Inositol para lactantes prematuros con síndrome de dificultad respiratoria o con riesgo de presentarlo
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised, placebo‐controlled trial | |
| Participants | Preterm infants (birth weight < 1500 grams) with a diagnosis of RDS, requiring mechanical ventilation. 24 infants were randomised to high concentration inositol formula (SC 30) (estimated PMA 27.7, SD 1.9) and 24 infants were randomised to a low concentration of inositol formula (SC 24). Randomisation ended when the high‐inositol formula was no longer available. Location: 2 NICUs in the US. Study period: October 1994 to June 1998. | |
| Interventions | The study group was enterally fed high‐inositol formula (2500 µmol/L inositol), while the control group was given low‐inositol formula (242 µmol/L) enterally. | |
| Outcomes | Neonatal deaths, infant deaths, infants with bacteraemia, necrotizing enterocolitis (radio graphically documented), IVH > grade 2, BPD (oxygen therapy > 30 days), duration of mechanical ventilation, ROP (reported in unpublished data from 1995). | |
| Notes | The results of this study have been reported 3 times; in abstract form in 1995 after 37 infants were enrolled; in a personal communication report to us in 1995 when 41 infants had been enrolled; and in a final published report in 2000 when 48 infants had been entered. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | Infants were allocated to one of the two groups by sequential random card selection. No information provided whether the cards were enclosed in opaque and numbered envelopes. |
| Blinding (performance bias and detection bias) | Unclear risk | Infants were blinded but no information provided whether the clinical staff and the researchers were. |
| Incomplete outcome data (attrition bias) | Low risk | Outcomes provided for all 48 infants randomised. |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us so we cannot judge if there were any deviations between the protocol and the final report. |
| Other bias | High risk | The results of this study have been reported 3 times: in abstract form in 1995 after 37 infants were enrolled; in a personal communication report to us in 1995 when 41 infants had been enrolled; and in a final published report in 2000 when 48 infants had been entered. |
| Methods | Randomised, placebo‐controlled, double‐blind trial. Enrolment from 1983 to 1985. | |
| Participants | Preterm infants (birth weight < 2000 grams) (mean PMA 29.5, SD 2.0 in the inositol group and 29.5, SD 2.1 in the placebo group) with a diagnosis of RDS, requiring mechanical ventilation. Location: 1 NICU in Helsinki, Finland. Study period: January 1983 to August 1985. | |
| Interventions | IV or supplemental inositol (120 to 160 mg/kg/day) or placebo (5% glucose) given daily for 10 days. | |
| Outcomes | Neonatal deaths, infant deaths, BPD (supplemental oxygen at 28 days and x‐ray findings compatible with BPD), IVH, ROP (ophthalmological exam at PMA of 9 and 13 months), NEC (clinical findings and abdominal x‐ray showing pneumatosis intestinalis and air in the portal circulation), and sepsis. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Low risk | "Infants were randomly and blindly assigned to be treated with inositol or placebo (glucose) after their parents had consented to their participation". For further details see "Blinding" below. |
| Blinding (performance bias and detection bias) | Low risk | Each set of solutions, containing either inositol or glucose (5% weight/volume each) had a code number. Only the pharmacist preparing the doses knew the contents of the drug packages. |
| Incomplete outcome data (attrition bias) | Low risk | Of the 83 infants who entered the trial, nine did not fulfil the entrance criteria and were excluded from the final analysis. An explanation was provided for each excluded infant. |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us so we cannot judge if there were any deviations between the protocol and the final report. |
| Other bias | High risk | The present report represents the third interim analysis and the researchers may have been influenced by the results of the two previous interim analyses. The study was not registered in a trials registry. |
| Methods | Randomised, placebo‐controlled, double‐blind trial, occurring between 1985 and 1989. | |
| Participants | Preterm infants (birth weight < 2000 grams and 24.0 to 31.9 weeks' PMA at birth) with evidence of RDS, requiring mechanical ventilation. | |
| Interventions | The study group received IV inositol 80 mg/kg body weight daily for 5 days, with repeated courses at day 10 and day 20 if necessary (infant continued to require ventilation, required supplemental O₂ or did not tolerate enteral feeds). The control group received 5% glucose. | |
| Outcomes | Neonatal death, infant death, BPD (supplemental oxygen at 28 days of age), BPD (supplemental oxygen at 38 weeks' PMA or the week of discharge from hospital), ROP (as per International Classification assessed from 4 to 6 weeks and ending at 12 months), IVH (all grades, grade > 2), NEC (no definition provided), and sepsis (no definition provided). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) | Unclear risk | 5% glucose was given as placebo, but no information provided on whether staff was blinded to study drugs or not. |
| Incomplete outcome data (attrition bias) | Low risk | 4 infants in the placebo group and 3 in the inositol group died before receiving any treatment, 2 had lethal malformations (1 in each group), and 3 did not have RDS (2 in the placebo group and 1 in the inositol group). These 12 infants were included only in the safety analysis. |
| Selective reporting (reporting bias) | Unclear risk | This study was not registered in a trials registry so we cannot judge if there were any deviations between the protocol and the final report. |
| Other bias | High risk | Interim analyses were to be performed after enrolment of 100, 200, 300 patients. Early termination of the trial was recommended by the monitoring committee after the second interim analysis, when the Chi² test revealed a significant increase in neonatal survival without BPD and no trend towards serious morbidity in 1 study group. 1 interim analysis previously reported, Hallman 1992 (published in Lung 1990;168 Suppl: 877 to 82). |
| Methods | Randomised, double‐masked, placebo‐controlled pharmacokinetic (PK) study. Enrolment between June 2006 and December 2007. The trial was conducted by the National Institutes of Child Health and Human Development Neonatal Research Network. 10 of the Neonatal Research Network Centers participated. | |
| Participants | Eligible subjects were of 23 0/7 to 29 6/7 weeks' PMA and ≥ 600 G BW, had no major congenital anomalies, were between 12 hours and 6 days of age at randomisation, and had received no human milk or formula feedings since birth. | |
| Interventions | Inositol was given as a single low (60 mg/kg) (N = 25) or high (120 mg/kg) (N = 24) dose of 5% myo‐inositol IV over 20 min in a 1:1:1 randomisation with placebo delivered in 1 of 2 volumes to maintain masking (5% glucose) (N = 25). Drug or placebo was dispensed from the respective pharmacies in unit doses labelled as 'inositol study drug', and all clinical and research personnel except the pharmacist were masked to the study group. | |
| Outcomes | Pharmacokinetic data for inositol (central volume of distribution, clearance, endogenous production, the half‐life, renal inositol excretion during the first 12 H and after 48 H and diuretic side effect. | |
| Notes | Abbott Nutrition Division, Abbott Laboratories, supplied the inositol drug used in the study. Portions of this study were presented at the 2010 Paediatric Academic Societies Annual Meeting, Vancouver, Canada, May 1–4, 2010 (Abstract 3737.387). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed centrally via computer within two pre‐specified PMA strata (23 0/7 to 26 6/7 weeks and 27 0/7 to 29 6/7 weeks). |
| Allocation concealment (selection bias) | Low risk | There was central allocation to study group. |
| Blinding (performance bias and detection bias) | Low risk | Drug or placebo was dispensed from the respective pharmacies in unit doses labelled as 'inositol study drug', and all clinical and research personnel except the pharmacist were masked to the study group. |
| Incomplete outcome data (attrition bias) | Low risk | Consent was obtained for 79 infants, 76 infants were randomised, and 74 infants received study drug. 2 infants did not complete the minimum of 4 specified blood samples (three post drug infusion), and their randomisation were replaced with 2 additional enrollees from the same centre and of the same gestational age (GA) stratum, per protocol. Available data from the 2 replaced infants were included in the PK and safety analyses. 1 infant received placebo instead of the assigned 120 mg/kg dose, and for the PK analysis, this infant’s serum and urine data were included in the placebo group. However, this subject’s data on adverse events and clinical outcomes were included as randomised (intention to treat). |
| Selective reporting (reporting bias) | Low risk | The study was registered with ClinicalTrials.gov (NCT00349726) and there do not appear to be any deviations from the protocol. |
| Other bias | Low risk | Appears free of other bias. |
| Methods | Prospective, parallel, randomised controlled trial. Infants enrolled in 14 centres in the Eunice Kennedy Shriver NICHD Neonatal Research Network. | |
| Participants | Infants ≤ 29 weeks' PMA (23 0/7 to 29 6/7 weeks' PMA), who weighed at least 400 G, and could receive study drug by 72 H after birth. | |
| Interventions | Myo‐inositol provided by Abbott Nutrition, Columbus, Ohio, USA as an isotonic, preservative and pyrogen‐free, sterile, 5% solution at 10, 40 or 80 mg/kg/day. Intravenous administration converted to enteral when feedings were established, and continued to the first of 10 weeks, 34 weeks' PMA, death or discharge. Total number randomised: 10 mg/kg N = 29; 40 mg/kg N = 30; 80 mg/kg N = 28 Placebo: 5% glucose. Total number randomised: N = 35. | |
| Outcomes | Adverse events were prospectively monitored from 24 hours prior to study drug until 7 days following the final dose (unless discharged sooner), and judged according to a neonatal toxicity table developed for the study. An unfavourable outcome was defined as either type 1 ROP or worse, in either eye, or surgical intervention for severe ROP in either eye. A favourable ROP outcome was assigned if the retinal vessels progressed to full vascularization in both eyes without meeting criteria for severe ROP, or if on 2 consecutive examinations the retinal vessels were in zone III. Infants who did not meet either criterion had all available examinations reviewed by an adjudication committee. Adjudication was conducted by a committee of 3 experienced ophthalmologists not involved with the study and masked to study group assignment. The final ROP status was judged separately in each eye as 'probably favourable', 'probably unfavourable' or 'cannot be determined', and the majority classification was assigned as the adjudicated outcome. At 18 to 22 months' corrected age, infants received a set of standardized examinations of neurologic function and development according to the NRN Follow‐Up Protocol (to be reported separately). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated. |
| Allocation concealment (selection bias) | Low risk | Computer generated and communicated to research pharmacist. |
| Blinding (performance bias and detection bias) | Low risk | Personal communication from the first author indicates blinded performance and detection bias for all outcomes. Ophthalmologists were blinded during the adjudication process. |
| Incomplete outcome data (attrition bias) | Low risk | Severe ROP data presented for 106 surviving infants and there were 15 deaths, which adds up to 121 infants not 122 infants, which was the number enrolled. |
| Selective reporting (reporting bias) | Low risk | The protocol was available to us and we did not notice any major deviations from the planned study. The study was registered as: NCT01030575. |
| Other bias | Low risk | Appears free of other bias. |
| Methods | Randomised clinical trial included infants enrolled from 18 neonatal intensive care centres throughout the USA from 17 April 2014 to 4 September 2015; final date of follow‐up was 12 February 2016. | |
| Participants | 638 infants < 28 weeks’ PMA, surviving for at least 12 hours, and admitted to 1 of the 18 Neonatal Research Network centres before 72 hours’ postnatal age. | |
| Interventions | A 40 mg/kg dose of myo‐inositol was given every 12 hours (initially intravenously, then enterally when feeding; (N = 317) or for up to 10 weeks. The active drug was an isotonic, sterile, pyrogen‐ and preservative‐free aqueous solution of 5% myo‐inositol (50 mg/mL) at neutral pH and was provided by Abbott Laboratories. A dose of 40 mg/kg every 12 hours was selected to achieve serum concentrations similar to those previously reported. A therapeutic duration of up to 10 weeks was chosen to sustain serum myo‐inositol levels similar to those found in utero throughout the period of normal retinal vascular development and because of the reported benefits in the treatment of ROP and survival. Placebo (N = 321) (5% glucose for IV use from pharmacy stock). | |
| Outcomes | The unfavourable primary outcome was type 1 ROP, which was defined as meeting the criteria for ophthalmological intervention to prevent retinal detachment, a more severe ROP type than ROP type 1 (e.g. aggressive posterior ROP or Rush disease), or death before the ROP outcome could be determined. | |
| Notes | The planned enrolment of 1760 participants would permit detection of an absolute reduction in death or type 1 ROP of 7% with 90% power. The trial was terminated early due to a statistically significantly higher mortality rate in the myo‐inositol group. The favourable primary outcome was survival with only milder ROP or no ROP. Infants were followed up as outpatients to determine the primary outcome up to a maximum of 55 weeks’ PMA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated and centrally administered randomisation. |
| Allocation concealment (selection bias) | Low risk | With the exception of pharmacists, who prepared the daily unit doses of myo‐inositol or placebo according to randomisation assignment, all other clinical and research personnel and families were blind to group assignment. |
| Blinding (performance bias and detection bias) | Low risk | See above for allocation concealment. |
| Incomplete outcome data (attrition bias) | Low risk | Outcome group: of the 317 infants randomised to receive myo‐inositol 313 received the intervention as randomised; 3 died prior to receiving the intervention and 1 was withdrawn prior to intervention. 313 included in primary analysis. Of the 321 infants randomised to placebo 315 received the intervention as randomised; 2 died prior to receiving intervention and 4 received < 2 doses of myo‐inositol. 320 included in the primary analysis of type 1 retinopathy of prematurity or death. 1 excluded from primary analysis. |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was available to us and we did not identify any deviations except that the study was terminated prior to reaching the full sample size because of safety concerns. The authors state: “No changes to the protocol occurred during the trial”. Registered as: NCT01954082. |
| Other bias | Low risk | Appears free of other bias. |
BW = birth weight
PMA = postmenstrual age
PK = pharmacokinetics
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal death (age < 28 days) Show forest plot | 3 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.31, 0.91] |
| Analysis 1.1  Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 1 Neonatal death (age < 28 days). | ||||
| 2 Infant death (age < one year) Show forest plot | 5 | 1115 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.71, 1.13] |
| Analysis 1.2  Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 2 Infant death (age < one year). | ||||
| 3 BPD (supplementary oxygen ar 36 weeks; PMA or death due to BPD)at 36 week's PMA Show forest plot | 2 | 666 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.87, 1.14] |
| Analysis 1.3  Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 3 BPD (supplementary oxygen ar 36 weeks; PMA or death due to BPD)at 36 week's PMA. | ||||
| 4 Bronchopulmonary dysplasia (at 28 to 30 days of age) Show forest plot | 3 | 343 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.54, 1.13] |
| Analysis 1.4  Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 4 Bronchopulmonary dysplasia (at 28 to 30 days of age). | ||||
| 5 Bronchopulmonary dysplasia (at 36 to 38 weeks PMA) Show forest plot | 2 | 737 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.90, 1.20] |
| Analysis 1.5  Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 5 Bronchopulmonary dysplasia (at 36 to 38 weeks PMA). | ||||
| 6 Retinopathy of prematurity, stage ≥ 3 or ≥ 2 Show forest plot | 3 | 810 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.75, 1.06] |
| Analysis 1.6  Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 6 Retinopathy of prematurity, stage ≥ 3 or ≥ 2. | ||||
| 6.1 ROP ≥ 3 | 2 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 0.67] |
| 6.2 Retinopathy of prematurity, stage ≥ 2 | 1 | 548 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.80, 1.13] |
| 7 Retinopathy of prematurity, any stage Show forest plot | 4 | 889 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.83, 1.07] |
| Analysis 1.7  Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 7 Retinopathy of prematurity, any stage. | ||||
| 8 Necrotizing enterocolitis (suspected or proven) Show forest plot | 5 | 1115 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.64, 1.39] |
| Analysis 1.8  Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 8 Necrotizing enterocolitis (suspected or proven). | ||||
| 9 Sepsis (early and/or late onset) Show forest plot | 4 | 1067 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.95, 1.54] |
| Analysis 1.9  Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 9 Sepsis (early and/or late onset). | ||||
| 10 Intraventricular haemorrhage, grade > 2 Show forest plot | 5 | 1103 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.58, 1.01] |
| Analysis 1.10  Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 10 Intraventricular haemorrhage, grade > 2. | ||||
| 11 Intraventricular haemorrhage, all grades Show forest plot | 3 | 427 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.59, 1.00] |
| Analysis 1.11  Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 11 Intraventricular haemorrhage, all grades. | ||||
| 12 Minor neural developmental impairment at one year corrected age Show forest plot | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.38, 1.86] |
| Analysis 1.12  Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 12 Minor neural developmental impairment at one year corrected age. | ||||
| 13 Major neural developmental impairment at one year corrected age Show forest plot | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.24, 1.16] |
| Analysis 1.13  Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 13 Major neural developmental impairment at one year corrected age. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death during hospital stay Show forest plot | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.34, 4.21] |
| Analysis 2.1  Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg) in preterm infants, Outcome 1 Death during hospital stay. | ||||
| 2 Bronchopulmonary dysplasia at 36 weeks PMA Show forest plot | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.74 [0.88, 8.48] |
| Analysis 2.2  Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg) in preterm infants, Outcome 2 Bronchopulmonary dysplasia at 36 weeks PMA. | ||||
| 3 Retinopathy of prematurity (infants who underwent surgery for ROP) Show forest plot | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.10, 1.22] |
| Analysis 2.3  Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg) in preterm infants, Outcome 3 Retinopathy of prematurity (infants who underwent surgery for ROP). | ||||
| 4 Necrotizing enterocolitis (stage 2A or worse) Show forest plot | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.12, 1.39] |
| Analysis 2.4  Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg) in preterm infants, Outcome 4 Necrotizing enterocolitis (stage 2A or worse). | ||||
| 5 Necrotizing enterocolitis (infants who underwent surgery for NEC) Show forest plot | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.08, 3.41] |
| Analysis 2.5  Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg) in preterm infants, Outcome 5 Necrotizing enterocolitis (infants who underwent surgery for NEC). | ||||
| 6 Sepsis (late onset) Show forest plot | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.71, 2.97] |
| Analysis 2.6  Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg) in preterm infants, Outcome 6 Sepsis (late onset). | ||||
| 7 Intraventricular haemorrhage (grade 3 or 4) Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.29, 3.90] |
| Analysis 2.7  Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg) in preterm infants, Outcome 7 Intraventricular haemorrhage (grade 3 or 4). | ||||
| 8 Hearing test (failed both ears) Show forest plot | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.09, 3.84] |
| Analysis 2.8  Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg) in preterm infants, Outcome 8 Hearing test (failed both ears). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Type 1 ROP or death before determination of ROP outcome using the adjudicated ROP outcome Show forest plot | 2 | 679 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.99, 1.67] |
| Analysis 3.1  Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 1 Type 1 ROP or death before determination of ROP outcome using the adjudicated ROP outcome. | ||||
| 2 Type 1 ROP Show forest plot | 1 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.89, 2.24] |
| Analysis 3.2  Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 2 Type 1 ROP. | ||||
| 3 Death before determination of ROP outcome Show forest plot | 1 | 638 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.02, 2.31] |
| Analysis 3.3  Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 3 Death before determination of ROP outcome. | ||||
| 4 Type 1 ROP including adjudicated ROP outcome Show forest plot | 2 | 605 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.82, 1.86] |
| Analysis 3.4  Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 4 Type 1 ROP including adjudicated ROP outcome. | ||||
| 5 Any ROP Show forest plot | 1 | 553 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.88, 1.13] |
| Analysis 3.5  Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 5 Any ROP. | ||||
| 6 ROP ≥ 2 ROP Show forest plot | 1 | 548 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.80, 1.13] |
| Analysis 3.6  Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 6 ROP ≥ 2 ROP. | ||||
| 7 All cause infant mortality to 55 week's PMA Show forest plot | 1 | 638 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.12, 2.48] |
| Analysis 3.7  Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 7 All cause infant mortality to 55 week's PMA. | ||||
| 8 All cause mortality (outcome collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth) Show forest plot | 2 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.91, 2.00] |
| Analysis 3.8  Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 8 All cause mortality (outcome collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth). | ||||
| 9 BPD (requiring oxygen at 36 week's PMA for oxygen saturation > 90%) Show forest plot | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.89, 1.18] |
| Analysis 3.9  Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 9 BPD (requiring oxygen at 36 week's PMA for oxygen saturation > 90%). | ||||
| 10 BPD or death by it prior to 37 weeks' PMA (outcomes collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth) Show forest plot | 2 | 616 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.87, 1.16] |
| Analysis 3.10  Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 10 BPD or death by it prior to 37 weeks' PMA (outcomes collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth). | ||||
| 11 Severe IVH (grade 3 or 4) Show forest plot | 2 | 690 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.65, 1.29] |
| Analysis 3.11  Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 11 Severe IVH (grade 3 or 4). | ||||
| 12 Cystic areas in the cerebral parenchyma measured through 28 d Show forest plot | 2 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.58, 2.85] |
| Analysis 3.12  Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 12 Cystic areas in the cerebral parenchyma measured through 28 d. | ||||
| 13 Early onset sepsis Show forest plot | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 3.13  Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 13 Early onset sepsis. | ||||
| 14 Late onset sepsis (> 72 hrs of age) Show forest plot | 2 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.00, 1.75] |
| Analysis 3.14  Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 14 Late onset sepsis (> 72 hrs of age). | ||||
| 15 Suspected or proven NEC Show forest plot | 2 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.55, 1.41] |
| Analysis 3.15  Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 15 Suspected or proven NEC. | ||||
| 16 Surgical NEC Show forest plot | 2 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.57, 2.58] |
| Analysis 3.16  Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 16 Surgical NEC. | ||||
| 17 Spontaneous gastro‐intestinal perforation Show forest plot | 2 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.48, 1.52] |
| Analysis 3.17  Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 17 Spontaneous gastro‐intestinal perforation. | ||||
| 18 Pulmonary haemorrhage Show forest plot | 1 | 638 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.59, 1.62] |
| Analysis 3.18  Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 18 Pulmonary haemorrhage. | ||||
| 19 PDA Show forest plot | 2 | 700 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.85, 1.14] |
| Analysis 3.19  Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 19 PDA. | ||||
| 20 PDA requiring indomethacin Show forest plot | 1 | 637 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.67, 1.22] |
| Analysis 3.20  Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 20 PDA requiring indomethacin. | ||||
| 21 PDA requiring surgery Show forest plot | 2 | 700 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.65, 1.42] |
| Analysis 3.21  Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 21 PDA requiring surgery. | ||||
| 22 Seizure treatment for ≥ 2 days Show forest plot | 2 | 700 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.43, 2.56] |
| Analysis 3.22  Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 22 Seizure treatment for ≥ 2 days. | ||||
| 23 Negative hearing screening in either ear at discharge Show forest plot | 2 | 472 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.92, 2.29] |
| Analysis 3.23  Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 23 Negative hearing screening in either ear at discharge. | ||||
| 24 Respiratory distress syndrome Show forest plot | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.91, 1.09] |
| Analysis 3.24  Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 24 Respiratory distress syndrome. | ||||
| 25 Sepsis, necrotizing enterocolitis, pneumonia or other infection as a cause of death Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.95, 1.93] |
| Analysis 3.25  Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 25 Sepsis, necrotizing enterocolitis, pneumonia or other infection as a cause of death. | ||||
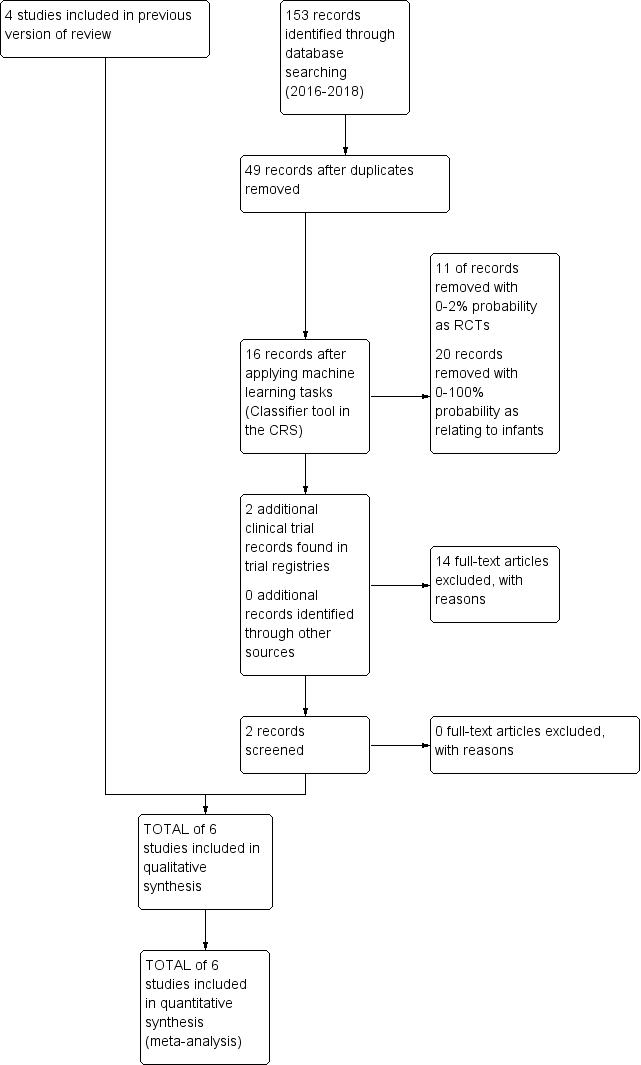
Study flow diagram: review update
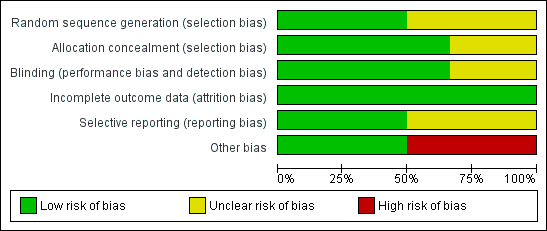
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Inositol supplementation (repeat doses in any amount and any duration of treatment) versus control, outcome: 1.5 Retinopathy of prematurity, stage ≥ 3.

Forest plot of comparison: 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, outcome: 3.4 Type 1 ROP including adjudicated ROP outcome.

Forest plot of comparison: 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, outcome: 3.14 Late onset sepsis (> 72 hours of age).
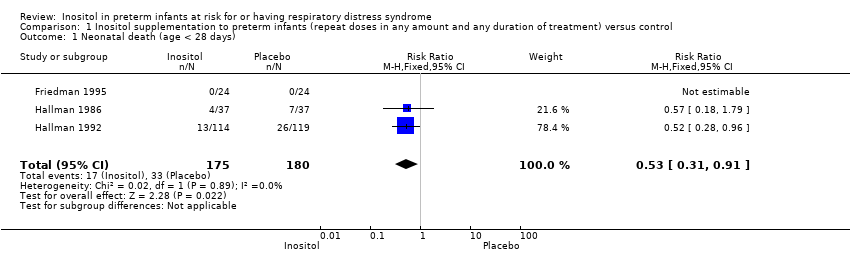
Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 1 Neonatal death (age < 28 days).
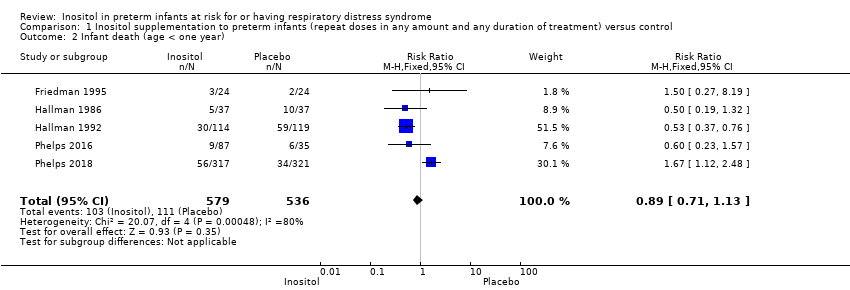
Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 2 Infant death (age < one year).

Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 3 BPD (supplementary oxygen ar 36 weeks; PMA or death due to BPD)at 36 week's PMA.

Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 4 Bronchopulmonary dysplasia (at 28 to 30 days of age).

Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 5 Bronchopulmonary dysplasia (at 36 to 38 weeks PMA).

Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 6 Retinopathy of prematurity, stage ≥ 3 or ≥ 2.
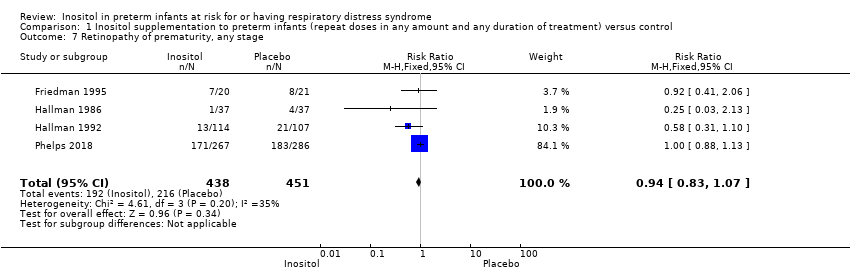
Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 7 Retinopathy of prematurity, any stage.

Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 8 Necrotizing enterocolitis (suspected or proven).

Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 9 Sepsis (early and/or late onset).

Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 10 Intraventricular haemorrhage, grade > 2.
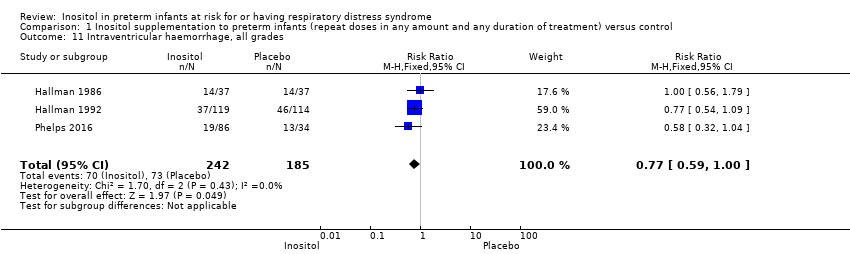
Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 11 Intraventricular haemorrhage, all grades.

Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 12 Minor neural developmental impairment at one year corrected age.
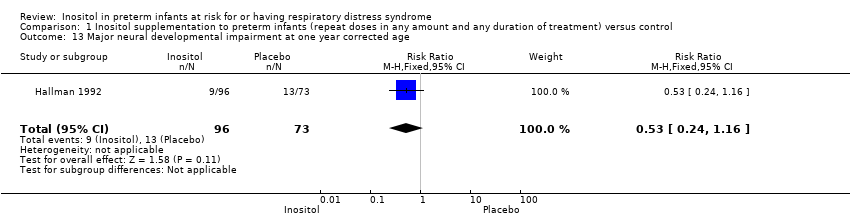
Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 13 Major neural developmental impairment at one year corrected age.
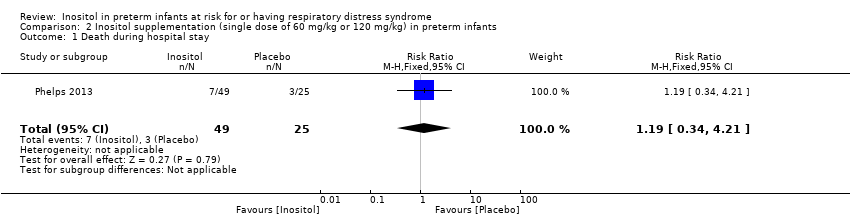
Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg) in preterm infants, Outcome 1 Death during hospital stay.

Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg) in preterm infants, Outcome 2 Bronchopulmonary dysplasia at 36 weeks PMA.
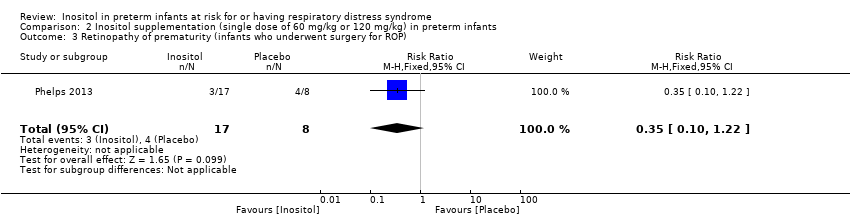
Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg) in preterm infants, Outcome 3 Retinopathy of prematurity (infants who underwent surgery for ROP).

Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg) in preterm infants, Outcome 4 Necrotizing enterocolitis (stage 2A or worse).
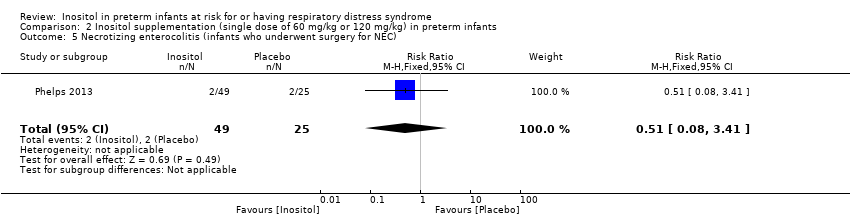
Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg) in preterm infants, Outcome 5 Necrotizing enterocolitis (infants who underwent surgery for NEC).
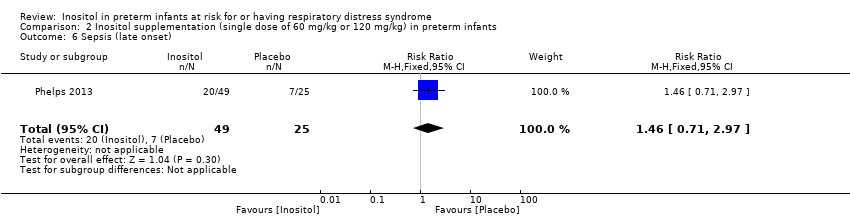
Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg) in preterm infants, Outcome 6 Sepsis (late onset).

Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg) in preterm infants, Outcome 7 Intraventricular haemorrhage (grade 3 or 4).

Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg) in preterm infants, Outcome 8 Hearing test (failed both ears).

Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 1 Type 1 ROP or death before determination of ROP outcome using the adjudicated ROP outcome.

Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 2 Type 1 ROP.
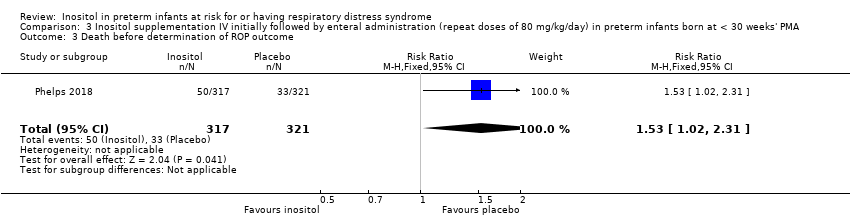
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 3 Death before determination of ROP outcome.

Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 4 Type 1 ROP including adjudicated ROP outcome.
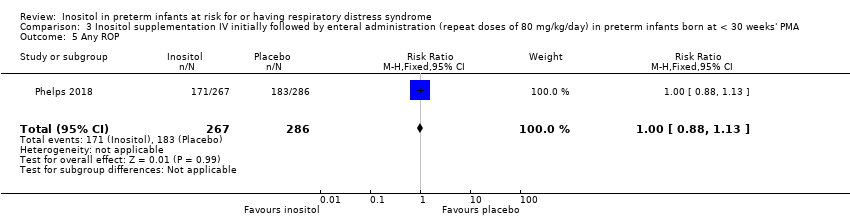
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 5 Any ROP.

Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 6 ROP ≥ 2 ROP.

Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 7 All cause infant mortality to 55 week's PMA.

Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 8 All cause mortality (outcome collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth).

Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 9 BPD (requiring oxygen at 36 week's PMA for oxygen saturation > 90%).

Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 10 BPD or death by it prior to 37 weeks' PMA (outcomes collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth).
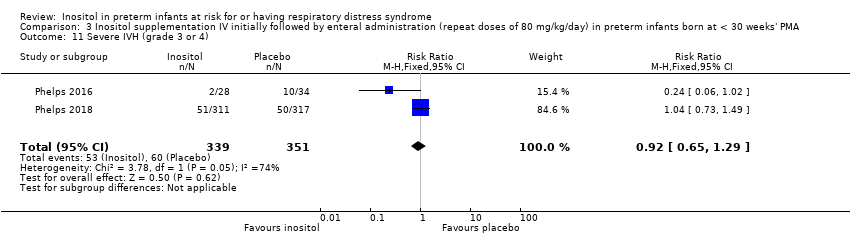
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 11 Severe IVH (grade 3 or 4).

Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 12 Cystic areas in the cerebral parenchyma measured through 28 d.

Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 13 Early onset sepsis.

Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 14 Late onset sepsis (> 72 hrs of age).

Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 15 Suspected or proven NEC.

Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 16 Surgical NEC.
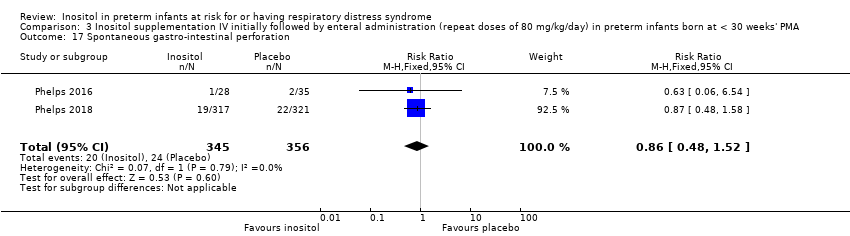
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 17 Spontaneous gastro‐intestinal perforation.

Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 18 Pulmonary haemorrhage.

Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 19 PDA.
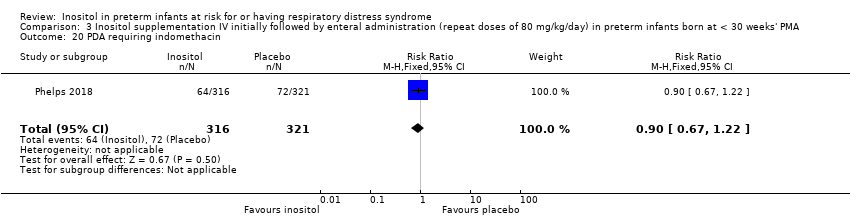
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 20 PDA requiring indomethacin.

Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 21 PDA requiring surgery.

Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 22 Seizure treatment for ≥ 2 days.
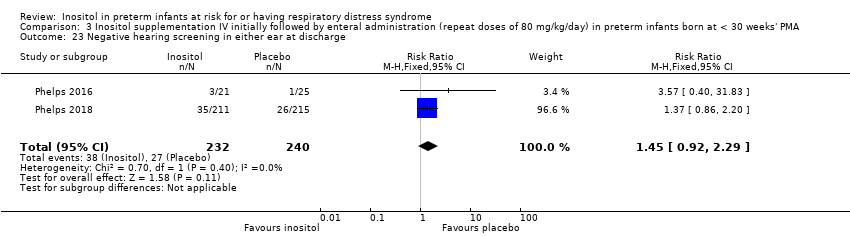
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 23 Negative hearing screening in either ear at discharge.
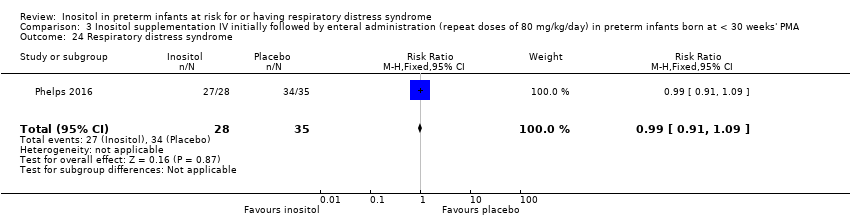
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 24 Respiratory distress syndrome.
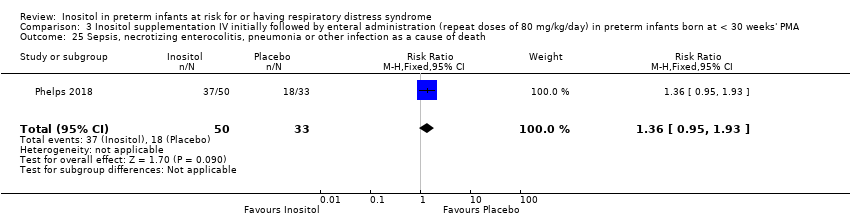
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 25 Sepsis, necrotizing enterocolitis, pneumonia or other infection as a cause of death.
| Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) compared to control for preterm infants at risk for or having respiratory distress syndrome | ||||||
| Patient or population: preterm infants at risk for or having respiratory distress syndrome | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with control | Risk with Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) | |||||
| Infant death (age < 1 year) | Study population | RR 0.89 | 1115 | Low | Design (risk of bias): the risk of bias for random sequence generation was low in 2 studies and unclear in 3 studies; the risk of bias for allocation concealment was low in 3 studies and unclear in 2 studies; the risk of bias regarding performance bias and detection bias was low in 3 studies and unclear in 2 studies. We downgraded the quality of the evidence by 1 step Heterogeneity/consistency across studies: there was high heterogeneity for RR (I² = 80 % ) and for RD (I² = 84%). We downgraded the quality of the evidence by 1 step Directness of the evidence: Studies were conducted in the target population. Precision of estimates: Results from 1115 infants have been reported in the studies to date and the confidence intervals around the point estimates for RR and RD were narrow. Presence of publication bias: As only 5 studies were included in the analysis we did not perform a funnel plot. | |
| 207 per 1000 | 184 per 1000 | |||||
| Bronchopulmonary dysplasia (at 36 to 38 weeks' PMA) | Study population | RR 1.04 | 737 | Moderate | Design (risk of bias): the risk of bias for random sequence generation was low in 1 study and unclear in 1 study; The risk of bias for allocation concealment was low in 1 study and unclear in 1 study; the risk of bias regarding performance bias and detection bias was low in 1 study and unclear in 1 study. We downgraded the quality of the evidence by 1 step Heterogeneity/consistency across studies: there was no heterogeneity for RR (I² = 0%) and for RD (I² = 0%) Directness of the evidence: studies were conducted in the target population Precision of estimates: to date the results from 737 infants have been reported in the studies and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 2 studies were included in the analysis we did not perform a funnel plot | |
| 459 per 1000 | 477 per 1000 | |||||
| ROP, stage ≥ 3 or ≥ 2 | Study population | RR 0.89 | 810 | Moderate | Design (risk of bias): the risk of bias for random sequence generation was low in 1 study and unclear in 2 studies; the risk of bias for allocation concealment was low in 1 study and unclear in 2 studies; the risk of bias regarding performance bias and detection bias was low in 1 study and unclear in 2 studies. We downgraded the quality of the evidence by 1 step Heterogeneity/consistency across studies: there was moderate heterogeneity for RR (I² = 63% ) and none for RD (I² = 23%) Directness of the evidence: Studies were conducted in the target population Precision of estimates: to date the results from 810 infants have been reported in the studies and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 3 studies were included in the analysis we did not perform a funnel plot | |
| 368 per 1000 | 328 per 1000 | |||||
| Sepsis (early or late onset) | Study population | RR 1.21 | 1067 | Moderate | Design (risk of bias): the risk of bias for random sequence generation was low in 2 studies and unclear in 2 studies; the risk of bias for allocation concealment was low in 3 studies and unclear in 1 study; the risk of bias regarding performance bias and detection bias was low in 3 studies and unclear in 1 study. We downgraded the quality of the evidence by 1 step Heterogeneity/consistency: across studies: There was no heterogeneity for RR (I² = 24% ) and low for RD (I² = 34%) Directness of the evidence: studies were conducted in the target population Precision of estimates: to date results from 1067 infants have been reported in the studies and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 4 studies were included in the analysis we did not perform a funnel plot | |
| 189 per 1000 | 229 per 1000 | |||||
| Necrotizing enterocolitis (suspected or proven) | Study population | RR 0.94 | 1115 | Moderate | Design (risk of bias): the risk of bias for random sequence generation was low in 2 studies and unclear in 3 studies; the risk of bias for allocation concealment was low in 3 studies and unclear in 2 studies; the risk of bias regarding performance bias and detection bias was low in 3 studies and unclear in 2 studies. We downgraded the quality of the evidence by 1 step Heterogeneity/consistency across studies: there was no heterogeneity for RR (I² = 0%) nor for RD (I² = 0%) Directness of the evidence: studies were conducted in the target population Precision of estimates: to date results from 1115 infants have been reported in the studies and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 5 studies were included in the analysis we did not perform a funnel plot | |
| 83 per 1000 | 78 per 1000 | |||||
| Intraventricular haemorrhage, grade > 2 | Study population | RR 0.77 | 1103 | Moderate | Design (risk of bias): the risk of bias for random sequence generation was low in 2 studies and unclear in 3 studies; the risk of bias for allocation concealment was low in 3 studies and unclear in 2 studies; the risk of bias regarding performance bias and detection bias was low in 3 studies and unclear in 2 studies. We downgraded the quality of the evidence by 1 step Heterogeneity/consistency across studies: there was low heterogeneity for RR (I² = 48% ) and for RD (I² = 42%) Directness of the evidence: studies were conducted in the target population Precision of estimates: to date the results from 1103 infants have been reported in the studies and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: As only 5 studies were included in the analysis we did not perform a funnel plot | |
| 177 per 1000 | 136 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA compared to placebo for preterm infants at risk for or having respiratory distress syndrome | ||||||
| Patient or population: preterm infants at risk for or having respiratory distress syndrome | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA | |||||
| Type 1 ROP or death before determination of ROP outcome using the adjudicated ROP outcome | Study population | RR 1.28 | 679 | Moderate | Design (risk of bias): the risk of bias for random sequence generation, for allocation concealment, for performance bias and detection bias was low in both studies Heterogeneity/consistency across studies: there was high heterogeneity for RR (I² = 79 %) and for RD (I² = 85%). We downgraded the quality of the evidence by 1 step Directness of the evidence: studies were conducted in the target population Precision of estimates: this outcome was reported for 679 infants and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: As only 2 studies were included in the analysis we did not perform a funnel plot | |
| 222 per 1000 | 284 per 1000 | |||||
| Type 1 ROP including adjudicated ROP outcome | Study population | RR 1.24 1.86) | 605 | Moderate | Design (risk of bias): the risk of bias for random sequence generation, for allocation concealment, for performance bias and detection bias was low in both studies Heterogeneity/consistency across studies: there was low heterogeneity for RR (I² = 46 %) and moderate for RD (I² = 54%). We downgraded the quality of the evidence by 1 step Directness of the evidence: studies were conducted in the target population Precision of estimates: this outcome was reported on for 605 infants and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 2 studies were included in the analysis we did not perform a funnel plot | |
| 120 per 1000 | 149 per 1000 | |||||
| All‐cause mortality (outcome collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth) | Study population | RR 1.35 2.00) | 701 | Moderate | Design (risk of bias): the risk of bias for random sequence generation, for allocation concealment, for performance bias and detection bias was low in both studies Heterogeneity/consistency across studies: there was moderate heterogeneity for RR (I² = 72%) and high for RD (I² = 84%). We downgraded the quality of the evidence by 1 step Directness of the evidence: studies were conducted in the target population Precision of estimates: this outcome was reported for 701 infants and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 2 studies were included in the analysis we did not perform a funnel plot | |
| 110 per 1000 | 148 per 1000 | |||||
| BPD or death by it prior to 37 weeks' PMA (outcomes collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth) | Study population | RR 1.01 1.16) | 616 | High | Design (risk of bias): the risk of bias for random sequence generation, for allocation concealment, for performance bias and detection bias was low in both studies Heterogeneity/consistency across studies: there was no heterogeneity for RR (I² = 0%) nor for RD (I² = 0%) Directness of the evidence: studies were conducted in the target population. Precision of estimates: this outcome was reported for 616 infants and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 2 studies were included in the analysis we did not perform a funnel plot | |
| 555 per 1000 | 561 per 1000 | |||||
| Severe IVH (grade 3 or 4) | Study population | RR 0.92 1.29) | 690 | Moderate | Design (risk of bias): The risk of bias for random sequence generation, for allocation concealment, for performance bias and detection bias was low in both studies Heterogeneity/consistency across studies: there was moderate heterogeneity for RR (I² = 74%) and high for RD (I² = 82%) Directness of the evidence: studies were conducted in the target population Precision of estimates: this outcome was reported for 690 infants and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 2 studies were included in the analysis we did not perform a funnel plot. | |
| 171 per 1000 | 157 per 1000 | |||||
| Late‐onset sepsis (> 72 hours of age) | Study population | RR 1.33 1.75) | 701 | HIgh | Design (risk of bias): the risk of bias for random sequence generation, for allocation concealment, for performance bias and detection bias was low in both studies Heterogeneity/consistency across studies: there was no heterogeneity for RR (I² = 0%) nor for RD (I² = 0%) Directness of the evidence: studies were conducted in the target population Precision of estimates: this outcome was reported for 701 infants and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 2 studies were included in the analysis we did not perform a funnel plot | |
| 191 per 1000 | 254 per 1000 | |||||
| Suspected or proven NEC | Study population | RR 0.88 1.41) | 701 | High | Design (risk of bias): the risk of bias for random sequence generation, for allocation concealment, for performance bias and detection bias was low in both studies Heterogeneity/consistency across studies: there was low heterogeneity for RR (I² = 36%) and moderate for RD (I² = 53%). Directness of the evidence: studies were conducted in the target population. Precision of estimates: this outcome was reported on in 701 infants and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 2 studies were included in the analysis we did not perform a funnel plot. | |
| 98 per 1000 | 87 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal death (age < 28 days) Show forest plot | 3 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.31, 0.91] |
| 2 Infant death (age < one year) Show forest plot | 5 | 1115 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.71, 1.13] |
| 3 BPD (supplementary oxygen ar 36 weeks; PMA or death due to BPD)at 36 week's PMA Show forest plot | 2 | 666 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.87, 1.14] |
| 4 Bronchopulmonary dysplasia (at 28 to 30 days of age) Show forest plot | 3 | 343 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.54, 1.13] |
| 5 Bronchopulmonary dysplasia (at 36 to 38 weeks PMA) Show forest plot | 2 | 737 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.90, 1.20] |
| 6 Retinopathy of prematurity, stage ≥ 3 or ≥ 2 Show forest plot | 3 | 810 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.75, 1.06] |
| 6.1 ROP ≥ 3 | 2 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 0.67] |
| 6.2 Retinopathy of prematurity, stage ≥ 2 | 1 | 548 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.80, 1.13] |
| 7 Retinopathy of prematurity, any stage Show forest plot | 4 | 889 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.83, 1.07] |
| 8 Necrotizing enterocolitis (suspected or proven) Show forest plot | 5 | 1115 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.64, 1.39] |
| 9 Sepsis (early and/or late onset) Show forest plot | 4 | 1067 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.95, 1.54] |
| 10 Intraventricular haemorrhage, grade > 2 Show forest plot | 5 | 1103 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.58, 1.01] |
| 11 Intraventricular haemorrhage, all grades Show forest plot | 3 | 427 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.59, 1.00] |
| 12 Minor neural developmental impairment at one year corrected age Show forest plot | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.38, 1.86] |
| 13 Major neural developmental impairment at one year corrected age Show forest plot | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.24, 1.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death during hospital stay Show forest plot | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.34, 4.21] |
| 2 Bronchopulmonary dysplasia at 36 weeks PMA Show forest plot | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.74 [0.88, 8.48] |
| 3 Retinopathy of prematurity (infants who underwent surgery for ROP) Show forest plot | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.10, 1.22] |
| 4 Necrotizing enterocolitis (stage 2A or worse) Show forest plot | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.12, 1.39] |
| 5 Necrotizing enterocolitis (infants who underwent surgery for NEC) Show forest plot | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.08, 3.41] |
| 6 Sepsis (late onset) Show forest plot | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.71, 2.97] |
| 7 Intraventricular haemorrhage (grade 3 or 4) Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.29, 3.90] |
| 8 Hearing test (failed both ears) Show forest plot | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.09, 3.84] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Type 1 ROP or death before determination of ROP outcome using the adjudicated ROP outcome Show forest plot | 2 | 679 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.99, 1.67] |
| 2 Type 1 ROP Show forest plot | 1 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.89, 2.24] |
| 3 Death before determination of ROP outcome Show forest plot | 1 | 638 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.02, 2.31] |
| 4 Type 1 ROP including adjudicated ROP outcome Show forest plot | 2 | 605 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.82, 1.86] |
| 5 Any ROP Show forest plot | 1 | 553 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.88, 1.13] |
| 6 ROP ≥ 2 ROP Show forest plot | 1 | 548 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.80, 1.13] |
| 7 All cause infant mortality to 55 week's PMA Show forest plot | 1 | 638 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.12, 2.48] |
| 8 All cause mortality (outcome collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth) Show forest plot | 2 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.91, 2.00] |
| 9 BPD (requiring oxygen at 36 week's PMA for oxygen saturation > 90%) Show forest plot | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.89, 1.18] |
| 10 BPD or death by it prior to 37 weeks' PMA (outcomes collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth) Show forest plot | 2 | 616 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.87, 1.16] |
| 11 Severe IVH (grade 3 or 4) Show forest plot | 2 | 690 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.65, 1.29] |
| 12 Cystic areas in the cerebral parenchyma measured through 28 d Show forest plot | 2 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.58, 2.85] |
| 13 Early onset sepsis Show forest plot | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Late onset sepsis (> 72 hrs of age) Show forest plot | 2 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.00, 1.75] |
| 15 Suspected or proven NEC Show forest plot | 2 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.55, 1.41] |
| 16 Surgical NEC Show forest plot | 2 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.57, 2.58] |
| 17 Spontaneous gastro‐intestinal perforation Show forest plot | 2 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.48, 1.52] |
| 18 Pulmonary haemorrhage Show forest plot | 1 | 638 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.59, 1.62] |
| 19 PDA Show forest plot | 2 | 700 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.85, 1.14] |
| 20 PDA requiring indomethacin Show forest plot | 1 | 637 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.67, 1.22] |
| 21 PDA requiring surgery Show forest plot | 2 | 700 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.65, 1.42] |
| 22 Seizure treatment for ≥ 2 days Show forest plot | 2 | 700 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.43, 2.56] |
| 23 Negative hearing screening in either ear at discharge Show forest plot | 2 | 472 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.92, 2.29] |
| 24 Respiratory distress syndrome Show forest plot | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.91, 1.09] |
| 25 Sepsis, necrotizing enterocolitis, pneumonia or other infection as a cause of death Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.95, 1.93] |

