Transarterial (chemo)embolisation versus no intervention or placebo for liver metastases
Information
- DOI:
- https://doi.org/10.1002/14651858.CD009498.pub4Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 12 March 2020see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Hepato-Biliary Group
- Copyright:
-
- Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
JK developed the concept for the project
RR and JK formulated the search strategy and carried out searches
MJS, DS, JWM, and MP searched clinical trials registers
MJS, DS, MMB, RR, JWM, MP, and RW performed the title & abstract screening
MJS, DS, MMB, RR, JWM, MP, and RW performed the full‐text screening
MJS, MMB, and RR performed data extraction
MJS, MMB, RR, and RW performed the analyses
MJS, RR, DS, and MB assessed the overall certainty of the evidence
MJS, DS, MMB, RR, JWM, MP, RW, and JK prepared the text of the review
All review authors approved the review for publication.
Sources of support
Internal sources
-
Kleijnen Systematic Reviews Ltd (KSR), UK.
-
KSR funded the updating of the review and producing Cochrane Reviews.
-
External sources
-
The Dutch Health Care Insurance Board (currently ZIN – The National Health Care Institute (Zorginstituut Nederland)), Netherlands.
This systematic review was funded by the Dutch Health Care Insurance Board (CVZ). CVZ commissioned a systematic review of the effectiveness of non‐surgical ablation methods for liver metastases
Declarations of interest
MJS: none
DS: none
MMB: works as a freelancer for a company that completes works for a number of pharmaceutical companies in an unrelated indication; she was unaware of any conflict of interest.
RR, RW, and JK: all declare that the company they work for or own completes work for a number of pharmaceutical companies in unrelated indications, and that they were unaware of any conflict of interest.
JWM: none
MP: received speaking honoraria and travel grants from Nestle, Nutricia, Roche, and Johnson & Johnson. He is unaware of any conflict of interest
Acknowledgements
We would like to thank Dimitrinka Nikolova for help and Christian Gluud for advice on preparing this systematic review.
Peer reviewers of the protocol of the review: Thomas Karlas, Germany, and Hans Christian Spangenberg, Germany
Peer reviewer of the previous update of the review: Kang Mo Kim, South Korea; Timothy Price, Australia; Luit Peninga, Denmark
Contact editor: Emil Eik Nielsen, Denmark; Omar Abdel‐Rahman, Egypt
Sign‐off editor: Christian Gluud, Denmark
Cochrane Abdomen and Endocine Network Associate Editor: Liz Bickerdike, UK
Cochrane Review Group funding acknowledgement: the Danish State is the largest single funder of the Cochrane Hepato‐Biliary Group through its investment in the Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen University Hospital, Denmark.
Disclaimer: the views and opinions expressed in this review are those of the authors and do not necessarily reflect those of the Danish State or the Copenhagen Trial Unit.
Version history
| Published | Title | Stage | Authors | Version |
| 2020 Mar 12 | Transarterial (chemo)embolisation versus no intervention or placebo for liver metastases | Review | Mateusz J Swierz, Dawid Storman, Robert P Riemsma, Robert Wolff, Jerzy W Mitus, Michal Pedziwiatr, Jos Kleijnen, Malgorzata M Bala | |
| 2013 Apr 30 | Transarterial (chemo)embolisation versus no intervention or placebo intervention for liver metastases | Review | Robert P Riemsma, Malgorzata M Bala, Robert Wolff, Jos Kleijnen | |
| 2012 Sep 12 | Transarterial (chemo)embolisation versus no intervention or placebo intervention for liver metastases | Review | Robert P Riemsma, Malgorzata M Bala, Robert Wolff, Jos Kleijnen | |
| 2011 Dec 07 | Transarterial (chemo)embolisation for liver metastases | Protocol | Robert P Riemsma, Malgorzata M Bala, Robert Wolff, Jos Kleijnen | |
Differences between protocol and review
-
Amended inclusion criteria for data on harm, from: 'Quasi‐randomised and observational studies that will come up with the search, will be considered only for the report of data on harm' to 'Relevant quasi‐randomised and other controlled studies that were identified in the searches were only considered for reporting of data on harm'
-
We removed the domains 'baseline imbalance' and 'early stopping of trials'. The argument for not considering baseline imbalance is that it may occur due to random error (play of chance), and that such random error is likely to be levelled out by conducting a meta‐analysis of several trials. The argument for not considering early stopping is that such trials – although they may overestimate intervention effects – are likely to be counterbalanced by trials finding no significant difference. By solely excluding trials that are stopped early, one would bias the meta‐analysis towards a neutral effect (hbg.cochrane.org/information‐authors)
-
We removed the 'for profit bias' as a domain in the 'risk of bias' section
-
In the "Secondary outcomes" section, we changed the wording from 'Failure or recurrence of tumours' to 'Failure to clear liver metastases or recurrence of liver metastases' and from 'Quality of life' to 'Health‐related quality of life'
-
We changed 'All adverse events and complications, separately and in total' from a primary outcome to 'Any adverse events or complications' as a secondary outcome
-
We extracted the data from the included study again, onto a separate extraction sheet, and then compared them with the previous extraction; so, this has been independently done by two review authors (originally data extraction was done by one review author (MMB) and was checked by a second review author (RR))
-
In the protocol, we planned to calculate pooled estimates using the random‐effects model (DerSimonian 1986) and the fixed‐effect model (Mantel 1959; Greenland 1985). We planned to present both results if there were discrepancies in the results. In this review, we used the fixed‐effect model as a sensitivity analysis.
-
We added 'Unit of analysis issues' and 'Assessment of reporting biases' subheadings and provided text
-
We were unable to perform all planned analyses due to lack of data
-
In comparison to the previous version of the review, we used the data from all three intervention groups in the included trial; i.e. including the comparison TACE versus no intervention
-
We constructed two additional 'Summary of findings' tables
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- *Colorectal Neoplasms;
- *Hepatic Artery;
- Antimetabolites, Antineoplastic [administration & dosage];
- Chemoembolization, Therapeutic [methods, mortality];
- Embolization, Therapeutic [*methods, mortality];
- Fluorouracil [administration & dosage];
- Infusions, Intra‐Arterial [methods];
- Liver Neoplasms [blood supply, *secondary, *therapy];
- Microspheres;
- Randomized Controlled Trials as Topic;
Medical Subject Headings Check Words
Female; Humans; Male;
PICOs

Flow chart for identification of included randomised trials for the original published review

Flow chart for identification of included randomised trials for the 2019 update

'Risk of bias' summary: review authors' judgements about each 'risk of bias' item for each included study

'Risk of bias' graph: review authors' judgements about each 'risk of bias' item presented as percentages across all included studies
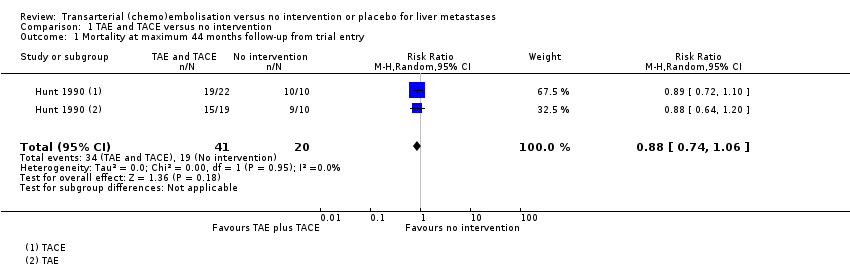
Comparison 1 TAE and TACE versus no intervention, Outcome 1 Mortality at maximum 44 months follow‐up from trial entry.

Comparison 1 TAE and TACE versus no intervention, Outcome 2 Evidence of extrahepatic disease.

Comparison 2 TAE versus no intervention or placebo, Outcome 1 Mortality at maximum 44 months follow‐up from trial entry.
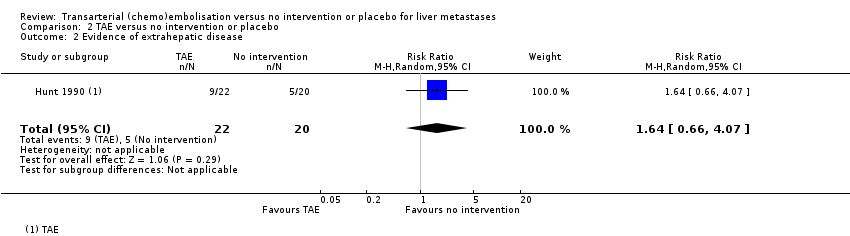
Comparison 2 TAE versus no intervention or placebo, Outcome 2 Evidence of extrahepatic disease.

Comparison 3 TACE versus no intervention or placebo, Outcome 1 Mortality at maximum 44 months follow‐up from trial entry.
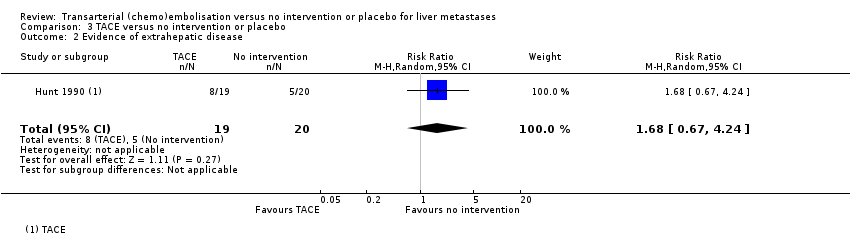
Comparison 3 TACE versus no intervention or placebo, Outcome 2 Evidence of extrahepatic disease.
| Transarterial embolisation and transarterial chemoembolisation compared with no intervention for liver metastases | ||||||
| Patient or population: people with liver metastases | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed riskwith no intervention | Corresponding risk with TAE plus TACE | |||||
| Mortality at 44 months from trial entry Number of participants | 950 per 1000 | 836 per 1000 | RR 0.88 | 61 (1 RCT) | ⊕⊝⊝⊝ | |
| Survival time Median (range) survival time after trial entry Survival time Median (range) survival time from diagnosis | The median (range) survival time after trial entry in the control group was 7.9 months (1 month to 26 months) | The median survival time after trial entry in the TAE group was 0.9 months lower and in the TACE group was 2.8 months higher. | ‐‐ | 61 | ⊕⊝⊝⊝ | Median survival after trial entry was 7.0 months (range 2 to 44) in the TAE group, 10.7 months (range 3 to 38) in the TACE group and 7.9 months (range 1 to 26) in the control group. Authors reported that the difference was not statistically significant. |
| The median (range) survival time after from diagnosis in the control group was 9.6 months (1 month to 27 months) | The median survival time from diagnosis in the TAE group was 0.9 months lower and in the TACE group was 3.4 months higher. | ‐‐ | 61 | ⊕⊝⊝⊝ | Median survival after diagnosis was 8.7 months (range 2 to 49 months) in the TAE group, 13.0 months (range 3 to 38 months) in the TACE group, and 9.6 months (range 1 to 27 months) in the control group. The trial authors reported the differences were not statistically significant. | |
| Failure to clear liver metastases or recurrence of liver metastases | Local recurrence was reported in 10 participants without any details about the group to which they had been allocated to. | ‐‐ | 61 | ⊕⊝⊝⊝ | Number of participants who developed evidence of extrahepatic disease was 9/22 participants in the TAE group, 8/19 participants in the TACE group, and 5/20 participants in the control group. | |
| Time to progression of liver metastases | Outcome not reported | ‐‐ | ||||
| Tumour response measures | Outcome not reported | ‐‐ | ||||
| Health‐related quality of life | Outcome not reported | ‐‐ | ||||
| Adverse events and complications | No adverse events reported | 82% of TAE recipients reported short‐term pain, nausea, vomiting, and pyrexia, which resolved with symptomatic treatment. All TACE recipients reported short‐term nausea with or without vomiting immediately after most of the treatment sessions, and short‐lived pain or discomfort. Single cases of local puncture site haematoma, wound infection, and deep vein thrombosis were reported. | ‐‐ | 61 | ⊕⊝⊝⊝ | |
| *The risk in the intervention group (corresponding risk and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; RCT: randomised clinical trial; OIS: optimal information size | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aDowngraded by two levels due to within‐study risk of bias: the trial did not describe sequence generation, allocation concealment, or blinding, and we had some concerns regarding selective outcome reporting. | ||||||
| Transarterial embolisation compared with no intervention for liver metastases | ||||||
| Patient or population: people with liver metastases | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk with no intervention | Corresponding risk with TAE | |||||
| Mortality at 44 months from trial entry Number of participants | 950 per 1000 | 865 per 1000 | RR 0.91 | 42 | ⊕⊝⊝⊝ | |
| Survival time Median (range) survival time after trial entry Survival time Median (range) survival time from diagnosis | The median (range) survival time after trial entry in the control group was 7.9 months (1 month to 26 months). | The median survival time after trial entry in the TAE group was 0.9 months lower. | ‐‐ | 42 | ⊕⊝⊝⊝ | Median survival from trial entry was 7.0 months (range 2 to 44 months) in the TAE group and 7.9 months (range 1 to 26 months) in the control group. The trial authors reported the differences were not statistically significant. |
| The median (range) survival time from diagnosis in the control group was 9.6 months (1 month to 27 months). | The median survival time from diagnosis in the TAE group was 0.9 months lower. | ‐‐ | 42 | ⊕⊝⊝⊝ | Median survival after diagnosis was 8.7 months (range 2 to 49 months) in the TAE group and 9.6 months (range 1 to 27 months) in the control group. The trial authors reported the differences were not statistically significant. | |
| Failure to clear liver metastases or recurrence of liver metastases | Local recurrence was reported in 10 participants without any details about the group to which they had been allocated to. | ‐‐ | 42 | ⊕⊝⊝⊝ | Number of participants who developed evidence of extrahepatic disease was 9/22 participants in the TAE group and 5/20 participants in the control group. | |
| Time to progression of liver metastases | Outcome not reported | ‐‐ | ||||
| Tumour response measures | Outcome not reported | ‐‐ | ||||
| Health‐related quality of life | Outcome not reported | ‐‐ | ||||
| Adverse events and complications | No adverse events reported | 18/22 (82%) of recipients reported short‐term pain, nausea, vomiting, and pyrexia, which resolved with symptomatic treatment. Single case of local puncture site haematoma | ‐‐ | 42 | ⊕⊝⊝⊝ | |
| *The risk in the intervention group (corresponding risk and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; RCT: randomised clinical trial; OIS: optimal information size | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aDowngraded by two levels due to within‐study risk of bias: the trial did not describe sequence generation, allocation concealment, or blinding, and we had some concerns regarding selective outcome reporting. | ||||||
| Transarterial chemoembolisation compared with no intervention for liver metastases | ||||||
| Patient or population: people with liver metastases | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk with no intervention | Corresponding risk with TACE | |||||
| Mortality at 44 months from trial entry Number of participants | 950 per 1000 | 789 per 1000 | RR 0.83 | 39 | ⊕⊝⊝⊝ | |
| Survival time Median (range) survival time after trial entry Survival time Median (range) survival time from diagnosis | The median (range) survival time after trial entry in the control group was 7.9 months (1 month to 26 months). | The median survival time after trial entry in the TACE group was 2.8 months higher. | ‐‐ | 39 | ⊕⊝⊝⊝ | Median survival from trial entry was 10.7 months (range 3 to 38 months) in the TACE group, and 7.9 months (range 1 to 26 months) in the control group. The trial authors reported the differences were not statistically significant |
| The median (range) survival time from diagnosis in the control group was 9.6 months (1 month to 27 months). | The median survival time from diagnosis in the TACE group was 3.4 months higher. | ‐‐ | 39 | ⊕⊝⊝⊝ | Median survival after diagnosis was 13.0 months (range 3 to 38 months) in the TACE group and 9.6 months (range 1 to 27 months) in the control group. The trial authors reported the differences were not statistically significant. | |
| Failure to clear liver metastases or recurrence of liver metastases | Local recurrence was reported in 10 participants without any details about the group to which they had been allocated to. | ‐‐ | 39 | ⊕⊝⊝⊝ | Number of participants who developed evidence of extrahepatic disease was 8/19 participants in the TACE group and 5/20 participants in the control group | |
| Time to progression of liver metastases | Outcome not reported | ‐‐ | ||||
| Tumour response measures | Outcome not reported | ‐‐ | ||||
| Health‐related quality of life | Outcome not reported | ‐‐ | ||||
| Adverse events and complications | No adverse events reported | All recipients reported short‐term nausea with or without vomiting immediately after most of the treatment sessions, and short‐lived pain or discomfort. Single cases of wound infection, and deep vein thrombosis. | ‐‐ | 39 | ⊕⊝⊝⊝ | |
| *The risk in the intervention group (corresponding risk and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; RCT: randomised clinical trial; OIS: optimal information size | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aDowngraded by two levels due to within‐study risk of bias: the trial did not describe sequence generation, allocation concealment, or blinding, and we had some concerns regarding selective outcome reporting. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at maximum 44 months follow‐up from trial entry Show forest plot | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.74, 1.06] |
| 2 Evidence of extrahepatic disease Show forest plot | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.70, 3.73] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at maximum 44 months follow‐up from trial entry Show forest plot | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.75, 1.10] |
| 2 Evidence of extrahepatic disease Show forest plot | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [0.66, 4.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at maximum 44 months follow‐up from trial entry Show forest plot | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.65, 1.07] |
| 2 Evidence of extrahepatic disease Show forest plot | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 1.68 [0.67, 4.24] |

