Anticoagulants and antiplatelet agents for preventing central venous haemodialysis catheter malfunction in patients with end‐stage kidney disease
Abstract
Background
Catheter malfunction, including thrombosis, is associated with reduced dialysis adequacy, as well as an increased risk of catheter‐related bacteraemia and mortality. The role of anticoagulants in the prevention of catheter malfunction remains uncertain.
Objectives
This review aimed to compare the prophylactic effect of different anticoagulant agents, preparations, doses and administration on the incidence of central venous haemodialysis catheter‐related malfunction and sepsis in patients with end‐stage kidney disease (ESKD).
Search methods
We searched the Cochrane Renal Group's Specialised Register to 7 January 2016 through contact with the Trials' Search Co‐ordinator using search terms relevant to this review.
Selection criteria
We included all randomised controlled trials (RCT) assessing anticoagulants compared with conventional care for the prevention of catheter malfunction in adult patients receiving haemodialysis for ESKD.
Data collection and analysis
The primary outcome was catheter malfunction defined as a catheter blood flow of 200 mL/min or less, or as defined by study authors. Secondary outcomes were catheter‐related bacteraemia, all‐cause mortality and bleeding events. Relative risks (RR) with 95% confidence intervals (CI) for individual studies were pooled using random effects models within treatment classes. Analyses were conducted by class, with subgroup analyses performed of individual agents within classes.
Main results
We included 27 studies (3003 participants) that were followed up for a median of six months. Study interventions included alternative anticoagulant locking solutions (19 studies, 2216 patients), systemic agents (6 studies, 664 patients) and low or no dose heparin (2 studies, 123 patients). The most common comparison treatment was a locking solution of heparin 5000 IU/mL, used in 17 studies. No significant effect on catheter malfunction was observed for alternative anticoagulant locking solutions (RR 0.96, 95% CI 0.74 to 1.26), systemic agents (RR 0.59, 95% CI 0.28 to 1.23), or low or no dose heparin (RR 0.90, 95% CI 0.10 to 8.31). A significant reduction on incidence of catheter‐related bacteraemia was observed for alternative anticoagulant locking solutions (RR 0.46, 95% CI 0.32 to 0.66) but not systemic agents (RR 2.41, 95% CI 0.89 to 6.55), and could not be assessed in reports of low or no dose heparin studies. No significant effect on all‐cause mortality was observed for alternative anticoagulant locking solutions (RR 0.88, 95% CI 0.54 to 1.43) or systemic agents (RR 0.78, 95% CI 0.37 to 1.65), and was not reported in studies of low or no dose heparin. Bleeding events were only reported in eight studies, including only 2/5 studies of systemic warfarin, with no clear effect demonstrated (RR 1.43, 95% CI 0.86 to 2.39). For individual agents, recombinant tissue plasminogen (rt‐PA) was the only locking solution shown to reduce catheter malfunction (RR 0.58, 95% CI 0.37 to 0.91) based on the results of a single study. No significant on catheter malfunction was observed for other individual classes of alternative anticoagulant locking solutions (citrate: RR 1.14, 95% CI 0.76 to 1.69; antibiotic: RR 1.48, 95% CI 0.79 to 2.77; ethanol: RR 0.88, 95% CI 0.21 to 3.67). On the other hand, all individual classes of alternative anticoagulant locking solutions, except ethanol, reduced catheter‐related bacteraemia (citrate: RR 0.49, 95% CI 0.36 to 0.68; antibiotic: RR 0.27, 95% CI 0.11 to 0.70; rt‐PA: RR 0.35, 95% CI 0.13 to 0.93; ethanol: RR 0.33, 95% CI 0.03 to 4.05). No significant effect on all‐cause mortality was observed for any individual agent within the class of alternative locking solutions. Studies were mainly of low quality and underpowered with an average participant number of 75 and study duration of six months. The interpretation of the study evidence was further limited by the variation in tested interventions and outcome reporting.
Authors' conclusions
The relative net benefit of anticoagulant therapies for prevention of catheter malfunction remains uncertain. Multiple agents appear to reduce catheter‐related bacteraemia although the lack of clear assessment of harms and the limitations of study quality mean these results should be interpreted with caution. Methodological approaches can be used to avoid methods of reporting unduly affecting on the results of meta‐analyses incorporating studies employed mixed reporting methods. Further high quality randomised studies, including safety outcomes, are needed.
PICOs
Plain language summary
Anticoagulants and antiplatelet agents for preventing central venous haemodialysis catheter malfunction in patients with end‐stage kidney disease
Background
Patients with end‐stage kidney disease require vascular access during haemodialysis. Central venous haemodialysis catheters are frequently used when permanent vascular access is not available. Catheter problems contribute to greater morbidity and mortality. Catheter malfunction leads to requirements for additional interventions, increased risk of catheter‐related infection and hospitalisation.
Standard care for the prevention of catheter malfunction is the use of heparin solutions as a post dialysis ‘lock’ in the catheter ports. The potential impact of heparin treatment on bleeding risk is an acknowledged concern. Therefore newer approaches have been proposed to seek improvements in catheter patency or treatment‐associated harm rates.
Study characteristics
This review focused on randomised controlled trials (RCTs) of anticoagulants compared with conventional care for the prevention of catheter malfunction patients receiving haemodialysis.
Key results
We found 27 studies, involving 3003 patients followed for an average six months, which assessed alternative anticoagulant locking solutions, systemic agents and low or no dose heparin. Catheter malfunction were not affected by any of these classes of agents. Subgroup analysis showed that the only agent reducing catheter malfunction was recombinant tissue plasminogen locking solution based on the results of a single study. A significant reduction was observed on the incidence of catheter‐related bacteraemia for alternative anticoagulant locking solutions. There was no evidence to suggest that alternative anticoagulants to heparin locking solutions had an effect on death rates or bleeding events, although only a small proportion of studies reported bleeding events.
Quality of the evidence
Further high quality information is needed on both potential benefits and safety of alternative approaches to maintaining dialysis access catheter function.
Authors' conclusions
Background
Central venous haemodialysis catheters are a necessary but problematic component of dialysis practice. Central venous catheters (CVC) are used for approximately 57% of incident dialysis patients in Australia among whom they are associated with significantly increased dialysis‐related mortality (Hariharan 2006; Polkinghorne 2013; Schwab 1999; USRDS 2009). CVC are associated with the risk of catheter‐related thrombosis which can result in catheter malfunction (Suhocki 1996).
Description of the condition
Catheter‐related thrombosis can be classified as either extrinsic or intrinsic (Beathard 2001) based on the site at which the thrombosis forms. The main consequences from catheter‐related thrombotic events are deep venous thrombosis (Vanherweghem 1994), shortened access life and requirement for extra procedures (Linenberger 2006), inadequate dialysis (Little 2001) and increased risk of sepsis (Timsit 1998). The incidence of catheter‐related thrombosis varies by catheter location (Trerotola 2000), sex, systemic prothrombotic states, site of insertion (subclavian compared with internal jugular) (Trerotola 2000), previous catheter‐related thrombosis and catheter malposition (Liangos 2006; Trerotola 2000).
Description of the intervention
Current guidelines recommend antithrombotic locking solutions to prevent catheter‐related malfunction in dialysis patients but do not refer to specific agents or concentrations in recognition of the lack of definitive evidence for individual regimens (UK Renal Association 2011; KDOQI 2006). Newer approaches including alternative anticoagulant containing locking solutions, antibiotic containing lock solutions, systematic anticoagulants, antiplatelet agents (Abdul‐Rahman 2007), catheter flushing regimes (Pepper 2007) and recombinant tissue‐type plasminogen activator (rt‐PA) (Schenk 2000) have been investigated to seek improvements in catheter patency or treatment‐associated harm rates.
How the intervention might work
Standard care in the prevention of catheter malfunction is the use of heparin solutions as a post dialysis ‘lock’ in the catheter ports. Heparin is a mucopolysaccharide with in vitro and in vivo anticoagulant properties. It exerts anticoagulant effect by deactivating activated factor X and inhibiting conversion of prothrombin to thrombin. Heparin locking solutions have been recommended for maintaining catheter patency, though the nominated wide concentration range of 1000 to 10,000 U/mL reflects the lack of evidence on optimal dosing (Besarab 2011). The potential impact on bleeding risk is an acknowledged concern (Moritz 2003; Yevzlin 2007). Other adverse events associated with heparin use include major bleeding, heparin‐induced thrombocytopenia and thrombosis, and osteoporosis (Thomas 2007).
Alternative approaches, therefore, have been tested to improve catheter function and reduce adverse events. These potentially include alternative anticoagulant based locking solutions (e.g. citrate locking solutions), systemic anticoagulation, and impregnated catheters. These may improve efficacy by their anticoagulant properties. For example, solutions containing 4% to 5% of trisodium citrates express anticoagulant activity (von Brecht 1986) by binding Ca²+ to prevent progression of the coagulation cascade (Pinnick 1983). Alternative approaches to reducing adverse events include reduced heparin or no heparin saline flushes.
Why it is important to do this review
Catheter thrombosis is associated with negative outcomes including reduced dialysis adequacy, requirement for repeated invasive interventions, increased risk of catheter‐related bacteraemia and higher rates of hospitalisation and mortality.
Objectives
This review aimed to compare the prophylactic effect of different anticoagulant agents, preparations, doses and administration on the incidence of central venous haemodialysis catheter‐related malfunction and sepsis in patients with ESKD.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) and quasi‐RCTs (studies in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) in which any locking solutions containing anticoagulant, systemic anticoagulants and antiplatelet agents were used for catheter‐related thrombosis prophylaxis. The first phase of randomised cross‐over studies were also included.
Types of participants
Inclusion criteria
Studies conducted in people with ESKD who require CVC for initiation or maintenance haemodialysis access were included. Studies enrolling patients who had been treated previously with anticoagulants for thrombotic events were included in the review.
Exclusion criteria
Studies that included patients who were receiving systemic therapeutic anticoagulation for non catheter‐related thromboembolic events were excluded. Patients with acute kidney injury were also excluded.
Types of interventions
The review compared the prophylactic effect of different anticoagulant or antiplatelet agents, preparations, doses and administration on the incidence of central venous haemodialysis catheter‐related malfunction and sepsis in patients with ESKD. We conducted comparisons of placebos or comparators versus:
-
anticoagulant catheter locking solutions;
-
systemic anticoagulants;
-
antiplatelet agents.
Types of outcome measures
Primary outcomes
-
Reported thrombotic outcomes: Incidence of catheter malfunction presumed due to thrombosis defined as a persistent inability to achieve a blood flow of greater than 200 mL/min despite positional changes of the patient and additional flushing or both, or as defined by the study authors.
Secondary outcomes
-
Incidence of major bleeding, defined as a reduction in haemoglobin of 20 g/L; bleeding requiring blood transfusion, bleeding requiring hospital admission, or as defined by the authors
-
Incidence of minor bleeding, defined as a reduction in haemoglobin of less than 20 g/L, change from baseline less than 19.9 g/L; bleeding not requiring hospital admission, bleeding not requiring blood transfusion, or as defined by the authors
-
Other thrombotic events, defined as thromboses in vessels in the region of the vascular catheter, or as defined by the study authors
-
Requirement for replacement of CVC
-
Requirement for thrombolytic agents
-
Infection related to vascular access, defined as catheter‐related exit site infection, bacteraemia in the absence of another clear source of infection, or as defined by the study authors
-
Thrombocytopenia, defined as a new platelet count less than 150,000/μL (150 x 10⁹/L), or as defined by the study authors
-
Hypocalcaemia, defined as a serum corrected calcium level less than 2.20 mmol/L, or as defined by the study authors
-
All‐cause mortality
-
Other adverse events including allergic reactions, urticaria, and anaphylaxis
-
Other adverse events, as defined by the study authors
-
Economic costs to health services funders.
Search methods for identification of studies
Electronic searches
We searched Cochrane Kidney and Transplant's Specialised Register to 7 January 2016 through contact with the Trials' Search Co‐ordinator using search terms relevant to this review. The Specialised Register contains studies identified from the following sources.
-
Quarterly searches of the Cochrane Central Register of Controlled Trials CENTRAL
-
Weekly searches of MEDLINE OVID SP
-
Handsearching of renal‐related journals and the proceedings of major renal conferences
-
Searching of the current year of EMBASE OVID SP
-
Weekly current awareness alerts for selected renal journals
-
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies as well as a list of handsearched journals, conference proceedings and current awareness alerts are available in the Specialised Register section of information about the Cochrane Kidney and Transplant.
See Appendix 1 for search terms.
Searching other resources
Relevant studies were also obtained from the following sources.
-
Reference lists of review articles, relevant studies and clinical practice guidelines.
-
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
Two authors independently reviewed the abstracts of all studies from the initial search. Those that meet the inclusion criteria were collated. Two authors independently applied the inclusion criteria to each full text article. A third author resolved any conflicts by acting as arbitrator. There were three ways we dealt with duplicate publications. Firstly, we used Endnote’s ‘find duplicate’ function to automatically remove duplicates. Secondly, we manually screened the duplications when we went through abstracts review. Thirdly, when one study produced more than one publication, we combined reports together and used the publication with the most complete data in the analyses. The number of duplicates is reported in the study flow chart.
Data extraction and management
Two authors independently extracted information using a standardised data collection form. These data were extrapolated from tables and graphs in published papers. If this is not possible, the study authors were contacted for further information. Extracted data included clinical measures such as participants' comorbidities, and length and frequency of dialysis. We also scrutinised information regarding interventions, different anticoagulants, and assess data related to our primary and secondary outcomes measures.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (seeAppendix 2).
-
Was there adequate sequence generation (selection bias)?
-
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study (detection bias)?
-
Participants and personnel
-
Outcome assessors
-
-
Were incomplete outcome data adequately addressed (attrition bias)?
-
Were reports of the study free of suggestion of selective outcome reporting (reporting bias)?
-
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For the primary outcome, incidence of thromboembolic events, and secondary outcomes with continuous variables (such as rates of bleeding and rates of other adverse effects), we treated data as continuous and use mean differences to estimate the difference that one treatment has on the average outcome to another. However, if results were reported as time to a first event, we used time‐to‐event data to measure treatment effect.
For binary outcomes, such as all‐cause mortality, we compared proportions and 95% confidence intervals, and calculate numbers‐needed‐to‐treat and numbers‐needed‐to‐harm to establish a standardised, clinically relevant measure of data.
An a priori decision was made to calculate summary estimates within three therapeutic classes (i.e. alternative anticoagulant locking solutions, systemic agents, and low or no dose heparin locking solutions) given the different mechanisms of action between classes and the plausible true heterogeneity of effect. Studies varied in the method of outcome reporting. We nominated heparin 5000 IU/ml as the comparison arm where possible, based on clinical practice despite the lack of evidence‐based recommendations from current guidelines on optimal heparin lock concentration (Besarab 2011).
Unit of analysis issues
We analysed outcomes at the individual patient level. If the unit of randomisation was not the same as the level of analysis, i.e. the patient, adjustments were made to address the potential impact of clustering on the outcome.
Dealing with missing data
Missing data were requested by written correspondence (e.g. emailing corresponding author) from the authors.
Assessment of heterogeneity
Heterogeneity was analysed using a Chi² test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I² test (Higgins 2003). I² values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
An a priori decision was made to calculate summary estimates within three therapeutic classes (i.e. alternative anticoagulant locking solutions, low or no dose heparin locking solutions and systemic agents) given the different mechanisms of action between classes and the plausible true heterogeneity of effect.
Assessment of reporting biases
If possible, funnel plots were to be used to assess for the potential existence of small study bias (Higgins 2011). If there was funnel plot asymmetry we planned to use the trim‐and‐fill method to estimate the volume of unpublished studies on this concept.
Data synthesis
Relative risks (RR) with 95% confidence intervals (CIs) were calculated for dichotomous outcomes. By preference we selected data reported as the number of patients with an event. However, where data was not available for the number of patients with an event, we included data reported as events per study and then as events per catheter day, by deriving a RR for each study and pooling the RRs as below.
Given the heterogeneity of the interventions involved, our pre‐specified preference was to use random effects models to derive the summary estimates. However, we observed a variety of methods of outcome reporting including reporting by patients and reporting of repeated events. The random effects model weights individual studies by confidence interval with the consequence that any given study will have greater influence on the overall result if repeated events measures are used compared with if patient counts are used. Having the results influenced by decisions on reporting method appears arbitrary and could have the overall effect of favouring smaller studies as these may be more likely to report event rates rather than counts, and so does not seem consistent with the conservative approach underlying our initial decision to use random effects models. We therefore modified our analysis methods as follows. Summary estimates of relative risks were derived from individual study risks in a two‐step process. Firstly random effects models were constructed pooling individual studies that reported by each method. Secondly the results of these models were weighted by average study sample size.
Analyses were performed using RevMan 5.3 if possible, i.e. where all studies reported the raw counts of participants experiencing an event. When this was not possible, analyses were performed using STATA 11.0.
Subgroup analysis and investigation of heterogeneity
Planned a priori subgroup analyses were used to explore possible sources of heterogeneity. Heterogeneity in prevention of catheter malfunction in alternative anticoagulant locking solutions could be related to different class of interventions, i.e. citrate, rt‐PA, LMWH and antibiotic locking solutions. Whether the use of a co‐intervention or not can also cause heterogeneity of the results, which was also analysed.
Sensitivity analysis
Sensitivity study was performed according to Cochrane methodology, i.e. by substituting alternative decisions or ranges of values for decisions that were arbitrary or unclear, including the omission of single studies whose inclusion alters the analysis outcome.
Results
Description of studies
Results of the search
The search identified 448 potentially relevant studies, of which 52 studies with 71 reports were reviewed in full text (Characteristics of included studies). Twenty seven studies were included, 16 studies excluded and there are nine studies awaiting assessment: seven were abstract‐only publications (Bonkain 2013; Clement 1998; Freudiger 1990; Geron 2008; Hemmelder 2003; Shi 2008; Sishir 2014); one study was recently completed and no data are available (ISRCTN27307877); and one study was identified just prior to publication and will be assessed in a future update of this review (Ray 1999) (Figure 1). There were no citations in languages other than English were identified.
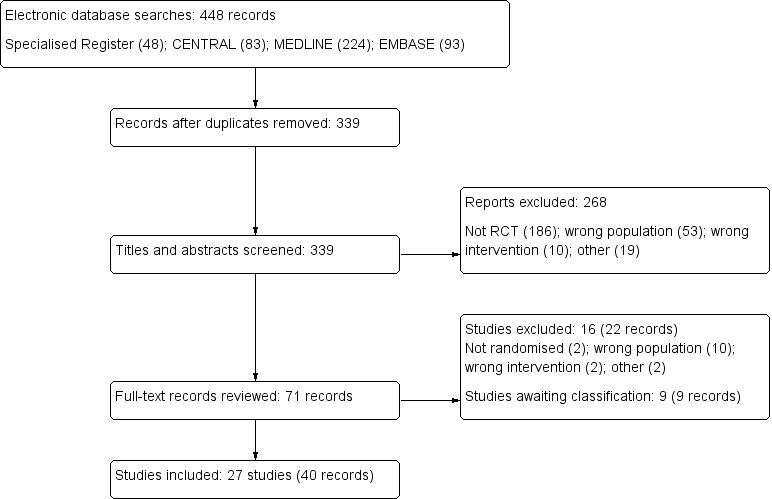
Study flow diagram.
Included studies
Interventions included alternative anticoagulant locking solutions (19 studies, 2216 patients) (AZEPTIC Study 2011; Betjes 2004; Bleyer 2005; Buturovic 1998; Campos 2011; CHARTS Study 2008; Corbett 2013; Dogra 2002; Filiopoulos 2011; HEALTHY‐CATH Study 2009; Hendrickx 2001; Lustig 2011; Malo 2010; Moran 2012; Nori 2006; Pervez 2002; Power 2009; PreCLOT Study 2006; Solomon 2010), systemic agents (aspirin (1 study, 180 patients) (Mozafar 2013); warfarin (5 studies, 479 patients) (Abdul‐Rahman 2007; Coli 2006; Mokrzycki 2001; Traynor 2001; Wilkieson 2011), and low or no dose heparin (2 studies, 123 patients (Hryszko 2013; Kaneko 2004).
Within the class of alternative anticoagulant locking solutions, agents tested included citrate locking solutions (14 studies, 1656 patients) (AZEPTIC Study 2011; Betjes 2004; Buturovic 1998; CHARTS Study 2008; Corbett 2013; Dogra 2002; Filiopoulos 2011; Hendrickx 2001; Lustig 2011; Moran 2012; Nori 2006; Pervez 2002; Power 2009; Solomon 2010); recombinant tissue plasminogen activator locking solutions (1 study, 225 patients) (PreCLOT Study 2006), antibiotic locking solutions (2 studies, 244 patients) (Bleyer 2005; Campos 2011), low molecular weight heparin (LMWH) locking solutions (1 study, 42 patients) (Malo 2010), and ethanol locking solution (1 study, 49 patients) (HEALTHY‐CATH Study 2009).
Excluded studies
Sixteen studies (22 reports) were excluded after review of the full article (Characteristics of excluded studies). Two studies were not randomised (Aslam 2008; Ota 1996); two studies were cross‐over studies with no extractable data (Meeus 2005; Schenk 2000);10 studies enrolled the wrong population (Betjes 2006; Caruana 1991; Gittins 2007; Hu 2011; Huraib 1994; Lange 2007; Oguzhan 2012; Plamondon 2005; Thomson 2011; Weijmer 2005); and two studies did not use anticoagulant or antiplatelet agents (Oran 2008; Saxena 2012).
Risk of bias in included studies
Risk of bias was variable as illustrated in overall Figure 2 and by individual study in Figure 3. In the majority of studies as reported, the risk of bias was unclear for random sequence generation and allocation concealment, unclear or high for blinding of participants or personnel, and for outcome assessment. Most studies as reported were low risk for incomplete outcome data reporting and high risk for selective reporting.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Random sequence generation was judged to be at low risk of bias in 12/27 (45%) studies (AZEPTIC Study 2011; Bleyer 2005; Dogra 2002; Filiopoulos 2011; HEALTHY‐CATH Study 2009; Hryszko 2013; Malo 2010; Moran 2012; Pervez 2002; PreCLOT Study 2006; Solomon 2010; Wilkieson 2011); unclear in 13/27 (48%) studies (Abdul‐Rahman 2007; Betjes 2004; Buturovic 1998; Campos 2011; Coli 2006; Corbett 2013; Hendrickx 2001; Kaneko 2004; Lustig 2011; Mokrzycki 2001; Mozafar 2013; Nori 2006; Traynor 2001) and at high risk of bias in 2/27 (7%) studies (CHARTS Study 2008; Power 2009).
Allocation concealment
Allocation concealment was judged to be of low risk in 5/27 (19%) studies (Dogra 2002; PreCLOT Study 2006; Solomon 2010;Wilkieson 2011; HEALTHY‐CATH Study 2009); unclear in 20/27 (74%) studies (Abdul‐Rahman 2007; Betjes 2004; Bleyer 2005; Buturovic 1998; Campos 2011; Coli 2006; Filiopoulos 2011; Hendrickx 2001; AZEPTIC Study 2011; Malo 2010; Mokrzycki 2001; Moran 2012; Nori 2006; Pervez 2002; Power 2009; Traynor 2001; Corbett 2013; Hryszko 2013; Lustig 2011; Mozafar 2013); and at high risk of bias in 2/27 (7%) studies (Kaneko 2004; CHARTS Study 2008).
Blinding
Blinding of participants and personnel (performance bias) was judged to be at low risk of bias in 8/27 (30%) studies (Abdul‐Rahman 2007; Bleyer 2005; Dogra 2002; Mokrzycki 2001; Mozafar 2013; PreCLOT Study 2006; Solomon 2010; Wilkieson 2011); unclear in 9/27 (33%) studies (Betjes 2004; Campos 2011; Coli 2006; Hendrickx 2001; Lustig 2011; Moran 2012; Pervez 2002; Power 2009; Traynor 2001) and at high risk of bias in 10/27 (37%) studies (AZEPTIC Study 2011; Buturovic 1998; CHARTS Study 2008; Corbett 2013; Filiopoulos 2011; HEALTHY‐CATH Study 2009; Hryszko 2013; Kaneko 2004; Malo 2010; Nori 2006).
Blinding of outcome assessors (detection bias) was judged to be of low risk in 3/27 (11%) studies (Dogra 2002; Solomon 2010; Wilkieson 2011), unclear in 15/27 (56%) studies (Abdul‐Rahman 2007; Betjes 2004; Bleyer 2005; Campos 2011; Coli 2006; Filiopoulos 2011; PreCLOT Study 2006; Hendrickx 2001; Lustig 2011; Mozafar 2013; Mokrzycki 2001; Nori 2006; Pervez 2002; Power 2009; Traynor 2001); and of high risk in 9/27 (33%) studies (Buturovic 1998; Kaneko 2004; CHARTS Study 2008; AZEPTIC Study 2011; Malo 2010; Moran 2012; HEALTHY‐CATH Study 2009; Corbett 2013; Hryszko 2013).
Incomplete outcome data
Incomplete data was judged to be of low risk in 18/27 (67%) studies (Betjes 2004; Campos 2011; Bleyer 2005; Coli 2006; Dogra 2002; PreCLOT Study 2006; CHARTS Study 2008; AZEPTIC Study 2011; Malo 2010; Mokrzycki 2001; Moran 2012; Nori 2006; Pervez 2002; Power 2009; Solomon 2010;Wilkieson 2011; HEALTHY‐CATH Study 2009; Mozafar 2013); and unclear in 3/27 (11%) studies (Corbett 2013; Lustig 2011; Filiopoulos 2011); and of high risk in 6/27 (22%) studies (Abdul‐Rahman 2007;Buturovic 1998; Hendrickx 2001; Kaneko 2004; Hryszko 2013; Traynor 2001).
Selective reporting
Low risk for selective reporting was defined as report with at least one catheter malfunction type outcome and safety outcome (bleeding), which was judged in 7/27 (26%) studies (PreCLOT Study 2006; CHARTS Study 2008; Mokrzycki 2001; Power 2009; Wilkieson 2011;Mozafar 2013; HEALTHY‐CATH Study 2009); unclear risk in 2/27 (7%) studies (Corbett 2013; Lustig 2011); and of high risk in 18/27 (67%) studies (Abdul‐Rahman 2007; Betjes 2004; Bleyer 2005; Buturovic 1998; Coli 2006; Campos 2011; AZEPTIC Study 2011; Malo 2010; Hryszko 2013; Dogra 2002; Moran 2012; Nori 2006; Pervez 2002; Solomon 2010; Filiopoulos 2011; Hendrickx 2001; Kaneko 2004; Traynor 2001).
Other potential sources of bias
Intention‐to‐treat analysis
Intention‐to‐treat analysis was used in 8/27 (30%) studies (Dogra 2002; Filiopoulos 2011; AZEPTIC Study 2011; Moran 2012; Power 2009; Solomon 2010; Wilkieson 2011; HEALTHY‐CATH Study 2009), and was not reported in 19/27 (70%).
Funding
Four studies were assessed as being at high risk of bias (AZEPTIC Study 2011; Bleyer 2005; Malo 2010; Mokrzycki 2001); 10 studies were judged to be at low risk of bias (Campos 2011; HEALTHY‐CATH Study 2009; Hryszko 2013; Moran 2012; Nori 2006; Pervez 2002; Power 2009; PreCLOT Study 2006; Solomon 2010; Wilkieson 2011), and bias was unclear in 13 studies (Abdul‐Rahman 2007; Betjes 2004; Buturovic 1998; CHARTS Study 2008; Coli 2006; Corbett 2013; Dogra 2002; Filiopoulos 2011; Hendrickx 2001; Kaneko 2004; Lustig 2011; Mozafar 2013; Traynor 2001).
Effects of interventions
Prevention of catheter malfunction
We were able to assess the incidence of catheter malfunction in 16/27 studies (1490 patients) with a median follow‐up of six months. There were 159 catheter malfunction events in 753 participants in the intervention group, and 199 events in 737 participants in the control group. In addition, catheter malfunction events were reported in different ways. Four studies assessed catheter loss, five studies assessed thrombosis, and one study assessed requirement for intervention to maintain catheter function (Table 1). There was no effect for alternative anticoagulant locking solutions (Figure 4 (9 studies, 908 patients): RR 0.96, 95% CI 0.74 to 1.26), systemic warfarin (Figure 4 (6 studies, 460 patients): RR 0.59, 95% CI 0.28 to 1.23), and low or no dose heparin (Figure 4 (2 studies, 123 patients): RR 0.90, 95% CI 0.10 to 8.31).

Catheter malfunction
| Study Definition of | Overall malfunction | Sub‐classification of catheter malfunction events | |||
| Loss due to | Catheter duration | Interventions | Venous occlusion | ||
| Alternative anticoagulant locking solutions | |||||
| BFR < 200 mL/min for non‐tunnelled and 250 mL/min for tunnelled | Int: 20/92 Cont: 14/95 | Int: 20/92 Cont: 14/95 | Not reported | Not reported | Not reported |
| BFR < 250 mL/min | Int: 13/32 Cont: 12/39 | Int: 25% Cont: 17.2% | Int: 55 days Cont: 90 days | Not reported | Not reported |
| BFR < 200 mL/min | Int: 13/42 Cont: 16/37 | Not reported | Not reported | Not reported | Not reported |
| BFR < 250 mL/min | Int: 9/59 Cont: 11/60 | Not reported | Not reported | Not reported | Not reported |
| Catheter removal due to flow difficulties | Not reported | Not reported | Not reported | Not reported | Not reported |
| BFR < 200 mL/min | Int: 5/10 Cont: 5/9 | Not reported | Not reported | Not reported | Int: 105 non‐occlusive clots Cont: 44 non‐occlusive clots |
| BFR < 250 mL/min | Int: 4/14 Cont: 6/19 | Not reported | Not reported | Not reported | Not reported |
| BFR < 200 mL/min | Int: 18/110 Cont: 36/115 | Not reported | Not reported | Int: 0/110 Cont: 1/115 | Not reported |
| Catheter loss due to occlusion | Int: 8/53 Cont: 3/54 | Int: 8/53 Cont: 3/54 | Not reported | Not reported | Int: 8/53 Cont: 3/54 |
| Systematic anticoagulants | |||||
| Catheter thrombosis | Int: 4/20 Cont: 9/19 | Not reported | Int: 75% survival at 12 months Cont: 36.8% survival at 12 months | Not reported | Int: 4/20 Cont: 9/19 |
| BFR < 300 mL/min | Int: 10/81 Cont: 33/63 | Not reported | Not reported | Not reported | Not reported |
| BFR < 300 mL/min | Int: 8/41 Cont: 8/44 | Int: 8/41 Cont: 8/44 | Not reported | Not reported | Nor reported |
| BFR < 250 mL/min | Int: 1/10 Cont: 1/8 | Not reported | Int: 188 days Cont: 356 days | Not reported | Not reported |
| BFR < 150 mL/min | Int: 8/41 Cont: 8/44 | Not reported | Not reported | Not reported | Not reported |
| No or low dose heparin locking solution | |||||
| Catheter thrombosis | Int: 0/37 Cont: 0/38 | Not reported | Not reported | Not reported | Int: 0/37 Cont: 0/38 |
| Catheter thrombosis or BFR < 140 mL/min | Int: 1/26 Cont: 1/22 | Not reported | Not reported | Not reported | Int: 1/26 Cont: 1/22 |
BFR ‐ blood flow rate; Cont ‐ control; Int ‐ intervention
Subgroup analysis of the impact of individual locking solution agents demonstrated that only rt‐PA was associated with a reduction of catheter malfunction (Figure 5 (1 study, 225 patients): RR 0.58, 95% CI 0.37 to 0.91). Citrate locking solutions of concentrations ranging from 4% to 46.7% did not significantly reduce catheter malfunction (Figure 5 (6 studies, 447 patients): RR 1.14, 95% CI 0.76 to 1.69), regardless of use in isolation or in conjunction with anti‐microbial solutions including gentamicin, taurolidine or methylene blue (regression coefficient for adjuvant antibiotics compared with none: 0.455, P = 0.167). High citrate concentration was not superior to low concentration (regression coefficient for citrate concentration 0.143, P = 0.605).

Catheter malfunction (subgroup analysis)
Among the studies of systemic anticoagulation, there was no suggestion of a dose related effect of warfarin on prevention of catheter malfunction although only five eligible studies were identified (regression coefficient for warfarin dose ‐0.992, P = 0.108). The dosage of warfarin and the target INRs in systemic anticoagulants studies was summarised in Table 2.
| Study | Number | Intervention arm | Control arm | Background care |
| 58 | Variable dose warfarin Target INR 1.5 to 2 | Placebo | Tinzaparin 40 to 50 IU/kg | |
| 144 | Variable dose warfarin Target INR 1.8 to 2.5 | Warfarin after catheter malfunction | Ticlopidine 250 mg/d | |
| 85 | Fixed dose warfarin 1 mg/d | Placebo | Heparin 5000 U/mL | |
| 18 | Fixed dose warfarin 1 mg/d | Placebo | Not reported | |
| 174 | Variable dose warfarin Target INR 1.5 to 1.9 | Placebo | Heparin 1000 to 10,000 U/mL |
INR ‐ international normalised ratio
Sensitivity analysis demonstrated the impact of citrate solutions remained non‐significant when a single study of citrate and taurolidine (Solomon 2010) was excluded (RR 0.85, 95% CI 0.61 to 1.18). Use of warfarin demonstrated a 42% reduction in the incidence of catheter malfunction, however, high heterogeneity among the five warfarin studies exist (I² = 77.6%, P = 0.001).
Prevention of catheter‐related bacteraemia
Catheter‐related bacteraemia was assessed in 16 studies (2214 patients, median follow‐up 4 months). The incidence of catheter‐related bacteraemia was reported as the rate per 1000 catheter days in 10 studies, the rate per patient in 14 studies and both in 8 studies. A significant reduction on the rate of catheter‐related bacteraemia was found from alternative anticoagulant locking solutions (Figure 6: RR 0.46, 95% CI 0.32 to 0.66), but not from systemic warfarin (Figure 7: RR 2.41, 95% CI 0.89 to 6.55).

Catheter‐related bacteraemia

Catheter‐related bacteraemia (subgroup analysis)
Subgroup analysis showed a reduction in the rate of catheter‐related bacteraemia by all the individual classes of alternative anticoagulant locking solutions (Figure 7) (citrate locking solutions: RR 0.49, 95% CI 0.36 to 0.68; antibiotic locking solutions: RR 0.27, 95% CI 0.11 to 0.70; rt‐PA locking solutions RR 0.35, 95% CI 0.13 to 0.93) except for ethanol locks (RR 0.33, 95% CI 0.03 to 4.05). The impact on catheter‐related bacteraemia was not affected by the addition or otherwise of antimicrobial solutions to citrate locking solution (P = 0.387).
Prevention of exit site infection
Exit site infection was assessed in eight studies (1199 participants, median follow‐up 4.4 months). The incidence of exit site infection was reported as the rate per patient in six studies and the rate per 1000 catheter days in two studies. No significant difference in the rate of exit site infection was found from alternative anticoagulant locking solutions (RR 0.95, 95% CI 0.58 to 1.58), nor from systemic warfarin (RR 0.79, 95% CI 0.29 to 2.16) (Figure 8).

Exit site infection
Subgroup analysis did not show a reduction in the rate of exit site infection by all the individual classes of alternative anticoagulant locking solutions (citrate locking solutions: RR 0.95, 95% CI 0.60 to 1.51; antibiotic locking solutions: RR 0.91, 95% CI 0.37 to 2.25; ethanol locks: RR 2.89, 95% CI 0.12 to 67.73) (Figure 9).

Exit site infection (subgroup analysis)
All‐cause mortality
All‐cause mortality was reported in 11 studies (1828 participants, median follow‐up 4.4 months) assessing alternative locking solutions and systemic warfarin. No treatment class improved mortality: alternative anticoagulant locking solutions (Analysis 1.1.1 (8 studies, 1425 participants): RR 0.88, 95% CI 0.54 to 1.43; I2 = 0%); warfarin Analysis 1.1.2 (3 studies, 403 participants): RR 0.78, 95% CI 0.37 to 1.65; I2 = 0%).
No individual alternative anticoagulant locking solutions showed survival benefits compared with standard heparin: citrate (Analysis 1.2.1 (6 studies, 1151 participants): RR 0.89, 95% CI 0.52 to 1.51; I2 = 1%); rt‐PA (Analysis 1.2.2 (1 study, 225 participants): RR 0.63, 95% CI 0.15 to 2.56); ethanol (Analysis 1.2.3 (1 study, 49 participants): RR 2.88, 95% CI 0.12 to 67.53).
Safety profile
The safety outcomes of the interventions were reported less often than efficacy outcomes. Only eight studies reported bleeding events, among which data were able to be pooled in seven studies (849 participants, median follow‐up 3.7 months) meaning these analyses maybe affected by bias associated with incomplete outcome and selective reporting. Two studies reported major bleeding only, four reported total bleeding only, and two studies reported both.
There was no significant differences in total bleeding events for alternative anticoagulant locking solutions (Analysis 1.3.1 (3 studies, 335 participants): RR 0.69, 95% CI 0.47 to 1.01; I2 = 0%), and systemic agents (Analysis 1.3.2 (3 studies, 439 participants): RR 1.30, 95% CI 0.93 to 1.83; I2 = 0%). Low dose heparin reduced bleeding events by 55% although this result was based on a single study (Analysis 1.3.3 (1 study, 75 participants): RR 0.45, 95% CI 0.21 to 0.96). Subgroup analysis did not show the effect on bleeding events by any individual classes of alternative anticoagulant locking solutions: citrate locking solutions (Analysis 1.4.1 (2 studies, 286 participants): RR 0.70, 95% CI 0.47 to 1.02; I2 = 0%); ethanol (Analysis 1.4.2 (1 study, 49 participants): RR 0.32, 95% CI 0.01 to 7.50); warfarin (Analysis 1.4.3 (2 studies, 259 participants): RR 1.43, 95% CI 0.86 to 2.39; I2 = 0%); aspirin (Analysis 1.4.4 (1 study, 180 participants): RR 1.21, 95% CI 0.77 to 1.90); rt‐PA (Analysis 1.4.5 (1 study, 225 participants): RR 0.85, 95% CI 0.43 to 1.68).
Five studies (633 participants) reported other adverse events, including two citrate studies (311 participants), one rt‐PA study (225 participants), one ethanol study (49 participants), and one low or no dose heparin study (48 participants). There were 53 adverse events in 335 participants in the intervention group, while 39 adverse events occurred in 298 participants in the control group. Participants receiving citrate locking solutions experienced more adverse events, including thrombocytopenia (1 study), intermittent nonspecific dizziness (1 study), metallic taste (1 study) and facial and/or digital paraesthesia (1 study).
Requirement for thrombolytic agents
The requirement for rescue thrombolytic agents due to catheter malfunction was reported in eight studies, among which data were able to be meta‐analysed in seven studies (1168 participants, median follow‐up 6 months). All of the studies assessed alternative anticoagulant locking solutions. Overall, no significant effect on the requirement for thrombolytic agents was observed (Figure 10: RR 0.87, 95% CI 0.55 to 1.38). With regards to individual alternative anticoagulant locking solutions, rt‐PA and LMWH reduced the use of thrombolytic agents by 79% and 47%, respectively (LMWH (1 study, 42 participants): RR 0.53, 95% CI 0.32 to 0.86; rt‐PA (1 study, 225 participants): RR 0.21, 95% CI 0.07 to 0.59), while use of citrates did not show a significant impact on the thrombolytic use (5 studies, 877 participants: RR 1.18, 95% CI 0.95 to 1.47) (Figure 11).
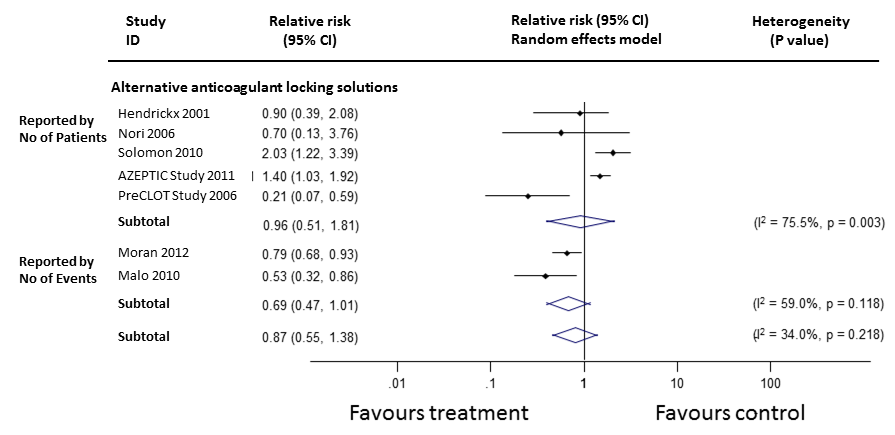
Requirement for thrombolytic agents

Requirement for thrombolytic agents (subgroup analysis)
Discussion
Summary of main results
This systematic review of RCTs assessing the relative effects of different strategies for prevention of catheter malfunction in adults with ESKD identified 27 relatively small studies, with an average of 75 participants and 6 months follow up. Newer approaches, including alternative anticoagulant locking solutions, systemic agents and low or no dose heparin, did not affect rates of catheter malfunction compared with usual care. The only individual agent demonstrating statistically significant improvement for catheter malfunction compared with conventional care was rt‐PA‐based locking solution in a result that was based on a single study. No significant effect on all‐cause mortality was observed for individual classes of anticoagulants. Use of rt‐PA and LMWH locking solutions reduced the use of thrombolytic agents but the results were based on a single study. The relative effectiveness of other interventions remains inconclusive and, of concern, the reporting of safety outcomes was infrequent. Specifically, bleeding rates were only reported in 8 studies, despite the use of anticoagulants in a patient population with recognised bleeding risk.
Citrate locking solutions, antibiotic locking solutions and rt‐PA locking solutions were associated with a significant reduction in catheter‐related bacteraemia. However, the additional use of antibiotic locks to citrate did not have an impact on incidence of catheter‐related bacteraemia. The effectiveness of an alternative mechanism for preventing infectious complications in haemodialysis patients with CVC, topical interventional strategies, was the subject of a Cochrane review last updated in 2010 (McCann 2010). Interventions that assessed included prophylactic topical antimicrobials, topical antiseptics, medicated and non‐medicated dressings. The review found mupirocin ointment appears effective in reducing the risk of catheter‐related bacteraemia, while the effect of povidone‐iodine ointment, polysporin ointment, topical honey and types of dressing on catheter‐related bacteraemia remain uncertain.
Among individual alternative anticoagulant agents, rt‐PA was effective in reducing catheter malfunction in a single study of Canadian centres for the prevention of catheter malfunction at no increased bleeding risk. These results are promising but, as a single study of 225 participants, require replication in other settings (PreCLOT Study 2006). The author’s cost‐effectiveness analysis found the incremental cost of rt‐PA was CAD 13,956 per episode of prevented catheter‐related bacteraemia in the setting where the rate of catheter‐related bacteraemia in the control group was 13%. Differences of background bacteraemia prevalence will obviously influence the cost‐effectiveness of the intervention.
Citrate solutions reduced catheter‐related bacteraemia, but there was no clear evidence they reduced catheter malfunction. Based on a single study, citrate did not show a reduction in bleeding events. There was a suggestion that patients receiving citrate locking solutions experienced more adverse events, although these were only reported in two studies with 311 participants with one of them showing statistically significant result. Cardiac arrhythmia rates were not reported in any despite safety concerns that high concentration citrate may promote the induction of cardiac arrhythmia via systemic hypocalcaemia. Future studies should of citrate solutions are warranted and should address the uncertainty for both efficacy and safety outcomes.
Ethanol locking solutions were not inferior to heparin locks in prevention of catheter malfunction in a single reported study. Ethanol appears to possess intrinsic anticoagulant activity and is therefore effective at restoring catheter patency (Pennington 1987). In addition, in vitro studies suggested that ethanol also has broad‐spectrum antimicrobial activity, which seems to be based on denaturization rather than a specific molecular target (Sherertz 2006). Our review did not show a benefit for ethanol on prevention of catheter‐related bacteraemia based on only one small study; future large studies should test it as a promising alternative locking solution to heparin.
Our analysis indicates it is possible low or no dose heparin may not be inferior to heparin locking in preventing catheter malfunction based on two small studies. The uptake of heparin locking solutions for temporary dialysis access catheters appears to have developed as the default without RCT evidence. The priority that should be assigned to future research of lower dosing heparin is unclear given the scarcity of information on the rates of adverse effects associated with current heparin usage.
The broad inclusiveness of interventions included in our review meant we anticipated heterogeneity of effects and hence planned to use random effects models. However we also found heterogeneity in the reporting methodologies. Individual studies variously reported numbers of patients experiencing events – the information conventionally used in meta‐analyses ‐ or by repeated event rates. Relative risks generated by the latter can be utilized in meta‐analysis but the narrower CI generated by repeated events mean that these studies have greater impact in the random effects models than if they had reported by individual patients. None of these studies made statistical adjustments for the potential lack of independence of repeated events in a given individual. We have developed a methodology for analysis in these situations.
Overall completeness and applicability of evidence
This is the first systematic review assessing all RCTs investigating anticoagulants for the prevention of catheter malfunction in adults undergoing haemodialysis. Twenty‐seven studies with a large variety of interventions involving six different catheter locking solutions and systemic agents were included in our review. The relative net benefit of anticoagulant therapies over conventional care for prevention of catheter malfunction remains uncertain. However, a significant reduction in catheter‐related bacteraemia was observed for citrate locking solutions, antibiotic locking solutions and rt‐PA locking solutions. Currently there is no adequate information on locking solutions collected in the Australian, USA or UK registries. The inclusion of locking solution type is warranted in large registries to facilitate the monitoring of bleeding rates and infrequent events such as cardiac arrhythmias.
Quality of the evidence
The studies were predominantly of low quality, and underpowered with an average participant number of 75 and study duration of 6 months. The interpretation of the study evidence was further limited by the variation in tested interventions and outcome reporting differences.
Potential biases in the review process
One limitation of this study was the reliance on the published data. Therefore, incomplete data reporting (attribution bias) in some studies could lead to potential loss of statistical power. In addition, assessment of potential net benefit, including efficacy and harms, was prevented by the limited safety reporting, notably of bleeding events.
Agreements and disagreements with other studies or reviews
The only agent that appeared to have prophylactic effect in our review is rt‐PA locking solution but the result was only based on a single study. This finding is consistent with those of a current systematic review (Firwana 2011) assessing three RCTs comparing rt‐PA versus heparin for locking dialysis catheters. Only one of these was eligible for our study with the other two being excluded because they were conducted in a paediatric population (Gittins 2007) and another because it included patients with both acute kidney injury and chronic kidney disease Schenk 2000.
Our review also found that use of citrate locking solutions significantly reduced incidence of catheter‐related bacteraemia but not for catheter malfunction or requirement for thrombolytic agents in 14 studies. The review is consistent in the main with a previous systematic review (Zhao 2014) which included 13 studies. We found citrate locking solutions had no impact on bleeding events (one eligible study), while Zhao 2014 identified reduced bleeding with citrate locking solutions which included two studies with the results driven by inclusion of a study assessing patients with both acute kidney injury and chronic kidney disease (Weijmer 2005).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Catheter malfunction (subgroup analysis)

Catheter‐related bacteraemia

Catheter‐related bacteraemia (subgroup analysis)

Exit site infection (subgroup analysis)

Requirement for thrombolytic agents

Requirement for thrombolytic agents (subgroup analysis)
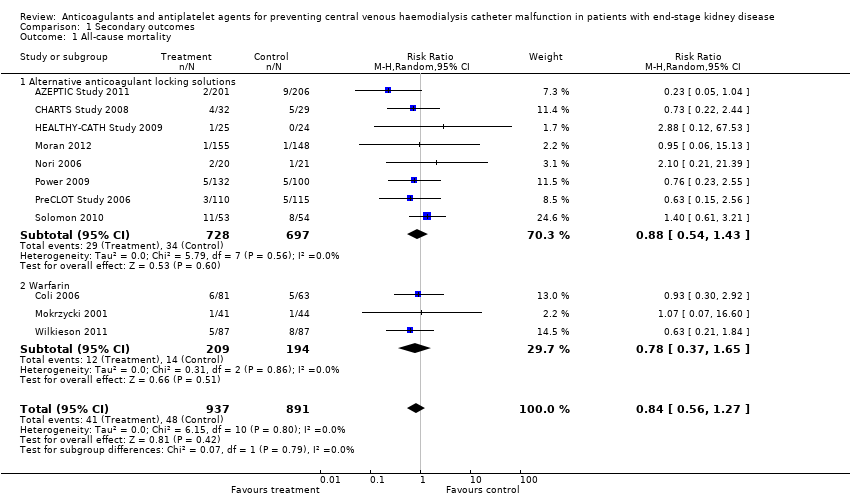
Comparison 1 Secondary outcomes, Outcome 1 All‐cause mortality.

Comparison 1 Secondary outcomes, Outcome 2 Subgroup analysis of all‐cause mortality in alternative anticoagulant locking solutions.

Comparison 1 Secondary outcomes, Outcome 3 Total bleeding events.

Comparison 1 Secondary outcomes, Outcome 4 Subgroup analysis of total bleeding events in alternative anticoagulant locking solutions.
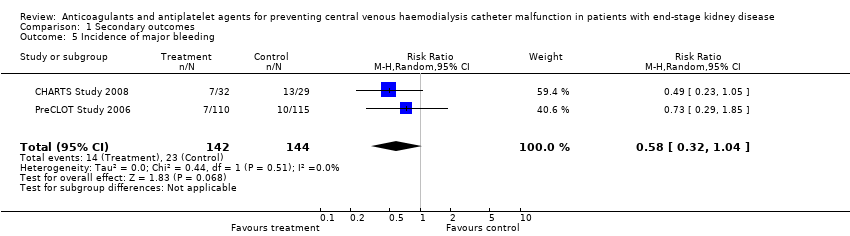
Comparison 1 Secondary outcomes, Outcome 5 Incidence of major bleeding.

Comparison 1 Secondary outcomes, Outcome 6 Incidence of minor bleeding.
| Study Definition of | Overall malfunction | Sub‐classification of catheter malfunction events | |||
| Loss due to | Catheter duration | Interventions | Venous occlusion | ||
| Alternative anticoagulant locking solutions | |||||
| BFR < 200 mL/min for non‐tunnelled and 250 mL/min for tunnelled | Int: 20/92 Cont: 14/95 | Int: 20/92 Cont: 14/95 | Not reported | Not reported | Not reported |
| BFR < 250 mL/min | Int: 13/32 Cont: 12/39 | Int: 25% Cont: 17.2% | Int: 55 days Cont: 90 days | Not reported | Not reported |
| BFR < 200 mL/min | Int: 13/42 Cont: 16/37 | Not reported | Not reported | Not reported | Not reported |
| BFR < 250 mL/min | Int: 9/59 Cont: 11/60 | Not reported | Not reported | Not reported | Not reported |
| Catheter removal due to flow difficulties | Not reported | Not reported | Not reported | Not reported | Not reported |
| BFR < 200 mL/min | Int: 5/10 Cont: 5/9 | Not reported | Not reported | Not reported | Int: 105 non‐occlusive clots Cont: 44 non‐occlusive clots |
| BFR < 250 mL/min | Int: 4/14 Cont: 6/19 | Not reported | Not reported | Not reported | Not reported |
| BFR < 200 mL/min | Int: 18/110 Cont: 36/115 | Not reported | Not reported | Int: 0/110 Cont: 1/115 | Not reported |
| Catheter loss due to occlusion | Int: 8/53 Cont: 3/54 | Int: 8/53 Cont: 3/54 | Not reported | Not reported | Int: 8/53 Cont: 3/54 |
| Systematic anticoagulants | |||||
| Catheter thrombosis | Int: 4/20 Cont: 9/19 | Not reported | Int: 75% survival at 12 months Cont: 36.8% survival at 12 months | Not reported | Int: 4/20 Cont: 9/19 |
| BFR < 300 mL/min | Int: 10/81 Cont: 33/63 | Not reported | Not reported | Not reported | Not reported |
| BFR < 300 mL/min | Int: 8/41 Cont: 8/44 | Int: 8/41 Cont: 8/44 | Not reported | Not reported | Nor reported |
| BFR < 250 mL/min | Int: 1/10 Cont: 1/8 | Not reported | Int: 188 days Cont: 356 days | Not reported | Not reported |
| BFR < 150 mL/min | Int: 8/41 Cont: 8/44 | Not reported | Not reported | Not reported | Not reported |
| No or low dose heparin locking solution | |||||
| Catheter thrombosis | Int: 0/37 Cont: 0/38 | Not reported | Not reported | Not reported | Int: 0/37 Cont: 0/38 |
| Catheter thrombosis or BFR < 140 mL/min | Int: 1/26 Cont: 1/22 | Not reported | Not reported | Not reported | Int: 1/26 Cont: 1/22 |
| BFR ‐ blood flow rate; Cont ‐ control; Int ‐ intervention | |||||
| Study | Number | Intervention arm | Control arm | Background care |
| 58 | Variable dose warfarin Target INR 1.5 to 2 | Placebo | Tinzaparin 40 to 50 IU/kg | |
| 144 | Variable dose warfarin Target INR 1.8 to 2.5 | Warfarin after catheter malfunction | Ticlopidine 250 mg/d | |
| 85 | Fixed dose warfarin 1 mg/d | Placebo | Heparin 5000 U/mL | |
| 18 | Fixed dose warfarin 1 mg/d | Placebo | Not reported | |
| 174 | Variable dose warfarin Target INR 1.5 to 1.9 | Placebo | Heparin 1000 to 10,000 U/mL | |
| INR ‐ international normalised ratio | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 11 | 1828 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.56, 1.27] |
| 1.1 Alternative anticoagulant locking solutions | 8 | 1425 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.54, 1.43] |
| 1.2 Warfarin | 3 | 403 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.37, 1.65] |
| 2 Subgroup analysis of all‐cause mortality in alternative anticoagulant locking solutions Show forest plot | 8 | 1425 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.54, 1.43] |
| 2.1 Citrate | 6 | 1151 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.52, 1.51] |
| 2.2 rt‐PA | 1 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.15, 2.56] |
| 2.3 Ethanol | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 2.88 [0.12, 67.53] |
| 3 Total bleeding events Show forest plot | 7 | 849 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.61, 1.25] |
| 3.1 Alternative anticoagulant locking solutions | 3 | 335 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.47, 1.01] |
| 3.2 Systemic agents | 3 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.93, 1.83] |
| 3.3 Low/no dose heparin | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.21, 0.96] |
| 4 Subgroup analysis of total bleeding events in alternative anticoagulant locking solutions Show forest plot | 7 | 1074 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.65, 1.19] |
| 4.1 Citrates | 2 | 286 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.47, 1.02] |
| 4.2 Ethanol | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.50] |
| 4.3 Warfarin | 2 | 259 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.86, 2.39] |
| 4.4 Aspirin | 1 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.77, 1.90] |
| 4.5 rt‐PA | 1 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.43, 1.68] |
| 4.6 Low/no dose heparin | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.21, 0.96] |
| 5 Incidence of major bleeding Show forest plot | 2 | 286 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.32, 1.04] |
| 6 Incidence of minor bleeding Show forest plot | 2 | 286 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.44, 1.50] |

