Transarterial (chemo)embolisation versus no intervention or placebo for liver metastases
Abstract
Background
The liver is affected by two of the most common groups of malignant tumours: primary liver tumours and liver metastases from colorectal carcinoma or other extrahepatic primary cancers. Liver metastases are significantly more common than primary liver cancer, and long‐term survival rate after radical surgical treatment is approximately 50%. However, R0 resection (resection for cure) is not feasible in the majority of people; therefore, other treatments have to be considered. One possible option is based on the concept that the blood supply to hepatic tumours originates predominantly from the hepatic artery. Transarterial chemoembolisation (TACE) of the hepatic artery can be achieved by administering a chemotherapeutic drug followed by vascular occlusive agents, and can lead to selective necrosis of the liver tumour while it may leave normal parenchyma virtually unaffected. This can also be performed without chemotherapy, which is called bland transarterial embolisation (TAE).
Objectives
To assess the beneficial and harmful effects of TAE or TACE compared with no intervention or placebo in people with liver metastases.
Search methods
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register, CENTRAL, MEDLINE, Embase, and four more databases (December 2019). We also searched two trials registers and the US Food and Drug Administration database (September 2019).
Selection criteria
Randomised clinical trials assessing beneficial and harmful effects of TAE or TACE compared with no intervention or placebo for liver metastases.
Data collection and analysis
We followed standard Cochrane methodological procedures. We extracted information on participant characteristics, interventions, study outcomes, study design, and trial methods. Two review authors independently extracted data and assessed risk of bias. We assessed the certainty of evidence with GRADE. We resolved disagreements by discussion.
Main results
We included one randomised clinical trial with 61 participants (43 male and 18 female) with colorectal cancer with liver metastases: 22 received transarterial embolisation (TAE; hepatic artery embolisation), 19 received transarterial chemoembolisation (TACE; 5‐fluorouracil hepatic artery infusion chemotherapy with degradable microspheres), and 20 received 'no active therapeutic intervention' as a control. Most tumours were synchronous, unresectable metastases involving up to 75% of the liver. Participants were followed for a minimum of seven months. The trial was at high risk of bias. Very‐low‐certainty evidence found inconclusive results for mortality at 44 months between the TAE and TACE versus no intervention groups (risk ratio (RR) 0.88, 95% confidence interval (CI) 0.74 to 1.06; 61 participants). Local recurrence was reported in 10 participants without any details about the group allocation. Very‐low‐certainty evidence found little or no difference in mortality between the TAE and no intervention groups (RR 0.91, 95% CI 0.75 to 1.10; 42 participants). Median survival was 7 months from trial entry (range 2 to 44 months) in the TAE group and 7.9 months (range 1 to 26 months) in the control group, and 8.7 months after diagnosis (range 2 to 49 months) in the TAE group and 9.6 months (range 1 to 27 months) in the control group. The trial authors reported the differences were not statistically significant. There were no reported side effects in the control group. In the TAE group, 18 participants experienced short‐term symptoms of 'post‐embolisation syndrome', which were relieved with symptomatic treatment; one participant also had a local puncture site haematoma. Very‐low‐certainty evidence found little or no difference in mortality between the TACE and no intervention groups (RR 0.83, 95% CI 0.65 to 1.07; 39 participants). Median survival in the TACE group was 10.7 months (range 3 to 38 months) from trial entry, and 13.0 months (range 3 to 38 months) after diagnosis. The trial authors reported that differences between groups were not statistically significant. All participants experienced short‐term nausea, with or without vomiting, immediately after treatment; one participant developed a wound infection, and one developed deep vein thrombosis. The trial did not measure failure to clear liver metastases, time to progression of liver metastases, tumour response measures, or health‐related quality of life.
Cancer Research Campaign, a non‐profit organisation, provided a grant for the trial; Pharmacia Ltd. delivered the Port‐a‐Cath arterial delivery systems and degradable starch microspheres.
We identified one ongoing trial comparing TACE plus chemotherapy versus chemotherapy alone in people with unresectable colorectal liver metastases who failed with first‐line chemotherapy (NCT03783559).
Authors' conclusions
Based on one, small randomised trial at high risk of bias, the evidence is very uncertain about the effect of TAE or TACE versus no active therapeutic intervention on mortality for people with liver metastases as the true effect may be substantially different. The trial did not measure failure to clear liver metastases, time to progression of liver metastases, tumour response measures, or health‐related quality of life. Short‐term, minor adverse events were recorded in the intervention groups only.
Large trials, following current standards of conduct and reporting, are required to explore the benefits and harms of TAE or TACE compared with no intervention or placebo in people with resectable and unresectable liver metastasis.
PICOs
Plain language summary
Transarterial embolisation, with or without chemotherapy, for liver metastases
Review question
Can transarterial embolisation, with (TACE) or without chemotherapy (TAE) destroy cancer metastases in the liver? Metastases are new cancer sites that spread to other parts of the body from the original site of the cancer.
We looked for randomised clinical trials (studies in which participants are allocated to groups by a play of chance) that assessed the benefits and harms of TAE or TACE, compared with no intervention or placebo, for people with liver metastases from cancer of any location. We were interested in the risk of death, survival time, recurrence rates, progression of the disease, health‐related quality of life, and adverse events (unwanted effects caused by the intervention).
Background
One of the most common sites of metastasis is the liver. Primary cancers of the liver, and metastases from colorectal cancer are the most common cancers affecting the liver. More than half of the people who have liver metastases die of complications.
Liver metastases can be destroyed by several methods. One method is based on the concept that the blood supply to liver (hepatic) tumours comes mainly from the hepatic artery. Transarterial chemoembolisation (TACE) of the hepatic artery involves the injection of a chemotherapeutic drug, followed by agents (small particles) that block the blood vessels. This interaction leads to death (necrosis) of the liver tumour, while leaving normal liver tissue virtually unaffected. The hepatic artery can also be blocked without chemotherapy, in which case it is called bland transarterial embolisation (TAE).
Search results and study characteristics
We last searched for evidence on 20 December 2019. We included one randomised trial from earlier searches. This trial randomly assigned people with colorectal liver metastases that could not be surgically removed, to one of three intervention groups: TAE (22 participants), TACE (19 participants), and a control group (20 participants) that received no active therapeutic intervention.
Cancer Research Campaign, a non‐profit organisation, provided a grant for the study; Pharmacia Ltd. delivered the Port‐a‐Cath arterial delivery systems and degradable starch microspheres.
Key results
Trial participants were followed for a minimum of seven months.
Mortality at 44 months from trial entry was 86% in the TAE group, 79% in the TACE group, and 95% in the control group. Median survival after trial entry was 7.0 months in the TAE group, 10.7 months in the TACE group, and 7.9 months in the control group. Median survival from diagnosis was 8.7 months in the TAE group, 13.0 months in the TACE group, and 9.6 months in the control group. Local recurrence was reported in 10 participants, without further details of their treatment group.
None of the participants in the control group reported side effects; 82% of the TAE group experienced short‐term pain, nausea, vomiting, and high temperature, which got better with symptomatic treatment, and there was one report of bruising at the puncture site. TACE recipients reported short‐term nausea, with or without vomiting, after most of the treatment sessions, and short‐lived pain or discomfort; there was one wound infection, and one case of deep vein thrombosis.
All the results were inconclusive between groups. The evidence from one small randomised clinical trial showed neither beneficial nor harmful effects of TAE or TACE compared to no intervention, in people with liver metastases, measured by mortality. We did not find data on the other outcomes of interest.
Quality of evidence
We judged the evidence to be of very low certainty. The identified trial was small, at high risk of bias, and with inconclusive results.
Authors' conclusions
Summary of findings
| Transarterial embolisation and transarterial chemoembolisation compared with no intervention for liver metastases | ||||||
| Patient or population: people with liver metastases | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed riskwith no intervention | Corresponding risk with TAE plus TACE | |||||
| Mortality at 44 months from trial entry Number of participants | 950 per 1000 | 836 per 1000 | RR 0.88 | 61 (1 RCT) | ⊕⊝⊝⊝ | |
| Survival time Median (range) survival time after trial entry Survival time Median (range) survival time from diagnosis | The median (range) survival time after trial entry in the control group was 7.9 months (1 month to 26 months) | The median survival time after trial entry in the TAE group was 0.9 months lower and in the TACE group was 2.8 months higher. | ‐‐ | 61 | ⊕⊝⊝⊝ | Median survival after trial entry was 7.0 months (range 2 to 44) in the TAE group, 10.7 months (range 3 to 38) in the TACE group and 7.9 months (range 1 to 26) in the control group. Authors reported that the difference was not statistically significant. |
| The median (range) survival time after from diagnosis in the control group was 9.6 months (1 month to 27 months) | The median survival time from diagnosis in the TAE group was 0.9 months lower and in the TACE group was 3.4 months higher. | ‐‐ | 61 | ⊕⊝⊝⊝ | Median survival after diagnosis was 8.7 months (range 2 to 49 months) in the TAE group, 13.0 months (range 3 to 38 months) in the TACE group, and 9.6 months (range 1 to 27 months) in the control group. The trial authors reported the differences were not statistically significant. | |
| Failure to clear liver metastases or recurrence of liver metastases | Local recurrence was reported in 10 participants without any details about the group to which they had been allocated to. | ‐‐ | 61 | ⊕⊝⊝⊝ | Number of participants who developed evidence of extrahepatic disease was 9/22 participants in the TAE group, 8/19 participants in the TACE group, and 5/20 participants in the control group. | |
| Time to progression of liver metastases | Outcome not reported | ‐‐ | ||||
| Tumour response measures | Outcome not reported | ‐‐ | ||||
| Health‐related quality of life | Outcome not reported | ‐‐ | ||||
| Adverse events and complications | No adverse events reported | 82% of TAE recipients reported short‐term pain, nausea, vomiting, and pyrexia, which resolved with symptomatic treatment. All TACE recipients reported short‐term nausea with or without vomiting immediately after most of the treatment sessions, and short‐lived pain or discomfort. Single cases of local puncture site haematoma, wound infection, and deep vein thrombosis were reported. | ‐‐ | 61 | ⊕⊝⊝⊝ | |
| *The risk in the intervention group (corresponding risk and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; RCT: randomised clinical trial; OIS: optimal information size | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aDowngraded by two levels due to within‐study risk of bias: the trial did not describe sequence generation, allocation concealment, or blinding, and we had some concerns regarding selective outcome reporting. | ||||||
| Transarterial embolisation compared with no intervention for liver metastases | ||||||
| Patient or population: people with liver metastases | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk with no intervention | Corresponding risk with TAE | |||||
| Mortality at 44 months from trial entry Number of participants | 950 per 1000 | 865 per 1000 | RR 0.91 | 42 | ⊕⊝⊝⊝ | |
| Survival time Median (range) survival time after trial entry Survival time Median (range) survival time from diagnosis | The median (range) survival time after trial entry in the control group was 7.9 months (1 month to 26 months). | The median survival time after trial entry in the TAE group was 0.9 months lower. | ‐‐ | 42 | ⊕⊝⊝⊝ | Median survival from trial entry was 7.0 months (range 2 to 44 months) in the TAE group and 7.9 months (range 1 to 26 months) in the control group. The trial authors reported the differences were not statistically significant. |
| The median (range) survival time from diagnosis in the control group was 9.6 months (1 month to 27 months). | The median survival time from diagnosis in the TAE group was 0.9 months lower. | ‐‐ | 42 | ⊕⊝⊝⊝ | Median survival after diagnosis was 8.7 months (range 2 to 49 months) in the TAE group and 9.6 months (range 1 to 27 months) in the control group. The trial authors reported the differences were not statistically significant. | |
| Failure to clear liver metastases or recurrence of liver metastases | Local recurrence was reported in 10 participants without any details about the group to which they had been allocated to. | ‐‐ | 42 | ⊕⊝⊝⊝ | Number of participants who developed evidence of extrahepatic disease was 9/22 participants in the TAE group and 5/20 participants in the control group. | |
| Time to progression of liver metastases | Outcome not reported | ‐‐ | ||||
| Tumour response measures | Outcome not reported | ‐‐ | ||||
| Health‐related quality of life | Outcome not reported | ‐‐ | ||||
| Adverse events and complications | No adverse events reported | 18/22 (82%) of recipients reported short‐term pain, nausea, vomiting, and pyrexia, which resolved with symptomatic treatment. Single case of local puncture site haematoma | ‐‐ | 42 | ⊕⊝⊝⊝ | |
| *The risk in the intervention group (corresponding risk and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; RCT: randomised clinical trial; OIS: optimal information size | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aDowngraded by two levels due to within‐study risk of bias: the trial did not describe sequence generation, allocation concealment, or blinding, and we had some concerns regarding selective outcome reporting. | ||||||
| Transarterial chemoembolisation compared with no intervention for liver metastases | ||||||
| Patient or population: people with liver metastases | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk with no intervention | Corresponding risk with TACE | |||||
| Mortality at 44 months from trial entry Number of participants | 950 per 1000 | 789 per 1000 | RR 0.83 | 39 | ⊕⊝⊝⊝ | |
| Survival time Median (range) survival time after trial entry Survival time Median (range) survival time from diagnosis | The median (range) survival time after trial entry in the control group was 7.9 months (1 month to 26 months). | The median survival time after trial entry in the TACE group was 2.8 months higher. | ‐‐ | 39 | ⊕⊝⊝⊝ | Median survival from trial entry was 10.7 months (range 3 to 38 months) in the TACE group, and 7.9 months (range 1 to 26 months) in the control group. The trial authors reported the differences were not statistically significant |
| The median (range) survival time from diagnosis in the control group was 9.6 months (1 month to 27 months). | The median survival time from diagnosis in the TACE group was 3.4 months higher. | ‐‐ | 39 | ⊕⊝⊝⊝ | Median survival after diagnosis was 13.0 months (range 3 to 38 months) in the TACE group and 9.6 months (range 1 to 27 months) in the control group. The trial authors reported the differences were not statistically significant. | |
| Failure to clear liver metastases or recurrence of liver metastases | Local recurrence was reported in 10 participants without any details about the group to which they had been allocated to. | ‐‐ | 39 | ⊕⊝⊝⊝ | Number of participants who developed evidence of extrahepatic disease was 8/19 participants in the TACE group and 5/20 participants in the control group | |
| Time to progression of liver metastases | Outcome not reported | ‐‐ | ||||
| Tumour response measures | Outcome not reported | ‐‐ | ||||
| Health‐related quality of life | Outcome not reported | ‐‐ | ||||
| Adverse events and complications | No adverse events reported | All recipients reported short‐term nausea with or without vomiting immediately after most of the treatment sessions, and short‐lived pain or discomfort. Single cases of wound infection, and deep vein thrombosis. | ‐‐ | 39 | ⊕⊝⊝⊝ | |
| *The risk in the intervention group (corresponding risk and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; RCT: randomised clinical trial; OIS: optimal information size | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aDowngraded by two levels due to within‐study risk of bias: the trial did not describe sequence generation, allocation concealment, or blinding, and we had some concerns regarding selective outcome reporting. | ||||||
Background
Description of the condition
The liver is affected by two of the most common groups of malignant tumours: primary liver tumours and liver metastases from colorectal carcinoma (Chakedis 2017; Forner 2018). Primary liver tumours arise from malignant cells within the liver, and hepatocellular carcinoma (HCC) represents the most common form of primary liver cancer (Forner 2018). Liver metastases are significantly more common than primary liver cancers (Bilchik 2000). Long‐term survival rates reported for people after radical surgical treatment is approximately 50%. However, resection with a clear border (R0) is not feasible in the majority of people (Nordlinger 2013). Liver metastases commonly originate from cancers of the lung, stomach, colon and rectum, and endometrium (Hugh 1997). In 35% of people with colorectal cancer, liver metastases are found on preoperative imaging, and 8% to 30% will subsequently develop liver metastases (Hugh 1997). Colorectal cancer is the second most common cancer in Europe, and its prevalence is rising (Ferlay 2013). Globally, the age‐adjusted annual incidence rate for colorectal cancer is 23.6 per 100,000 in men, and 16.3 per 100,000 in women (GCO 2018). Very high incidence is observed in North America (age‐adjusted 26.2 per 100,000), Australia and New Zealand (age‐adjusted 36.7), northern Europe (age‐adjusted 32.1), and western Europe (age‐adjusted 28.8 (GCO 2018)). Lower incidences are observed in Africa (age‐adjusted from 6.4 in Western Africa to 13.4 in Southern Africa) and South‐Central Asia (age‐adjusted 4.9). Globally, age‐adjusted mortality for colorectal cancer is 8.9 per 100,000; this rate is higher in countries with a higher incidence, and lower in countries with a lower incidence (GCO 2018). Globally, in 2013, approximately 414,000 men and 357,000 women died of colorectal cancer, making it the fourth leading cause of cancer death in men and the third for women (Global Burden of Disease Cancer Collaboration 2017). In the USA, approximately 51,370 Americans die of colorectal cancer each year, accounting for approximately 9% of all cancer deaths (Jemal 2010). In the USA, five‐year survival after diagnosis of colorectal cancer is 64.5% (Howlader 2018). In all high‐income countries analysed together in 2005, the estimated survival was 55% (Parkin 2005), and in low‐ and middle‐income countries analysed together, it was 39% (Parkin 2005), with the lowest rate reported for Sub‐Saharan Africa (13% for males and 14% for females). In Europe, the relative survival rate for five years was 57% for colon cancer and 56% for rectal cancer (Holleczek 2015). Although improvement in long‐term survival has been reported since the 2000s, CONCORD‐3 analysis shows significant disparities in colorectal cancer treatment outcomes worldwide.
Globally, lung cancer has been the most common cancer; every fifth person with cancer dies of lung cancer (1.76 million deaths, 18.4% of the total (GCO 2018)). The age‐standardised incidence rate is 22.5 per 100,00 people, and the mortality rate is 18.6 per 100,000 people of both sexes; the highest estimated age‐standardised incidence rates are observed in Polynesia (38.1 per 100,000 (GCO 2018)). In all high‐income countries analysed together, the estimated survival after the diagnosis of lung cancer is 12% among men and women (Torre 2016).
Endometrial cancer is the most common gynaecological malignancy, mostly affecting women in the postmenopausal age group. Diagnoses of endometrial cancer have increased worldwide in recent years (Siegel 2016). For instance, in 2016, an estimated 60,050 women in the USA were diagnosed, with an estimated 10,470 related deaths. Endometrial cancer accounts for 26.0 new cases per 100,000 women per year, and 4.6 deaths per 100,000 women per year (Howlader 2018). In all, 66.9% of women with uterine cancer are diagnosed at the local stage. However, in one‐third of women, lymph nodes or distant metastases are present. The five‐year relative survival rate in women with distant metastases is 16% (Howlader 2018).
Although the incidence has declined over the last decades, almost 0.8 million new cases of stomach cancer were estimated to have occurred in 2018 (GCO 2018). Standardised incidence rates are higher in men than in women, ranging from 4.7 in Eastern Africa to 32.1 in Eastern Asia per 100,000 men, and from 4.0 in Eastern Africa to 13.2 in Eastern Asia per 100,000 women (GCO 2018). Thanks to improvements in surgical techniques and radiotherapy, and the introduction of neoadjuvant therapy regimens, overall survival after gastric cancer treatment has improved over the past two decades (Song 2017).
For people with liver metastases, surgical resection may cure the disease, but only a limited number of these people qualify for resection (Bilchik 2000; Bipat 2007). Other options for people with unresectable liver metastases include chemotherapy delivered intra‐arterially (5‐fluorouracil), called regional chemotherapy; systemic chemotherapy (5‐fluorouracil, irinotecan, oxaliplatin, leucovorin, capecitabine); and monoclonal antibodies (such as bevacizumab or cetuximab (Riemsma 2009)). Additional methods include local tumour ablative techniques, transarterial (chemo)embolisation, percutaneous ethanol injection, microwave coagulation, laser‐induced thermotherapy, radiofrequency ablation, and cryosurgical ablation (Riemsma 2009).
Description of the intervention
Transarterial chemoembolisation (TACE) is defined as selective administration of chemotherapy directly to the hepatic artery that supplies the tumour, followed by embolisation of the same artery. This results in selective ischaemic and cytotoxic effects on the liver metastases, without having an effect on surrounding hepatic tissue (Vogl 2007; Fiorentini 2014). Embolisation can be achieved with agents acting either temporarily, like microspheres, degradable starch microspheres, collagen and gelatine sponge; or permanently, like polyvinyl alcohol (Vogl 2007). It can also be performed without chemotherapy; this procedure is called bland transarterial embolisation (TAE). Common contraindications for TACE include portal vein thrombosis, high grade liver dysfunction, and hepatorenal syndrome. TACE is most widely used as primary treatment for unresectable HCC, aiming to reduce tumour growth, but randomised trials and meta‐analyses assessing survival have found no significant effect on mortality (Oliveri 2011; EASL 2018).
How the intervention might work
All local ablative techniques offer the advantages of preserving the uninvolved liver parenchyma and eliminating the morbidity and mortality associated with major hepatic surgery. Chemoembolisation is based on the concept of different sources of blood supply to hepatic tumours (mainly the hepatic artery) and normal tissue (mainly the portal vein (Breedis 1954; Vogl 2003)). Therefore, stopping the blood supply by embolisation of the hepatic artery can lead to the necrosis of the tumour without damage to the normal hepatic tissue (Vogl 2003). This ischaemic damage increases vascular permeability, and is associated with higher levels of penetration of the cytotoxic drug into the tumour, without systemic effects (Wallace 1990).
Why it is important to do this review
For people with liver metastases, local or regional treatment methods can provide local control, but the long‐term outcomes of some of these interventions are uncertain. Systematic reviews may help to establish the trade‐off between benefits and harms associated with different non‐surgical ablation methods for the treatment of all forms of liver tumours (primary and metastases). Systematic reviews published so far focus mostly on primary liver tumours or colorectal cancer liver metastases (Llovet 2003; Decadt 2004; Lopez 2006; ASERNIP‐S 2006; Sutherland 2006; Cho 2016; Levy 2018; Kacmaz 2019). The methods used in these publications lack clarity on how the review authors addressed the risk of systematic errors (bias) and the risks of random errors (play of chance (Oliveri 2011)). To date, our review is the only published systematic review comparing TAE or TACE with no intervention or placebo (sham) in people with liver metastases.
Objectives
To assess the beneficial and harmful effects of transarterial embolisation (TAE) or transarterial chemoembolisation (TACE) compared with no intervention or placebo in people with liver metastases.
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials assessing the beneficial and harmful effects of transarterial embolisation (TAE) or transarterial chemoembolisation (TACE) versus no intervention or placebo, regardless of publication status, language, or blinding. We planned to include trials only if they assessed our outcomes of interest.
By excluding non‐randomised studies, we are aware that we are putting more focus on potential benefits and may overlook late occurring or rare harms, which are often missed in randomised clinical trials (Storebø 2018). If we demonstrate benefits from using TAE or TACE in people with liver metastases, a systematic review of adverse events of TAE and TACE in people with liver metastases should be performed (Storebø 2018). We only considered relevant quasi‐randomised and other controlled studies that were identified in the searches as sources of data on harms.
Types of participants
People with liver metastases, irrespective of the primary location of the tumour
Types of interventions
Experimental intervention
Transarterial embolisation (TAE) or transarterial chemoembolisation (TACE)
Control intervention
No intervention or placebo (sham)
Co‐interventions were allowed if provided equally to the experimental and control groups in individual randomised trials.
Types of outcome measures
Primary outcomes
-
All‐cause mortality at last follow‐up
-
Survival time
Secondary outcomes
-
Failure to clear liver metastases, or recurrence of liver metastases
-
Time to progression of liver metastases
-
Tumour response measures (complete response, partial response, stable disease, disease progression)
-
Health‐related quality of life
-
Any adverse events or complications – the International Conference on Harmonization (ICH) Guidelines defined adverse events as serious and non‐serious (ICH‐CPG 1997). A serious fatal or non‐fatal adverse event was any event that led to death, was life‐threatening, required inpatient hospitalisation or prolongation of existing hospitalisation, or resulted in persistent or significant disability, and any important medical event that may have jeopardised the person or required an intervention to prevent it. All other adverse events were considered non‐serious.
Search methods for identification of studies
Electronic searches
We searched: the Cochrane Hepato‐Biliary Group (CHBG) Controlled Trials Register (maintained and searched internally by the CHBG Information Specialist via the Cochrane Register of Studies Web; in December 2019); the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 12), in the Cochrane Library, searched 20 December 2019; MEDLINE Ovid (1946 to December 2019); Embase Ovid (1974 to December 2019); Science Citation Index Expanded (Web of Science; 1900 to December 2019); Conference Proceedings Citation Index – Science (Web of Science; 1990 to December 2019); Latin American Caribbean Health Sciences Literature (LILACS; Bireme; 1982 to December 2019), and the Cumulative Index to Nursing and Allied Health Literature (CINAHL EBSCO; 1981 to December 2019 (Royle 2003)). In addition, we searched ClinicalTrials.gov, the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP; www.who.int/ictrp/en/), and all US Food and Drug Administration (FDA) approvals and investigational device exemptions, as found on www.fda.gov/ on 7 September 2019.
Up to 5 June 2017, searches for this review were part of a global search for a full review of non‐surgical ablation methods in people with liver metastases or primary malignant liver tumours (Riemsma 2009). These search strategies with time spans are given in Appendix 1. We developed a new, focused search strategy when we updated the searches on 28 June 2018 (Appendix 2).
Searching other resources
We searched reference lists of relevant reviews (such as Cho 2016, Levy 2018, and Kacmaz 2019), Health Technology Assessment (HTA) reports (such as ASERNIP‐S 2006), relevant Cochrane Reviews, and the included trial.
Data collection and analysis
We performed the systematic review according to the recommendations of Cochrane (Higgins 2011).
Selection of studies
Two review authors, in pairs (MJS, DS, MMB, RR, JWM, MP and RW), independently screened the titles and abstracts of identified studies. We resolved any differences in opinion by discussion, or if necessary, by consultation with a third review author. Two review authors, in pairs (MJS, DS, MMB, RR, JWM, MP and RW), independently screened the full‐text reports; differences in opinion were resolved as mentioned above.
Data extraction and management
We extracted relevant data on participant characteristics, interventions, comparisons, study outcome measures, time of follow‐up, trial design, and methods. We used a data extraction form that was previously developed for another review on non‐surgical ablation methods. Data extraction was originally done by MMB and checked by RR. Upon this review update, the extraction was repeated independently by MJS, compared with previous extraction, and discussed among review authors.
Assessment of risk of bias in included studies
We assessed the risk of bias in the included study based on the domains described below (Schulz 1995; Moher 1998; Kjaergard 2001; Gluud 2008; Wood 2008; Higgins 2011; Savović 2012a; Savović 2012b; Hrobjartsson 2013; Hrobjartsson 2014a; Hrobjartsson 2014b; Savović 2018). If we identify more trials in future updates, we plan to assess each trial separately. We considered the risk of bias in relation to the overall reliability of the evidence. This was originally done by MMB and checked by RR. Upon the review update, MJS independently repeated the assessment, compared it with the previous assessment, and discussed the assessment among the review authors.
Allocation sequence generation
-
Low risk of bias: study authors performed sequence generation using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice were adequate if an independent person, not otherwise involved in the study, performed them
-
Unclear risk of bias: the study authors did not specify the method of sequence generation
-
High risk of bias: the sequence generation method was not random
Allocation concealment
-
Low risk of bias: participant allocation could not have been foreseen in advance of, or during, enrolment. A central and independent randomisation unit controlled allocation. Investigators were unaware of the allocation sequence (e.g. if the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes)
-
Unclear risk of bias: study authors did not describe the method used to conceal the allocation, so intervention allocations may have been foreseen before, or during, enrolment
-
High risk of bias: it is likely that investigators who assigned participants knew the allocation sequence
Blinding of participants and personnel
-
Low risk of bias: either of the following: no blinding or incomplete blinding, but review authors judged that the outcome was unlikely to have been influenced by lack of blinding; or blinding of participants and key study personnel ensured, and it was unlikely that the blinding could have been broken
-
Unclear risk of bias: either of the following: insufficient information to permit judgement of low risk or high risk; or the trial did not address this outcome
-
High risk of bias: either of the following: no blinding or incomplete blinding, and the outcome was likely to have been influenced by lack of blinding; or blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome was likely to have been influenced by lack of blinding
Blinding of outcome assessment
-
Low risk of bias: either of the following: no blinding of outcome assessment, but review authors judged that the outcome measurement was not likely to be influenced by lack of blinding; or blinding of outcome assessment ensured, and unlikely that the blinding could have been broken
-
Unclear risk of bias: either of the following: insufficient information to permit judgement of low risk or high risk; or the trial did not address this outcome
-
High risk of bias: either of the following: no blinding of outcome assessment, and the outcome measurement was likely to be influenced by lack of blinding; or blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement was likely to be influenced by lack of blinding
Incomplete outcome data
-
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. The study used sufficient methods, such as multiple imputation, to handle missing data
-
Unclear risk of bias: information was insufficient to assess whether missing data, in combination with the method used to handle missing data, were likely to induce bias on the results
-
High risk of bias: results were likely to be biased due to missing data
Selective outcome reporting
-
Low risk of bias: the trial reported the following predefined outcomes: all‐cause mortality, adverse events, and failure to clear liver metastases or recurrence of liver metastases. If the original trial protocol was available, the outcomes were those called for in that protocol. If the trial protocol was obtained from a trial registry (e.g. www.ClinicalTrials.gov), the outcomes sought should have been those enumerated in the original protocol, if the trial protocol was registered before or at the time the trial was begun. If the trial protocol was registered after the trial was begun, we did not consider those outcomes to be reliable.
-
Unclear risk of bias: study authors did not report all predefined outcomes fully, or it was unclear whether or not study authors recorded data on these outcomes
-
High risk of bias: study authors did not report one or more predefined outcomes
Other bias
-
Low risk of bias: the trial appeared free of other factors that could have put it at risk of bias
-
Unclear risk of bias: the trial may or may not have been free of other factors that could have put it at risk of bias
-
High risk of bias: other factors in the trial could have put it at risk of bias
We judged a trial to be at low overall risk of bias if we assessed it at low risk of bias in all of the above domains. We judged a trial to be at high overall risk of bias if we assessed it as unclear or at high risk of bias in one or more of the above domains.
Measures of treatment effect
For dichotomous variables, we calculated the risk ratio (RR) with 95% confidence interval (CI). For continuous variables, we planned to calculate the mean difference with 95% CI when outcomes were measured with the same scales, or standardised mean difference with 95% CI for outcomes such as quality of life, when different scales could be used. For outcomes such as death, measured with a hazard ratio, we planned to use the generic inverse variance method for the meta‐analysis.
Unit of analysis issues
Participants randomised per intervention group, in a randomised clinical trial with parallel‐group design. For trials with multiple intervention groups, we planned to collect data for all intervention groups that met the inclusion criteria of our protocol. If the control group was a common comparator in a comparison, we divided the participants by the number of relevant intervention groups in order to avoid counting the same data multiple times. In the case of cross‐over trials, we planned to use data from the first trial period only.
Dealing with missing data
We analysed data using the intention‐to‐treat principle, that is, we included the total number of randomised participants in the analyses.
Assessment of heterogeneity
We planned to check if the included trials were similar enough to combine their results before commencing statistical pooling of the data. We planned to assess heterogeneity using Chi² and I² statistics (Higgins 2011). We planned to discuss any plausible causes of heterogeneity. We planned to use the random‐effects model (DerSimonian 1986) and the fixed‐effect model (Mantel 1959; Greenland 1985), and compare the results of both models in order to explore heterogeneity. We planned to present both sets of results if heterogeneity was present (I2 > 0). Otherwise, we planned to report the results of the random‐effects model.
Assessment of reporting biases
We planned to use a funnel plot to explore reporting bias, had we identified at least ten trials (Egger 1997; Macaskill 2001).
Data synthesis
We followed the instructions given in the Cochrane Handbook for Systematic Reviews of Interventions, and used Review Manager 5 for the analyses (Higgins 2011; Review Manager 2014).
We planned to calculate relevant measures of effect, that is, hazard ratios and risk ratios. Whenever possible, we planned to calculate hazard ratios using the methods described by Parmar and Tierney (Parmar 1998; Tierney 2007). We planned to extract information on, for example, hazard ratios, P values, events ratios, curve data, and follow‐up, and enter them into a Microsoft Office Excel 2003 spreadsheet to calculate the log hazard ratios and their standard errors (Tierney 2007). If we could not meta‐analyse the data, for example, in the case of extreme heterogeneity, we planned to present results in a narrative way as well as in a forest plot, without the estimate, to show the variance of effects (Egger 1997).
If we identified cross‐over trials, we planned to use the results of the first period only (before cross‐over), as if they were parallel trials.
We planned to group the trials by intervention, participant characteristics, and outcomes, and to describe the most important characteristics of the trials, including a detailed review of the methodological shortcomings of a trial.
We planned to use funnel plots to identify possible small‐trial biases, such as publication bias (Egger 1997). Furthermore, we planned to discuss the possible implications of our findings if bias was present. None of this was possible due to lack of data.
When possible, we planned to examine the apparent significant beneficial and harmful intervention effects using Trial Sequential Analysis (TSA (Thorlund 2011; TSA 2011; Wetterslev 2017)), to evaluate if these apparent effects could have been caused by random error ('play of chance’ (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009; Wetterslev 2009; Thorlund 2010; Wetterslev 2017)). We did not carry out TSA, as we only included one trial.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses, when possible, based on prognostic indicators such as age, sex, tumour size, location of primary tumour, and use of any co‐interventions. In addition, we planned to summarise the separate outcomes after intervention at six months or less, at six to 12 months, and at one year or longer.
Sensitivity analysis
We planned to use both fixed‐ and random‐effects models and compare the results in a sensitivity analysis if we had concerns of small study‐effects (Mantel 1959; Greenland 1985). We also planned to compare studies at low risk of bias versus trials at high risk of bias as defined in the Risk of bias in included studies section. However, we lacked the data needed to conduct sensitivity analyses.
'Summary of findings' tables
We used GRADE methods and GRADEPro GDT software to evaluate the certainty of evidence for our primary (mortality at last follow‐up; survival time) and secondary outcomes (failure to clear liver metastases or recurrence of liver metastases; time to progression of liver metastases; tumour response measures; health‐related quality of life; and any adverse events or complications). We considered within‐study risk of bias (study limitations) based on individual domains, as well as overall assessment, indirectness of evidence (population, intervention, control, outcomes), unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses), imprecision of effect estimate, and risk of publication bias. We calculated the optimal information size (OIS) which is the minimum number of trial participants required for an individual trial to be adequately powered. Comparing the number of participants included in a certain comparison to the OIS is useful for assessing imprecision (Guyatt 2011). We defined the levels of certainty in the evidence as high, moderate, low, or very low (Higgins 2011; GRADEpro GDT).
-
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
-
Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
-
Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
-
Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
Results
Description of studies
See the Characteristics of included studies table.
Results of the search
Up to 5 June 2017, searches for this review were part of a global search for a full review of non‐surgical ablation methods in people with liver metastases or primary malignant liver tumours (Riemsma 2009). These search strategies with time spans are given in Appendix 1; they produced 13,409 references. After excluding duplicates, we screened 13,382 references. Based on titles and abstracts, we found 13,047 references irrelevant for the current review, resulting in 335 full papers to be retrieved. We found one trial that met the inclusion criteria for the present review (Hunt 1990). We excluded 334 references because they did not describe randomised clinical trials (149), they examined another intervention not relevant for this review (142), the population was not relevant for this review (30), the comparison was not relevant for this review (12), or study authors examined outcomes not relevant for this review (1). We identified no ongoing trials. We did not run separate searches for non‐randomised studies; we checked eligibility only if studies were identified during searches for randomised trials; none of these studies were eligible for inclusion. A summary of the searches is provided in Figure 1.

Flow chart for identification of included randomised trials for the original published review
When updating the searches on 28 June 2018, we developed the new search strategies given in Appendix 2. We also searched ClinicalTrials.gov, the clinical trials registry WHO ICTRP (www.who.int/ictrp/en/), and all US FDA approvals and investigational device exemptions found on www.fda.gov/.
For the current update, we reran the searches on 20 December 2019 (Appendix 2). We searched ClinicalTrials.gov, WHO, and FDA databases on 7 September 2019. As outlined in the indications for study flow diagrams in Cochrane Review updates, we reported cumulative results for the updates (Stovold 2014). Altogether, all updates considered, we found 14,771 references, 4269 of which we screened manually, after we removed duplicate records. We excluded 4257 references based on the title and abstract and screened 12 full‐text publications. We identified one ongoing study (NCT03783559). A summary of the updated searches is provided in Figure 2.

Flow chart for identification of included randomised trials for the 2019 update
Included studies
We included one randomised trial with three intervention groups: transarterial embolisation (TAE; hepatic artery embolisation), transarterial chemoembolisation (TACE; hepatic artery infusion chemotherapy with degradable microspheres), and a control group, described as receiving no active therapeutic intervention (Hunt 1990).
The trial included 61 participants with unresectable colorectal liver metastases: 22 received TAE, 19 received TACE, and 20 received the control treatment. The trial included 43 male and 18 female participants. Mean age was not reported, but only people between 18 and 75 years old were eligible. Participants were followed for a minimum of seven months.
We pooled data from the trial's three intervention groups into three comparisons:
-
TAE or TACE versus the control group
-
TAE versus the control group
-
TACE versus the control group
In the TAE group, participants underwent hepatic arteriography with subsequent selective cannulation of the main hepatic artery (or right and left hepatic arteries when possible) and embolisation (performed with the use of homologous lyophilised dura mater to occlude smaller peripheral vessels, and gel foam to occlude the larger proximal arterial branches). Completeness of embolisation was confirmed on arteriography. Complete embolisation was achieved in 18 people; partial embolisation of the right hepatic artery only was achieved in one; embolisation was attempted and failed in three people.
In the TACE group, participants also underwent hepatic arteriography to define the anatomy before the catheter was placed intraoperatively (arteriotomy in the gastroduodenal artery was performed and the catheter was inserted up to the origin of the gastroduodenal artery from the hepatic artery). Participants received four injections on four consecutive days, followed by two injections on two consecutive days every 28 days. Each of the first four injections had 500 mg 5‐fluorouracil (5‐FU) mixed with 900 mg degradable starch microspheres (DSM). Subsequent injections had 500 mg 5‐fluorouracil (5‐FU) mixed with 300 mg to 900 mg DSM, depending on individual need. Participants with successful cannulation received a median of 7 (range 2 to 34) monthly treatments. In 14 participants, complete perfusion following cannulation was achieved; in three participants, only partial hepatic perfusion was achieved and in two participants, cannulation was attempted and failed. In the control group, participants received no active therapeutic intervention.
The mean size of the tumours was not reported; 42 participants had synchronous metastases and 19 had metachronous metastases. All of the tumours were unresectable, but the reasons were not reported. The diagnosis was confirmed by histological assessment, or by a combination of imaging procedures. The study authors did not report baseline characteristics of the groups, but stated that they were comparable for age, sex, primary carcinoma site, Duke’s staging, histology of the primary tumour, proportion of synchronous or metachronous metastases, and the percentage of liver involvement.
The study reported mixed‐funding: Cancer Research Campaign, a non‐profit organisation, provided a grant for the study; Pharmacia Ltd. delivered the Port‐a‐Cath arterial delivery systems and degradable starch microspheres.
Excluded studies
We excluded 13 studies in total: 12 studies because of irrelevant for the review comparison and one study because of irrelevant outcomes (Characteristics of excluded studies).
Risk of bias in included studies
Overall, we judged the trial to be at high risk of bias (See Figure 3 and Figure 4). For details of risk of bias judgements, see Characteristics of included studies.

'Risk of bias' summary: review authors' judgements about each 'risk of bias' item for each included study

'Risk of bias' graph: review authors' judgements about each 'risk of bias' item presented as percentages across all included studies
Allocation
The trial was described as a randomised clinical trial. However, data regarding sequence generation or allocation concealment were insufficient (unclear risk of bias).
Blinding
Information was insufficient to assess whether participants, physicians, or outcome assessors were properly blinded (unclear risk of bias).
Incomplete outcome data
We judged the analysis methods as appropriate. There were no missing outcome data (low risk of bias).
Selective reporting
There was no protocol of the reported trial. Authors reported on mortality, survival time, adverse events, and local recurrence. Local recurrence was reported in 10 participants, but the group to which they were randomised was not reported. In addition, not all log rank tests' results were reported (high risk of bias).
Other potential sources of bias
It was not possible to assess whether the trial was free of other biases (unclear risk of bias).
Effects of interventions
See: Summary of findings for the main comparison Transarterial embolisation and transarterial chemoembolisation compared with no intervention for liver metastases; Summary of findings 2 Transarterial embolisation compared with no intervention for liver metastases; Summary of findings 3 Transarterial chemoembolisation compared with no intervention for liver metastases
Transarterial embolisation (TAE) and transarterial chemoembolisation (TACE) versus no intervention
Primary outcomes
Mortality at last follow‐up
As the trial did not specifically report the last follow‐up, we assumed the longest reported survival from trial entry to be the last follow‐up of the trial (i.e. 44 months from trial entry).
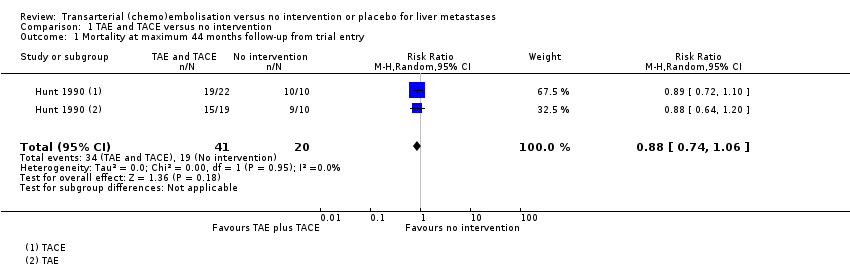
There were inconclusive results for mortality between the TAE and TACE and no intervention groups (risk ratio (RR) 0.88, 95% confidence interval (CI) 0.74 to 1.06; 61 participants; very‐low‐certainty evidence; Analysis 1.1).
Survival time
Median survival from trial entry was 7.0 months (range from 2 to 44) in the TAE group, 10.7 months (range from 3 to 38) in the TACE group, and 7.9 months (range from 1 to 26) in the control group. Median survival after diagnosis was 8.7 months (range 2 to 49) in TAE, 13.0 months (range 3 to 38) in TACE and 9.6 months (range 1 to 27) in the control group. Trial authors reported the differences to be not statistically significant.
We were not able to make a separate analysis for survival time as the available data were presented in form of median (range).
Secondary outcomes
Failure to clear liver metastases or recurrence of liver metastases
Local recurrence was reported in 10 participants without any details about the group to which they had been allocated.
The review reported some information on development of extrahepatic disease. The results for extrahepatic disease between the TAE and TACE and the no intervention groups were inconclusive (RR 1.61, 95% CI 0.70 to 3.73; 61 participants; very‐ low‐certainty evidence; Analysis 1.2).
Time to progression of liver metastases
Time to progression of liver metastases was not measured.
Tumour response measures
Tumour response measures (complete response, partial response, stable disease, disease progression) were not measured.
Health‐related quality of life
Health‐related quality of life was not measured.
Any adverse events or complications
This outcome is reported in the following below separate TAE and TACE intervention group comparisons.
TAE versus no intervention
Primary outcomes
Mortality at last follow‐up
Mortality was 86% (19/22) in the TAE group versus 95% (19/20) in the control group. There was little or no difference in mortality between the TAE and no intervention groups (RR 0.91, 95% CI 0.75 to 1.1; 42 participants; very‐low‐certainty evidence; Analysis 2.1). As this comparison included data from only one trial, and given the fact that the number of events was high, we checked the results using Fisher's exact test, which produced a two‐sided P value of 0.6079, confirming that the difference was not statistically significant.
Survival time
Median survival from trial entry was 7.0 months (range 2 to 44 months) in the TAE group and 7.9 months (range 1 to 26 months) in the control group. The trial authors reported the differences were not statistically significant.
Median survival after diagnosis was 8.7 months (range 2 to 49 months) in the TAE group and 9.6 months (range 1 to 27 months) in the control group. The trial authors reported the differences were not statistically significant.
We were not able to make a separate analysis for survival time as the available data were presented in form of median (range).
Secondary outcomes
Failure to clear liver metastases or recurrence of liver metastases
The trial reported local recurrence in 10 participants without any details about which of the three groups they had been allocated to.
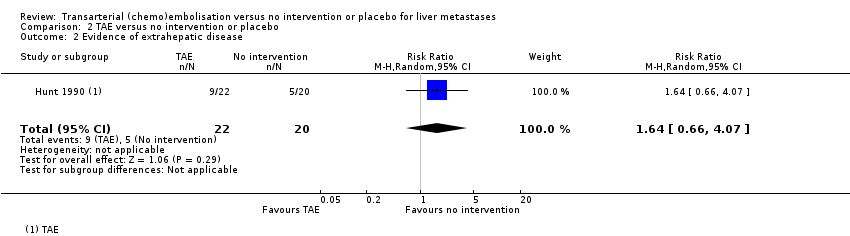
Additionally, nine out of 22 (41%) participants in the TAE group and five out of 20 (25%) participants in the control group developed extrahepatic disease. The results for development of extrahepatic disease were inconclusive between the TAE and no intervention groups (RR 1.64, 95% CI 0.66 to 4.07; 42 participants; very‐low‐certainty evidence; Analysis 2.2).
Time to progression of liver metastases
Time to progression of liver metastases was not measured.
Tumour response measures
Tumour response measures (complete response, partial response, stable disease, disease progression) were not measured.
Health‐related quality of life
Health‐related quality of life was not measured.
Any adverse events or complications
There were no reported adverse events in the control group. In the TAE group, 18 participants experienced symptoms of 'post‐embolisation syndrome'. This comprised pain, nausea, vomiting, and pyrexia; none of these required more than symptomatic treatment, and symptoms lasted between one and five days. One participant also had a local puncture site haematoma. No other adverse events were reported.
TACE versus no intervention
Primary outcomes
Mortality at last follow‐up
Mortality was 79% (15/19) in the TACE group versus 95% (19/20) in the control group. There was little or no difference in mortality between the TACE and no intervention groups (RR 0.83, 95% CI 0.65 to 1.07; 39 participants; very‐low‐certainty evidence; Analysis 3.1). As this comparison included data from only one trial, and given the fact that the number of events was high, we checked the results using Fisher's exact test, which produced a two‐sided P value of 0.1818, confirming the difference was not statistically significant.
Survival time
Median survival from trial entry was 10.7 months (range 3 to 38 months) in the TACE group and 7.9 months (range 1 to 26 months) in the control group. The trial authors reported the differences were not statistically significant.
Median survival after diagnosis was 13.0 months (range 3 to 38 months) in the TACE group and 9.6 months (range 1 to 27 months) in the control group. The trial authors reported the differences were not statistically significant.
We were not able to make a separate analysis for survival time as the available data were presented in form of median (range).
Secondary outcomes
Failure to clear liver metastases or recurrence of liver metastases
The trial reported local recurrence in 10 participants without any details about which of the three groups they had been allocated to.
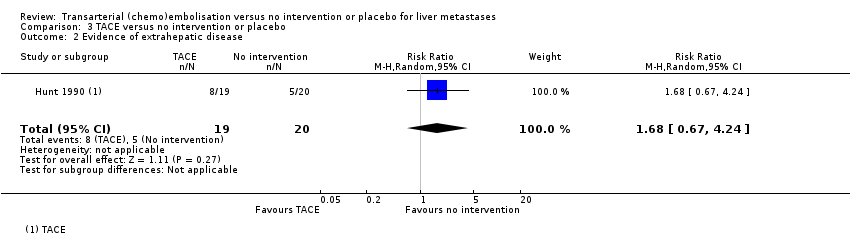
Eight out of 19 (42%) participants in the TACE group and five out of 20 (25%) participants in the control group developed extrahepatic disease. There was little to no difference in development of extrahepatic disease between the TACE and no intervention groups (RR 1.68, 95% CI 0.67 to 4.24; 39 participants; very‐low‐certainty evidence; Analysis 3.2).
Time to progression of liver metastases
Time to progression of liver metastases was not measured.
Tumour response measures
Tumour response measures (complete response, partial response, stable disease, disease progression) were not measured.
Health‐related quality of life
Health‐related quality of life was not measured.
Any adverse events or complications
In the TACE group, one participant developed a wound infection and another developed deep vein thrombosis. In general, treatment sessions were well tolerated; however, nausea with or without vomiting, lasting between 30 and 60 minutes, developed in all participants immediately after most of the treatment sessions. The participants occasionally experienced short‐lived pain or discomfort. The authors reported that no alopecia, haematological toxicity, or other adverse effects were observed.
Subgroup analysis and investigation of heterogeneity
We were not able to perform any subgroup analyses as only one trial was included. However, the trial authors did conduct subgroup analyses for survival. We included the results above.
Sensitivity analysis
We lacked data for our planned sensitivity analyses.
Certainty of the evidence
We created summary of findings Table for the main comparison for TAE and TACE versus no intervention, summary of findings Table 2 for TAE versus no intervention, and summary of findings Table 3 for TACE versus no intervention. The tables summarise all the primary and secondary review outcomes. We assessed the evidence at very low certainty because of risk of bias in the trial (downgraded by two levels because of lack of description of sequence generation, allocation concealment, and blinding, and because of concerns regarding selective outcome reporting), indirectness (downgraded by one level because the trial included people with mostly unresectable liver metastases), and imprecision (downgraded by two levels because the 95% CI included both benefit and harm; and the sample size was small, not reaching the optimal information size (calculated OIS ranged from 69 to 166)). We were unable to statistically assess for publication bias because there was only one included trial.
Discussion
Summary of main results
Evidence for the effectiveness of transarterial embolisation (TAE) versus no intervention and transarterial chemoembolisation (TACE) versus no intervention in people with liver metastases is based on one small randomised trial at high risk of bias. The results with respect to all‐cause mortality are inconclusive. Participants were followed for 44 months from trial entry. The authors reported some non‐serious adverse events related to the performed procedures in both the TAE and TACE groups. We did not find data on failure to clear liver metastases, time to progression of liver metastases, tumour response measures, or health‐related quality of life.
Overall completeness and applicability of evidence
Our past and present search strategies were comprehensive and included a wide range of databases and registries. We additionally searched reference lists of the included trials and of relevant review articles.
Quality of the evidence
The included trial did not report sufficient details about the trial design related to sequence generation, allocation concealment, and blinding, and we had concerns regarding selective outcome reporting (downgraded by two levels). Additionally, we observed some indirectness of the evidence as the trial included people with mostly unresectable liver metastases (downgraded by one level). Furthermore, there were problems with imprecision because the 95% confidence interval (CI) included both benefit and harm, and the sample size was small, not reaching the optimal information size (downgraded by two levels). Therefore, the main limitation of this review is the very‐low‐certainty evidence it provides. Additionally, the included trial lacked a protocol.
Potential biases in the review process
The process of the review was rigorous. The review was preceded by the publication of a protocol that described all review methods, which the review authors followed during the review preparation. All of the review authors were appropriately trained and had previous experience in review preparation. We performed comprehensive searches for both published and unpublished studies. We extracted all relevant data and assessed both risk of bias in the included study and the overall certainty of the evidence. However, because only one trial was found, we may suspect publication bias, which we cannot assess formally because the minimum number for reasonable assessment of publication bias is 10 trials (Higgins 2011).
Agreements and disagreements with other studies or reviews
We did not find any other published reviews comparing TAE or TACE with no intervention for people with liver metastases.
There are several reviews that compare TAE or TACE with other interventions in people with liver metastases, however, none of these included comparisons with no intervention or placebo (Carter 2009; Yang 2012; Richardson 2013; Akinwande 2017; Levy 2018). These reviews compared TAE with drug‐eluting beads (DEB‐TACE (Carter 2009)); TAE plus drug‐eluting beads with irinotecan (DEBIRI (Richardson 2013)); or they included mainly observational studies of TACE, DEB‐TACE, or TAE and DEBIRI (Yang 2012; Akinwande 2017; Levy 2018).
Consensus on the management of metastatic colorectal cancer in Central America and the Caribbean concluded that chemoembolisation of colorectal liver metastases may be considered a consolidation treatment; however, for now, it should be further investigated in randomised clinical trials (Lopez 2018). For liver metastases from neuroendocrine tumours, current recommendations are that TAE or TACE may be used to treat liver metastases in situations where surgery is not feasible, regardless of the origin of the original tumour (Pavel 2012; Ramage 2012; Frilling 2014).
Society of Interventional Radiology guidelines recommend TACE for people with surgically unresectable metastases, which are not amenable to thermal ablation, involve less than 50% to 60% of the liver volume, and have more than 80% of the tumour burden located in the liver; however, they do not provide any detailed guidance (Gaba 2017).

Flow chart for identification of included randomised trials for the original published review

Flow chart for identification of included randomised trials for the 2019 update

'Risk of bias' summary: review authors' judgements about each 'risk of bias' item for each included study

'Risk of bias' graph: review authors' judgements about each 'risk of bias' item presented as percentages across all included studies
Comparison 1 TAE and TACE versus no intervention, Outcome 1 Mortality at maximum 44 months follow‐up from trial entry.

Comparison 1 TAE and TACE versus no intervention, Outcome 2 Evidence of extrahepatic disease.

Comparison 2 TAE versus no intervention or placebo, Outcome 1 Mortality at maximum 44 months follow‐up from trial entry.
Comparison 2 TAE versus no intervention or placebo, Outcome 2 Evidence of extrahepatic disease.

Comparison 3 TACE versus no intervention or placebo, Outcome 1 Mortality at maximum 44 months follow‐up from trial entry.
Comparison 3 TACE versus no intervention or placebo, Outcome 2 Evidence of extrahepatic disease.
| Transarterial embolisation and transarterial chemoembolisation compared with no intervention for liver metastases | ||||||
| Patient or population: people with liver metastases | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed riskwith no intervention | Corresponding risk with TAE plus TACE | |||||
| Mortality at 44 months from trial entry Number of participants | 950 per 1000 | 836 per 1000 | RR 0.88 | 61 (1 RCT) | ⊕⊝⊝⊝ | |
| Survival time Median (range) survival time after trial entry Survival time Median (range) survival time from diagnosis | The median (range) survival time after trial entry in the control group was 7.9 months (1 month to 26 months) | The median survival time after trial entry in the TAE group was 0.9 months lower and in the TACE group was 2.8 months higher. | ‐‐ | 61 | ⊕⊝⊝⊝ | Median survival after trial entry was 7.0 months (range 2 to 44) in the TAE group, 10.7 months (range 3 to 38) in the TACE group and 7.9 months (range 1 to 26) in the control group. Authors reported that the difference was not statistically significant. |
| The median (range) survival time after from diagnosis in the control group was 9.6 months (1 month to 27 months) | The median survival time from diagnosis in the TAE group was 0.9 months lower and in the TACE group was 3.4 months higher. | ‐‐ | 61 | ⊕⊝⊝⊝ | Median survival after diagnosis was 8.7 months (range 2 to 49 months) in the TAE group, 13.0 months (range 3 to 38 months) in the TACE group, and 9.6 months (range 1 to 27 months) in the control group. The trial authors reported the differences were not statistically significant. | |
| Failure to clear liver metastases or recurrence of liver metastases | Local recurrence was reported in 10 participants without any details about the group to which they had been allocated to. | ‐‐ | 61 | ⊕⊝⊝⊝ | Number of participants who developed evidence of extrahepatic disease was 9/22 participants in the TAE group, 8/19 participants in the TACE group, and 5/20 participants in the control group. | |
| Time to progression of liver metastases | Outcome not reported | ‐‐ | ||||
| Tumour response measures | Outcome not reported | ‐‐ | ||||
| Health‐related quality of life | Outcome not reported | ‐‐ | ||||
| Adverse events and complications | No adverse events reported | 82% of TAE recipients reported short‐term pain, nausea, vomiting, and pyrexia, which resolved with symptomatic treatment. All TACE recipients reported short‐term nausea with or without vomiting immediately after most of the treatment sessions, and short‐lived pain or discomfort. Single cases of local puncture site haematoma, wound infection, and deep vein thrombosis were reported. | ‐‐ | 61 | ⊕⊝⊝⊝ | |
| *The risk in the intervention group (corresponding risk and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; RCT: randomised clinical trial; OIS: optimal information size | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aDowngraded by two levels due to within‐study risk of bias: the trial did not describe sequence generation, allocation concealment, or blinding, and we had some concerns regarding selective outcome reporting. | ||||||
| Transarterial embolisation compared with no intervention for liver metastases | ||||||
| Patient or population: people with liver metastases | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk with no intervention | Corresponding risk with TAE | |||||
| Mortality at 44 months from trial entry Number of participants | 950 per 1000 | 865 per 1000 | RR 0.91 | 42 | ⊕⊝⊝⊝ | |
| Survival time Median (range) survival time after trial entry Survival time Median (range) survival time from diagnosis | The median (range) survival time after trial entry in the control group was 7.9 months (1 month to 26 months). | The median survival time after trial entry in the TAE group was 0.9 months lower. | ‐‐ | 42 | ⊕⊝⊝⊝ | Median survival from trial entry was 7.0 months (range 2 to 44 months) in the TAE group and 7.9 months (range 1 to 26 months) in the control group. The trial authors reported the differences were not statistically significant. |
| The median (range) survival time from diagnosis in the control group was 9.6 months (1 month to 27 months). | The median survival time from diagnosis in the TAE group was 0.9 months lower. | ‐‐ | 42 | ⊕⊝⊝⊝ | Median survival after diagnosis was 8.7 months (range 2 to 49 months) in the TAE group and 9.6 months (range 1 to 27 months) in the control group. The trial authors reported the differences were not statistically significant. | |
| Failure to clear liver metastases or recurrence of liver metastases | Local recurrence was reported in 10 participants without any details about the group to which they had been allocated to. | ‐‐ | 42 | ⊕⊝⊝⊝ | Number of participants who developed evidence of extrahepatic disease was 9/22 participants in the TAE group and 5/20 participants in the control group. | |
| Time to progression of liver metastases | Outcome not reported | ‐‐ | ||||
| Tumour response measures | Outcome not reported | ‐‐ | ||||
| Health‐related quality of life | Outcome not reported | ‐‐ | ||||
| Adverse events and complications | No adverse events reported | 18/22 (82%) of recipients reported short‐term pain, nausea, vomiting, and pyrexia, which resolved with symptomatic treatment. Single case of local puncture site haematoma | ‐‐ | 42 | ⊕⊝⊝⊝ | |
| *The risk in the intervention group (corresponding risk and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; RCT: randomised clinical trial; OIS: optimal information size | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aDowngraded by two levels due to within‐study risk of bias: the trial did not describe sequence generation, allocation concealment, or blinding, and we had some concerns regarding selective outcome reporting. | ||||||
| Transarterial chemoembolisation compared with no intervention for liver metastases | ||||||
| Patient or population: people with liver metastases | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk with no intervention | Corresponding risk with TACE | |||||
| Mortality at 44 months from trial entry Number of participants | 950 per 1000 | 789 per 1000 | RR 0.83 | 39 | ⊕⊝⊝⊝ | |
| Survival time Median (range) survival time after trial entry Survival time Median (range) survival time from diagnosis | The median (range) survival time after trial entry in the control group was 7.9 months (1 month to 26 months). | The median survival time after trial entry in the TACE group was 2.8 months higher. | ‐‐ | 39 | ⊕⊝⊝⊝ | Median survival from trial entry was 10.7 months (range 3 to 38 months) in the TACE group, and 7.9 months (range 1 to 26 months) in the control group. The trial authors reported the differences were not statistically significant |
| The median (range) survival time from diagnosis in the control group was 9.6 months (1 month to 27 months). | The median survival time from diagnosis in the TACE group was 3.4 months higher. | ‐‐ | 39 | ⊕⊝⊝⊝ | Median survival after diagnosis was 13.0 months (range 3 to 38 months) in the TACE group and 9.6 months (range 1 to 27 months) in the control group. The trial authors reported the differences were not statistically significant. | |
| Failure to clear liver metastases or recurrence of liver metastases | Local recurrence was reported in 10 participants without any details about the group to which they had been allocated to. | ‐‐ | 39 | ⊕⊝⊝⊝ | Number of participants who developed evidence of extrahepatic disease was 8/19 participants in the TACE group and 5/20 participants in the control group | |
| Time to progression of liver metastases | Outcome not reported | ‐‐ | ||||
| Tumour response measures | Outcome not reported | ‐‐ | ||||
| Health‐related quality of life | Outcome not reported | ‐‐ | ||||
| Adverse events and complications | No adverse events reported | All recipients reported short‐term nausea with or without vomiting immediately after most of the treatment sessions, and short‐lived pain or discomfort. Single cases of wound infection, and deep vein thrombosis. | ‐‐ | 39 | ⊕⊝⊝⊝ | |
| *The risk in the intervention group (corresponding risk and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; RCT: randomised clinical trial; OIS: optimal information size | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aDowngraded by two levels due to within‐study risk of bias: the trial did not describe sequence generation, allocation concealment, or blinding, and we had some concerns regarding selective outcome reporting. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at maximum 44 months follow‐up from trial entry Show forest plot | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.74, 1.06] |
| 2 Evidence of extrahepatic disease Show forest plot | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.70, 3.73] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at maximum 44 months follow‐up from trial entry Show forest plot | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.75, 1.10] |
| 2 Evidence of extrahepatic disease Show forest plot | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [0.66, 4.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at maximum 44 months follow‐up from trial entry Show forest plot | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.65, 1.07] |
| 2 Evidence of extrahepatic disease Show forest plot | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 1.68 [0.67, 4.24] |

