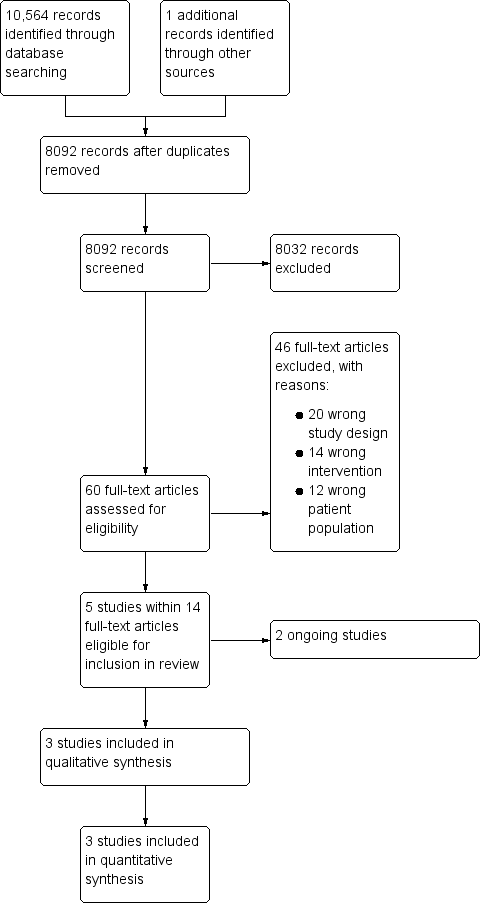
Prophylactic platelet transfusions prior to surgery for people with a low platelet count
References
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Study design: randomised, double‐blind, parallel‐group controlled trial Setting and country: outpatient university hospital, USA Number of centres: 1 Study duration (start to end): unclear Follow‐up duration: 4 weeks Power calculation: unclear Publication type: abstract | |
| Participants | Inclusion criteria: CLD with thrombocytopenia Exclusion criteria: ITP, drug‐induced thrombocytopenia, HIV, hepatocellular carcinoma, haemangioma, autoimmune thrombocytopenia, use of steroids, MDS Number screened: unclear Number randomised: 65 Number analysed: unclear Number excluded: unclear Baseline characteristics
| |
| Interventions | Procedure: liver biopsy Platelet transfusion (n = 18)
Romiplostim (n = 23)
Eltrombopag (n = 24)
| |
| Outcomes | Review outcomes
Other outcomes
| |
| Notes | Trial registration: none found Sources of funding: not reported Conflicts of interest: unclear Ethical approval: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Published as an abstract, no information available |
| Allocation concealment (selection bias) | Unclear risk | Published as an abstract, no information available |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind trial |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind trial |
| Incomplete outcome data (attrition bias) | Unclear risk | Published as an abstract, no information available |
| Selective reporting (reporting bias) | Unclear risk | Published as an abstract, no information available |
| Other bias | Unclear risk | Published as an abstract, no information available |
| Methods | Study design: randomised, open‐label, parallel‐controlled trial Setting and country: dental clinic, Mount Sinai Hospital, USA Number of centres: 1 Study duration (start to end): study enrolment occurred between October 2005 and May 2007 Follow‐up duration: 24 hours after surgery Study power calculation: no Publication type: full | |
| Participants | Inclusion criteria: adults with biopsy‐confirmed liver cirrhosis or clinical/radiological evidence of cirrhosis, requiring dental extraction, having a platelet count of 30 × 109/L to 50 × 109/L, INR of 2.0 to 3.0, or a combination of these Exclusion criteria: presence of other bleeding disorders besides cirrhosis such as renal dysfunction (creatinine 2.0 mg/dL) or HIV, receipt of blood transfusion within 2 weeks before the study, recent acute decompensation of liver cirrhosis, malignancy excluding hepatocellular carcinoma in the absence of portal vein thrombosis, treatment with antiplatelet medications (aspirin, non‐steroidal anti‐inflammatory drugs or clopidogrel) within 10 days before the extraction and documented allergy to desmopressin Pretreatment: no difference between groups with regard to the age, gender, the severity of liver disease as reflected by MELD score, number of teeth removed and percentage of surgical extractions Number screened: 43 enrolled. 2 participants assigned to the blood transfusion group withdrew their consent for the study; 1 participant wanted to be assigned to the desmopressin group, and the other participant changed his mind about having the dental extraction done at that time. 5 participants, 4 in the desmopressin group and 1 in the transfusion group, had blood drawn after being enrolled in the study but before their surgical procedure that resulted in laboratory values outside of the study parameters. These 5 participants underwent the surgical procedure but were not included in the analysis. Number excluded: 5 participants underwent the surgical procedure but were not included in the analysis. Baseline characteristics Platelet transfusion group
Alternatives to platelet transfusions group
| |
| Interventions | Surgical procedure: dental extraction Platelet transfusion
Alternatives to platelet transfusions
| |
| Outcomes | Primary outcome:
Secondary outcomes:
| |
| Notes | Trial registration number:NCT00816127 Source of funding: Icahn School of Medicine at Mount Sinai, New York City, NY Conflicts of interest: unclear Ethical approval: yes, "The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in the approval by the Mount Sinai Institutional Review Board." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization list was generated by one member of the biomathematics team using computer software for randomization." |
| Allocation concealment (selection bias) | Low risk | Quote: "same member of the biomathematics team, not involved in patient enrollment, prepared sealed white randomization envelopes prelabeled with an ID number. The content of the envelope (patient's assignment to the DDAVP [desmopressin] or blood transfusion group) matched the information in the randomization plan for that particular ID number (DDAVP or blood transfusion group). The content of the envelope was not accessible to the research coordinator who enrolled patients or to any member of the team at the time of enrollment." Judgement comment: sealed white envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label design, no blinding |
| Blinding of outcome assessment (detection bias) | High risk | No blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | In all, 43 participants were enrolled in the study and randomised: 21 in desmopressin group and 22 in transfusion group. 36 participants (17 in desmopressin group and 19 in transfusion group) completed the study procedures. 2 participants assigned to the blood transfusion group withdrew their consent for the study; 1 participant wanted to be assigned to the desmopressin group, and the other participant changed his mind about having the dental extraction done at that time. 5 participants, 4 in desmopressin group and 1 in transfusion group, had blood drawn after being enrolled in the study but before their surgical procedure that resulted in laboratory values outside of the study parameters. These 5 participants underwent the surgical procedure but were not included in the analysis. None of these participants experienced bleeding after the procedure. 7 participants randomised were not included in the analysis. 3 in transfusion arm and 4 in desmopressin arm. This is a relative large (up to 19%) number of participants randomised who were not included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Only outcome listed on clinical trials.gov was: "Necessity of rescue blood transfusion in patients who received DDAVP [desmopressin] or blood transfusion prior to dental extraction." No other secondary outcomes listed. This outcome was reported. However, no protocol to assess if other outcomes was planned. |
| Other bias | Low risk | No other biases found |
| Methods | Study design: parallel‐group, open‐label, RCT Setting and country: ICU University hospital, the Netherlands Number of centres: 1 Study duration (start to end): July 2007 to October 2009 Follow‐up duration: Not reported Power calculation: yes, "Although a sample size of 152 patients was considered necessary to find a difference of 15% in bleeding between groups, the study was prematurely terminated." Publication type: full | |
| Participants | Inclusion criteria: participants planned for bedside PDT with mild coagulation disorders defined as PT 14.7–20.0 seconds or platelet counts 40–100 × 109/L or active treatment with aspirin at any dose, or a combination of these Exclusion criteria: aged < 18 years; need for surgical tracheotomy; contraindications to transfusion of blood products and use of clopidogrel. Participant would also be excluded from participation if the attending physician insisted on the need for transfusion of FFP or platelets (or both) before the procedure. Number screened: 355 Number randomised: 72 Number analysed: 64, 4 in each arm did not undergo PDT Number excluded: 283 (refused consent 27, surgical procedure 53, clopidogrel 13, PT > 20 seconds, 11, normal coagulation 179) Baseline characteristics Platelet transfusion group
No platelet transfusion group
| |
| Interventions | Procedure: PDT Platelet transfusion group
No platelet transfusion group
| |
| Outcomes | Primary outcomes
Secondary outcome
Review outcomes reported by the trial
| |
| Notes | Trial registration number: Current Controlled Trials ISRCTN31808827 Source of funding: Academic Medical Centre (AMC) (The Netherlands), Department of Intensive Care Conflicts of interest: authors declared no conflicts of interest Ethical approval: yes. "The study was approved by the local ethics committee." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated randomisation scheme was used." |
| Allocation concealment (selection bias) | Low risk | Quote: "Each assignment ("correction" or "no correction") was recorded on a piece of paper folded three times and enclosed in a consecutively numbered, opaque, sealed envelope." |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label design RCT |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label design RCT |
| Incomplete outcome data (attrition bias) | Low risk | CONSORT flow diagram clearly reported. All participants who underwent the procedure were included in the analysis. 4 in each arm did not undergo the procedure. |
| Selective reporting (reporting bias) | High risk | No protocol available, but registered ISRCTN31808827. Primary outcome measures: volume of blood loss during PDT2; intensity of intratracheal bleeding; time until no blood is visible in tracheal aspirates. All these were all reported in the published paper. Secondary outcome measures: amount of blood products used during and after tracheotomy – not clearly reported in the published paper |
| Other bias | Unclear risk | Trial stopped early due to increase resistance to recruitment and low rate of bleeding in either arm of the study. Quote: "Although a sample size of 152 patients was considered necessary to find a difference of 15% in bleeding between groups, the study was prematurely terminated." |
ALT: alanine transaminase; CLD: chronic liver disease; FFP: fresh frozen plasma; ICU: intensive care unit; INR: international normalised ratio; IQR: interquartile range; ITP: immune thrombocytopenia; IV: intravenous; MDS: myelodysplastic syndrome; MELD: Model for End‐Stage Liver Disease; n: number of participants; PDT: percutaneous dilational tracheotomy; PT: prothrombin time; RCT: randomised controlled trial; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Wrong study design, observational retrospective cohort | |
| Wrong intervention, no platelet transfusions | |
| Wrong study design, single‐centre observational cohort | |
| Wrong participant population, not receiving surgery or a procedure | |
| Wrong study design, observational retrospective cohort | |
| Wrong study design, single‐centre observational cohort | |
| Wrong study design, single‐centre observational cohort | |
| Wrong study design, single‐centre observational cohort | |
| wrong intervention, thrombelastography‐guided blood product use | |
| Wrong study design, observational retrospective cohort | |
| Wrong intervention, no platelet transfusions | |
| Wrong study design, observational cohort | |
| Wrong participant population (not thrombocytopenic prior to surgery) | |
| Wrong participant population (bleeding) | |
| Wrong study design, observational cohort | |
| Wrong participant population (bleeding) | |
| Wrong intervention, thrombelastography‐guided blood product use | |
| Wrong study design, case series | |
| Wrong intervention, thrombelastography‐guided blood product use | |
| Wrong study design, case series | |
| Wrong participant population, not receiving surgery or a procedure | |
| Wrong study setting, measuring thrombopoietin levels | |
| Wrong intervention, no platelet transfusions | |
| Wrong participant population (bleeding) | |
| Wrong participant population (not thrombocytopenic prior to surgery) | |
| Wrong intervention, thrombelastography‐guided blood product use | |
| Wrong intervention, no platelet transfusions | |
| Wrong participant population, not receiving surgery or a procedure | |
| Wrong study design, observational study | |
| Wrong intervention, thrombelastography‐guided blood product use | |
| Wrong study design, single‐centre non‐randomised study | |
| Wrong intervention, thrombelastography‐guided blood product use | |
| Wrong study design, observational cohort study | |
| Wrong participant population, not receiving surgery or a procedure | |
| Wrong study design, observational cohort | |
| Wrong study design, case series | |
| Wrong study design, retrospective cohort | |
| Wrong intervention, blood transfusion ratios in trauma (PROPPR study) | |
| Wrong intervention, thrombelastography‐guided blood product use | |
| Wrong study design, observational cohort | |
| Wrong participant population (not thrombocytopenic prior to surgery) | |
| Wrong participant population (not thrombocytopenic prior to surgery) | |
| Wrong intervention, thrombelastography‐guided blood product use | |
| Wrong participant population (not thrombocytopenic prior to surgery) | |
| Wrong intervention, no platelet transfusions | |
| Wrong study design, observational cohort study |
Characteristics of ongoing studies [ordered by study ID]
Jump to:
| Trial name or title | Prophylactic platelet transfusion prior to central venous catheter placement in patients with thrombocytopenia |
| Methods | Study design: parallel‐group, single‐blind, randomised controlled trial Country: the Netherlands Number of centres: multicentre Planned starting date: February 2016 Planned completion date: October 2019 Follow‐up points: directly after CVC insertion, 1 hour, 24 hours |
| Participants | Target number of participants: 462 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention arm: 1 unit of platelets Control arm: no platelets or placebo treatment |
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Starting date | February 2016 |
| Contact information | APJ Vlaar |
| Notes | Trial registration date: January 2016 |
| Trial name or title | Point‐of‐care versus standard coagulation tests versus restrictive strategy to guide transfusion in chronic liver failure patients requiring central venous line: prospective randomized trial (POCKET) |
| Methods | Allocation: randomised Endpoint classification: safety/efficacy study Intervention model: parallel assignment (3 arms) Masking: double‐blind (participant, caregiver) Primary purpose: treatment |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Arm 1: coagulogram‐based protocol Arm based on standard coagulation tests protocol to guide blood transfusion before central venous catheterisation. The possible components to be used include fresh frozen plasma, platelets (random or apheresis), cryoprecipitate (or a combination of these), based on INR), PTT, platelet count, fibrinogen (or a combination of these).
Arm 2: thromboelastometry‐based protocol The interventions for this protocol included transfusion of fresh frozen plasma, platelets (random or aphaeresis) cryoprecipitate (or a combination of these), based on rotational thromboelastometry.
Arm 3: restrictive strategy The interventions for this protocol include transfusion of fresh frozen plasma or platelets (random or aphaeresis), or both, based on INR and platelet count.
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Starting date | September 2014 |
| Contact information | Leonardo L Rocha, MD +55‐11‐21511500, [email protected] Thiago D Correa, MD, PhD +55‐11‐21511500, [email protected] |
| Notes | Estimated enrolment: 165 Estimated study completion date: January 2019 Estimated primary completion date: December 2018 (final data collection date for primary outcome measure) Trial registration: NCT02311985 Location of trial: Hospital Israelita Albert Einstein, Sao Paulo, Brazil |
CVC: central venous catheter; ICU: intensive care unit; IgA: immunoglobulin A; INR: international normalised ratio; PTT: partial thromboplastin time; RBC: red blood cell; WHO: World Health Organization.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality within 30 days of surgery Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.1 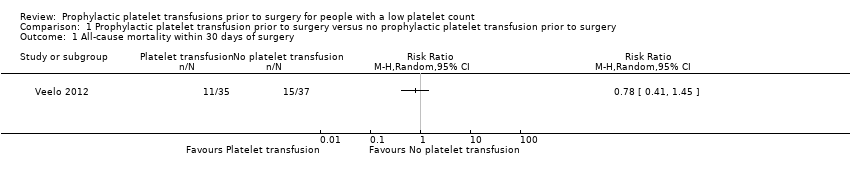 Comparison 1 Prophylactic platelet transfusion prior to surgery versus no prophylactic platelet transfusion prior to surgery, Outcome 1 All‐cause mortality within 30 days of surgery. | ||||
| 2 Number of participants with major procedure‐related bleeding within 7 days of surgery Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.2 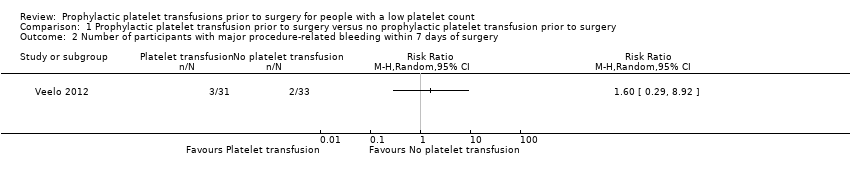 Comparison 1 Prophylactic platelet transfusion prior to surgery versus no prophylactic platelet transfusion prior to surgery, Outcome 2 Number of participants with major procedure‐related bleeding within 7 days of surgery. | ||||
| 3 Number of participants with minor procedure‐related bleeding within 7 days of surgery Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.3 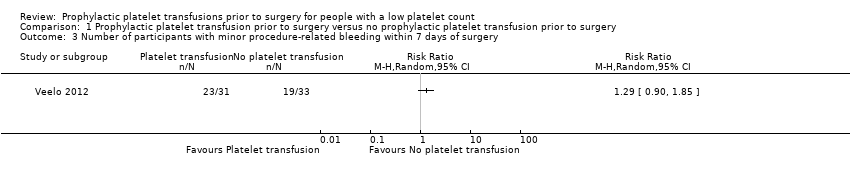 Comparison 1 Prophylactic platelet transfusion prior to surgery versus no prophylactic platelet transfusion prior to surgery, Outcome 3 Number of participants with minor procedure‐related bleeding within 7 days of surgery. | ||||
| 4 Proportion of participants requiring additional interventions to stop bleeding within 7 days of surgery Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.4 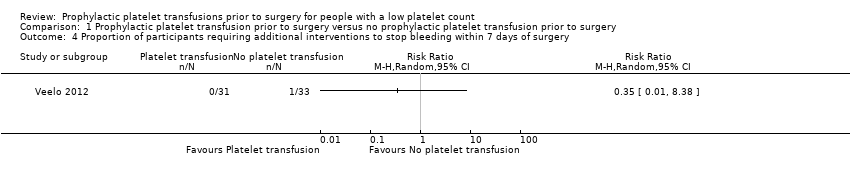 Comparison 1 Prophylactic platelet transfusion prior to surgery versus no prophylactic platelet transfusion prior to surgery, Outcome 4 Proportion of participants requiring additional interventions to stop bleeding within 7 days of surgery. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with minor procedure‐related bleeding within 7 days of surgery Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.1 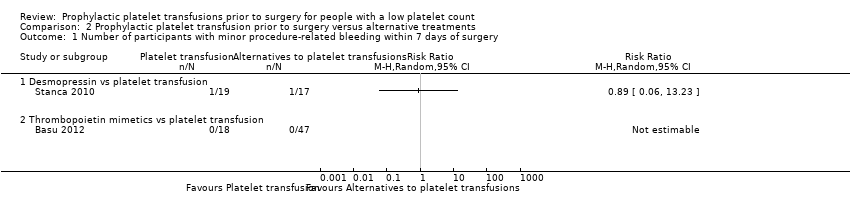 Comparison 2 Prophylactic platelet transfusion prior to surgery versus alternative treatments, Outcome 1 Number of participants with minor procedure‐related bleeding within 7 days of surgery. | ||||
| 1.1 Desmopressin vs platelet transfusion | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Thrombopoietin mimetics vs platelet transfusion | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Proportion of participants requiring additional interventions to stop bleeding within 7 days of surgery Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.2 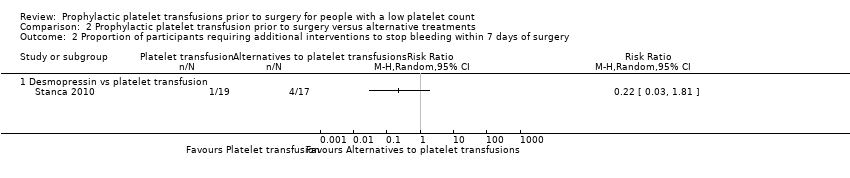 Comparison 2 Prophylactic platelet transfusion prior to surgery versus alternative treatments, Outcome 2 Proportion of participants requiring additional interventions to stop bleeding within 7 days of surgery. | ||||
| 2.1 Desmopressin vs platelet transfusion | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Serious adverse events (transfusion related adverse effects within 24 hours of the transfusion) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.3 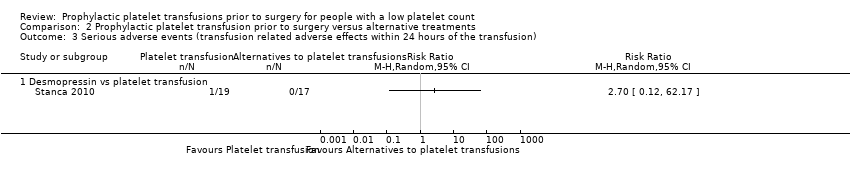 Comparison 2 Prophylactic platelet transfusion prior to surgery versus alternative treatments, Outcome 3 Serious adverse events (transfusion related adverse effects within 24 hours of the transfusion). | ||||
| 3.1 Desmopressin vs platelet transfusion | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
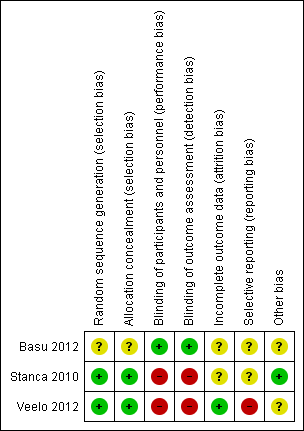
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Prophylactic platelet transfusion prior to surgery versus no prophylactic platelet transfusion prior to surgery, Outcome 1 All‐cause mortality within 30 days of surgery.

Comparison 1 Prophylactic platelet transfusion prior to surgery versus no prophylactic platelet transfusion prior to surgery, Outcome 2 Number of participants with major procedure‐related bleeding within 7 days of surgery.

Comparison 1 Prophylactic platelet transfusion prior to surgery versus no prophylactic platelet transfusion prior to surgery, Outcome 3 Number of participants with minor procedure‐related bleeding within 7 days of surgery.

Comparison 1 Prophylactic platelet transfusion prior to surgery versus no prophylactic platelet transfusion prior to surgery, Outcome 4 Proportion of participants requiring additional interventions to stop bleeding within 7 days of surgery.

Comparison 2 Prophylactic platelet transfusion prior to surgery versus alternative treatments, Outcome 1 Number of participants with minor procedure‐related bleeding within 7 days of surgery.

Comparison 2 Prophylactic platelet transfusion prior to surgery versus alternative treatments, Outcome 2 Proportion of participants requiring additional interventions to stop bleeding within 7 days of surgery.

Comparison 2 Prophylactic platelet transfusion prior to surgery versus alternative treatments, Outcome 3 Serious adverse events (transfusion related adverse effects within 24 hours of the transfusion).
| Prophylactic platelet transfusion prior to surgery versus no prophylactic platelet transfusion prior to surgery | ||||||
| Patient or population: people with a low platelet count Setting: surgery Intervention: platelet transfusion Comparison: no platelet transfusion | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no platelet transfusion | Risk with platelet transfusion | |||||
| All‐cause mortality within 30 days of surgery | Study population | RR 0.78 | 72 | ⊕⊝⊝⊝ | — | |
| 405 per 1000 | 316 per 1000 | |||||
| Mortality secondary to bleeding within 30 days of surgery – not reported | — | — | — | — | — | — |
| Mortality secondary to thromboembolism within 30 days of surgery – not reported | — | — | — | — | — | — |
| Mortality secondary to infection within 30 days of surgery – not reported | — | — | — | — | — | — |
| Number of participants with major bleeding within 7 days of surgery (surgical site bleeding requiring a second intervention or reoperation or surgical site bleeding that causes a haematoma or haemarthrosis of sufficient size to delay mobilisation or wound healing) | Study population | RR 1.60 | 64 | ⊕⊝⊝⊝ | — | |
| 61 per 1000 | 97 per 1000 | |||||
| The number of participants with minor procedure‐related bleeding within 7 days of surgery | Study population | RR 1.29 | 64 | ⊕⊝⊝⊝ | — | |
| 576 per 1000 | 743 per 1000 | |||||
| Serious adverse events (surgery‐related adverse effects within 30 days) | No events occurred in either study arm | 64 | ⊕⊝⊝⊝ | — | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aOnly adults in the intensive care unit were included in this trial (downgraded one level for indirectness). bThe confidence intervals included a serious risk of harm or benefit (downgraded two levels for imprecision). cThis is a subjective outcome and the trial was unblinded (downgraded one level for risk of bias). dThe confidence intervals included a risk of harm or benefit (downgraded one level for imprecision). | ||||||
| Prophylactic platelet transfusion prior to surgery versus alternative treatments | ||||||
| Patient or population: people with a low platelet count Setting: surgery Intervention: platelet transfusion Comparison: desmopressin | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with desmopressin | Risk with platelet transfusion | |||||
| All‐cause mortality within 30 days of surgery – not reported | — | — | — | — | — | — |
| Mortality secondary to bleeding within 30 days of surgery – not reported | — | — | — | — | — | — |
| Mortality secondary to thromboembolism within 30 days of surgery – not reported | — | — | — | — | — | — |
| Mortality secondary to infection within 30 days of surgery – not reported | — | — | — | — | — | — |
| Number of participants with major bleeding within 7 days of surgery (bleeding that required ≥ 2 units of whole blood/red blood cells within 24 hours of the bleeding) | No events in either study arm | 36 | ⊕⊝⊝⊝ | — | ||
| Number of participants with minor procedure‐related bleeding within 7 days of surgery | Study population | RR 0.89 | 36 | ⊕⊝⊝⊝ | — | |
| 59 per 1000 | 52 per 1000 | |||||
| Serious adverse events (transfusion‐related adverse effects within 24 hours of the transfusion) | Study population | RR 2.70 | 36 | ⊕⊝⊝⊝ | — | |
| 0 per 1000 | 0 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aOpen‐label trial (downgraded one level for risk of bias). bStudy only included adults with chronic liver disease (downgraded one level for indirectness). cConfidence intervals included a serious risk or benefit or treatment (downgraded one level for imprecision, as already downgraded one level for indirectness and risk of bias). | ||||||
| Different platelet count thresholds for administering a prophylactic platelet transfusion prior to surgery | ||||||
| Patient or population: people with a low platelet count Setting: surgery Intervention: platelet transfusion Comparison: TPO mimetic | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with TPO mimetic | Risk with platelet transfusion | |||||
| All‐cause mortality within 30 days of surgery – not reported | — | — | — | — | — | — |
| Mortality secondary to bleeding within 30 days of surgery – not reported | — | — | — | — | — | — |
| Mortality secondary to thromboembolism within 30 days of surgery – not reported | — | — | — | — | — | — |
| Mortality secondary to infection within 30 days of surgery – not reported | — | — | — | — | — | — |
| Number of participants with major bleeding within 7 days of surgery | No bleeding in any of the study arms | 65 | ⊕⊝⊝⊝ | — | ||
| Number of participants with minor procedure‐related bleeding within 7 days of surgery | No bleeding occurred in any of the study arms | 65 | ⊕⊝⊝⊝ | — | ||
| Serious adverse events – not reported | — | — | — | — | — | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio; TPO: thrombopoietin. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aStudy only included adults with chronic liver disease (downgraded one level for indirectness). bNo events occurred (downgraded two levels for imprecision). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality within 30 days of surgery Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Number of participants with major procedure‐related bleeding within 7 days of surgery Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Number of participants with minor procedure‐related bleeding within 7 days of surgery Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Proportion of participants requiring additional interventions to stop bleeding within 7 days of surgery Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with minor procedure‐related bleeding within 7 days of surgery Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Desmopressin vs platelet transfusion | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Thrombopoietin mimetics vs platelet transfusion | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Proportion of participants requiring additional interventions to stop bleeding within 7 days of surgery Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Desmopressin vs platelet transfusion | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Serious adverse events (transfusion related adverse effects within 24 hours of the transfusion) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Desmopressin vs platelet transfusion | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |