Inositol para lactantes prematuros con síndrome de dificultad respiratoria o con riesgo de presentarlo
Resumen
Antecedentes
El inositol es un nutriente esencial necesario para el crecimiento y la supervivencia de las células humanas en cultivo. El inositol promueve la maduración de varios componentes de surfactante y puede tener un rol fundamental en la vida fetal y en la neonatal temprana. Una disminución en los niveles de inositol en los lactantes con síndrome de dificultad respiratoria (SDR) puede ser un signo de que la enfermedad será grave.
Objetivos
Evaluar la efectividad y la seguridad de los suplementos de inositol en lactantes prematuros con o sin síndrome de dificultad respiratoria (SDR) para reducir los resultados neonatales adversos como: mortalidad (muerte neonatal e infantil), displasia broncopulmonar (DBP), retinopatía del prematuro (RP), hemorragia intraventricular (HIV), leucomalacia periventricular (LPV), enterocolitis necrosante (ECN) y sepsis.
Métodos de búsqueda
Se utilizó la estrategia de búsqueda estándar del Grupo Cochrane de Neonatología para buscar en el Registro Cochrane Central de Ensayos Controlados (CENTRAL 2018, número 11), MEDLINE vía PubMed (1966 hasta 5 de noviembre 2018), Embase (1980 hasta 5 de noviembre 2018) y en CINAHL (1982 hasta 5 de noviembre 2018). También se buscaron ensayos controlados aleatorios y ensayos cuasialeatorios en las bases de datos de ensayos clínicos (ECA), las actas de congresos y las listas de referencias de los artículos recuperados.
Criterios de selección
Se incluyeron todos los ensayos controlados aleatorios que compararon la administración de suplementos de inositol a lactantes prematuros con un grupo de control que recibió placebo o ninguna intervención. Los resultados incluyeron: muerte neonatal, mortalidad infantil, displasia broncopulmomar (DBP), retinopatía del prematuro (RP), hemorragia intraventricular (HIV), enterocolitis necrosante (ECN) y sepsis.
Obtención y análisis de los datos
Los tres autores de la revisión resumieron, de forma independiente, los datos sobre los resultados neonatales y resolvieron los desacuerdos mediante discusión y consenso. Los resultados se informaron como cociente de riesgos (CR) típico, diferencia de riesgos (DR) y número necesario a tratar para lograr un resultado beneficioso adicional (NNTB), o número necesario a tratar para lograr un resultado perjudicial adicional (NNTH). Se utilizaron los criterios GRADE para evaluar la calidad de la evidencia.
Resultados principales
Se identificaron seis ensayos controlados aleatorios publicados, con un total de 1177 lactantes. La calidad de los estudios varió para la comparación "Administración de suplementos de inositol en lactantes prematuros (dosis repetidas en cualquier cantidad y con cualquier duración del tratamiento) versus control" y en varios ensayos se habían realizado análisis provisionales de los resultados de interés. En esta comparación, se encontró que la muerte neonatal se redujo significativamente (CR típico 0,53; IC del 95%: 0,31 a 0,91; DR típica: ‐0,09; IC del 95%: ‐0,16 a ‐0,01; NNTB 11; IC del 95%: 6 a 100; tres ensayos, 355 neonatos). No se redujeron las muertes infantiles (CR típico 0,89; IC del 95%: 0,71 a 1,13; DR típica ‐0,02; IC del 95%: ‐0,07 a 0,02; cinco ensayos, 1115 lactantes) (evidencia de calidad baja). El estadio 2 o mayor, o el estadio 3 o mayor de la RP no se redujo significativamente (CR típico 0,89; IC del 95%: 0,75 a 1,06; DR típica ‐0,04; IC del 95%: ‐0,10 a 0,02; tres ensayos, 810 lactantes) (evidencia de calidad moderada). No hubo hallazgos significativos para la RP (cualquier estadio), la ECN (presunta o confirmada), la sepsis, el grado de HIV mayor de II (evidencia de calidad moderada). En la comparación "Administración i.v. inicial de suplementos de inositol seguida de administración enteral (dosis repetidas de 80 mg/kg/día) en lactantes prematuros con menos de 30 semanas de la fecha de la última menstruación (FUM) en comparación con placebo en lactantes prematuros con síndrome de dificultad respiratoria o en riesgo de presentar este síndrome", se incluyeron los resultados de dos estudios de alta calidad (N = 760 neonatos). El reclutamiento del estudio más grande (N = 638) se interrumpió debido a una tasa mayor de muertes en el grupo de inositol. No se disminuyó la calificación de la calidad del estudio. Los metanálisis de los resultados "RP tipo 1 o muerte antes de la determinación del resultado RP mediante el resultado RP adjudicada", "RP tipo 1 que incluye el resultado RP adjudicada", "Mortalidad por todas las causas (resultado obtenido a través del primer evento: muerte, alta hospitalaria, traslado al hospital o 120 días después del parto)" y "HIV grave (grado 3 o 4)" no mostraron resultados significativos (evidencia de calidad moderada). No hubo hallazgos significativos para el resultado "DBP o muerte por esta enfermedad antes de las 37 semanas de la fecha de la última menstruación (resultados obtenidos a través del primer evento: muerte, alta hospitalaria, traslado al hospital o 120 días después del parto)", "Sepsis de aparición tardía (> 72 horas de vida)", y "ECN presunta o confirmada" (evidencia de calidad alta).
Conclusiones de los autores
Según la evidencia de los ensayos controlados aleatorios hasta la fecha, la administración de suplementos de inositol no produce reducciones importantes en las tasas de mortalidad infantil, RP estadio 3 o mayor, RP tipo 1, HIV grados 3 o 4, DBP, ECN ni en la sepsis. Estas conclusiones se basan principalmente en dos ensayos controlados aleatorios recientes en neonatos con menos de 30 semanas de la fecha de la última menstruación (N = 760), la población más vulnerable. Actualmente, los suplementos de inositol no se deben administrar de forma habitual como parte del tratamiento nutricional de los lactantes prematuros con o sin SDR. Es importante que se realice un seguimiento de los lactantes que han participado en los ensayos incluidos en esta revisión para evaluar cualquier efecto de la administración de suplementos de inositol sobre los resultados a largo plazo en la infancia. No se recomienda la realización de ensayos adicionales en neonatos.
PICO
Resumen en términos sencillos
Inositol para lactantes prematuros con síndrome de dificultad respiratoria o con riesgo de presentarlo
Pregunta de la revisión
¿La administración de suplementos de inositol reduce los resultados adversos en los lactantes prematuros con o sin síndrome de dificultad respiratoria (SDR)?
Antecedentes
El inositol es un nutriente esencial para las células que se encuentra en altas concentraciones en la leche (especialmente en la leche de las madres cuyos bebés nacieron prematuros). Una disminución en los niveles de inositol en los lactantes con síndrome de dificultad respiratoria (SDR) puede ser un signo de que la enfermedad será grave. Se cree que el inositol es un nutriente importante para el desarrollo antes y después del parto.
Fecha de la búsqueda
Se realizaron búsquedas relevantes el 5 de noviembre 2018.
Características de los estudios
Seis ensayos controlados aleatorios publicados, con 1177 lactantes, cumplieron con los criterios de inclusión. Esta actualización incluye los resultados de dos estudios de alta calidad realizados en 760 lactantes de menos de 30 semanas de la fecha de la última menstruación (FUM).
Resultados clave
En la actualización anterior de la revisión, en 2015, se determinó que la evidencia inicial sobre la administración de suplementos de inositol en lactantes prematuros con SDR era promisoria. La administración de suplementos con inositol disminuyó las tasas de mortalidad y de hemorragia cerebral, con una reducción importante en los trastornos oculares. El inositol no mostró efectos adversos graves. Se indicó realizar estudios de investigación adicionales para confirmar estos hallazgos preliminares. Esta investigación fue publicada a partir de dos estudios de alta calidad que incluyeron a 760 lactantes con menos de 30 semanas de la FUM, la población más vulnerable. Todos los resultados indican que no hay reducción en los resultados adversos asociados con la administración de suplementos de inositol, incluida la mortalidad infantil, los trastornos oculares, la hemorragia cerebral, las infecciones, los trastornos pulmonares crónicos y los trastornos gastrointestinales. Por lo tanto, no se recomienda la administración de suplementos de inositol en lactantes prematuros. Se debe hacer un seguimiento durante la infancia de los lactantes incorporados en estos estudios para evaluar cualquier tipo de trastorno del desarrollo neurológico.
Calidad de la evidencia
Según los criterios GRADE (un método para calificar la calidad de los ensayos que apoyan cada resultado), varió la calidad de la evidencia, aunque fue moderada a alta para los resultados importantes en los análisis de dosis altas repetidas de inositol en lactantes con menos de 30 semanas de la fecha de la última menstruación.
Conclusiones de los autores
Summary of findings
| Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) compared to control for preterm infants at risk for or having respiratory distress syndrome | ||||||
| Patient or population: preterm infants at risk for or having respiratory distress syndrome | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with control | Risk with Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) | |||||
| Infant death (age < 1 year) | Study population | RR 0.89 | 1115 | Low | Design (risk of bias): the risk of bias for random sequence generation was low in 2 studies and unclear in 3 studies; the risk of bias for allocation concealment was low in 3 studies and unclear in 2 studies; the risk of bias regarding performance bias and detection bias was low in 3 studies and unclear in 2 studies. We downgraded the quality of the evidence by 1 step Heterogeneity/consistency across studies: there was high heterogeneity for RR (I² = 80 % ) and for RD (I² = 84%). We downgraded the quality of the evidence by 1 step Directness of the evidence: Studies were conducted in the target population. Precision of estimates: Results from 1115 infants have been reported in the studies to date and the confidence intervals around the point estimates for RR and RD were narrow. Presence of publication bias: As only 5 studies were included in the analysis we did not perform a funnel plot. | |
| 207 per 1000 | 184 per 1000 | |||||
| Bronchopulmonary dysplasia (at 36 to 38 weeks' PMA) | Study population | RR 1.04 | 737 | Moderate | Design (risk of bias): the risk of bias for random sequence generation was low in 1 study and unclear in 1 study; The risk of bias for allocation concealment was low in 1 study and unclear in 1 study; the risk of bias regarding performance bias and detection bias was low in 1 study and unclear in 1 study. We downgraded the quality of the evidence by 1 step Heterogeneity/consistency across studies: there was no heterogeneity for RR (I² = 0%) and for RD (I² = 0%) Directness of the evidence: studies were conducted in the target population Precision of estimates: to date the results from 737 infants have been reported in the studies and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 2 studies were included in the analysis we did not perform a funnel plot | |
| 459 per 1000 | 477 per 1000 | |||||
| ROP, stage ≥ 3 or ≥ 2 | Study population | RR 0.89 | 810 | Moderate | Design (risk of bias): the risk of bias for random sequence generation was low in 1 study and unclear in 2 studies; the risk of bias for allocation concealment was low in 1 study and unclear in 2 studies; the risk of bias regarding performance bias and detection bias was low in 1 study and unclear in 2 studies. We downgraded the quality of the evidence by 1 step Heterogeneity/consistency across studies: there was moderate heterogeneity for RR (I² = 63% ) and none for RD (I² = 23%) Directness of the evidence: Studies were conducted in the target population Precision of estimates: to date the results from 810 infants have been reported in the studies and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 3 studies were included in the analysis we did not perform a funnel plot | |
| 368 per 1000 | 328 per 1000 | |||||
| Sepsis (early or late onset) | Study population | RR 1.21 | 1067 | Moderate | Design (risk of bias): the risk of bias for random sequence generation was low in 2 studies and unclear in 2 studies; the risk of bias for allocation concealment was low in 3 studies and unclear in 1 study; the risk of bias regarding performance bias and detection bias was low in 3 studies and unclear in 1 study. We downgraded the quality of the evidence by 1 step Heterogeneity/consistency: across studies: There was no heterogeneity for RR (I² = 24% ) and low for RD (I² = 34%) Directness of the evidence: studies were conducted in the target population Precision of estimates: to date results from 1067 infants have been reported in the studies and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 4 studies were included in the analysis we did not perform a funnel plot | |
| 189 per 1000 | 229 per 1000 | |||||
| Necrotizing enterocolitis (suspected or proven) | Study population | RR 0.94 | 1115 | Moderate | Design (risk of bias): the risk of bias for random sequence generation was low in 2 studies and unclear in 3 studies; the risk of bias for allocation concealment was low in 3 studies and unclear in 2 studies; the risk of bias regarding performance bias and detection bias was low in 3 studies and unclear in 2 studies. We downgraded the quality of the evidence by 1 step Heterogeneity/consistency across studies: there was no heterogeneity for RR (I² = 0%) nor for RD (I² = 0%) Directness of the evidence: studies were conducted in the target population Precision of estimates: to date results from 1115 infants have been reported in the studies and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 5 studies were included in the analysis we did not perform a funnel plot | |
| 83 per 1000 | 78 per 1000 | |||||
| Intraventricular haemorrhage, grade > 2 | Study population | RR 0.77 | 1103 | Moderate | Design (risk of bias): the risk of bias for random sequence generation was low in 2 studies and unclear in 3 studies; the risk of bias for allocation concealment was low in 3 studies and unclear in 2 studies; the risk of bias regarding performance bias and detection bias was low in 3 studies and unclear in 2 studies. We downgraded the quality of the evidence by 1 step Heterogeneity/consistency across studies: there was low heterogeneity for RR (I² = 48% ) and for RD (I² = 42%) Directness of the evidence: studies were conducted in the target population Precision of estimates: to date the results from 1103 infants have been reported in the studies and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: As only 5 studies were included in the analysis we did not perform a funnel plot | |
| 177 per 1000 | 136 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA compared to placebo for preterm infants at risk for or having respiratory distress syndrome | ||||||
| Patient or population: preterm infants at risk for or having respiratory distress syndrome | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA | |||||
| Type 1 ROP or death before determination of ROP outcome using the adjudicated ROP outcome | Study population | RR 1.28 | 679 | Moderate | Design (risk of bias): the risk of bias for random sequence generation, for allocation concealment, for performance bias and detection bias was low in both studies Heterogeneity/consistency across studies: there was high heterogeneity for RR (I² = 79 %) and for RD (I² = 85%). We downgraded the quality of the evidence by 1 step Directness of the evidence: studies were conducted in the target population Precision of estimates: this outcome was reported for 679 infants and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: As only 2 studies were included in the analysis we did not perform a funnel plot | |
| 222 per 1000 | 284 per 1000 | |||||
| Type 1 ROP including adjudicated ROP outcome | Study population | RR 1.24 1.86) | 605 | Moderate | Design (risk of bias): the risk of bias for random sequence generation, for allocation concealment, for performance bias and detection bias was low in both studies Heterogeneity/consistency across studies: there was low heterogeneity for RR (I² = 46 %) and moderate for RD (I² = 54%). We downgraded the quality of the evidence by 1 step Directness of the evidence: studies were conducted in the target population Precision of estimates: this outcome was reported on for 605 infants and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 2 studies were included in the analysis we did not perform a funnel plot | |
| 120 per 1000 | 149 per 1000 | |||||
| All‐cause mortality (outcome collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth) | Study population | RR 1.35 2.00) | 701 | Moderate | Design (risk of bias): the risk of bias for random sequence generation, for allocation concealment, for performance bias and detection bias was low in both studies Heterogeneity/consistency across studies: there was moderate heterogeneity for RR (I² = 72%) and high for RD (I² = 84%). We downgraded the quality of the evidence by 1 step Directness of the evidence: studies were conducted in the target population Precision of estimates: this outcome was reported for 701 infants and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 2 studies were included in the analysis we did not perform a funnel plot | |
| 110 per 1000 | 148 per 1000 | |||||
| BPD or death by it prior to 37 weeks' PMA (outcomes collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth) | Study population | RR 1.01 1.16) | 616 | High | Design (risk of bias): the risk of bias for random sequence generation, for allocation concealment, for performance bias and detection bias was low in both studies Heterogeneity/consistency across studies: there was no heterogeneity for RR (I² = 0%) nor for RD (I² = 0%) Directness of the evidence: studies were conducted in the target population. Precision of estimates: this outcome was reported for 616 infants and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 2 studies were included in the analysis we did not perform a funnel plot | |
| 555 per 1000 | 561 per 1000 | |||||
| Severe IVH (grade 3 or 4) | Study population | RR 0.92 1.29) | 690 | Moderate | Design (risk of bias): The risk of bias for random sequence generation, for allocation concealment, for performance bias and detection bias was low in both studies Heterogeneity/consistency across studies: there was moderate heterogeneity for RR (I² = 74%) and high for RD (I² = 82%) Directness of the evidence: studies were conducted in the target population Precision of estimates: this outcome was reported for 690 infants and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 2 studies were included in the analysis we did not perform a funnel plot. | |
| 171 per 1000 | 157 per 1000 | |||||
| Late‐onset sepsis (> 72 hours of age) | Study population | RR 1.33 1.75) | 701 | HIgh | Design (risk of bias): the risk of bias for random sequence generation, for allocation concealment, for performance bias and detection bias was low in both studies Heterogeneity/consistency across studies: there was no heterogeneity for RR (I² = 0%) nor for RD (I² = 0%) Directness of the evidence: studies were conducted in the target population Precision of estimates: this outcome was reported for 701 infants and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 2 studies were included in the analysis we did not perform a funnel plot | |
| 191 per 1000 | 254 per 1000 | |||||
| Suspected or proven NEC | Study population | RR 0.88 1.41) | 701 | High | Design (risk of bias): the risk of bias for random sequence generation, for allocation concealment, for performance bias and detection bias was low in both studies Heterogeneity/consistency across studies: there was low heterogeneity for RR (I² = 36%) and moderate for RD (I² = 53%). Directness of the evidence: studies were conducted in the target population. Precision of estimates: this outcome was reported on in 701 infants and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 2 studies were included in the analysis we did not perform a funnel plot. | |
| 98 per 1000 | 87 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
Antecedentes
Descripción de la afección
Dado que más niños prematuros sobreviven más allá del período neonatal, se espera un aumento de la incidencia de complicaciones a largo plazo, tales como la displasia broncopulmonar (DBP) y la retinopatía del prematuro (RP). Aún quedan por comprender la participación relativa de factores de riesgo como el barotrauma, la oxigenoterapia y el estado nutricional. Recientemente, el interés se ha centrado en la administración de suplementos de mioinositol (inositol) a lactantes prematuros para prevenir la DBP y la RP (Hallman 1986; Hallman 1992). El inositol es un alcohol de azúcar de seis carbonos que se encuentra con frecuencia en los tejidos de los mamíferos en su forma libre como el fosfolípido fosfatidilinositol, y en las membranas celulares como una fosfoinositida (Dawson 1961; Hasan 1974). El inositol es un nutriente esencial necesario para el crecimiento y la supervivencia de las células humanas en cultivo (Eagle 1957). Los efectos de la carencia y la administración de suplementos en animales se han estudiado ampliamente (Egberts 1986; Guarner 1992; Hallman 1984). El inositol promueve la maduración de los fosfolípidos surfactantes fosfatidilcolina y fosfatidilinositol, y la síntesis del fosfatidilinositol en los neumonocitos tipo II parece depender de las concentraciones extracelulares de inositol (Hallman 1980; Hallman 1984). Los cambios en la composición del surfactante pulmonar del feto de rata se correlacionan con los cambios en los niveles de inositol en plasma, y la administración de suplementos incrementa hasta la normalidad los niveles fosfolípidos en crías de rata con carencia (Egberts 1986; Guarner 1992; Hallman 1980).
Descripción de la intervención
El inositol se administra por vía intravenosa siempre y cuando el lactante no reciba alimentación oral completa. Cuando el lactante progresa a la alimentación completa, el inositol se administra por vía oral o a través de una sonda orogástrica.
De qué manera podría funcionar la intervención
En los lactantes humanos con síndrome de dificultad respiratoria (SDR), un descenso precoz en los niveles de inositol sérico predice una evolución más grave del síndrome (Hallman 1985). La administración de suplementos de inositol incrementa la cantidad de fosfatidilcolina saturada en el surfactante de los recién nacidos y produce un incremento en la concentración sérica de inositol (Hallman 1987). En los humanos, los niveles de inositol libre en el suero de los neonatos prematuros son de dos a 20 veces más altos que los niveles en suero materno o de adultos (Bromberger 1986; Burton 1974; Lewin 1978). Estudios en recién nacidos indican una síntesis endógena de inositol durante la vida fetal (Bromberger 1986; Pereira 1990). La leche materna tiene una alta concentración de inositol, y la leche pretérmino es la fuente más rica. Los lactantes que son amamantados tienen niveles más altos de inositol sérico en comparación con los que no son amamantados durante la primera o segunda semana de vida (Bromberger 1986; Pereira 1990). Estos hechos sugieren un rol fundamental del inositol en la vida fetal y neonatal temprana. Se han publicado varios estudios que evalúan los niveles séricos de inositol en lactantes prematuros (Bromberger 1986; Hallman 1987; Lewin 1978; Pereira 1990), así como los efectos de la administración de suplementos de inositol. Sin embargo, en el momento de la revisión Cochrane original (Howlett 1997), solo dos ensayos controlados aleatorios (ECA) publicados sobre la administración de suplementos de inositol (Hallman 1986 y un análisis intermedio de Hallman 1992 publicado en 1990) habían sido sometidos a una revisión sistemática (Soll 1992). Debido a que se dispone de evidencia adicional, se justifica la realización de otra revisión crítica de la administración de suplementos de inositol que incluya todos los ensayos conocidos hasta la fecha. El mantenimiento de concentraciones de inositol similares a las que existen de forma natural en el útero puede reducir las tasas de RP y DBP en los lactantes prematuros.
Por qué es importante realizar esta revisión
Esta revisión es la actualización de 2019 de una revisión existente: "Inositol para el síndrome de dificultad respiratoria en lactantes prematuros", publicada por primera vez en la Biblioteca Cochrane en 1997 (Howlett 1997) y actualizada en 2003, 2012 y 2015 (Howlett 2003; Howlett 2012; Howlett 2015).
Objetivos
Evaluar la efectividad y la seguridad de los suplementos de inositol en lactantes prematuros con o sin síndrome de dificultad respiratoria (SDR) para la reducción de resultados neonatales adversos como: mortalidad (muerte neonatal e infantil), DBP, RP, hemorragia intraventricular (HIV), leucomalacia periventricular (LPV), enterocolitis necrosante (ECN) y sepsis.
Métodos
Criterios de inclusión de estudios para esta revisión
Tipos de estudios
Ensayos controlados aleatorios o cuasialeatorios con un grupo de control que recibió placebo, inositol a dosis bajas o ninguna intervención.
Tipos de participantes
Lactantes prematuros (< 37 semanas de la fecha de la última menstruación) o lactantes con bajo peso al nacer (< 2500 gramos) o ambos.
Tipos de intervenciones
Administración de suplementos de inositol por vía enteral o intravenosa.
Tipos de medida de resultado
Resultados primarios
-
Muerte
-
-
Muerte neonatal (muerte < 28 días después del parto)
-
Muerte durante la estancia hospitalaria (agregado como un resultado en 2015)
-
Muerte infantil (muerte durante el primer año de vida)
-
RP tipo 1 y muerte antes de determinar el resultado de RP (agregado como un resultado en 2019)
-
Mortalidad por todas las causas (resultados obtenidos hasta las 55 semanas de la FUM (agregado como un resultado en 2019)
-
Mortalidad por todas las causas (resultados obtenidos a través del primer evento; muerte, alta hospitalaria, traslado al hospital o 120 días después del parto [agregado como un resultado en 2019])
-
Resultados secundarios
-
Displasia broncopulmonar (DBP)
-
-
DBP con dependencia de oxígeno a los 28 días de vida (con 30 días o un mes si esa edad fue utilizada por los autores) con una radiografía compatible con DBP
-
DBP con dependencia de oxígeno a las 36 semanas de la FUM (con 38 semanas de la FUM si fue utilizada por los autores) con una radiografía compatible con DBP (Shennan 1988)
-
DBP que requiere oxígeno a las 36 semanas de la fecha de la última menstruación para una saturación de oxígeno superior al 90% (agregado como un resultado en 2019)
-
-
Retinopatía del prematuro (RP)
-
-
RP de cualquier estadio (ICROP 1984)
-
RP estadio 1 a 2
-
RP estadio ≥ 3
-
RP (número de lactantes que requirieron cirugía por RP) (agregado como un resultado en 2015)
-
RP tipo 1 (definida como que cumple con los criterios de intervención oftalmológica para prevenir el desprendimiento de retina) (agregado como un resultado en 2019)
-
-
Enterocolitis necrosante (ECN) (Bell 1978)
-
ECN (lactantes que requieren cirugía) (agregado como un resultado en 2015)
-
Sepsis (signos clínicos de sepsis y cultivos bacterianos positivos de fluidos corporales normalmente estériles o de material de autopsia). La sepsis temprana y tardía se combinaron en algunos análisis
-
Hemorragia intraventricular (HIV de cualquier grado (Papile 1978)
-
HIV grado > 2
-
Leucomalacia periventricular (LPV)
-
Deterioro del desarrollo a los 12 meses, 18 meses o más tarde en la vida (evaluado mediante un instrumento validado)
-
Examen de audición (deficiencia en uno o ambos oídos) (agregado como un resultado en 2015)
-
Sepsis, enterocolitis necrosante, neumonía u otra infección como causa de muerte (agregado como un resultado en 2019)
-
Cualquier efecto adverso informado por los autores (agregado como un resultado en 2015)
Métodos de búsqueda para la identificación de los estudios
We used the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal search strategy for specialized register).
Búsquedas electrónicas
We conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL 2018, Issue 11) in the Cochrane Library; MEDLINE via PubMed (1966 to 5 November 2018); Embase (1980 to 5 November 2018); and CINAHL (1982 to 5 November 2018).
We used the following search terms: inositol, plus database‐specific limiters for RCTs and neonates (see Appendix 1 for the full search strategies for each database). We did not apply language restrictions.
We searched clinical trials registries for ongoing or recently completed trials (ClinicalTrials.gov; the World Health Organization’s International Trials Registry and Platform, and the ISRCTN Registry).
We searched trials registries (ClinicalTrials.gov and www.controlled‐trials.com). We searched content on the Pediatric Academic Societies' (PAS) web site published between 2000 and 2018. We searched the Web of Science in August 2011 and in September 2014 using Hallman 1992 as the starting point.
Búsqueda de otros recursos
We searched personal files in August 2011, September 2014 and November 2018. We searched the reference lists of any articles selected for inclusion in this review in order to identify additional relevant articles.
Obtención y análisis de los datos
We used the standardized review methods of Cochrane Neonatal to assess the methodological quality of the studies.
For the original review and previous updates of the review the main comparison has been inositol supplementation versus control (Comparison 1) and we included studies under this comparison that provided repeated doses of inositol (by IV or enteral route) to the infants. For the update in 2015, we identified one dose‐finding study in which infants were supplemented with a single dose of inositol (Phelps 2013). We did not consider it appropriate to include the results of this study in the meta‐analyses of repeat doses of inositol and we changed the first comparison to 'inositol supplementation (repeat doses) versus control' (Comparison 1) and added a second comparison, 'inositol supplementation (single dose) versus control' (Comparison 2). These different dosing regimens were not known at the protocol stage and we have made a deviation from the protocol and included a single dose of inositol in our review as those analyses provide important information. The two new studies we included in this update enrolled infants with a PMA up to 29 6/7 weeks (Phelps 2016); and infants with a PMA less than 28 0/7 weeks (Phelps 2018). The three other studies included in Comparison 1 enrolled some infants with a PMA beyond 30 weeks (Friedman 1995; Hallman 1986; Hallman 1992). As most of the adverse outcomes included in this review are highly influenced by PMA with higher rates in infants of low PMA, we therefore performed a separate comparison for studies that used multiple doses of inositol and enrolled infants less than 30 weeks' PMA (Comparison 3).
Selección de los estudios
We applied machine learning using the Cochrane Classifier tool in the Cochrane Register of Studies (CRS) to remove reports with the least (0% to 2%) probability of being randomised controlled trials and with the least (0% to 100%) probability of having infants in the population.
For this update the three review authors (AH, AO, NP) reviewed the titles (and abstracts when available) in CENTRAL (in the Cochrane Library), MEDLINE, Embase, Web of Science, PAS Abstracts (PAS Abstracts‐AAP.org) and handsearched printouts. We retrieved any article that a review author felt met the inclusion criteria or warranted having its reference list searched. We attempted to locate additional unpublished information from published studies.
Extracción y manejo de los datos
We developed data extraction forms. The three review authors (AH, AO, NP) independently abstracted information on each study and checked for any discrepancies. AO pooled the results. Data abstraction included: the time period and geographical location of the study, baseline characteristics of the patients, inclusion and exclusion criteria, preparation, route of administration and dosing regime of inositol and placebo. We abstracted information on outcomes and numbers of affected infants. Outcomes included neonatal and infant deaths. We abstracted the total number of infants with 1) BPD at 28 to 30 days of life (oxygen requirements above the concentration in room air at 28 days of life and a chest roentgenogram compatible with BPD) and 2) BPD at 36 to 38 weeks' PMA (oxygen requirements above the concentration in room air at 36 to 38 weeks' PMA and a chest roentgenogram compatible with BPD), as well as information on ROP (stage 0 to 2; ≥ 3); type 1 ROP, IVH (all grades and grade > 2); NEC; and sepsis (early and late onset). We abstracted any adverse effects reported by the authors.
Evaluación del riesgo de sesgo de los estudios incluidos
Three review authors (AH, AO, NP) independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool for the following domains (Higgins 2011).
• Sequence generation (selection bias)
• Allocation concealment (selection bias)
• Blinding of participants and personnel (performance bias)
• Blinding of outcome assessment (detection bias)
• Incomplete outcome data (attrition bias)
• Selective reporting (reporting bias)
• Any other bias
We resolved any disagreements by discussion or by involving a third assessor to achieve consensus. See Appendix 2 for a more detailed description of risk of bias for each domain.
Medidas del efecto del tratamiento
The statistical analyses followed the recommendations of the Cochrane Neonatal Review Group. We calculated a treatment effect using Review Manager 5 software, supplied by Cochrane (Review Manager 2014). The treatment effect estimates included typical relative risk (RR), risk difference (RD), number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) for dichotomous outcomes; and mean difference (MD) for continuous outcomes. We have reported all estimates of treatment effects with 95% confidence intervals (CI).
Cuestiones relativas a la unidad de análisis
We expected only to encounter data reported as dichotomous or continuous for the whole population randomised.
Manejo de los datos faltantes
In the event of missing data, we planned to contact the authors for clarification. For previous updates, we have contacted authors, but for the update in 2012 we found no need to do so. For this 2019 update, we contacted Dr D Phelps, and she and Dr T Nolen provided clarifying information for the Phelps 2016 study.
Evaluación de la heterogeneidad
We performed heterogeneity tests including the I² test to assess the appropriateness of pooling the data. The degree of heterogeneity was roughly categorised according to Higgins and co‐workers as I² less than 25% equals no heterogeneity, 25% to 49% equals low heterogeneity, 50% to 74% equals moderate heterogeneity, and of 75% or more equals high heterogeneity (Higgins 2003).
Evaluación de los sesgos de notificación
To ascertain the possibility of publication bias, we had planned to perform a funnel plot for the primary outcome of infant death. Because of the small number of studies (< 10) included in all the analyses in the review this was not done.
Síntesis de los datos
Meta‐analyses were performed using Review Manager 2014. For estimates of typical RR and RD we used the Mantel‐Haenszel method. For measured quantities we used the inverse variance method. All meta‐analyses were done using the fixed‐effect model.
Quality of the evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes.
For Comparison 1 we included the following outcomes in the Summary of findings table 1: infant death (age < 1 year); bronchopulmonary dysplasia (at 36 to 38 weeks' PMA); ROP (stage ≥ 3 or ≥ 2); sepsis (early or late onset); necrotizing enterocolitis (suspected or proven); and intraventricular haemorrhage grade > 2).
For Comparison two we did not construct a 'Summary of findings' table, as only one study was identified and the study had a small number of infants enrolled (N = 74).
For Comparison three, we included the following outcomes in the Summary of findings table 2: 'Type 1 ROP or death before determination of ROP outcome using the adjudicated ROP outcome'; 'Type 1 ROP including adjudicated ROP outcome'; 'All‐cause mortality (outcomes collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth)'; 'BPD or death by it prior to 37 weeks' PMA (outcomes collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth)'; 'Severe IVH (grade 3 or 4)'; 'Late‐onset sepsis (> 72 hours of age)'; and 'Suspected or proven NEC'.
The three review authors independently assessed the quality of evidence for each of the outcomes above. We considered evidence from randomised controlled trials as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias): consistency across studies, directness of evidence, precision of estimates, and presence of publication bias. We used the GRADEpro Guideline Development Tool (GRADEpro GDT) to create tables to report the quality of evidence.
The GRADE approach yields an assessment of the quality of a body of evidence using one of four grades.
-
High: we are very confident that the true effect lies close to that of the estimate of the effect.
-
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
-
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
-
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate.
Análisis de subgrupos e investigación de la heterogeneidad
We did perform one subgroup analysis. In Comparison three we included studies that used 'Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA'. As noted under Assessment of heterogeneity we performed heterogeneity tests including the I² test to assess the appropriateness of pooling the data. The degree of heterogeneity was roughly categorised according to Higgins and colleagues as I² less than 25% equals no heterogeneity, 25% to 49% equals low heterogeneity, 50% to 74% equals moderate heterogeneity, and 75% or above equals high heterogeneity (Higgins 2003).
Análisis de sensibilidad
We did not perform sensitivity analyses.
Results
Description of studies
The results of the searches are shown in the Study flow diagram (Figure 1). For study details please refer to the table Characteristics of included studies.
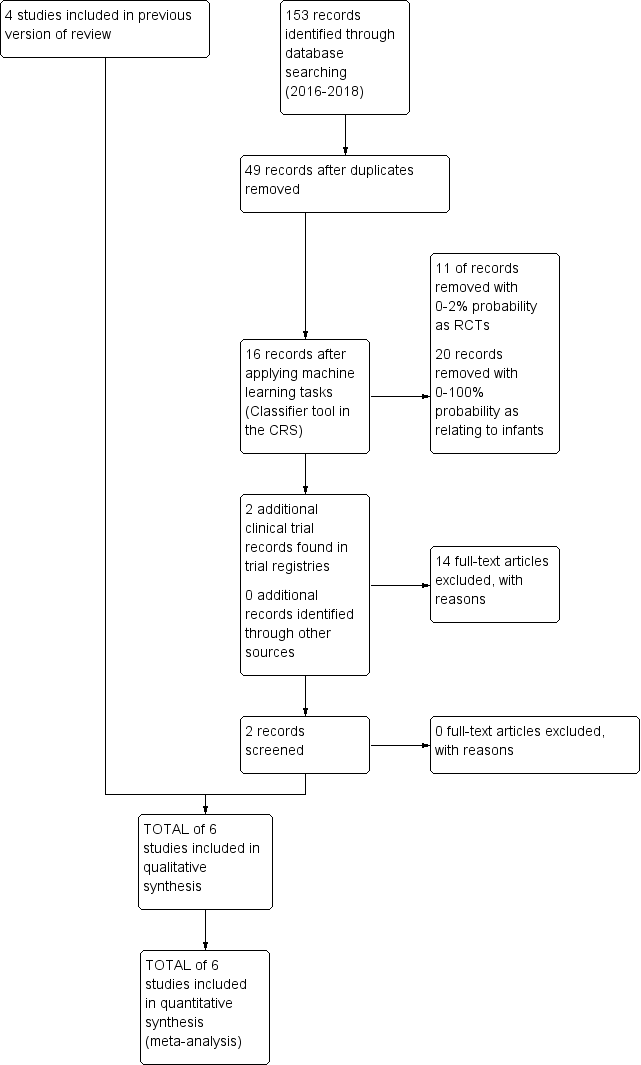
Study flow diagram: review update
Hallman 1986 was a randomised, placebo‐controlled, double blind single‐centre study performed in Helsinki, Finland.
-
Objective: to assess the effects of inositol supplementation provided for 10 days.
-
Population: 74 preterm infants (37 in each group) with RDS, birth weight (BW) < 2000 g.
-
Intervention: both IV (75% of the intragastric dose when enteral inositol could not be given) and enteral (intragastric) (160 mg/kg/day, divided in four doses) inositol were used, and the control group received a placebo (5% glucose).
-
Outcomes assessed: neonatal death, infant death, BPD (at 28 days), NEC, ROP, IVH, and sepsis.
Hallman 1992 was a placebo‐controlled, randomised, double blind trial conducted in Helsinki, Finland.
-
Objective: to assess the effects of IV inositol supplementation in the first five days of life in preterm infants with RDS.
-
Population: 221 infants (24 to 32 weeks' PMA, BW < 2000 g, age two to 10 hours and mechanically ventilated) were enrolled, of which 119 were randomised to receive inositol. All enrolled infants were stratified according to whether they had received surfactant as part of another ongoing study.
-
Intervention: inositol or placebo (glucose) was given as a 5% solution IV The dosage was 80 mg/kg of body weight per day, given for five days.
-
Outcomes assessed: neonatal death, infant death, BPD, ROP, patent ductus arteriosus (PDA), IVH, NEC, infection and neurodevelopmental impairment at 12 months' corrected age.
Friedman 1995 was a placebo‐controlled, randomised trial conducted in two units in the USA.
-
Objective: to examine the relationship between the intake of sugar inositol, serum inositol levels and ROP in LBW infants.
-
Population: 48 preterm infants (BW < 1500 g with severe lung disease) were enrolled, of which 24 were randomised to receive either standard enteral feeds (SC 24 242 µmol/L of inositol) or supplemented formula high in inositol (SC 30 2500 µmol/L of inositol).
-
Intervention: 24 infants received formula containing 242 µmol/L of inositol (control group) and 24 infants received high‐concentration inositol (2500 µmol/L of inositol). Duration not stated.
-
Outcomes: neonatal death, infant death, BPD, NEC, IVH and ROP.
Phelps 2013 was a multi‐centre, randomised, placebo‐controlled trial conducted in 10 units belonging to the National Institutes of Child Health and Human Development Neonatal Research Network.
-
Objective: to describe the pharmacokinetics (PK) of a single IV dose of inositol in 23 to 29 weeks' gestational age infants.
-
Population: 74 infants were randomised in PMA strata (23 to 26 weeks' PMA (N = 37) or 27 to 29 weeks' PMA (N = 37)) to receive either inositol or 5% glucose.
-
Intervention: infants received a single dose of 5% myon‐inositol (60 mg/kg or 120 mg/kg) (N = 49) within six days of birth and before enteral feeds began, or 5% glucose (N = 25).
-
Outcomes: inositol was measured over 96 hours in serum and timed urine collections. "Morbidity and mortality were prospectively recorded through discharge or 120 days of postnatal age." Outcomes included: death during hospital stay, BPD at 36 weeks' PMA, ROP (number of infants, who underwent surgery for ROP), NEC (stage II A or worse), NEC (number of infants who underwent surgery), sepsis (late onset), IVH (grade 3 or 4) and hearing test (failed both ears).
Phelps 2016 was a multi‐centre, randomised, placebo‐controlled trial conducted in 14 units belonging to the National Institutes of Child Health and Human Development Neonatal Research Network.
-
Objective: to assess the safety and pharmacokinetics of daily inositol to select a dose providing serum levels previously associated with benefit, and to learn if accumulation occurred when administered throughout the normal period of retinal vascularization.
-
Population: infants ≤ 29 weeks' PMA (23 0/7 to 29 weeks' PMA), who weighed at least 400 g, and could receive study drug by 72 h after birth (N = 122)
-
Intervention: myo‐inositol (5% solution) at 10, 40 or 80 mg/kg/day was given IV and converted to enteral when feedings were established, and continued to the first of 10 weeks, 34 weeks' PMA, death or discharge. Total number randomised: 10 mg/kg (N = 29); 40 mg/kg (N = 30); 80 mg/kg (N = 28). Placebo: 5% glucose (N = 35).
-
Outcomes: an unfavourable outcome was defined as either type 1 ROP or worse, in either eye, or surgical intervention for severe ROP in either eye. The final ROP status was judged separately in each eye as 'probably favourable', 'probably unfavourable' or 'cannot be determined', and the majority classification was assigned as the adjudicated outcome. Additional outcomes included: death , BPD, RDS, PDA, IVH. seizures, cystic areas in brain parenchyma, sepsis (early and late onset), NEC (suspected or proven), NEC requiring surgery, spontaneous gastrointestinal (GI) perforation and hearing screen failed (either ear). At 18 to 22 months' corrected age, infants received a set of standardized examinations of neurologic function and development according to the NRN Follow‐Up Protocol (to be reported separately).
Phelps 2018 was a multi‐centre, randomised, placebo‐controlled trial conducted in 18 units belonging to the National Institutes of Child Health and Human Development Neonatal Research Network.
-
Objective: to test the adverse events and efficacy of myo‐inositol to reduce type 1 ROP among infants younger than 28 weeks' PMA.
-
Population: 638 infants < 28 weeks' PMA were randomised to receive either myo‐inositol or placebo.
-
Intervention: A 40 mg/kg dose of myo‐inositol was given every 12 hours (initially IV, then enterally when feeding; N = 317), or placebo (N = 321) for up to 10 weeks.
-
Outcomes: type 1 ROP or death before determination of ROP outcome was designated as unfavourable. The designated favourable outcome was survival without type 1 ROP. Other included outcomes were: type 1 ROP, any ROP, ROP ≥ 2 ROP, all‐cause mortality to 55 weeks' PMA, all‐cause mortality (outcomes collected to the first event: death, hospital discharge, hospital transfer, or 120 days after birth), BPD defined as requiring oxygen at 36 weeks' PMA for oxygen saturation > 90%, BPD or death caused by it prior to 37 weeks' gestation (outcomes collected through the first event: death, hospital discharge, hospital transfer, or 120 days after birth, severe IVH (grade 3 or 4), late onset sepsis (> 72 h of age), suspected or proven NEC, surgical NEC, spontaneous GI perforation without NEC, pulmonary haemorrhage, PDA, PDA requiring indomethacin, PDA requiring surgery, seizure treatment(≥ 2 days), hearing screen failed (either ear), and cystic areas in brain parenchyma,
We did not identify any ongoing studies.
Results of the search
The searches in November 2018 identified two additional studies (Phelps 2016; Phelps 2018). For details see 'Study flow diagram: review update' (Figure 1).
Included studies
The review currently includes six studies (Friedman 1995; Hallman 1986; Hallman 1992; Phelps 2013; Phelps 2016; Phelps 2018). The total number of infants randomised in these studies was 1177, an increase in sample size from the previous update — Howlett 2015 — of 760 infants.
Excluded studies
For this update of the review we did not identify any additional studies for exclusion.
Risk of bias in included studies
For details see 'Risk of bias' graph (Figure 2) and 'Risk of bias' summary (Figure 3). Three studies reported that the randomisation sequence was generated by computer (Phelps 2013; Phelps 2016; Phelps 2018); and in three studies, it was unclear how the randomisation sequence was generated (Friedman 1995; Hallman 1986; Hallman 1992).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Two reports lacked written information on allocation concealment (Friedman 1995; Hallman 1992). In the studies by Hallman 1986,Phelps 2013, Phelps 2016 and Phelps 2018 the allocation was conducted centrally.
Blinding
Friedman 1995 did not provide any information on whether the clinical staff and the researchers were blinded. In the study by Hallman 1986 the clinicians and the researchers were blinded to which solution (inositol or glucose) the infants received. Only the pharmacist preparing the doses knew the contents of the drug packages. In the study by Hallman 1992, 5% glucose was given as placebo, but no information was provided on whether staff were blinded to study drugs or not. In the study by Phelps 2013 the drug or placebo was dispensed from the respective pharmacies in unit doses labelled as 'inositol study drug', and all clinical and research personnel except the pharmacist were masked to the study drug. In the two most recent studies by Phelps (Phelps 2016; Phelps 2018), care takers and outcome assessors were blinded to the intervention and the outcome assessments.
Incomplete outcome data
Incomplete outcome data were addressed and the reports seemed free of incomplete data in all studies.
Selective reporting
The studies by Phelps 2013,Phelps 2016 and Phelps 2018 were entered into a trials registry and there did not appear to be any differences between the published protocol and the full report.
The study protocols were not available to us for the studies by Friedman 1995,Hallman 1986 and Hallman 1992, so we can not judge if there were any deviations from the study as planned and the final report.
Other potential sources of bias
Three studies undertook interim analyses (Friedman 1995; Hallman 1986; Hallman 1992). Thus the code must have been broken and that might have influenced decisions on when to close the studies. In two studies (Phelps 2013, Phelps 2016), interim analyses were not undertaken. The Phelps 2018 study was terminated early due to a statistically significantly higher mortality rate in the myo‐inositol group. At 18 months, trial enrolment and treatment were suspended because of a manufacturing issue (later identified as glass lamellae in the third lot of drug, which was never used). Glass lamellae were subsequently found in 1.9% of stored vials of lot two of the trial drug. Detailed analyses revealed that there were no differences in the outcomes for infants treated with myo‐inositol between the two lots of the trial drug. Because the trial did not enrol as many infants as the preplanned sample size, it was underpowered to make conclusions regarding the efficacy and safety of myo‐inositol (Phelps 2018). We did not consider this early stopping of the trial a source of bias.
Effects of interventions
See: Summary of findings for the main comparison Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) compared to control for preterm infants at risk for or having respiratory distress syndrome (Comparison 1); Summary of findings 2 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at less than 30 weeks' PMA compared to placebo for preterm infants at risk for or having respiratory distress syndrome (Comparison 3)
Effects of intervention section The updated literature search detected six published reports (Friedman 1995; Hallman 1986; Hallman 1992; Phelps 2013; Phelps 2016; Phelps 2018). Hallman 1986, Hallman 1992 and Friedman 1995 have all been published as interim analyses, when fewer neonates were enrolled than in the final publication.
Inositol supplementation IV or enterally (repeat doses of any amount and duration) versus control (Comparison 1)
Primary outcomes
Neonatal death, age < 28 days (Outcome 1.1)
Neonatal death was reported in three studies (N = 355) (Friedman 1995; Hallman 1986; Hallman 1992). There was a significant reduction in death prior to 28 days of age in the inositol compared to the control group (typical RR 0.53, 95% CI 0.31 to 0.91; typical RD −0.09, 95% CI −0.16 to −0.01; NNTB 11, 95% CI 6 to 100); I² was 0% for RR (none) and 58% (moderate) for RD (Analysis 1.1).
Infant death, age < one year (Outcome 1.2)
Infant death was reported in five studies (N = 1115) (Friedman 1995; Hallman 1986; Hallman 1992; Phelps 2016; Phelps 2018). There was no significant change in infant deaths in the inositol compared to the control group (typical RR 0.89, 95% CI 0.71 to 1.13; typical RD −0.02, 95% CI −0.07 to 0.02). I² was 80% for RR and 84% for RD (both high) (Analysis 1.2). The certainty of the evidence according to GRADE was low.
Secondary outcomes
BPD (supplementary oxygen at 36 weeks' PMA or death due to BPD at 36 weeks' PMA) (Outcome 1.3)
BPD according to this definition was reported in two studies (N = 666) (Phelps 2016; Phelps 2018). There was no significant difference in BPD in the inositol compared to the control group (typical RR 1.00, 95% CI 0.87 to 1.14; typical RD −0.00, 95% CI −0.08 to 0.07); I² test was 0% (none) for both RR and RD (Analysis 1.3).
Bronchopulmonary dysplasia (BPD) at 28 to 30 days (Outcome 1.4)
Three studies (N = 343) examined the effect of inositol on BPD at 28 to 30 days (Friedman 1995; Hallman 1986; Hallman 1992). There was no significant difference between the groups (typical RR 0.78, 95% CI 0.54 to 1.13; typical RD −0.06, 95% CI −0.15 to 0.03); I² was 49% (low) for RR and 31% (low) for RD (Analysis 1.4).
Bronchopulmonary dysplasia (BPD) at 36 to 38 weeks' PMA (Outcome 1.5)
Two studies (N = 737) reported on this outcome (Hallman 1992; Phelps 2018). There was no significant difference between the inositol supplementation group and the control group (RR 1.04, 95% CI 0.90 to 1.20; RD 0.02, 95% CI −0.05 to 0.09); I² was 0% (none) for both RR and RD (Analysis 1.5). The certainty of the evidence according to GRADE was moderate.
Retinopathy of prematurity (ROP), stage ≥ 3 or ≥ 2 (Outcome 1.6)
Three studies (N = 810) reported on this outcome (Friedman 1995; Hallman 1992; Phelps 2018) (Figure 4). There was no significant difference in ROP stage 3 or more or stage 2 or more in the inositol compared to the control group (typical RR 0.89, 95% CI 0.75 to 1.06; typical RD −0.04, 95% CI −0.10 to 0.02); I² was 63% (moderate) for RR and 23% (none) for RD (Analysis 1.6).
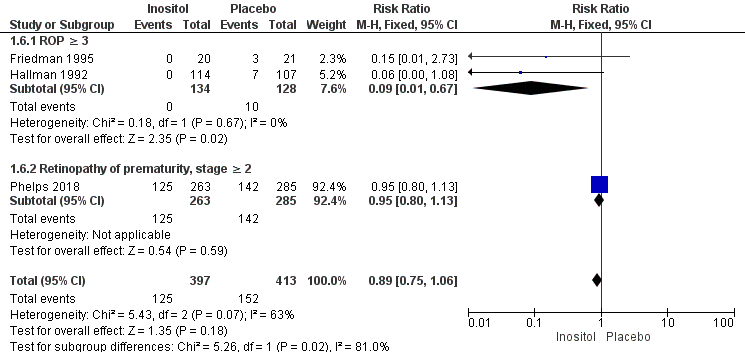
Forest plot of comparison: 1 Inositol supplementation (repeat doses in any amount and any duration of treatment) versus control, outcome: 1.5 Retinopathy of prematurity, stage ≥ 3.
For the subgroup of ROP stage 3 or more, two studies (N = 262) reported on this outcome (Friedman 1995; Hallman 1992). There was a significantly lower incidence of ROP stage 3 or more in the inositol compared to the control group (typical RR 0.09, 95% CI 0.01 to 0.67; typical RD −0.08, 95% CI −0.13 to −0.03; I² was 0% (none) for RR and 0% (none) for RD; NNTB 13 (95% CI 8 to 33) (Outcome 1.61).
For the subgroup of ROP stage 2 or more, one study (N = 548) reported on this outcome (Phelps 2018). There was no significant difference in the incidence of ROP stage 2 or more in the inositol compared to the control group (typical RR 0.95, 95% CI 0.80 to 1.13; typical RD −0.02, 95% CI −0.11 to 0.06); the I² test was not applicable as there was only one study in the analysis (Outcome 1.62). The certainty of the evidence according to GRADE was moderate.
Retinopathy of prematurity (ROP), all (any) stages (Outcome 1.7)
Four trials (N = 889) reported the effect of inositol on the outcome of ROP, all stages (Friedman 1995; Hallman 1986; Hallman 1992; Phelps 2018). There was no significant difference in the incidence of ROP, any stage, in the inositol compared to the control group (typical RR 0.94, 95% CI 0.83 to 1.07; typical RD −0.03, 95% CI −0.09 to 0.03). I² was 35% (low) for RR and 0% (none) for RD (Analysis 1.7).
Necrotizing enterocolitis (NEC) ‒ suspected or proven (Outcome 1.8)
Five studies (N = 1115) reported on this outcome (Friedman 1995; Hallman 1986; Hallman 1992; Phelps 2016;Phelps 2018). The incidence of NEC was not significantly influenced by the use of inositol supplementation (typical RR 0.94, 95% CI 0.64 to 1.39; typical RD −0.00, 95% CI −0.04 to 0.03); I² was 0% (none) for both RR and RD (Analysis 1.8). The certainty of the evidence according to GRADE was moderate.
Sepsis (early and/or late onset (Outcome 1.9))
Four studies (N = 1067) reported on this outcome (Hallman 1986; Hallman 1992; Phelps 2016; Phelps 2018). There was no significant effect of the use of inositol supplementation (typical RR 1.21, 95% CI 0.95 to 1.54; typical RD 0.04, 95% CI −0.01 to 0.09); I² was 24% (none) for RR and 34% (low) for RD (Analysis 1.9). The certainty of the evidence according to GRADE was moderate.
Intraventricular haemorrhage (IVH), grade > 2 (Outcome 1.10)
Five trials (N = 1103) reported on this outcome (Friedman 1995; Hallman 1986; Hallman 1992; Phelps 2016; Phelps 2018). There was no significant difference in the incidence of IVH grade greater than 2 following treatment with inositol (typical RR 0.77, 95% CI 0.58 to 1.01, P = 0.06; typical RD −0.04, 95% CI −0.09 to 0.00; P = 0.06); I² was 48% (low) for RR and 42% (low) for RD (Analysis 1.10). The certainty of the evidence according to GRADE was moderate.
Intraventricular haemorrhage (IVH), all grades (Outcome 1.11)
Three studies (N = 427) reported on this outcome (Hallman 1986; Hallman 1992; Phelps 2016). There was no significant effect of inositol on IVH, all grades (typical RR 0.77, 95% CI 0.59 to 1.00, P = 0.05; typical RD −0.09, 95% CI −0.19 to 0.00); P = 0.05. The I² was 0% (none) for both RR and RD (Analysis 1.11).
Minor neural developmental impairment at one year corrected age (impairment defined as sensorimotor abnormality and/or developmental delay) (Outcome 1.12)
One study (N = 169) reported on this outcome (Hallman 1992). There was no significant effect of inositol (RR 0.84, 95% CI 0.38 to 1.86; RD −0.02, 95% CI −0.12 to 0.08). Tests for heterogeneity were not applicable (Analysis 1.12).
Major neural developmental impairment at one year corrected age (impairment defined as sensory deficit, cerebral palsy, developmental delay, severe hypotonia) (Outcome 1.13)
One study (N = 169) reported on this outcome (Hallman 1992). There was no significant effect of inositol (RR 0.53, 95% CI 0.24 to 1.16; RD −0.08, 95% CI −0.19 to 0.02). Tests for heterogeneity were not applicable (Analysis 1.13).
Periventricular leukomalacia (PVL)
This outcome was not reported in our included studies. Cystic areas in the cerebral parenchyma measured through 28 days of life are reported under Comparison 3 (Analysis 3.12).
Inositol supplementation (single dose) versus control (Comparison 2)
One study compared inositol supplementation in a single dose of 60 mg/kg or 120 mg/kg with placebo (Phelps 2013). We combined the outcomes for the two groups that received a different dose of inositol. As only one study was included under this comparison, tests for heterogeneity were not applicable for any of the outcomes listed below.
Primary outcome
Death during hospital stay (Outcome 2.1)
One study reported on this outcome in 74 infants (Phelps 2013). There was no significant effect of inositol (RR 1.19, 95% CI 0.34 to 4.21; RD 0.02, 95% CI −0.14 to 0.18) (Analysis 2.1).
Secondary outcomes
Bronchopulmonary dysplasia (BPD) at 36 weeks' PMA (Outcome 2.2)
One study reported on this outcome in 65 infants (Phelps 2013). There was no significant effect of inositol on this outcome for RR (2.74, 95% CI 0.88 to 8.48; P = 0.08) but the RD was 0.23 (95% CI 0.03 to 0.43; P = 0.03) with NNTB of 4 (95% CI 2 to 33) (Analysis 2.2).
Retinopathy of prematurity (ROP), infants who underwent surgery for ROP (Outcome 2.3)
One study reported on this outcome in 25 infants (Phelps 2013). There was no significant effect of inositol (RR 0.35, 95% CI 0.10 to 1.22; RD −0.32, 95% CI −0.71 to 0.07) (Analysis 2.3).
Necrotizing enterocolitis (NEC), stage 2A or worse (Outcome 2.4)
One study reported on this outcome in 74 infants (Phelps 2013). There was no significant effect of inositol (RR 0.41, 95% CI 0.12 to 1.39; RD −0.12, 95% CI −0.29 to 0.06) (Analysis 2.4).
Necrotizing enterocolitis (NEC), infants who underwent surgery for NEC (Outcome 2.5) (Analysis 2.5)
One study (Phelps 2013) reported on this outcome in 74 infants. There was no significant effect of inositol (RR 0.51, 95% CI 0.08 to 3.41; RD −0.04, 95% CI −0.16 to 0.08). Analysis 2.5
Sepsis, late onset (Outcome 2.6)
One study reported on this outcome in 74 infants (Phelps 2013). There was no significant effect of inositol (RR 1.46, 95% CI 0.71 to 2.97; RD 0.13, 95% CI −0.10 to 0.35) (Analysis 2.6).
Intraventricular haemorrhage (IVH), grade 3 or 4 (Outcome 2.7)
One study reported on this outcome in 72 infants (Phelps 2013). There was no significant effect of inositol (RR 1.06, 95% CI 0.29 to 3.90; RD 0.01, 95% CI −0.15 to 0.17) (Analysis 2.7)
Hearing test (failed both ears) (Outcome 2.8) (Analysis 2.8)
One study reported on this outcome in 57 infants (Phelps 2013). There was no significant effect of inositol (RR 0.58, 95% CI 0.09 to 3.84; RD −0.04, 95% CI −0.19 to 0.11) (Analysis 2.8).
Inositol supplementation; IV initially, followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA (Comparison 3)
Tests for heterogeneity (I² test) were not applicable for analyses which had only one included study.
Type 1 ROP or death before determination of ROP outcome using adjudicated ROP outcome (Outcome 3.1)
Two studies reported on this outcome (N = 679) (Phelps 2016; Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (typical RR 1.28, 95% CI 0.99 to 1.67; typical RD 0.06, 95% CI −0.00 to 0.13); I² 79% for RR and 85% for RD (both high) (Analysis 3.1). The certainty of the evidence according to GRADE was moderate.
Type 1 ROP (Outcome 3.2)
One study reported on this outcome (N = 511) (Phelps 2018).There was no significant effect of inositol compared to placebo for this outcome (RR 1.41, 95% CI 0.89 to 2.24; RD 0.04, 95% CI −0.01 to 0.10) (Analysis 3.2).
Death before determination of ROP outcome (Outcome 3.3)
One study reported on this outcome (N = 638) (Phelps 2018). There was a significantly higher incidence of death before determination of ROP outcome in the inositol group compared with the placebo group (RR 1.53, 95% CI 1.02 to 2.31, P = 0.04; RD 0.05, 95% CI 0.00 to 0.11, P = 0.04); NNTH 33 (95% CI 9 to infinity) (Analysis 3.3).
Type 1 ROP including adjudicated ROP outcome (Outcome 3.4)
Two studies reported on this outcome (N = 605) (Phelps 2016; Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (typical RR 1.24, 95% CI 0.82 to 1.86; typical RD 0.03, 95% CI −0.03 to 0.08); I² 46 % (low) for RR and 54% (moderate) for RD (Analysis 3.4). The certainty of the evidence according to GRADE was moderate (Figure 5).

Forest plot of comparison: 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, outcome: 3.4 Type 1 ROP including adjudicated ROP outcome.
Any ROP (Outcome 3.5)
One study reported on this outcome (N = 553) (Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (RR 1.00, 95% CI 0.88 to 1.13; RD 0.00, 95% CI −0.08 to 0.08) (Analysis 3.5).
ROP stage ≥ 2 ROP (Outcome 3.6)
One study reported on this outcome (N = 548) (Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (RR 0.95, 95% CI 0.80 to 1.13; RD −0.02, 95% CI −0.11 to 0.06) (Analysis 3.6).
All‐cause infant mortality to 55 weeks' PMA (Outcome 3.7)
One study reported on this outcome (N = 638) (Phelps 2018). There was a significant higher mortality in the inositol group compared to the placebo group (RR 1.67, 95% CI 1.12 to 2.48; RD 0.07, 95% CI 0.02 to 0.12); NNTH 14 (95% CI 8 to 50) (Analysis 3.7).
All‐cause mortality (outcome collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth (Outcome 3.8))
Two studies reported on this outcome (N = 701) (Phelps 2016; Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (typical RR 1.35, 95% CI 0.91 to 2.00; typical RD 0.04, 95% CI −0.01 to 0.09); I² = 72% (moderate) for RR; and 84% (high) for RD (Analysis 3.8). The certainty of the evidence according to GRADE was moderate.
BPD (requiring oxygen at 36 weeks' PMA for oxygen saturation > 90%) (Outcome 3.9)
One study reported on this outcome (N = 560) (Phelps 2018).There was no significant effect of inositol compared to placebo for this outcome (RR 1.02, 95% CI 0.89 to 1.18; RD 0.01, 95% CI −0.07 to 0.09) (Analysis 3.9).
BPD or death by it prior to 37 weeks' PMA (outcomes collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth (Outcome 3.10)
Two studies reported on this outcome (N = 616) (Phelps 2016; Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (typical RR 1.01, 95% CI 0.87 to 1.16; typical RD 0.00, 95% CI −0.07 to 0.08); I² = 0% (none) for both RR and RD (Analysis 3.10). The certainty of the evidence according to GRADE was high.
Severe IVH (grade 3 or 4) (Outcome 3.11)
Two studies reported on this outcome (N = 690) (Phelps 2016; Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (typical RR 0.92, 95% CI 0.65 to 1.29; typical RD −0.01, 95% CI −0.07 to 0.04); I² = 74% (moderate) for RR and 82% (high) for RD (Analysis 3.11). The certainty of the evidence according to GRADE was moderate.
Cystic areas in the cerebral parenchyma measured through 28 d (Outcome 3.12)
Two studies reported on this outcome (N = 225) (Phelps 2016; Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (typical RR, 1.29, 95% CI 0.58 to 2.85; typical RD 0.03, 95% CI −0.05 to 0.10); I² = 0% for both RR and RD (Analysis 3.12).
Early onset sepsis (Outcome 3.13)
One study reported on this outcome (N = 63) (Phelps 2016). There was no significant effect of inositol compared to placebo for this outcome (RR, not estimable as there were no outcomes in either group; RD 0.00, 95% CI −0.06 to 0.06) (Analysis 3.13).
Late onset sepsis (> 72 hours of age) (Outcome 3.14)
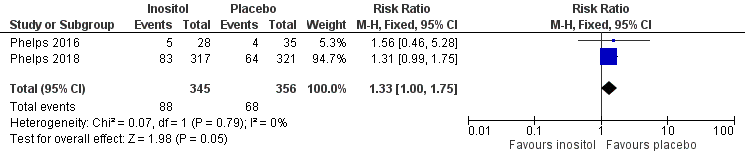
Two studies reported on this outcome (N = 701) (Phelps 2016; Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (typical RR 1.33, 95% CI 1.00 to 1.75; typical RD 0.06, 95% CI 0.00 to 0.12; P = 0.05 for both RR and RD); I² = 0% (none) for both RR and RD (Analysis 3.14). The certainty of the evidence according to GRADE was high. (Figure 6)
Forest plot of comparison: 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, outcome: 3.14 Late onset sepsis (> 72 hours of age).
Suspected or proven NEC (Outcome 3.15)
Two studies reported on this outcome (N = 701) (Phelps 2016; Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (typical RR 0.88, 95% CI 0.55 to 1.41; typical RD −0.01, 95% CI −0.05 to 0.03); I² = 36% (low) for RR; and 53% (moderate) for RD (Analysis 3.15). The certainty of the evidence according to GRADE was high.
Surgical NEC (Outcome 3.14) (Analysis 3.16)
Two studies reported on this outcome (N = 701) (Phelps 2016; Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (typical RR 1.21, 95% CI 0.57 to 2.58; typical RD 0.01, 95% CI −0.02 to 0.04); I² = 51% (moderate) for RR; and 69% (moderate) for RD. Analysis 3.16
Spontaneous gastro‐intestinal perforation (Outcome 3.17)
Two studies reported on this outcome (N = 701) (Phelps 2016; Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (typical RR 0.86, 95% CI 0.48 to 1.52; typical RD −0.01, 95% CI −0.05 to 0.03); I² = 0% (none) for both RR and RD. Analysis 3.17
Pulmonary haemorrhage (Outcome 3.18)
One study reported on this outcome (N = 638) (Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (RR 0.98, 95% CI 0.59 to 1.62; RD −0.00, 95% CI −0.05 to 0.04) (Analysis 3.18).
PDA (Outcome 3.19)
Two studies reported on this outcome (N = 700) (Phelps 2016; Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (typical RR 0.98, 95% CI 0.85 to 1.14; typical RD −0.01, 95% CI −0.08 to 0.07); I² = 0% (none) for both RR and RD (Analysis 3.19).
PDA requiring indomethacin (Outcome 3.20)
One study reported on this outcome (N = 637) (Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (RR 0.90, 95% CI 0.67 to 1.22; RD −0.02, 95% CI −0.09 to 0.04) (Analysis 3.20).
PDA requiring surgery (Outcome 3.21)
Two studies reported on this outcome (N = 700) (Phelps 2016; Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (RR 0.96, 95% CI 0.65 to 1.42; RD −0.00, 95% CI −0.05 to 0.04); I² = 0% (none) for both RR and RD (Analysis 3.21).
Seizures (treatment for ≥ 2 days) (Outcome 3.22)
Two studies reported on this outcome (N = 700) (Phelps 2016; Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (typical RR 1.04, 95% CI 0.43 to 2.56; typical RD 0.00, 95% CI −0.02 to 0.02); I² = 7% (none) for RR and 38% (low) for RD (Analysis 3.22).
Negative hearing screen in either ear at discharge (Outcome 3.23)
Two studies reported on this outcome (N = 472) (Phelps 2016; Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (typical RR 1.45, 95% CI 0.92 to 2.29; typical RD 0.05, 95% CI −0.01 to 0.11); I² = 0% (none) for both RR and RD (Analysis 3.23).
Respiratory distress syndrome (Outcome 3.24)
One study (Phelps 2016) reported on this outcome (N = 63). There was no significant effect of inositol compared to placebo for this outcome (RR 0.99, 95% CI 0.91 to 1.09; RD −0.01, 95% CI −0.10 to 0.08) (Analysis 3.24).
Sepsis, necrotizing enterocolitis, pneumonia or other infection as a cause of death (Outcome 3.25)
One study reported on this outcome among deaths (N = 83) (Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (RR 1.36, 95% CI 0.95 to 1.93; RD 0.19, 95% CI −0.01 to 0.40).
Discusión
Resumen de los resultados principales
En la actualización anterior de esta revisión en 2015 (Howlett 2015), se demostraron reducciones estadísticamente significativas en las muertes neonatales, la muerte infantil y la HIV de grado mayor de 2 con dosis repetidas de suplementos de inositol, y se encontró una reducción significativa en la RP estadio 3 o mayor. No hubo una reducción significativa de la DBP ni un aumento significativo de los efectos potencialmente adversos como la sepsis, la ECN o el deterioro neurológico a los 12 meses de vida corregidos. Se indicó que los resultados de la revisión, que mostraron resultados significativos prometedores a favor del inositol, se deberían interpretar con cautela (el número de neonatos incorporados en dos de los ensayos revisados fue pequeño, y las estimaciones del efecto en los ensayos individuales y en los metanálisis no fueron muy precisas, como indican los intervalos de confianza amplios). Se indicó que se requerían ensayos controlados aleatorios multicéntricos futuros sobre la administración de suplementos de inositol para confirmar los efectos beneficiosos sugeridos en la revisión (Howlett 2015), así como para evaluar los posibles efectos adversos de los resultados a corto y largo plazo. Se identificó un estudio grande en curso sobre dosis repetidas de inositol en lactantes prematuros (NCT01954082), que ya se ha publicado (Phelps 2018). En este estudio se programó incorporar a 1760 lactantes prematuros con menos de 28 0/7 semanas de la FUM. El resultado primario del estudio fue la incidencia de supervivencia sin RP grave a través de la determinación de RP aguda y final hasta las 55 semanas de FUM. Se indicó que este estudio confirmaría o refutaría los resultados prometedores de los estudios de administración repetida de suplementos de inositol incluidos en la actualización de 2015 de esta revisión (Howlett 2015).
En esta actualización de la revisión se incluyen dos estudios adicionales. Phelps 2016 incorporó a 122 neonatos, y Phelps 2018, a 638 neonatos. Los neonatos fueron reclutados de 14 y 18 centros de la Eunice Kennedy Shriver NICHD Neonatal Research Network en los Estados Unidos, respectivamente. El tamaño de la muestra de la presente revisión aumentó en 760 lactantes hasta un total de 1177. El reclutamiento planificado de 1760 participantes en el estudio Phelps 2018 habría permitido detectar una reducción absoluta de la mortalidad o de la RP tipo 1 del 7%, con un poder estadístico del 90%. El ensayo se interrumpió de manera precoz debido a una tasa de mortalidad significativamente mayor en el grupo de mioinositol. Los dos estudios incorporaron neonatos de menos de 30 semanas de FUM, mientras que los estudios de Friedman 1995, Hallman 1986 y Hallman 1992 incorporaron algunos lactantes que eran más maduros. Por lo tanto, se hicieron dos comparaciones: "Administración i.v. o enteral de suplementos de inositol (dosis repetidas de cualquier cantidad y duración) versus control (Comparación 1)" y "Administración i.v. inicial de suplementos de inositol seguida de administración enteral (dosis repetidas de 80 mg/kg/día) en lactantes prematuros con < 30 semanas de la FUM" (Comparación 3). Se mantuvo una comparación previa a la actualización de la revisión: "Administración de suplementos de inositol (dosis única) versus control" (Comparación 2). No se identificaron ensayos nuevos para esta comparación.
Hay diferencias significativas en los resultados de la Comparación 1 en esta actualización en comparación con la versión de 2015 (Howlett 2015). Para la "Administración i.v. o enteral de suplementos de inositol (dosis repetidas de cualquier cantidad y duración) versus control" (Comparación 1), los resultados de la muerte neonatal se mantuvieron significativos, ya que los dos estudios nuevos (Phelps 2016 y Phelps 2018) no informaron sobre ese resultado. Para los demás resultados, incluidas las muertes infantiles, la HIV de grado mayor de 2 y la RP estadio 2 o mayor, o 3 o mayor, dejó de haber diferencias estadísticamente significativas entre los grupos con inositol y con placebo.
No se incluyeron ensayos nuevos en la Comparación 2 y, por lo tanto, no se detectaron nuevos hallazgos.
En la comparación 3 ("Administración i.v. inicial de suplementos de inositol seguida de administración enteral [dosis repetidas de 80 mg/kg/día] en lactantes prematuros con < 30 semanas de la FUM"), no hubo diferencias estadísticamente significativas entre el grupo de mioinositol (80 mg/kg/día) y el grupo de placebo. En los análisis se incluyeron 24 resultados: RP tipo 1 o muerte antes de la determinación del resultado RP mediante el resultado de RP adjudicado, RP tipo 1 que incluye el resultado de RP adjudicado, mortalidad por todas las causas (resultado obtenido a través del primer evento: muerte, alta hospitalaria, traslado al hospital o 120 días después del parto), DBP o muerte antes de las 37 semanas de la FUM (resultado obtenido a través del primer evento: muerte, alta hospitalaria, traslado al hospital o 120 días después del parto), HIV grave (grado 3 o 4); sepsis de aparición tardía (> 72 horas de vida) y ECN presunta o confirmada.
Compleción y aplicabilidad general de las pruebas
En esta revisión, el tamaño de la muestra aumentó en 760 lactantes, todos con menos de 30 semanas de la FUM al parto. Esta es la población que tiene más probabilidades de resultados adversos relacionados con el parto prematuro. Para el resultado "Muerte infantil" en la Comparación 1 hubo una alta heterogeneidad para el CR y la DR. Esa comparación incluyó una población mixta de lactantes de diferentes FUM y el tratamiento con inositol varió, por lo que se puede esperar heterogeneidad. La Comparación 2 incluyó solo un ensayo con un tamaño de muestra pequeño, y las pruebas de heterogeneidad no fueron aplicables. La Comparación 3 incluyó dos poblaciones muy similares de la Eunice Kennedy Shriver NICHD Neonatal Research Network (Phelps 2016; Phelps 2018). Los neonatos de un subgrupo (tratados con 80 mg/kg/día de mioinositol) del ensayo Phelps 2016 y todos los neonatos del ensayo Phelps 2018 recibieron el mismo tratamiento, y la mayoría de los resultados se evaluaron e informaron de manera similar. Los dos estudios se realizaron relativamente cerca en el tiempo. Por lo tanto, es sorprendente que los resultados de los dos estudios difieran, con heterogeneidad alta o moderada en cuatro resultados: RP tipo 1 o muerte antes de la determinación del resultado RP mediante el resultado de RP adjudicado (CR, I² = 79% ‒ alta; DR, I² = 85% ‒ alta), mortalidad por todas las causas (resultado obtenido a través del primer evento: muerte, alta hospitalaria, traslado al hospital o 120 días después del parto) (CR, I² = 72% ‒ moderada; DR, I² = 84% ‒ alta), HIV grave (grado 3 o 4) (I² = 74% ‒ moderada; DR = 82% ‒ alta) y ECN quirúrgica (I² = 51% ‒ moderada; DR, I² = 69% ‒ moderada).
Calidad de la evidencia
Aunque los seis estudios incluidos fueron ensayos controlados aleatorios, en tres de ellos se realizó un análisis intermedio que podría haber alterado el cegamiento de los investigadores antes de que se complete el ensayo. Se consideró que la calidad de los estudios hacía que los metanálisis fuesen apropiados. Los dos nuevos estudios incluidos en esta revisión fueron de alta calidad (Phelps 2016; Phelps 2018). Sin embargo, en estos dos estudios las estimaciones puntuales de muchos resultados van en direcciones diferentes, lo que explica la heterogeneidad señalada anteriormente en Completitud y aplicabilidad general de la evidencia. En los análisis, el estudio Phelps 2018 tiene una mayor ponderación debido al mayor tamaño de la muestra (N = 638) en comparación con el estudio Phelps 2016 (N = 122). Para la Comparación 1, la certeza de la evidencia según los criterios GRADE fue baja para el resultado muerte infantil (edad < 1 año) y moderada para la displasia broncopulmonar (a las 36 a 38 semanas de la FUM), la RP (estadio ≥ 3 o ≥ 2), la sepsis (inicio temprano o tardío), la enterocolitis necrosante (presunta o confirmada) y la HIV (grado > 2). No se realizaron evaluaciones con criterios GRADE para la Comparación 2. Para la Comparación 3, la certeza de la evidencia según los criterios GRADE fue alta para la DBP o la muerte antes de las 37 semanas de la FUM (resultados obtenidos a través del primer evento: muerte, alta hospitalaria, traslado al hospital o 120 días después del parto); sepsis de aparición tardía (> 72 horas de vida) y la ECN presunta o confirmada. La certeza de la evidencia según los criterios GRADE fue moderada para la RP tipo 1 o la muerte antes de la determinación del resultado RP mediante el resultado de RP adjudicado, la RP tipo 1 que incluye el resultado de RP adjudicado, la mortalidad por todas las causas (resultado obtenido a través del primer evento: muerte, alta hospitalaria, traslado al hospital o 120 días después del parto) y la hemorragia intraventricular grave (grado 3 o 4).
El estudio Phelps 2018 terminó de manera precoz debido a una tasa de mortalidad significativamente más alta en el grupo de mioinositol. A los 18 meses se suspendió el reclutamiento y el tratamiento del ensayo debido a un problema de fabricación (posteriormente identificado como láminas de vidrio en el tercer lote del fármaco, que nunca se utilizó). Posteriormente, se encontraron láminas de vidrio en el 1,9% de los viales almacenados del lote 2 del fármaco del ensayo. Los análisis detallados mostraron que no hubo diferencias en los resultados de los lactantes tratados con mioinositol entre los dos lotes del fármaco del ensayo. Debido a que el ensayo no incluyó tantos neonatos como el tamaño de la muestra preplanificada, no tuvo el poder estadístico suficiente para establecer conclusiones con respecto a la eficacia y la seguridad del mioinositol (Phelps 2018). Esta interrupción temprana del ensayo no se consideró como una fuente de sesgo.
Sesgos potenciales en el proceso de revisión
No se conocen sesgos potenciales en el proceso de revisión.
Acuerdos y desacuerdos con otros estudios o revisiones
No se conoce de otras revisiones sistemáticas o metanálisis sobre este tema. El apartado de «Discusión» del estudio Phelps 2018 es directamente relevante para esta actualización de la revisión. Se cita (se han insertado las referencias del primer autor en lugar de los números de referencia Phelps y colaboradores utilizados en su texto):
"En este ensayo de administración de suplementos de mioinositol para mejorar la supervivencia sin RP tipo 1 en lactantes extremadamente prematuros, el mioinositol no redujo las tasas de RP tipo 1, y el ensayo se interrumpió precozmente debido a un aumento significativo e inesperado de la mortalidad. Los resultados beneficiosos anteriores del mioinositol no se observaron en el ensayo actual; sin embargo, existen varias diferencias relevantes entre los estudios actuales y anteriores de inositol (Hallman 1990; Hallman 1986; Hallman 1992). En los ensayos anteriores los corticosteroides prenatales no se utilizaron ampliamente, ni se dispuso de surfactante. Los estudios anteriores trataron a los lactantes durante tres a diez días con mioinositol durante la fase aguda del síndrome de dificultad respiratoria, mientras que el presente ensayo trató a los lactantes durante diez semanas para apoyar el desarrollo vascular de la retina.
"En los ensayos anteriores (Hallman 1990; Hallman 1986; Hallman 1992), los lactantes eran más maduros al nacer (media, 27 a 29 semanas de edad gestacional). Una explicación podría ser que el efecto beneficioso del mioinositol sobre las tasas de hemorragia intraventricular, displasia broncopulmonar y RP en los estudios anteriores puede haber sido resultado del efecto beneficioso previsto del mioinositol sobre la función surfactante (Hallman 1990; Hallman 1986; Hallman 1987). Se puede esperar que la reducción de la gravedad del síndrome de dificultad respiratoria reduzca estas morbilidades. Por lo tanto, el efecto beneficioso del mioinositol sobre la función surfactante en el ensayo actual puede haber sido superado por los efectos beneficiosos de los corticosteroides prenatales, el surfactante exógeno y el apoyo ventilatorio no invasivo que se utilizan actualmente.
"La dosis de mioinositol para producir concentraciones séricas fue similar a la de los estudios anteriores (Hallman 1987, Hallman 1992, Phelps 2016). Sin embargo, la combinación de un tratamiento más prolongado y la inclusión de lactantes de edad gestacional más temprana puede haber dado lugar a un aumento inesperado de la mortalidad a través de mecanismos aún desconocidos. Los datos in vitro han demostrado un aumento de la infección de los macrófagos por algunas bacterias intracelulares debido a su capacidad de utilizar el mioinositol como fuente de energía (Manske 2016).
"Un problema adicional en este ensayo fue su interrupción cuando se encontraron partículas (posteriormente identificadas como láminas de vidrio) en el tercer lote del fármaco, que nunca se utilizó. Las partículas de vidrio son una razón que se cita con frecuencia para retirar los medicamentos. La presencia de láminas dentro de los viales de vidrio depende del proceso de fabricación del vidrio y de las características químicas del medicamento, en particular si es ácido o cáustico (Guadagnino 2012; Zhao 2014).
"Las láminas de vidrio que se encontraron posteriormente en el 1,9% de los viales almacenados en el lote dos del fármaco del ensayo plantearon la interrogante de si el efecto nocivo observado del mioinositol podría haberse debido a estas partículas. Sin embargo, los análisis detallados mostraron que no hubo diferencias en los resultados para los lactantes tratados con mioinositol entre los dos lotes del fármaco del ensayo".
Phelps 2018 no discutió por qué las estimaciones puntuales de muchos de los resultados para el grupo de dosis de 80 mg/kg/día en el estudio Phelps 2016 difirieron de los resultados para el grupo de dosis de 80 mg/kg/día utilizada en todos los lactantes en el estudio Phelps 2018. Los datos de los dos estudios dieron lugar a heterogeneidad para muchos de los resultados cuando se incluyeron en los metanálisis de la Comparación 3. Sin embargo, para el resultado de "sepsis de aparición tardía (72 horas de edad)", las estimaciones puntuales para los dos estudios fueron en la misma dirección, el CR típico fue 1,33 (IC del 95%: 1,00 a 1,75; P = 0,05) y la DR fue 0,06 (IC del 95%: 0,00 a 0,12; P = 0,05); I² = 0% para el CR y para la DR. La sepsis, la enterocolitis necrosante, la neumonía u otras infecciones como causa de muerte fueron mayores (sin alcanzar significación estadística) en el grupo de mioinositol en comparación con el grupo placebo en el estudio Phelps 2018; CR 1,36 (IC del 95%: 0,95 a 1,93; P = 0,09; DR 0,19 [IC del 95%: ‐0,01 a 0,40]; P = 0,07). En el estudio Phelps 2016, "la infección se informó como una causa primaria de muerte en el grupo de 40 mg/kg para el 17% de los sujetos, en comparación con el 0% al 3% como causa de muerte para otros grupos de dosis (P < 0,01 para la comparación entre todos los grupos de dosis)". Los cuatro grupos en el estudio fueron inositol 10 mg/kg/día, 40 mg/kg/día, 80 mg/kg/día y placebo 0 mg/kg/día. Estos resultados indican una posible asociación entre la ingesta de mioinositol y un mayor riesgo de sepsis de inicio tardío en los lactantes prematuros.
Las tendencias hacia un mayor riesgo de sepsis de inicio tardío y un mayor riesgo de muerte por infección en el grupo de mioinositol en comparación con el grupo placebo pueden apoyar los datos in vitro que han demostrado que la infección de los macrófagos, por algunas bacterias intracelulares, está reforzada por su capacidad de utilizar el mioinositol como fuente de energía (Manske 2016).
Study flow diagram: review update
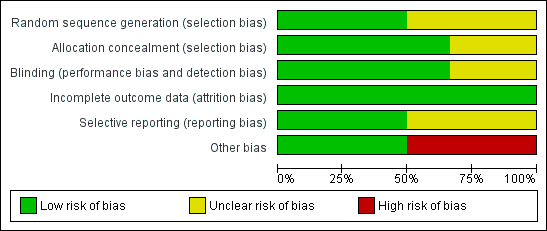
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Inositol supplementation (repeat doses in any amount and any duration of treatment) versus control, outcome: 1.5 Retinopathy of prematurity, stage ≥ 3.

Forest plot of comparison: 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, outcome: 3.4 Type 1 ROP including adjudicated ROP outcome.

Forest plot of comparison: 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, outcome: 3.14 Late onset sepsis (> 72 hours of age).
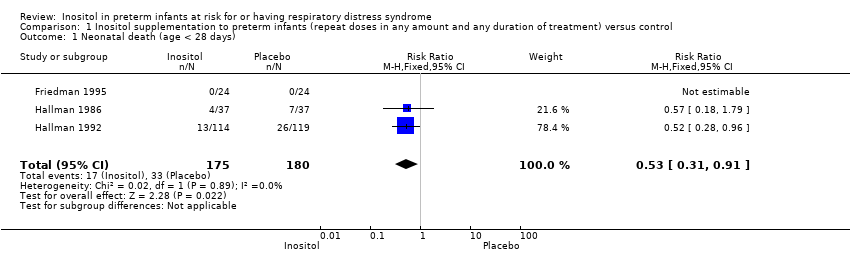
Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 1 Neonatal death (age < 28 days).
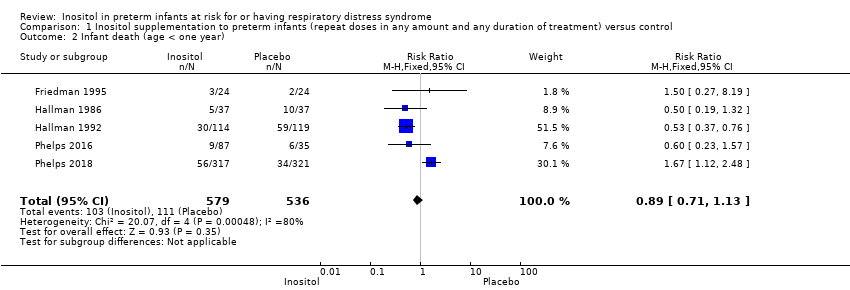
Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 2 Infant death (age < one year).

Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 3 BPD (supplementary oxygen ar 36 weeks; PMA or death due to BPD)at 36 week's PMA.

Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 4 Bronchopulmonary dysplasia (at 28 to 30 days of age).

Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 5 Bronchopulmonary dysplasia (at 36 to 38 weeks PMA).

Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 6 Retinopathy of prematurity, stage ≥ 3 or ≥ 2.
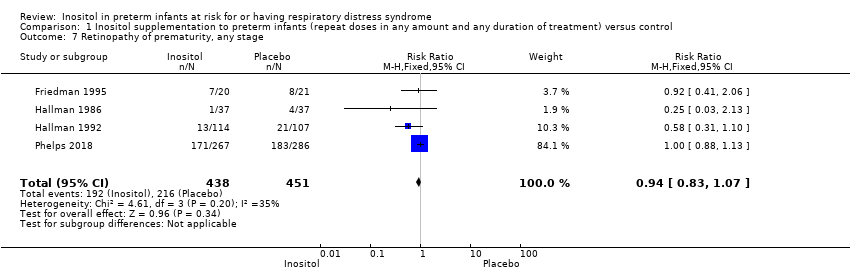
Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 7 Retinopathy of prematurity, any stage.

Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 8 Necrotizing enterocolitis (suspected or proven).

Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 9 Sepsis (early and/or late onset).

Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 10 Intraventricular haemorrhage, grade > 2.
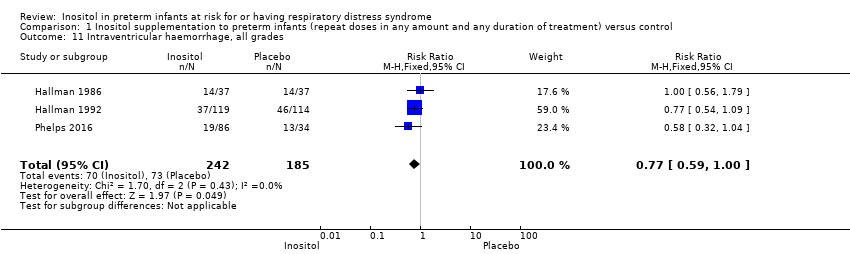
Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 11 Intraventricular haemorrhage, all grades.

Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 12 Minor neural developmental impairment at one year corrected age.
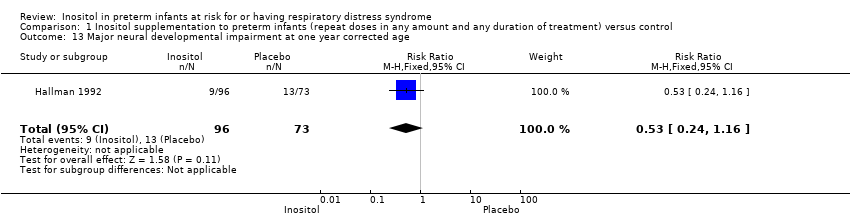
Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 13 Major neural developmental impairment at one year corrected age.
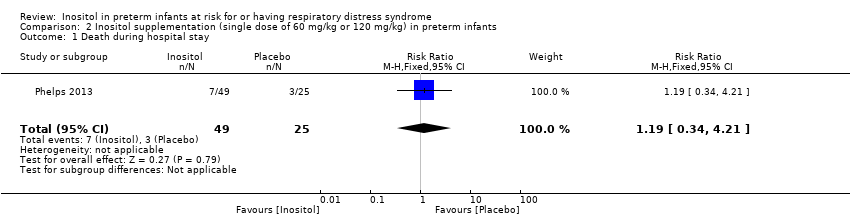
Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg) in preterm infants, Outcome 1 Death during hospital stay.

Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg) in preterm infants, Outcome 2 Bronchopulmonary dysplasia at 36 weeks PMA.
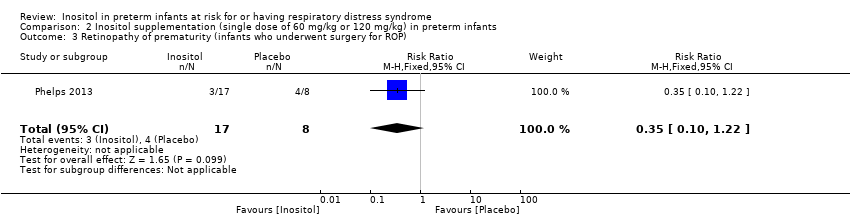
Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg) in preterm infants, Outcome 3 Retinopathy of prematurity (infants who underwent surgery for ROP).

Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg) in preterm infants, Outcome 4 Necrotizing enterocolitis (stage 2A or worse).
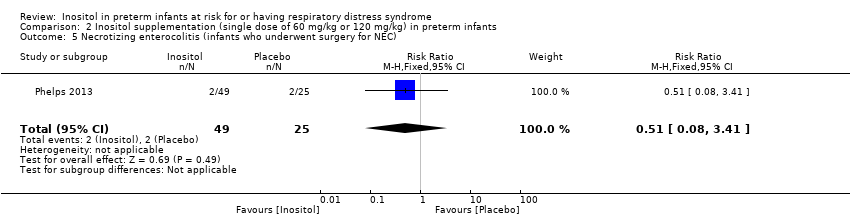
Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg) in preterm infants, Outcome 5 Necrotizing enterocolitis (infants who underwent surgery for NEC).
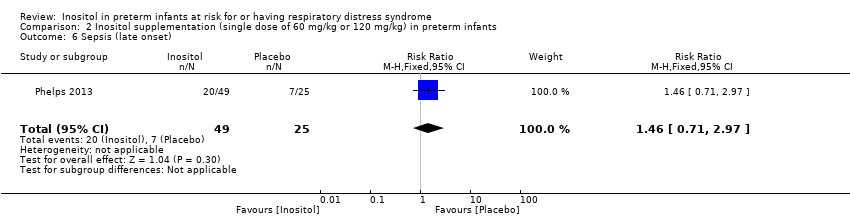
Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg) in preterm infants, Outcome 6 Sepsis (late onset).

Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg) in preterm infants, Outcome 7 Intraventricular haemorrhage (grade 3 or 4).

Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg) in preterm infants, Outcome 8 Hearing test (failed both ears).

Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 1 Type 1 ROP or death before determination of ROP outcome using the adjudicated ROP outcome.

Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 2 Type 1 ROP.
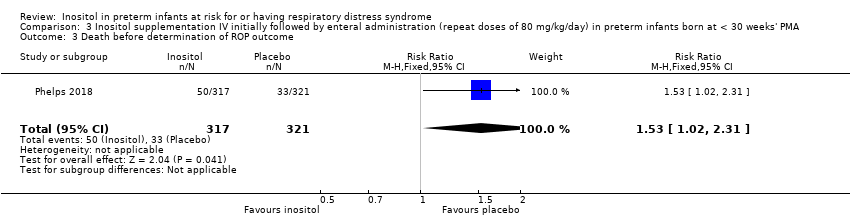
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 3 Death before determination of ROP outcome.

Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 4 Type 1 ROP including adjudicated ROP outcome.
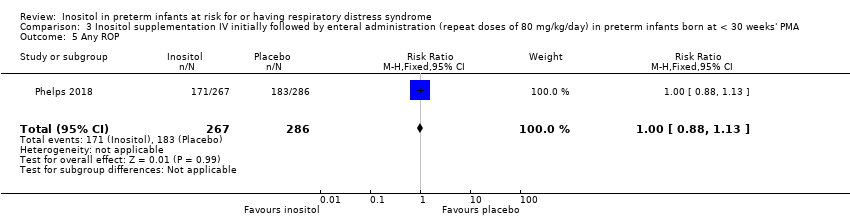
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 5 Any ROP.

Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 6 ROP ≥ 2 ROP.

Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 7 All cause infant mortality to 55 week's PMA.

Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 8 All cause mortality (outcome collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth).

Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 9 BPD (requiring oxygen at 36 week's PMA for oxygen saturation > 90%).

Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 10 BPD or death by it prior to 37 weeks' PMA (outcomes collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth).
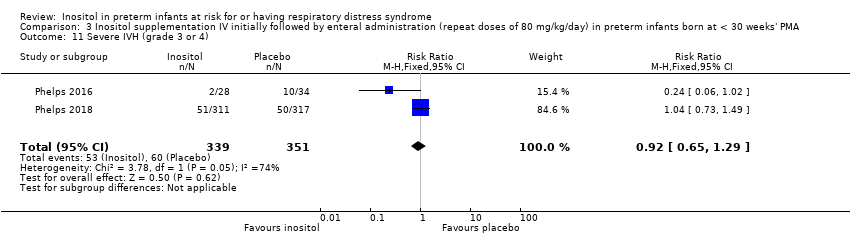
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 11 Severe IVH (grade 3 or 4).

Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 12 Cystic areas in the cerebral parenchyma measured through 28 d.

Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 13 Early onset sepsis.

Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 14 Late onset sepsis (> 72 hrs of age).

Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 15 Suspected or proven NEC.

Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 16 Surgical NEC.
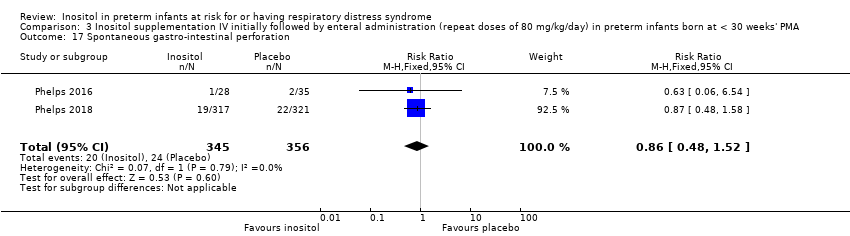
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 17 Spontaneous gastro‐intestinal perforation.

Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 18 Pulmonary haemorrhage.

Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 19 PDA.
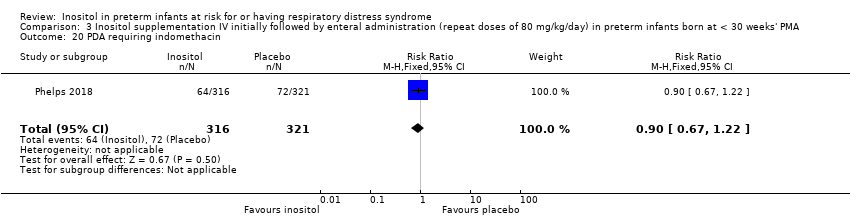
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 20 PDA requiring indomethacin.

Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 21 PDA requiring surgery.

Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 22 Seizure treatment for ≥ 2 days.
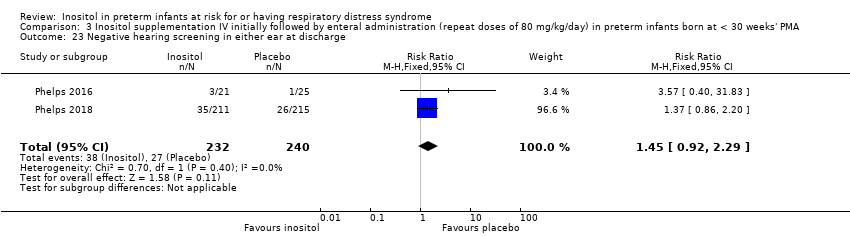
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 23 Negative hearing screening in either ear at discharge.
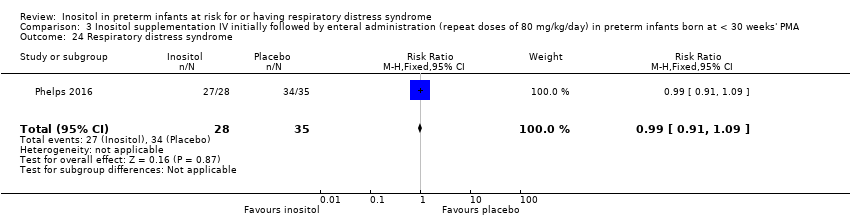
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 24 Respiratory distress syndrome.
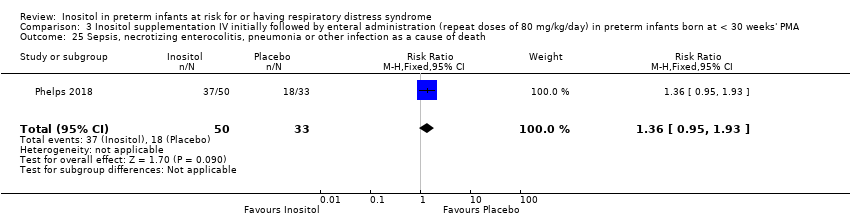
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 25 Sepsis, necrotizing enterocolitis, pneumonia or other infection as a cause of death.
| Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) compared to control for preterm infants at risk for or having respiratory distress syndrome | ||||||
| Patient or population: preterm infants at risk for or having respiratory distress syndrome | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with control | Risk with Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) | |||||
| Infant death (age < 1 year) | Study population | RR 0.89 | 1115 | Low | Design (risk of bias): the risk of bias for random sequence generation was low in 2 studies and unclear in 3 studies; the risk of bias for allocation concealment was low in 3 studies and unclear in 2 studies; the risk of bias regarding performance bias and detection bias was low in 3 studies and unclear in 2 studies. We downgraded the quality of the evidence by 1 step Heterogeneity/consistency across studies: there was high heterogeneity for RR (I² = 80 % ) and for RD (I² = 84%). We downgraded the quality of the evidence by 1 step Directness of the evidence: Studies were conducted in the target population. Precision of estimates: Results from 1115 infants have been reported in the studies to date and the confidence intervals around the point estimates for RR and RD were narrow. Presence of publication bias: As only 5 studies were included in the analysis we did not perform a funnel plot. | |
| 207 per 1000 | 184 per 1000 | |||||
| Bronchopulmonary dysplasia (at 36 to 38 weeks' PMA) | Study population | RR 1.04 | 737 | Moderate | Design (risk of bias): the risk of bias for random sequence generation was low in 1 study and unclear in 1 study; The risk of bias for allocation concealment was low in 1 study and unclear in 1 study; the risk of bias regarding performance bias and detection bias was low in 1 study and unclear in 1 study. We downgraded the quality of the evidence by 1 step Heterogeneity/consistency across studies: there was no heterogeneity for RR (I² = 0%) and for RD (I² = 0%) Directness of the evidence: studies were conducted in the target population Precision of estimates: to date the results from 737 infants have been reported in the studies and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 2 studies were included in the analysis we did not perform a funnel plot | |
| 459 per 1000 | 477 per 1000 | |||||
| ROP, stage ≥ 3 or ≥ 2 | Study population | RR 0.89 | 810 | Moderate | Design (risk of bias): the risk of bias for random sequence generation was low in 1 study and unclear in 2 studies; the risk of bias for allocation concealment was low in 1 study and unclear in 2 studies; the risk of bias regarding performance bias and detection bias was low in 1 study and unclear in 2 studies. We downgraded the quality of the evidence by 1 step Heterogeneity/consistency across studies: there was moderate heterogeneity for RR (I² = 63% ) and none for RD (I² = 23%) Directness of the evidence: Studies were conducted in the target population Precision of estimates: to date the results from 810 infants have been reported in the studies and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 3 studies were included in the analysis we did not perform a funnel plot | |
| 368 per 1000 | 328 per 1000 | |||||
| Sepsis (early or late onset) | Study population | RR 1.21 | 1067 | Moderate | Design (risk of bias): the risk of bias for random sequence generation was low in 2 studies and unclear in 2 studies; the risk of bias for allocation concealment was low in 3 studies and unclear in 1 study; the risk of bias regarding performance bias and detection bias was low in 3 studies and unclear in 1 study. We downgraded the quality of the evidence by 1 step Heterogeneity/consistency: across studies: There was no heterogeneity for RR (I² = 24% ) and low for RD (I² = 34%) Directness of the evidence: studies were conducted in the target population Precision of estimates: to date results from 1067 infants have been reported in the studies and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 4 studies were included in the analysis we did not perform a funnel plot | |
| 189 per 1000 | 229 per 1000 | |||||
| Necrotizing enterocolitis (suspected or proven) | Study population | RR 0.94 | 1115 | Moderate | Design (risk of bias): the risk of bias for random sequence generation was low in 2 studies and unclear in 3 studies; the risk of bias for allocation concealment was low in 3 studies and unclear in 2 studies; the risk of bias regarding performance bias and detection bias was low in 3 studies and unclear in 2 studies. We downgraded the quality of the evidence by 1 step Heterogeneity/consistency across studies: there was no heterogeneity for RR (I² = 0%) nor for RD (I² = 0%) Directness of the evidence: studies were conducted in the target population Precision of estimates: to date results from 1115 infants have been reported in the studies and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 5 studies were included in the analysis we did not perform a funnel plot | |
| 83 per 1000 | 78 per 1000 | |||||
| Intraventricular haemorrhage, grade > 2 | Study population | RR 0.77 | 1103 | Moderate | Design (risk of bias): the risk of bias for random sequence generation was low in 2 studies and unclear in 3 studies; the risk of bias for allocation concealment was low in 3 studies and unclear in 2 studies; the risk of bias regarding performance bias and detection bias was low in 3 studies and unclear in 2 studies. We downgraded the quality of the evidence by 1 step Heterogeneity/consistency across studies: there was low heterogeneity for RR (I² = 48% ) and for RD (I² = 42%) Directness of the evidence: studies were conducted in the target population Precision of estimates: to date the results from 1103 infants have been reported in the studies and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: As only 5 studies were included in the analysis we did not perform a funnel plot | |
| 177 per 1000 | 136 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA compared to placebo for preterm infants at risk for or having respiratory distress syndrome | ||||||
| Patient or population: preterm infants at risk for or having respiratory distress syndrome | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA | |||||
| Type 1 ROP or death before determination of ROP outcome using the adjudicated ROP outcome | Study population | RR 1.28 | 679 | Moderate | Design (risk of bias): the risk of bias for random sequence generation, for allocation concealment, for performance bias and detection bias was low in both studies Heterogeneity/consistency across studies: there was high heterogeneity for RR (I² = 79 %) and for RD (I² = 85%). We downgraded the quality of the evidence by 1 step Directness of the evidence: studies were conducted in the target population Precision of estimates: this outcome was reported for 679 infants and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: As only 2 studies were included in the analysis we did not perform a funnel plot | |
| 222 per 1000 | 284 per 1000 | |||||
| Type 1 ROP including adjudicated ROP outcome | Study population | RR 1.24 1.86) | 605 | Moderate | Design (risk of bias): the risk of bias for random sequence generation, for allocation concealment, for performance bias and detection bias was low in both studies Heterogeneity/consistency across studies: there was low heterogeneity for RR (I² = 46 %) and moderate for RD (I² = 54%). We downgraded the quality of the evidence by 1 step Directness of the evidence: studies were conducted in the target population Precision of estimates: this outcome was reported on for 605 infants and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 2 studies were included in the analysis we did not perform a funnel plot | |
| 120 per 1000 | 149 per 1000 | |||||
| All‐cause mortality (outcome collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth) | Study population | RR 1.35 2.00) | 701 | Moderate | Design (risk of bias): the risk of bias for random sequence generation, for allocation concealment, for performance bias and detection bias was low in both studies Heterogeneity/consistency across studies: there was moderate heterogeneity for RR (I² = 72%) and high for RD (I² = 84%). We downgraded the quality of the evidence by 1 step Directness of the evidence: studies were conducted in the target population Precision of estimates: this outcome was reported for 701 infants and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 2 studies were included in the analysis we did not perform a funnel plot | |
| 110 per 1000 | 148 per 1000 | |||||
| BPD or death by it prior to 37 weeks' PMA (outcomes collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth) | Study population | RR 1.01 1.16) | 616 | High | Design (risk of bias): the risk of bias for random sequence generation, for allocation concealment, for performance bias and detection bias was low in both studies Heterogeneity/consistency across studies: there was no heterogeneity for RR (I² = 0%) nor for RD (I² = 0%) Directness of the evidence: studies were conducted in the target population. Precision of estimates: this outcome was reported for 616 infants and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 2 studies were included in the analysis we did not perform a funnel plot | |
| 555 per 1000 | 561 per 1000 | |||||
| Severe IVH (grade 3 or 4) | Study population | RR 0.92 1.29) | 690 | Moderate | Design (risk of bias): The risk of bias for random sequence generation, for allocation concealment, for performance bias and detection bias was low in both studies Heterogeneity/consistency across studies: there was moderate heterogeneity for RR (I² = 74%) and high for RD (I² = 82%) Directness of the evidence: studies were conducted in the target population Precision of estimates: this outcome was reported for 690 infants and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 2 studies were included in the analysis we did not perform a funnel plot. | |
| 171 per 1000 | 157 per 1000 | |||||
| Late‐onset sepsis (> 72 hours of age) | Study population | RR 1.33 1.75) | 701 | HIgh | Design (risk of bias): the risk of bias for random sequence generation, for allocation concealment, for performance bias and detection bias was low in both studies Heterogeneity/consistency across studies: there was no heterogeneity for RR (I² = 0%) nor for RD (I² = 0%) Directness of the evidence: studies were conducted in the target population Precision of estimates: this outcome was reported for 701 infants and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 2 studies were included in the analysis we did not perform a funnel plot | |
| 191 per 1000 | 254 per 1000 | |||||
| Suspected or proven NEC | Study population | RR 0.88 1.41) | 701 | High | Design (risk of bias): the risk of bias for random sequence generation, for allocation concealment, for performance bias and detection bias was low in both studies Heterogeneity/consistency across studies: there was low heterogeneity for RR (I² = 36%) and moderate for RD (I² = 53%). Directness of the evidence: studies were conducted in the target population. Precision of estimates: this outcome was reported on in 701 infants and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 2 studies were included in the analysis we did not perform a funnel plot. | |
| 98 per 1000 | 87 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal death (age < 28 days) Show forest plot | 3 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.31, 0.91] |
| 2 Infant death (age < one year) Show forest plot | 5 | 1115 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.71, 1.13] |
| 3 BPD (supplementary oxygen ar 36 weeks; PMA or death due to BPD)at 36 week's PMA Show forest plot | 2 | 666 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.87, 1.14] |
| 4 Bronchopulmonary dysplasia (at 28 to 30 days of age) Show forest plot | 3 | 343 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.54, 1.13] |
| 5 Bronchopulmonary dysplasia (at 36 to 38 weeks PMA) Show forest plot | 2 | 737 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.90, 1.20] |
| 6 Retinopathy of prematurity, stage ≥ 3 or ≥ 2 Show forest plot | 3 | 810 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.75, 1.06] |
| 6.1 ROP ≥ 3 | 2 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 0.67] |
| 6.2 Retinopathy of prematurity, stage ≥ 2 | 1 | 548 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.80, 1.13] |
| 7 Retinopathy of prematurity, any stage Show forest plot | 4 | 889 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.83, 1.07] |
| 8 Necrotizing enterocolitis (suspected or proven) Show forest plot | 5 | 1115 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.64, 1.39] |
| 9 Sepsis (early and/or late onset) Show forest plot | 4 | 1067 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.95, 1.54] |
| 10 Intraventricular haemorrhage, grade > 2 Show forest plot | 5 | 1103 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.58, 1.01] |
| 11 Intraventricular haemorrhage, all grades Show forest plot | 3 | 427 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.59, 1.00] |
| 12 Minor neural developmental impairment at one year corrected age Show forest plot | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.38, 1.86] |
| 13 Major neural developmental impairment at one year corrected age Show forest plot | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.24, 1.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death during hospital stay Show forest plot | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.34, 4.21] |
| 2 Bronchopulmonary dysplasia at 36 weeks PMA Show forest plot | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.74 [0.88, 8.48] |
| 3 Retinopathy of prematurity (infants who underwent surgery for ROP) Show forest plot | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.10, 1.22] |
| 4 Necrotizing enterocolitis (stage 2A or worse) Show forest plot | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.12, 1.39] |
| 5 Necrotizing enterocolitis (infants who underwent surgery for NEC) Show forest plot | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.08, 3.41] |
| 6 Sepsis (late onset) Show forest plot | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.71, 2.97] |
| 7 Intraventricular haemorrhage (grade 3 or 4) Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.29, 3.90] |
| 8 Hearing test (failed both ears) Show forest plot | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.09, 3.84] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Type 1 ROP or death before determination of ROP outcome using the adjudicated ROP outcome Show forest plot | 2 | 679 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.99, 1.67] |
| 2 Type 1 ROP Show forest plot | 1 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.89, 2.24] |
| 3 Death before determination of ROP outcome Show forest plot | 1 | 638 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.02, 2.31] |
| 4 Type 1 ROP including adjudicated ROP outcome Show forest plot | 2 | 605 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.82, 1.86] |
| 5 Any ROP Show forest plot | 1 | 553 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.88, 1.13] |
| 6 ROP ≥ 2 ROP Show forest plot | 1 | 548 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.80, 1.13] |
| 7 All cause infant mortality to 55 week's PMA Show forest plot | 1 | 638 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.12, 2.48] |
| 8 All cause mortality (outcome collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth) Show forest plot | 2 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.91, 2.00] |
| 9 BPD (requiring oxygen at 36 week's PMA for oxygen saturation > 90%) Show forest plot | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.89, 1.18] |
| 10 BPD or death by it prior to 37 weeks' PMA (outcomes collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth) Show forest plot | 2 | 616 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.87, 1.16] |
| 11 Severe IVH (grade 3 or 4) Show forest plot | 2 | 690 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.65, 1.29] |
| 12 Cystic areas in the cerebral parenchyma measured through 28 d Show forest plot | 2 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.58, 2.85] |
| 13 Early onset sepsis Show forest plot | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Late onset sepsis (> 72 hrs of age) Show forest plot | 2 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.00, 1.75] |
| 15 Suspected or proven NEC Show forest plot | 2 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.55, 1.41] |
| 16 Surgical NEC Show forest plot | 2 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.57, 2.58] |
| 17 Spontaneous gastro‐intestinal perforation Show forest plot | 2 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.48, 1.52] |
| 18 Pulmonary haemorrhage Show forest plot | 1 | 638 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.59, 1.62] |
| 19 PDA Show forest plot | 2 | 700 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.85, 1.14] |
| 20 PDA requiring indomethacin Show forest plot | 1 | 637 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.67, 1.22] |
| 21 PDA requiring surgery Show forest plot | 2 | 700 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.65, 1.42] |
| 22 Seizure treatment for ≥ 2 days Show forest plot | 2 | 700 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.43, 2.56] |
| 23 Negative hearing screening in either ear at discharge Show forest plot | 2 | 472 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.92, 2.29] |
| 24 Respiratory distress syndrome Show forest plot | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.91, 1.09] |
| 25 Sepsis, necrotizing enterocolitis, pneumonia or other infection as a cause of death Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.95, 1.93] |

