Inositol in preterm infants at risk for or having respiratory distress syndrome
Abstract
Background
Inositol is an essential nutrient required by human cells in culture for growth and survival. Inositol promotes maturation of several components of surfactant and may play a critical role in fetal and early neonatal life.
Objectives
To assess the effectiveness and safety of supplementary inositol in preterm infants with or without respiratory distress syndrome (RDS) in reducing adverse neonatal outcomes.
Search methods
The Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, CINAHL, Clinicaltrials.gov and Controlled‐trials.com were searched in September 2014. The reference lists of identified randomised controlled trials (RCTs), personal files and Web of Science were searched.
Selection criteria
All RCTs of inositol supplementation of preterm infants compared with a control group that received a placebo or no intervention were included. Outcomes of interest were neonatal death, infant death, bronchopulmonary dysplasia (BPD), retinopathy of prematurity (ROP), intraventricular haemorrhage (IVH), necrotizing enterocolitis (NEC) and sepsis.
Data collection and analysis
Data on neonatal outcomes were abstracted independently by the three review authors and any discrepancy was resolved through consensus. Outcomes were reported as relative risk (RR), risk difference (RD) and number needed to treat to benefit (NNTB) or to harm (NNTH).
Main results
Four published RCTs and one ongoing RCT were identified. Study quality varied and interim analyses had occurred in all trials of repeat doses of inositol that provided data for the outcomes of interest in this review. In these trials neonatal death was found to be significantly reduced (3 trials, 355 neonates; typical RR 0.53, 95% CI 0.31 to 0.91; typical RD ‐0.09, 95% CI ‐0.17 to ‐0.03; NNTB 11, 95% CI 6 to 33). Infant deaths were reduced (3 trials, 355 infants; typical RR 0.55, 95% CI 0.40 to 0.77; typical RD ‐0.18, 95% CI ‐0.27 to ‐0.08; NNTB 6, 95% CI 4 to 13). ROP stage ≥ 3 was significantly reduced (2 trials, 262 infants; typical RR 0.09, 95% CI 0.01 to 0.67; typical RD ‐0.08, 95% CI ‐0.13 to ‐0.03; NNTB 13, 95% CI 8 to 33) and IVH grade > II was significantly decreased (3 trials, 355 infants; typical RR 0.53, 95% CI 0.31 to 0.90; typical RD ‐0.09, 95% CI ‐0.16 to ‐0.02; NNTB 11, 95% CI 6 to 50). Neither sepsis nor NEC differed significantly between groups. One study (74 infants) that administered a single dose of inositol (60 or 120 mg/kg) found no significant differences in adverse outcomes using RR, but an increased RD for BPD at 36 weeks postmenstrual age (RD 0.23, 95% CI 0.03 to 0.43; NNTH 4, 95% CI 2 to 33). This result should be interpreted with caution as only one dose of inositol was given and only the RD, but not the RR, was significant. One ongoing large study of repeat doses of inositol in preterm infants was identified.
Authors' conclusions
Inositol supplementation results in statistically significant and clinically important reductions in important short‐term adverse neonatal outcomes. A large size multi‐centre randomised controlled trial is currently ongoing and the trial will likely confirm or refute the findings from this systematic review.
PICOs
Plain language summary
Supplementing preterm babies who have respiratory distress with the nutrient inositol may reduce death and disability
Review question
Does the administration of supplementary inositol reduce adverse outcomes in preterm infants with or without respiratory distress syndrome (RDS)?
Background
Inositol is an essential nutrient for cells, with high concentrations in breast milk (particularly in the breast milk of mothers whose babies have been born early). A drop in inositol levels in babies with respiratory distress syndrome (RDS) can be a sign that their illness will be severe.
Study characteristics
Four published randomised controlled trials met our inclusion criteria.
Results
We found that the initial evidence regarding inositol supplementation in preterm babies with RDS is promising. Supplementation lowered rates of death and bleeding in the brain, with an important reduction in eye problems as well. Inositol did not have serious adverse effects. Further research is warranted to confirm these preliminary findings. Such research is currently ongoing in the USA.
Authors' conclusions
Background
Description of the condition
As more preterm infants survive beyond the neonatal period, the incidence of long‐term complications such as bronchopulmonary dysplasia (BPD) and retinopathy of prematurity (ROP) can be expected to increase. The relative contributions of risk factors such as barotrauma, oxygen therapy and nutritional status have yet to be fully understood. Interest has recently focused on the use of myo‐inositol (inositol) supplementation in preterm infants for the prevention of BPD and ROP (Hallman 1986; Hallman 1992). Inositol is a six‐carbon sugar alcohol found widely throughout mammalian tissues in its free form as the phospholipid phosphatidylinositol, and in cell membranes as a phosphoinositide (Dawson 1961; Hasan 1974). Inositol is an essential nutrient required by human cells in culture for growth and survival (Eagle 1957). The effects of deprivation and supplementation in animals have been studied extensively (Egberts 1986; Guarner 1992; Hallman 1984). Inositol promotes maturation of the surfactant phospholipids phosphatidylcholine and phosphatidylinositol, and the synthesis of phosphatidylinositol in type II pneumocytes appears to be dependent on extracellular inositol concentrations (Hallman 1980; Hallman 1984). Compositional changes in fetal rat lung surfactant correlate with changes in plasma inositol levels, and supplementation increases phospholipid levels to normal in the deprived rat pup (Egberts 1986; Guarner 1992; Hallman 1980).
Description of the intervention
Inositol is administered intravenously as long as the infant is not on full oral feeds. When the infant progresses to full feeds inositol is given orally or via an oro‐gastric tube.
How the intervention might work
In human infants with respiratory distress syndrome (RDS), a premature drop in serum inositol levels predicts a more severe course of the syndrome (Hallman 1985). Inositol supplementation increases the amount of saturated phosphatidylcholine in surfactant in newborns and produces a rise in serum inositol concentration (Hallman 1987). In humans, free inositol levels in sera from preterm neonates are 2 to 20 times higher than are levels in maternal or adult sera (Bromberger 1986; Burton 1974; Lewin 1978). Studies in newborns suggest an endogenous synthesis of inositol during fetal life (Bromberger 1986; Pereira 1990). Human milk has a high concentration of inositol, with preterm milk being the richest source. Infants who are breast fed have higher serum inositol levels compared to those that are not breastfed at one to two weeks of life (Bromberger 1986; Pereira 1990). These facts suggest a critical role for inositol in fetal and early neonatal life. Several studies have been published assessing serum inositol levels in the preterm human infant (Bromberger 1986; Hallman 1987; Lewin 1978; Pereira 1990) as well as the effects of inositol supplementation. However, at the time of our original Cochrane review (Howlett 1997) only two published randomised controlled trials (RCTs) of inositol supplementation (Hallman 1986, and an interim analysis of Hallman 1992 published in 1990) had been subjected to systematic review (Soll 1992). As additional evidence has become available, another critical overview of the use of inositol supplementation that includes all known trials to date was warranted. Maintaining inositol concentrations similar to those occurring naturally in utero may reduce the rates of ROP and BPD in preterm infants.
Why it is important to do this review
This review is an update, in 2014, of an existing review 'Inositol for respiratory distress syndrome in preterm infants' which was first published in The Cochrane Library in 1997 (Howlett 1997) and updated in 2003 (Howlett 2003) and in 2012 (Howlett 2012).
Objectives
To assess the effectiveness and safety of supplementary inositol in preterm infants with or without respiratory distress syndrome (RDS) in reducing adverse neonatal outcomes including: death (neonatal and infant deaths), BPD, ROP, intraventricular haemorrhage (IVH), periventricular leukomalacia (PVL), necrotizing enterocolitis (NEC) and sepsis.
Infants were preterm (< 37 weeks postmenstrual age (PMA)) or low birth weight (LBW) (< 2500 g), or both.
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised controlled trials with a control group that received a placebo, low dose inositol or no intervention.
Types of participants
Preterm infants (< 37 weeks PMA) or LBW (< 2500 g) infants.
Types of interventions
Supplementation with inositol either enterally or intravenously.
Types of outcome measures
Primary outcomes
-
Death
-
-
Neonatal death (death < 28 days postnatal age)
-
Death during hospital stay (added as an outcome in 2014)
-
Infant death (death during the first year of life)
-
Secondary outcomes
-
Bronchopulmonary dysplasia (BPD)
-
-
BPD oxygen dependency at 28 days of age (including 30 days or one month if that age was used by the authors) with a roentgenogram compatible with BPD
-
BPD oxygen dependency at 36 weeks PMA (including 38 weeks PMA if used by the authors) with a roentgenogram compatible with BPD (Shennan 1988)
-
-
Retinopathy of prematurity (ROP)
-
-
ROP any stage (ICROP 1984)
-
ROP stage 1 to 2
-
ROP stage ≥ 3
-
ROP (number of infants who required surgery for ROP) (added as an outcome in 2014)
-
-
Necrotizing enterocolitis (NEC) (Bell 1978)
-
NEC (infants requiring surgery) (added as an outcome in 2014)
-
Sepsis (clinical signs of sepsis and positive bacterial cultures from normally sterile body fluids or from autopsy material)
-
Intraventricular haemorrhage (IVH) any grade (Papile 1978)
-
IVH all grades
-
IVH grade > 2
-
Periventricular leukomalacia (PVL)
-
Developmental impairment at 12 months, 18 months or later in life (assessed using a validated instrument)
-
Hearing test (failed both ears) (added as an outcome in 2014)
-
Any adverse effects reported by the authors (added as an outcome in 2014)
Search methods for identification of studies
We used the Cochrane Neonatal Review Group's search strategy to identify studies. We searched the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE and EMBASE in July 2003 using keywords: (inositol and infant‐newborn) AND (random allocation) OR (controlled trial) OR (randomised trial (RCT)). We searched the reference lists of identified RCTs, personal files and Science Citation Index. We obtained unpublished additional information from the authors of one RCT published in abstract form.
We used the same search strategy in December 2007 and again in August 2011. We searched trials registries (http://clinicaltrials.gov and http://www.controlled‐trials.com) in August 2011. In September 2014 we searched the same resources.
Electronic searches
We searched the Pediatric Academic Societies (PAS) website (PASabstracts2view) from years 2000 to 2014. The Web of Science was searched in August 2011 and in September 2014 using Hallman 1992 as the starting point. We searched Google Scholar for the first 200 hits.
Searching other resources
We searched personal files in August 2011 and September 2014.
Data collection and analysis
We used the standardized review methods of the Cochrane Neonatal Review Group to assess the methodological quality of the studies.
For the original review and previous updates of the review the main comparison has been inositol supplementation versus control (Comparison 1) and we included studies under this comparison that provided repeated doses of inositol to the infants. For this update, we identified one dose‐finding study in which infants were supplemented with a single dose of inositol (Phelps 2013). We did not consider it appropriate to include the results of this study in the meta‐analyses of repeat doses of inositol and we have changed the first comparison to: inositol supplementation (repeat doses) versus control (Comparison 1) and added a second comparison: iInositol supplementation (single dose) versus control (Comparison 2). These different dosing regimens were not known at the protocol stage and we have made a deviation from the protocol and included a single dose of inositol in our review as those analyses provide important information.
Selection of studies
For this update the three review authors reviewed the titles (and abstracts when available) in CENTRAL (The Cochrane Library), MEDLINE, EMBASE, Web of Science, PAS abstracts2view and handsearch printouts. We retrieved any article that a review author felt met the inclusion criteria or warranted having its reference list searched. We attempted to locate additional unpublished information from published studies.
Data extraction and management
We developed data extraction forms. The three review authors independently abstracted information on each study and checked for any discrepancies. AO pooled the results. Data abstraction included: the time period and geographical location of the study, baseline characteristics of the patients, inclusion and exclusion criteria, preparation, route of administration and dosing regime of inositol and placebo. Information on outcomes and numbers of affected infants was abstracted. Outcomes included neonatal and infant deaths. The total number of infants with BPD at 28 to 30 days of life (oxygen requirements above the concentration in room air at 28 days of life and a chest roentgenogram compatible with BPD); BPD at 36 to 38 weeks PMA (oxygen requirements above the concentration in room air at 36 to 38 weeks PMA and a chest roentgenogram compatible with BPD) was abstracted as well as information on ROP (stage 0 to 2; ≥ 3), IVH (all grades and grade > 2), NEC and sepsis. Any adverse effects reported by the authors were to be abstracted.
Assessment of risk of bias in included studies
An assessment of the quality of the included studies was performed independently by the three review authors (AH, AO, NP). The methodological criteria used to appraise each paper were: the concealment of treatment allocation, blinding of intervention, blinding of observers, and exclusion and withdrawals. Each criterion was graded 'A', 'B' or 'C'. 'A' indicates a low risk of bias, where the plausibly postulated bias is unlikely to seriously alter the results; 'C' indicates a high risk of bias, where the plausibly postulated bias seriously weakens confidence in the results. A criterion was graded 'B' where it was partially met or where no data were available such that some doubt was raised about possible bias. Each paper was graded independently by two review authors with disagreements resolved by discussion. Methodological assessments were not conducted blind to author, institution, journal of publication or results, as the review authors were familiar with most of the studies. In addition, the results sections of articles often included methodological information. The quality of included trials was evaluated independently by the review authors using the following criteria.
-
Blinding of randomisation?
-
Blinding of intervention?
-
Blinding of outcome measure assessment?
-
Completeness of follow‐up?
There were three potential answers to these questions: yes, can't tell, no.
For the update in 2011 and the subsequent update in 2014, the following questions (based on the questions in the risk of bias table) were evaluated by the three review authors.
Selection bias
Random sequence generation
For each included study, we categorized the risk of selection bias as follows.
Low risk: adequate (any truly random process e.g. random number table; computer random number generator).
High risk: inadequate (any non‐random process e.g. odd or even date of birth; hospital or clinic record number).
Unclear risk: no or unclear information provided.
Allocation concealment
For each included study, we categorized the risk of bias regarding allocation concealment as follows.
Low risk: adequate (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes).
High risk: inadequate (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth).
Unclear risk: no or unclear information provided .
Blinding of participants and personnel and blinding of outcome assessment
Performance bias
For each included study, we categorized the methods used to blind study personnel from knowledge of which intervention a participant received. As our study population consisted of neonates they would all be blinded to the study intervention.
Low risk: adequate for personnel (a placebo that could not be distinguished from the active drug was used in the control group).
High risk: inadequate, personnel aware of group assignment.
Uncelar risk: no or unclear information provided.
Detection bias
For each included study, we categorized the methods used to blind outcome assessors from knowledge of which intervention a participant received. As our study population consisted of neonates they would all be blinded to the study intervention. Blinding was assessed separately for different outcomes or classes of outcomes. We categorized the methods used with regards to detection bias as follows.
Low risk: adequate; follow‐up was performed with assessors blinded to group.
High risk: inadequate; assessors at follow‐up were aware of group assignment;assignment.
Unclear risk: no or unclear information provided.
Incomplete outcome data
Attrition bias
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total number of randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorized the methods with respect to the risk attrition bias as follows.
Low risk: adequate (< 10% missing data).
High risk: inadequate (> 10% missing data).
Unclear risk: no or unclear information provided.
Selective reporting
Reporting bias
For each included study, we described how we investigated the risk of selective outcome reporting bias and what we found. We assessed the methods as follows.
Low risk: adequate (where it is clear that all of the study's pre‐specified outcomes and all expected outcomes of interest to the review have been reported).
High risk: inadequate (where not all the study's pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported).
Unclear risk: no or unclear information provided (the study protocol was not available).
Other bias
For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as follows.
Low risk: no concerns of other bias raised.
High risk: concerns raised about multiple looks at the data with the results made known to the investigators, difference in number of patients enrolled in abstract and final publications of the paper.
Unclear: concerns raised about potential sources of bias that could not be verified by contacting the authors.
If needed, we planned to explore the impact of the level of bias through undertaking sensitivity analyses.
For the original review, independent quality assessments were conducted by two review authors (AH, AO), who were not blinded to authors, institution or journal of publication.
The update in 2011 and the update in 2014 were conducted by three authors (AH, AO, NP).
Measures of treatment effect
The statistical analyses followed the recommendations of the Cochrane Neonatal Review Group. A treatment effect was calculated using Review Manager, software supplied by The Cochrane Collaboration (RevMan 2014). The treatment effect estimates included relative risk (RR), risk difference (RD), number needed to treat to benefit (NNTB) or number needed to treat to harm (NNTH) for dichotomous outcomes; and mean difference (MD) for continuous outcomes. All estimates of treatment effects were reported with 95% confidence intervals (CI).
Unit of analysis issues
We expected only to encounter data reported as dichotomous or continuous for the whole population randomised.
Dealing with missing data
In the event of missing data we planned to contact the authors for clarification. For previous updates we have contacted authors, but for the update in 2012 we found no need to do so. For this update in 2014 we did contact Dr D Phelps and she did provide clarifying information.
Assessment of heterogeneity
We performed heterogeneity tests including the I2 test to assess the appropriateness of pooling the data. The degree of heterogeneity was roughly categorized according to Higgins and co‐workers (Higgins 2003) as I2 < 25% none, 25% to 49% low, 50% to 74% moderate, and ≥ 75% high heterogeneity.
Assessment of reporting biases
To ascertain the possibility of publication bias, we had planned to perform a funnel plot for the primary outcome of infant death. Because of the small number of studies (< 10) included in the review this was not done.
Data synthesis
Meta‐analyses were performed using RevMan 2014. For estimates of typical RR and RD we used the Mantel‐Haenszel method. For measured quantities we used the inverse variance method. All meta‐analyses were done using the fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We did not perform subgroup analyses.
Sensitivity analysis
We did not perform sensitivity analyses.
Results
Description of studies
For details please refer to Characteristics of included studies.
Hallman 1986 was a randomised, placebo‐controlled, double blind single centre study performed in Helsinki, Finland.
-
Objective: to assess the effects of inositol supplementation provided for 10 days.
-
Population: 74 preterm infants (37 in each group) with RDS, birth weight (BW) < 2000 g.
-
Intervention: both intravenous (75% of the intragastric dose when enteral inositol could not be given) and enteral (intragastric) (160 mg/kg/day, divided in four doses) inositol were used, and the control group received a placebo (5% glucose).
-
Outcomes assessed: neonatal death, infant death, BPD (at 28 days), NEC, ROP, IVH, and sepsis.
Hallman 1992 was a placebo‐controlled, randomised, double blind trial conducted in Helsinki, Finland.
-
Objective: to assess the effects of intravenous inositol supplementation in the first five days of life in preterm infants with RDS.
-
Population: 221 infants (PMA 24 to 32 weeks, BW < 2000 g, age 2 to 10 hours and mechanically ventilated) were enrolled, of which 119 were randomised to receive inositol. All enrolled infants were stratified according to whether they had received surfactant as part of another ongoing study.
-
Intervention: inositol or placebo (glucose) was given as a 5% solution intravenously. The dosage was 80 mg/kg of body weight per day, given for five days.
-
Outcomes assessed: neonatal death, infant death, BPD, ROP, patent ductus arteriosus (PDA), IVH, NEC, infection and neurodevelopmental impairment at 12 months corrected age.
Friedman 1995 was a placebo‐controlled, randomised trial conducted in two units in the United States (US).
-
Objective: to examine the relationship between the intake of sugar inositol, serum inositol levels and ROP in LBW infants.
-
Population: 48 preterm infants (BW < 1500 g with severe lung disease) were enrolled, of which 24 were randomised to receive either standard enteral feeds (SC 24 242 µmol/L of inositol) or supplemented formula high in inositol (SC 30 2500 µmol/L of inositol).
-
Intervention: 24 infants received formula containing 242 µmol/L of inositol (control group) and 24 infants received high concentration inositol (2500 µmol/L of inositol). Duration not stated.
-
Outcomes: neonatal death, infant death, BPD, NEC, IVH and ROP.
Phelps 2013 was a multi‐centre, randomised, placebo‐controlled trial conducted in 10 units belonging to the National Institutes of Child Health and Human Development Neonatal Research Network.
-
Objective: to describe the pharmacokinetics (PK) of a single intravenous (IV) dose of inositol in 23 to 29 week gestational age infants.
-
Population: 74 infants were randomised in PMA strata (23 to 26 weeks (n = 37) or 27 to 29 weeks (n = 37)) to receive either inositol or 5% glucose.
-
Intervention: infants received a single dose of 5% myo‐inositol (60 mg/kg or 120 mg/kg) (n = 49) within six days of birth and before enteral feeds began, or 5% glucose (n = 25).
-
Outcomes: inositol was measured over 96 hours in serum and timed urine collections. "Morbidity and mortality were prospectively recorded through discharge or 120 days of postnatal age." Outcomes included: death during hospital stay, BPD at 36 weeks PMA, ROP (number of infants, who underwent surgery for ROP), NEC (stage IIA or worse), NEC (number of infants who underwent surgery), sepsis (late onset), IVH (grade 3 or 4) and hearing test (failed both ears).
NCT01954082 is an ongoing study ('Inositol to reduce retinopathy of prematurity (INS‐3)') involving several units in the US (for details please see Characteristics of ongoing studies).
Results of the search
The searches on 2 September 2014 identified one dose‐finding study (Phelps 2013), one ongoing large RCT (NCT01954082) and one study awaiting further classification as it was published in abstract form only and did not contribute results for any of the outcomes of interest for this review (Phelps 2012NCT01954082).
Included studies
The searches conducted in September, 2014 identified two additional published trials (Phelps 2012NCT01954082; Phelps 2013) and one ongoing trial (NCT01954082). We listed the trial by Phelps 2012NCT01954082 under trials awaiting classification as no data for the clinical outcomes of interest for this review were reported in the abstract presented at the PAS meeting in 2012
Excluded studies
For this update of the review we did not identify any additional studies for exclusion.
Risk of bias in included studies
Only one of the studies reported on how the randomisation sequence was generated (Phelps 2013).
Allocation
The reports often lacked written information on allocation concealment. In the study by Phelps 2013 the allocation was conducted centrally as is the case for the ongoing study by the same group (NCT01954082).
Blinding
Friedman 1995 did not provide any information on whether the clinical staff and the researchers were blinded. In the study by Hallman 1986 the clinicians and the researchers were blinded to which solution (inositol or glucose) the infants received. Only the pharmacist preparing the doses knew the contents of the drug packages. In the study by Hallman 1992 5% glucose was given as placebo, but no information was provided on whether staff were blinded to study drugs or not. In the study by Phelps 2013 the drug or placebo was dispensed from the respective pharmacies in unit doses labelled as 'inositol study drug', and all clinical and research personnel except the pharmacist were masked to the study drug.
Incomplete outcome data
Incomplete outcome data were addressed and the reports seemed free of selective reporting.
Selective reporting
Only Phelps 2013 had been entered into a trials registry and there did not appear to be any differences between the published protocol and the full report.
Other potential sources of bias
All studies except Phelps 2013 undertook and reported on interim analyses. Thus the code must have been broken and that might have influenced decisions on when to close the studies.
Effects of interventions
The updated literature search detected five published reports (Friedman 1995; Hallman 1986; Hallman 1992; Phelps 2013; Phelps 2012NCT01954082). Hallman 1986; Hallman 1992 and Friedman 1995 have all been published as interim analyses, when fewer neonates were enrolled than in the final publication. One study is ongoing (NCT01954082).
Inositol supplementation (repeat doses) versus control (Comparison 1)
Primary outcomes
Neonatal death, age < 28 days (Outcome 1.1) (Analysis 1.1)
Neonatal death was reported in three studies (n = 355) (Friedman 1995; Hallman 1986; Hallman 1992). There was a significant reduction in death prior to 28 days of age in the inositol compared to the control group (typical RR 0.53, 95% CI 0.31 to 0.91; typical RD ‐0.09, 95% CI ‐0.16 to ‐0.01; typical NNTB 11, 95% CI 6 to 100) (Figure 1). I2 was 0% for RR and 58% for RD.

Forest plot of comparison: 1 Inositol supplementation versus control, outcome: 1.1 Neonatal death (age < 28 days).
Infant death, age < one year (Outcome 1.2) (Analysis 1.2)
Infant death was reported in three studies (n = 355) (Friedman 1995; Hallman 1986; Hallman 1992). There was a significant reduction in infant deaths in the inositol compared to the control group (typical RR 0.55, 95% CI 0.40 to 0.77; typical RD ‐0.18, 95% CI ‐0.27 to ‐0.08; typical NNTB 6, 95% CI 4 to 13) (Figure 2). I2 was 0% for RR and 72% for RD.
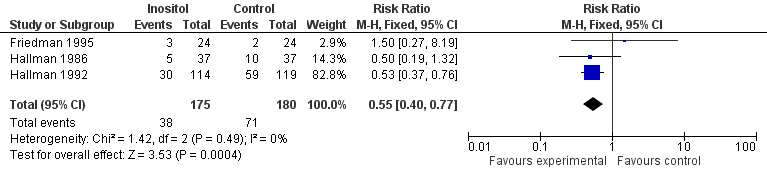
Forest plot of comparison: 1 Inositol supplementation versus control, outcome: 1.2 Infant death (age < one year).
Secondary outcomes
Bronchopulmonary dysplasia (BPD) at 28 to 30 days (Outcome 1.3) (Analysis 1.3)
Three studies (n = 343) (Friedman 1995; Hallman 1986; Hallman 1992) examined the effect of inositol on BPD at 28 to 30 days. There was no significant difference between the groups (typical RR 0.78, 95% CI 0.54 to 1.13; typical RD ‐0.06, 95% CI ‐0.15 to 0.03). I2 was 49% for RR and 31% for RD.
Bronchopulmonary dysplasia (BPD) at 36 to 38 weeks PMA (Outcome 1.4) (Analysis 1.4)
Only one study (n = 177) (Hallman 1992) reported on this outcome. There was no significant difference between the inositol supplementation group and the control group (RR 1.30, 95% CI 0.64 to 2.64; RD 0.04, 95% CI ‐0.07 to 0.15). The test for heterogeneity was not applicable.
Retinopathy of prematurity (ROP), stage ≥ 3 (Outcome 1.5) (Analysis 1.5)
Two studies (n = 262) (Friedman 1995; Hallman 1992) reported on this outcome. There was a significant reduction in ROP stage ≥ 3 in the inositol compared to the control group (typical RR 0.09, 95% CI 0.01 to 0.67; typical RD ‐0.08, 95% CI ‐0.13 to ‐0.03; typical NNTB 13, 95% CI 8 to 33) (Figure 3). I2 was 0% for both RR and RD.

Forest plot of comparison: 1 Inositol supplementation versus control, outcome: 1.5 Retinopathy of prematurity, stage ≥ 3.
Retinopathy of prematurity (ROP), all stages (Outcome 1.6) (Analysis 1.6)
Three trials (n = 336) (Friedman 1995; Hallman 1986; Hallman 1992) reported the effect of inositol on the outcome of ROP, all stages. There was a trend towards a reduced incidence of ROP, any stage, in the inositol compared to the control group (typical RR 0.62, 95% CI 0.38 to 1.01; typical RD ‐0.08, 95% CI ‐0.15 to ‐0.00; P = 0.05) (Figure 4). I2 was 0% for both RR and RD.
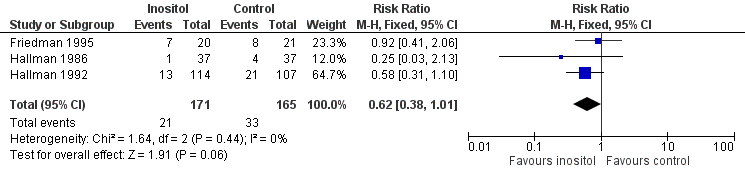
Forest plot of comparison: 1 Inositol supplementation versus control, outcome: 1.6 Retinopathy of prematurity, any stage.
Necrotizing enterocolitis (NEC) (Outcome 1.7) (Analysis 1.7)
Three studies (n = 355) (Friedman 1995; Hallman 1986; Hallman 1992) reported on this outcome. The incidence of NEC was not significantly influenced by the use of inositol supplementation (typical RR 1.17, 95% CI 0.51 to 2.70; typical RD 0.01, 95% CI‐0.04 to 0.06). I2 was 0% for both RR and RD.
Sepsis (Outcome 1.8) (Analysis 1.8)
Two studies (n = 307) (Hallman 1986; Hallman 1992) reported on this outcome. There was no significant effect of the use of inositol supplementation (typical RR 0.83, 95% CI 0.51 to 1.37; typical RD ‐0.03, 95% CI ‐0.11 to 0.05). I2 was 0% for both RR and RD.
Intraventricular haemorrhage (IVH), grade > 2 (Outcome 1.9) (Analysis 1.9)
Three trials (n = 355) (Friedman 1995; Hallman 1986; Hallman 1992) reported on this outcome. There was a significant reduction in the incidence of IVH grade > 2 following treatment with inositol (typical RR 0.53, 95% CI 0.31 to 0.90; typical RD ‐0.09, 95% CI ‐0.16 to ‐0.02; typical NNTB 11, 95% CI 6 to 50) (Figure 5). The I2 was 0% for both RR and RD.

Forest plot of comparison: 1 Inositol supplementation versus control, outcome: 1.9 Intraventricular haemorrhage, grade > 2.
Intraventricular haemorrhage (IVH), all grades (Outcome 1.10) (Analysis 1.10)
Two studies (n = 307) (Hallman 1986; Hallman 1992) reported on this outcome. There was no significant effect of inositol on IVH, all grades (typical RR 0.82, 95% CI 0.61 to 1.11; typical RD ‐0.07, 95% CI ‐0.18 to 0.04). The I2 was 0% for both RR and RD.
Minor neural developmental impairment at one year corrected age (impairment defined as sensorimotor abnormality and/or developmental delay) (Outcome 1.11) (Analysis 1.11)
One study (n = 169) (Hallman 1992) reported on this outcome. There was no significant effect of inositol (RR 0.84, 95% CI 0.38 to 1.86; RD ‐0.02, 95% CI ‐0.12 to 0.08). Tests for heterogeneity were not applicable.
Major neural developmental impairment at one year corrected age (impairment defined as sensory deficit, cerebral palsy, developmental delay, severe hypotonia) (Outcome 1.12) (Analysis 1.12)
One study (n = 169) (Hallman 1992) reported on this outcome. There was no significant effect of inositol (RR 0.53, 95% CI 0.24 to 1.16; RD ‐0.08, 95% CI ‐0.19 to 0.02). Tests for heterogeneity were not applicable.
Periventricular leukomalacia (PVL)
This outcome was not reported in our included studies.
Inositol supplementation (single dose) versus control (Comparison 2)
One study (Phelps 2013) compared inositol supplementation in a single dose of 60 or 120 mg/kg with placebo. We combined the outcomes for the two groups that received a different dose of inositol. As only one study was included under this comparison, tests for heterogeneity were not applicable for any of the outcomes listed below.
Primary outcome
Death during hospital stay (Outcome 2.1) (Analysis 2.1)
One study (Phelps 2013) reported on this outcome in 74 infants. There was no significant effect of inositol (RR 1.19, 95% CI 0.34 to 4.21; RD 0.02, 95% CI ‐0.14 to 0.18).
Secondary outcomes
Bronchopulmonary dysplasia (BPD) at 36 weeks PMA (Outcome 2.2) (Analysis 2.2)
One study (Phelps 2013) reported on this outcome in 65 infants. There was no significant effect of inositol on this outcome for RR (2.74, 95% CI 0.88 to 8.48; P = 0.08) but the RD was 0.23 (95% CI 0.03 to 0.43; P = 0.03) with NNTB of 4 (95% CI 2 to 33).
Retinopathy of prematurity (ROP), infants who underwent surgery for ROP (Outcome 2.3) (Analysis 2.3)
One study (Phelps 2013) reported on this outcome in 25 infants. There was no significant effect of inositol (RR 0.35, 95% CI 0.10 to 1.22; RD ‐0.32, 95% CI ‐0.71 to 0.07).
Necrotizing enterocolitis (NEC), stage 2A or worse (Outcome 2.4) (Analysis 2.4)
One study (Phelps 2013) reported on this outcome in 74 infants. There was no significant effect of inositol (RR 0.41, 95% CI 0.12 to 1.39; RD ‐0.12, 95% CI ‐0.29 to 0.06).
Necrotizing enterocolitis (NEC), infants who underwent surgery for NEC (Outcome 2.5) (Analysis 2.5)
One study (Phelps 2013) reported on this outcome in 74 infants. There was no significant effect of inositol (RR 0.51, 95% CI 0.08 to 3.41; RD ‐0.04, 95% CI ‐0.16 to 0.08).
Sepsis, late onset (Outcome 2.6) (Analysis 2.6)
One study (Phelps 2013) reported on this outcome in 74 infants. There was no significant effect of inositol (RR 1.46, 95% CI 0.71 to 2.97; RD 0.13, 95% CI ‐0.10 to 0.35).
Intraventricular haemorrhage (IVH), grade 3 or 4 (Outcome 2.7) (Analysis 2.7)
One study (Phelps 2013) reported on this outcome in 72 infants. There was no significant effect of inositol (RR 1.06, 95% CI 0.29 to 3.90; RD 0.01, 95% CI ‐0.15 to 0.17).
Hearing test (failed both ears) (Outcome 2.8) (Analysis 2.8)
One study (Phelps 2013) reported on this outcome in 57 infants. There was no significant effect of inositol (RR 0.58, 95% CI 0.09 to 3.84; RD ‐0.04, 95% CI ‐0.19 to 0.11).
Discussion
Summary of main results
Statistically significant reductions in neonatal deaths, infant deaths and IVH grade > 2 in infants were demonstrated with repeat doses of inositol supplementation, and a striking reduction was found in ROP stage ≥ 3. There was no significant reduction in BPD. There was no significant increase in potentially adverse effects such as sepsis, NEC or neurological impairment at 12 months corrected age. For neonatal deaths and infants deaths there was no significant heterogeneity for RR (I2 = 0%), but there was for RD (I2 = 58% for neonatal deaths and 72% for infant deaths). There was no significant heterogeneity for either RR or RD for ROP ≥ stage 3 or all stages (I2 = 0% for both RR and RD).
A 'Single dose intravenous inositol pharmacokinetics in preterm infants' study has been completed in the US (Phelps 2013). As inositol was administered in a single dose of either 60 mg/kg or 120 mg/kg or we did not think it was appropriate to combine the results of this study with the results of the other studies that used repeat doses of inositol. In this study there were no significant results noted for death during hospital stay, the number infants who underwent surgery for ROP, NEC (stage 2A or worse, or number of infants who underwent surgery), late onset sepsis, IVH (grade 3 or 4) or number of infants who failed hearing tests on both ears. There was no statistically significant difference in the incidence of BPD at 36 weeks PMA for RR, but an increased RD (NNTH 4, 95% CI 2 to 33) was noted.
Overall completeness and applicability of evidence
The number of studies available for analysis remains small. These results suggest the need for additional RCTs of inositol supplementation. The numbers of neonates enrolled in two of the reviewed trials were quite small. The estimates of effect, both in the individual trials and in the meta‐analyses, are not very precise, as indicated by the large confidence intervals. Future multi‐centre RCTs of inositol supplementation are required both to confirm the benefits suggested in this review and to assess possible adverse effects on short and long‐term outcomes.
A large multi‐centre study is being undertaken in the US (NCT01954082). This study will enrol 1760 preterm infants < 28 0/7 weeks PMA. It is likely that this study will confirm or refute the promising results of the repeat inositol supplementation studies included in this review. The primary outcome of this study is incidence of survival without severe ROP through acute and final ROP determination up to 55 weeks PMA.
Quality of the evidence
While all four included studies were RCTs, three of them had an interim analysis that may have unblinded the researchers before the trial was completed. Future studies should avoid this potential source of bias. We felt that the quality of the studies was such that a meta‐analysis was appropriate.
Potential biases in the review process
We are not aware of any potential bias in our review process.
Agreements and disagreements with other studies or reviews
We are not aware of any other systematic reviews or meta‐analyses regarding this topic.

Forest plot of comparison: 1 Inositol supplementation versus control, outcome: 1.1 Neonatal death (age < 28 days).

Forest plot of comparison: 1 Inositol supplementation versus control, outcome: 1.2 Infant death (age < one year).

Forest plot of comparison: 1 Inositol supplementation versus control, outcome: 1.5 Retinopathy of prematurity, stage ≥ 3.

Forest plot of comparison: 1 Inositol supplementation versus control, outcome: 1.6 Retinopathy of prematurity, any stage.

Forest plot of comparison: 1 Inositol supplementation versus control, outcome: 1.9 Intraventricular haemorrhage, grade > 2.

Comparison 1 Inositol supplementation versus control, Outcome 1 Neonatal death (age < 28 days).

Comparison 1 Inositol supplementation versus control, Outcome 2 Infant death (age < one year).

Comparison 1 Inositol supplementation versus control, Outcome 3 Bronchopulmonary dysplasia (at 28 to 30 days of age).

Comparison 1 Inositol supplementation versus control, Outcome 4 Bronchopulmonary dysplasia (at 36 to 38 weeks PMA).

Comparison 1 Inositol supplementation versus control, Outcome 5 Retinopathy of prematurity, stage ≥ 3.
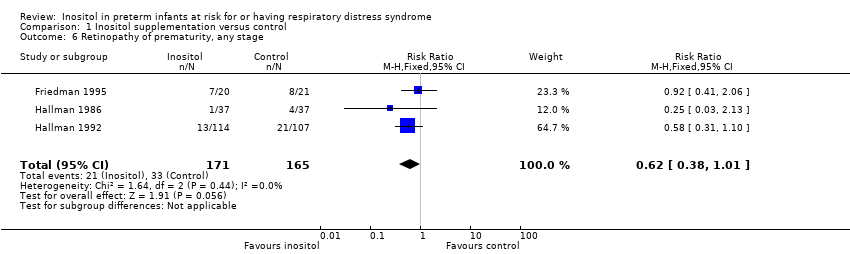
Comparison 1 Inositol supplementation versus control, Outcome 6 Retinopathy of prematurity, any stage.

Comparison 1 Inositol supplementation versus control, Outcome 7 Necrotizing enterocolitis.

Comparison 1 Inositol supplementation versus control, Outcome 8 Sepsis.

Comparison 1 Inositol supplementation versus control, Outcome 9 Intraventricular haemorrhage, grade > 2.

Comparison 1 Inositol supplementation versus control, Outcome 10 Intraventricular haemorrhage, all grades.

Comparison 1 Inositol supplementation versus control, Outcome 11 Minor neural developmental impairment at one year corrected age.

Comparison 1 Inositol supplementation versus control, Outcome 12 Major neural developmental impairment at one year corrected age.
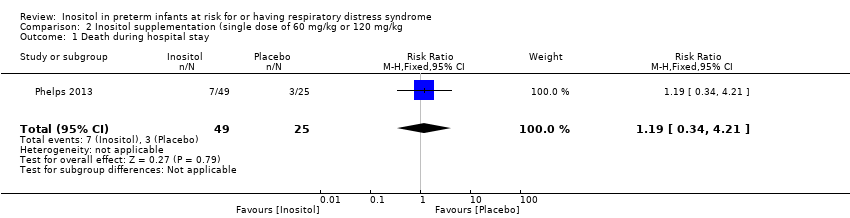
Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg, Outcome 1 Death during hospital stay.

Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg, Outcome 2 Bronchopulmonary dysplasia at 36 weeks PMA.

Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg, Outcome 3 Retinopathy of prematurity (infants who underwent surgery for ROP).

Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg, Outcome 4 Necrotizing enterocolitis (stage 2A or worse).
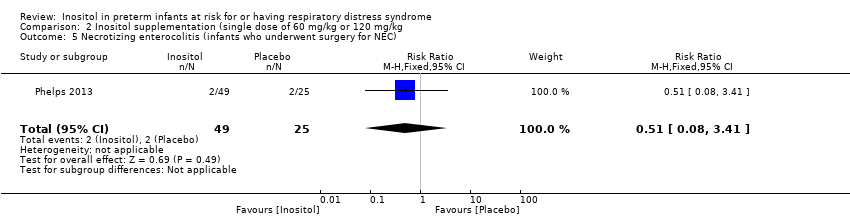
Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg, Outcome 5 Necrotizing enterocolitis (infants who underwent surgery for NEC).
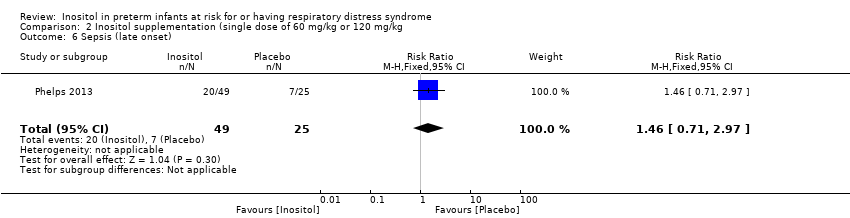
Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg, Outcome 6 Sepsis (late onset).

Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg, Outcome 7 Intraventricular haemorrhage (grade 3 or 4).

Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg, Outcome 8 Hearing test (failed both ears).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal death (age < 28 days) Show forest plot | 3 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.31, 0.91] |
| 2 Infant death (age < one year) Show forest plot | 3 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.40, 0.77] |
| 3 Bronchopulmonary dysplasia (at 28 to 30 days of age) Show forest plot | 3 | 343 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.54, 1.13] |
| 4 Bronchopulmonary dysplasia (at 36 to 38 weeks PMA) Show forest plot | 1 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.64, 2.64] |
| 5 Retinopathy of prematurity, stage ≥ 3 Show forest plot | 2 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 0.67] |
| 6 Retinopathy of prematurity, any stage Show forest plot | 3 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.38, 1.01] |
| 7 Necrotizing enterocolitis Show forest plot | 3 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.51, 2.70] |
| 8 Sepsis Show forest plot | 2 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.51, 1.37] |
| 9 Intraventricular haemorrhage, grade > 2 Show forest plot | 3 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.31, 0.90] |
| 10 Intraventricular haemorrhage, all grades Show forest plot | 2 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.61, 1.11] |
| 11 Minor neural developmental impairment at one year corrected age Show forest plot | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.38, 1.86] |
| 12 Major neural developmental impairment at one year corrected age Show forest plot | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.24, 1.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death during hospital stay Show forest plot | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.34, 4.21] |
| 2 Bronchopulmonary dysplasia at 36 weeks PMA Show forest plot | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.74 [0.88, 8.48] |
| 3 Retinopathy of prematurity (infants who underwent surgery for ROP) Show forest plot | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.10, 1.22] |
| 4 Necrotizing enterocolitis (stage 2A or worse) Show forest plot | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.12, 1.39] |
| 5 Necrotizing enterocolitis (infants who underwent surgery for NEC) Show forest plot | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.08, 3.41] |
| 6 Sepsis (late onset) Show forest plot | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.71, 2.97] |
| 7 Intraventricular haemorrhage (grade 3 or 4) Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.29, 3.90] |
| 8 Hearing test (failed both ears) Show forest plot | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.09, 3.84] |

