Canada.
-
Mount Sinai Hospital, Toronto, Ontario, Canada.
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA.
The Cochrane Neonatal Review Group has been funded in part with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN267200603418C
Declarations of interest
Dr Alexandra Howlett ‐ none.
Dr Arne Ohlsson ‐ none.
Dr Nishad Plakkal ‐ none.
Acknowledgements
We are thankful to Dr Friedman who provided us with additional unpublished information related to the abstract publication. We are grateful to Dr Phelps who drew our attention to her ongoing study in 2007. For this update we obtained clarifying information regarding the Phelps 2012NCT01954082; Phelps 2013 and NCT01954082 studies.
The editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN267200603418C.
Version history
| Published | Title | Stage | Authors | Version |
| 2019 Jul 08 | Inositol in preterm infants at risk for or having respiratory distress syndrome | Review | Alexandra Howlett, Arne Ohlsson, Nishad Plakkal | |
| 2015 Feb 04 | Inositol in preterm infants at risk for or having respiratory distress syndrome | Review | Alexandra Howlett, Arne Ohlsson, Nishad Plakkal | |
| 2012 Mar 14 | Inositol for respiratory distress syndrome in preterm infants | Review | Alexandra Howlett, Arne Ohlsson, Nishad Plakkal | |
| 2003 Oct 20 | Inositol for respiratory distress syndrome in preterm infants | Review | Alexandra Howlett, Arne Ohlsson | |
Differences between protocol and review
For the original review and previous updates of the review the main comparison has been 'inositol supplementation versus control' (Comparison 1) and we included studies under this comparison that provided repeated doses of inositol to the infants. For this update we identified one dose‐finding study in which infants were supplemented with a single dose of inositol (Phelps 2013). We did not consider it appropriate to include the results of this study in the meta‐analyses of repeated doses of inositol and we have changed the first comparison to read: 'inositol supplementation (repeat doses) versus control' (Comparison 1) and added a second comparison that reads 'inositol supplementation (single dose) versus control' (Comparison 2). These different dosing regimens were not known at the protocol stage and we have made a deviation from the protocol and included single doses of inositol in our review as those analyses provide important information. The infants in the study by Phelps 2013 were not included based on whether they had respiratory distress syndrome or not. To justify inclusion of this study we changed the title of the review to 'Inositol in preterm infants at risk for or having respiratory distress syndrome'. For this update we changed the objectives to read: to assess the effectiveness and safety of supplementary inositol in preterm infants with or without respiratory distress syndrome (RDS) in reducing adverse neonatal outcomes. Outcomes were not reported in an identical manner in the repeat doses of inositol studies and the single dose of inositol study (Phelps 2013). We accepted the outcomes and their definitions reported in the Phelps 2013 study.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans; Infant, Newborn;
PICO

Forest plot of comparison: 1 Inositol supplementation versus control, outcome: 1.1 Neonatal death (age < 28 days).
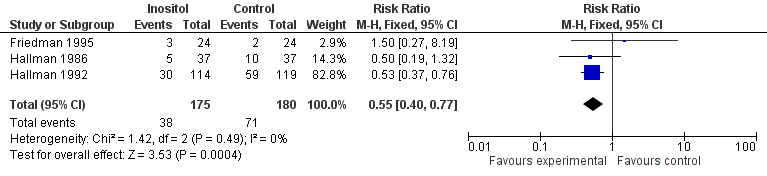
Forest plot of comparison: 1 Inositol supplementation versus control, outcome: 1.2 Infant death (age < one year).

Forest plot of comparison: 1 Inositol supplementation versus control, outcome: 1.5 Retinopathy of prematurity, stage ≥ 3.
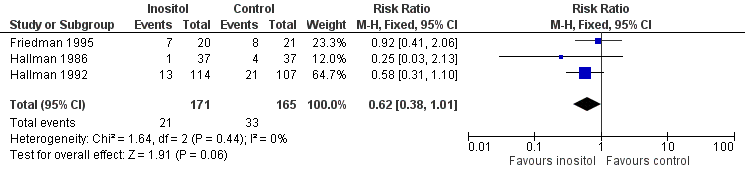
Forest plot of comparison: 1 Inositol supplementation versus control, outcome: 1.6 Retinopathy of prematurity, any stage.

Forest plot of comparison: 1 Inositol supplementation versus control, outcome: 1.9 Intraventricular haemorrhage, grade > 2.
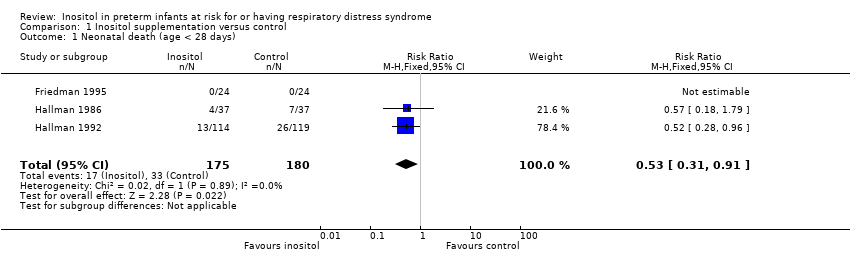
Comparison 1 Inositol supplementation versus control, Outcome 1 Neonatal death (age < 28 days).
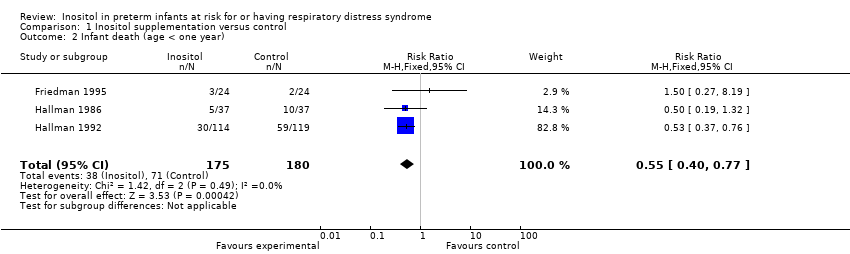
Comparison 1 Inositol supplementation versus control, Outcome 2 Infant death (age < one year).

Comparison 1 Inositol supplementation versus control, Outcome 3 Bronchopulmonary dysplasia (at 28 to 30 days of age).

Comparison 1 Inositol supplementation versus control, Outcome 4 Bronchopulmonary dysplasia (at 36 to 38 weeks PMA).

Comparison 1 Inositol supplementation versus control, Outcome 5 Retinopathy of prematurity, stage ≥ 3.
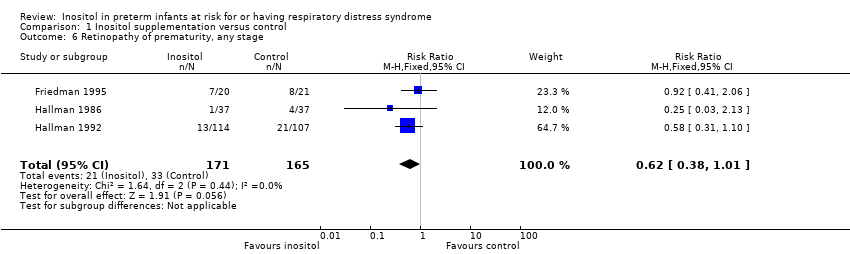
Comparison 1 Inositol supplementation versus control, Outcome 6 Retinopathy of prematurity, any stage.
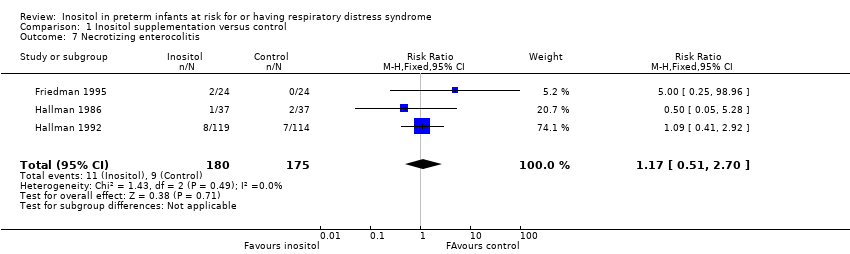
Comparison 1 Inositol supplementation versus control, Outcome 7 Necrotizing enterocolitis.
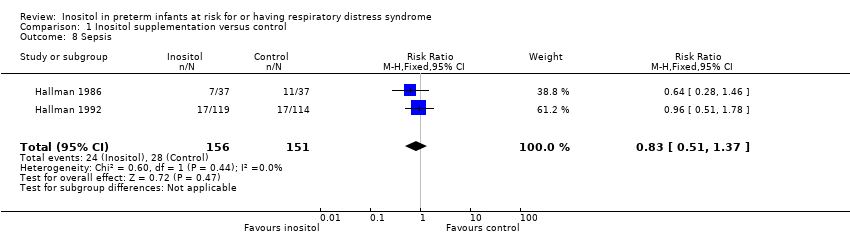
Comparison 1 Inositol supplementation versus control, Outcome 8 Sepsis.

Comparison 1 Inositol supplementation versus control, Outcome 9 Intraventricular haemorrhage, grade > 2.

Comparison 1 Inositol supplementation versus control, Outcome 10 Intraventricular haemorrhage, all grades.

Comparison 1 Inositol supplementation versus control, Outcome 11 Minor neural developmental impairment at one year corrected age.
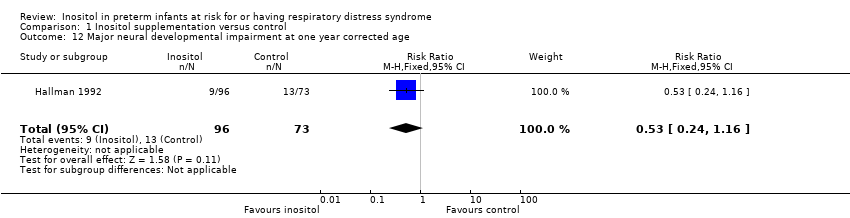
Comparison 1 Inositol supplementation versus control, Outcome 12 Major neural developmental impairment at one year corrected age.
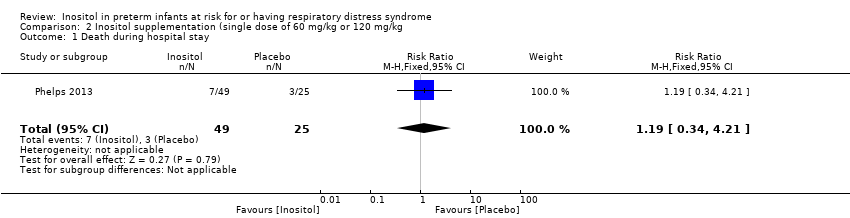
Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg, Outcome 1 Death during hospital stay.

Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg, Outcome 2 Bronchopulmonary dysplasia at 36 weeks PMA.

Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg, Outcome 3 Retinopathy of prematurity (infants who underwent surgery for ROP).

Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg, Outcome 4 Necrotizing enterocolitis (stage 2A or worse).
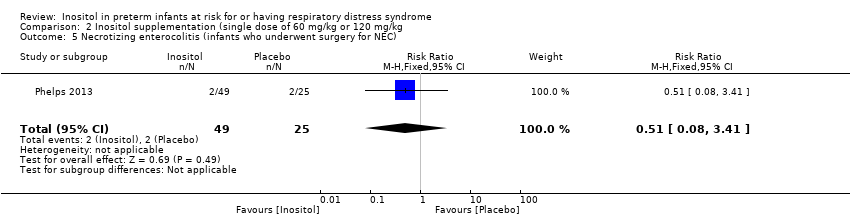
Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg, Outcome 5 Necrotizing enterocolitis (infants who underwent surgery for NEC).
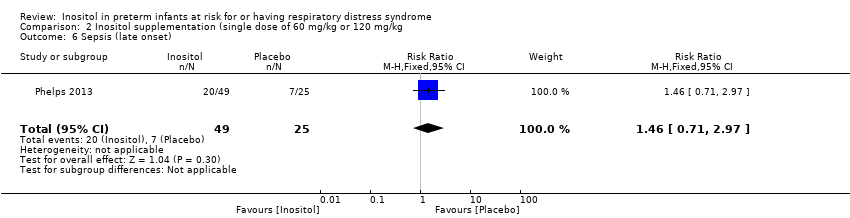
Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg, Outcome 6 Sepsis (late onset).

Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg, Outcome 7 Intraventricular haemorrhage (grade 3 or 4).

Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg, Outcome 8 Hearing test (failed both ears).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal death (age < 28 days) Show forest plot | 3 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.31, 0.91] |
| 2 Infant death (age < one year) Show forest plot | 3 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.40, 0.77] |
| 3 Bronchopulmonary dysplasia (at 28 to 30 days of age) Show forest plot | 3 | 343 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.54, 1.13] |
| 4 Bronchopulmonary dysplasia (at 36 to 38 weeks PMA) Show forest plot | 1 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.64, 2.64] |
| 5 Retinopathy of prematurity, stage ≥ 3 Show forest plot | 2 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 0.67] |
| 6 Retinopathy of prematurity, any stage Show forest plot | 3 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.38, 1.01] |
| 7 Necrotizing enterocolitis Show forest plot | 3 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.51, 2.70] |
| 8 Sepsis Show forest plot | 2 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.51, 1.37] |
| 9 Intraventricular haemorrhage, grade > 2 Show forest plot | 3 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.31, 0.90] |
| 10 Intraventricular haemorrhage, all grades Show forest plot | 2 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.61, 1.11] |
| 11 Minor neural developmental impairment at one year corrected age Show forest plot | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.38, 1.86] |
| 12 Major neural developmental impairment at one year corrected age Show forest plot | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.24, 1.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death during hospital stay Show forest plot | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.34, 4.21] |
| 2 Bronchopulmonary dysplasia at 36 weeks PMA Show forest plot | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.74 [0.88, 8.48] |
| 3 Retinopathy of prematurity (infants who underwent surgery for ROP) Show forest plot | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.10, 1.22] |
| 4 Necrotizing enterocolitis (stage 2A or worse) Show forest plot | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.12, 1.39] |
| 5 Necrotizing enterocolitis (infants who underwent surgery for NEC) Show forest plot | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.08, 3.41] |
| 6 Sepsis (late onset) Show forest plot | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.71, 2.97] |
| 7 Intraventricular haemorrhage (grade 3 or 4) Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.29, 3.90] |
| 8 Hearing test (failed both ears) Show forest plot | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.09, 3.84] |

