Blood pressure lowering efficacy of coenzyme Q10 for primary hypertension
References
References to studies included in this review
Jump to:
References to studies excluded from this review
Jump to:
Additional references
Jump to:
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Randomized, placebo‐controlled, crossover trial. 2 week washout period, 10 week treatment period, 2 week treatment suspension, 10 week crossover treatment. | |
| Participants | 18 patients (4 women, 14 men) with essential hypertension, WHO stages 1 and 2, average age 55.9 years (range 42‐66 years). Patients older than 70 years, renal failure or body weight > 90 kg excluded. | |
| Interventions | Intervention: Monotherapy with 100 mg oral coenzyme Q10 daily for 10 weeks Control: Placebo | |
| Outcomes | Resting supine SBP and DBP at 10 weeks | |
| Notes | Sources of funding not stated. Small study size. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote "...patients were randomly assigned... then each patient in group A crossed over to placebo treatment and each patient in group B crossed over to CoQ treatment..." Does not describe the sequence generation process. |
| Allocation concealment? | High risk | Insufficient information provided. Does not describe how allocation concealment was ensured. |
| Blinding? | High risk | No information provided as to whether blinding was achieved. |
| Incomplete outcome data addressed? | Low risk | No missing outcome data. |
| Free of selective reporting? | High risk | BP reported was stated as end of treatment. BPs at other times were not reported. |
| Free of other bias? | High risk | Patients could have been selected based on previous response to coenzyme Q10. BP standard deviations are lower and not as variable as would be expected. |
| Methods | Randomized, placebo‐controlled, double‐blind trial. | |
| Participants | 59 patients (52 men) patients with known coronary heart disease and essential hypertension (receiving medications for more than 1 year) | |
| Interventions | Intervention: Monotherapy with 60 mg CoQ twice daily (120 mg/day) Control: Placebo (Vitamin B complex) | |
| Outcomes | Resting supine SBP and DBP and heart rate at 4 and 8 weeks | |
| Notes | Sources of funding not stated. Q‐gel capsules provided free of cost by Tischon Corporation, USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Quote: "Each was individually randomised by the pharmacist..." Does not describe the sequence generation process. |
| Allocation concealment? | High risk | Does not describe allocation concealment |
| Blinding? | High risk | Quote: "The subjects in both groups remain blinded... and both the groups met separately." Meeting separately would lead to loss of blinding. Quote: "... to receive either coenzyme Q10 or vitamin capsules supplied in identical containers by the Heart Research Laboratory blinded to physicians and technicians examining the blood." |
| Incomplete outcome data addressed? | High risk | Author reports placebo group n=32 with 3 patients lost to follow‐up (n=29), however, table of results during follow‐up displays n=28. Quote (from correspondence): "It is possible that data may have been available for only 28. I don't remember exactly." |
| Free of selective reporting? | High risk | Patients were seen weekly and BP data was only reported at 4 and 8 weeks. |
| Free of other bias? | High risk | BP standard deviations are lower and not as variable as would be expected. |
| Methods | Randomized, placebo‐controlled, double‐blind trial. | |
| Participants | 52 patients with essential hypertension (BP > 150/90 mmHg) were selected at random from the outpatient clinic of The Center for adult Diseases in Osaka, Japan. 20 patients (8 men and 12 women, mean age 60 years) with low coenzyme Q10 and low SDH‐Q reductase activity were accepted. Conventional hypertension therapies were continued without change. | |
| Interventions | Intervention: Monotherapy with 33.3 mg CoQ 3x daily (100 mg/day) Control: Placebo | |
| Outcomes | SBP and DBP at 2 week intervals: (no description of position of patient or method of BP measurement). | |
| Notes | Sources of funding not stated. Limited to patients with low coenzyme Q10 levels, who could be particularly responsive to BP lowering effect of intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "A total of 20 patients was randomized..." Does not describe the sequence generation process. |
| Allocation concealment? | Unclear risk | "The capsules were numbered and the code was kept... until all trial had been over" Not clear whether numbers were random or in sequence. |
| Blinding? | Unclear risk | Quote [direct quotation, note typographical errors]: "The capsules were numbered and the code was kept... until all trial had been over... After all data were fixed in each case, key code was opened and the change of blood pressure was compared between coQ group and placebo group." Comment: insufficient information about how key codes were assigned. |
| Incomplete outcome data addressed? | Low risk | No missing outcome data. |
| Free of selective reporting? | Low risk | BP data at all time points was provided. |
| Free of other bias? | Low risk | SD data is as would be expected. |
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Washout period was only 10 days. | |
| No placebo control. | |
| No placebo control. | |
| No placebo control. | |
| Trial is not randomized, no parallel placebo group. Washout period is only 1‐2 weeks. | |
| Included mostly patients with normal blood pressure, baseline BP ranged from 127/75 to 136/80 mmHg and therefore, did not meet hypertension criteria. | |
| No placebo control. | |
| Treatment period was less than 3 weeks (longest post‐dose period was 8 hours). |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SBP Show forest plot | 3 | 96 | Mean Difference (Fixed, 95% CI) | ‐10.72 [‐13.77, ‐7.67] |
| Analysis 1.1  Comparison 1 Coenzyme Q10 vs placebo, Outcome 1 SBP. | ||||
| 2 DBP Show forest plot | 3 | 96 | Mean Difference (Fixed, 95% CI) | ‐6.64 [‐8.10, ‐5.17] |
| Analysis 1.2  Comparison 1 Coenzyme Q10 vs placebo, Outcome 2 DBP. | ||||
| 3 Heart rate Show forest plot | 1 | 58 | Mean Difference (Fixed, 95% CI) | ‐12.0 [‐15.19, ‐8.81] |
| Analysis 1.3 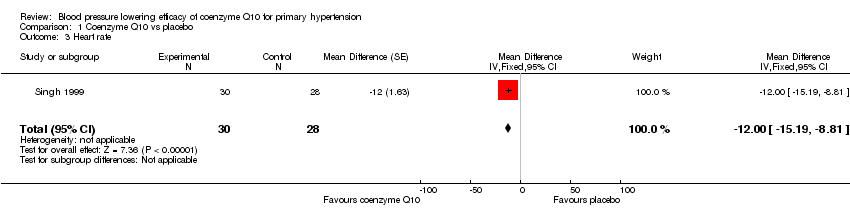 Comparison 1 Coenzyme Q10 vs placebo, Outcome 3 Heart rate. | ||||
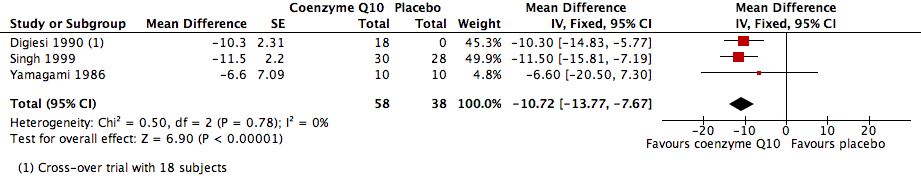
Forest plot of comparison: 1 Coenzyme Q10 vs placebo, outcome: 1.1 SBP.

Forest plot of comparison: 1 Coenzyme Q10 vs placebo, outcome: 1.2 DBP.
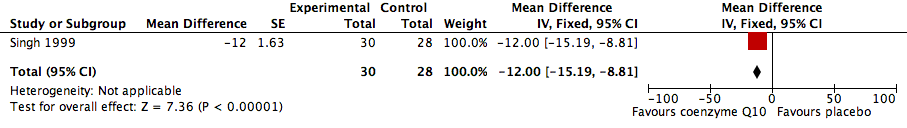
Forest plot of comparison: 1 Coenzyme Q10 vs placebo, outcome: 1.3 Heart rate.

Comparison 1 Coenzyme Q10 vs placebo, Outcome 1 SBP.

Comparison 1 Coenzyme Q10 vs placebo, Outcome 2 DBP.

Comparison 1 Coenzyme Q10 vs placebo, Outcome 3 Heart rate.
| Patient population: patients with essential arterial hypertension (SBP > 140 mmHg or DBP > 90 mmHg) Settings: primary care in Japan, Italy and India Intervention: coenzyme Q10 Comparison: placebo | ||||
| Outcomes | Relative effect (95% CI) | Number of participants (studies) | Quality of evidence (GRADE) | Comments |
| Systolic blood pressure (SBP) | ‐10.72 [‐13.77, ‐7.67] | 96 (3) | Low | |
| Diastolic blood pressure (DBP) | ‐6.64 [‐8.10, ‐5.17] | 96 (3) | Low | |
| Change in heart rate (HR) | ‐12.00 [‐15.19, ‐8.81] | 58 (1) | Low | |
| Number of withdrawals due to adverse effects compared to placebo | unknown, reason for withdrawals not reported in Singh 1999 | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SBP Show forest plot | 3 | 96 | Mean Difference (Fixed, 95% CI) | ‐10.72 [‐13.77, ‐7.67] |
| 2 DBP Show forest plot | 3 | 96 | Mean Difference (Fixed, 95% CI) | ‐6.64 [‐8.10, ‐5.17] |
| 3 Heart rate Show forest plot | 1 | 58 | Mean Difference (Fixed, 95% CI) | ‐12.0 [‐15.19, ‐8.81] |

