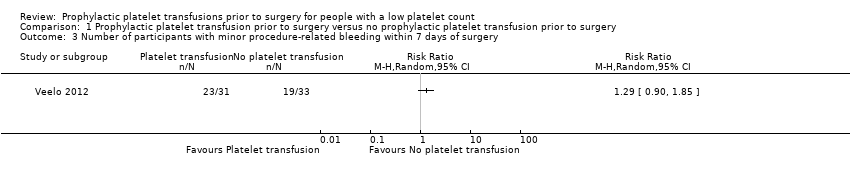
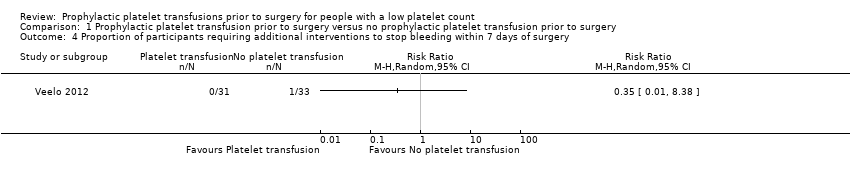
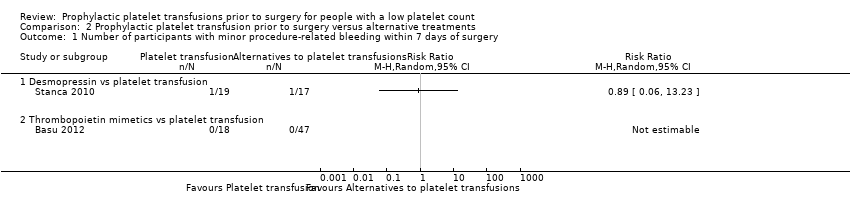
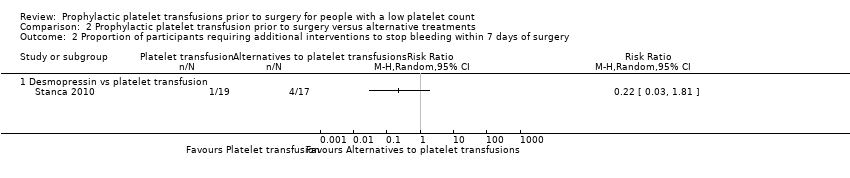
Related content
Related reviews and protocols
Michael JR Desborough, Andreas V Hadjinicolaou, Anna Chaimani, Marialena Trivella, Paresh Vyas, Carolyn Doree, Sally Hopewell, Simon J Stanworth, Lise J Estcourt | 31 October 2016
Helga Dodillet, Karl‐Anton Kreuzer, Ina Monsef, Nicole Skoetz | 30 September 2017
Xia Zhang, Yunhai Chuai, Wei Nie, Aiming Wang, Guanghai Dai | 27 November 2017
Yan Zenga, Xin Duan, Jiajun Xu, Xun Ni | 6 July 2011
Michael Desborough, Lise J Estcourt, Carolyn Doree, Marialena Trivella, Sally Hopewell, Simon J Stanworth, Michael F Murphy | 22 August 2016
Lise J Estcourt, Simon J Stanworth, Carolyn Doree, Sally Hopewell, Marialena Trivella, Michael F Murphy | 18 November 2015
Jonathan Huber, Simon J Stanworth, Carolyn Doree, Patricia M Fortin, Marialena Trivella, Susan J Brunskill, Sally Hopewell, Kirstin L Wilkinson, Lise J Estcourt | 28 November 2019
Lise J Estcourt, Michael JR Desborough, Sally Hopewell, Carolyn Doree, Simon J Stanworth | 2 December 2015
Lise Estcourt, Simon Stanworth, Carolyn Doree, Sally Hopewell, Michael F Murphy, Alan Tinmouth, Nancy Heddle | 16 May 2012
Lise J Estcourt, Simon Stanworth, Carolyn Doree, Marialena Trivella, Sally Hopewell, Patricia Blanco, Michael F Murphy | 27 October 2015