Blood pressure lowering efficacy of coenzyme Q10 for primary hypertension
Abstract
Background
Blood pressure is a commonly measured risk factor for non‐fatal and fatal cardiovascular adverse events such as heart attacks and strokes. Clinical trials have suggested that coenzyme Q10, a non‐prescription nutritional supplement, can effectively lower blood pressure (BP). When this review was completed and published in October 2009, it concluded that "due to the possible unreliability of the 3 included studies, it is uncertain whether or not coenzyme Q10 reduces blood pressure in the long‐term management of primary hypertension."
Objectives
To determine the blood pressure lowering effect of coenzyme Q10 in primary hypertension.
Search methods
We searched the Hypertension Group Specialised Register (1946 to November 2015), The Cochrane Central Register of Controlled Trials (The Cochrane Library 2015, Issue 10), MEDLINE (1946 to November 2015), MEDLINE In‐Process (accessed 10 November 2015), EMBASE (1974 to November 2015), Web of Science (1899 to November 2015), CINAHL (1970 to November 2015), and ClinicalTrials.gov (accessed 10 November 2015). We also searched reference lists of articles for relevant clinical trials in any language.
Selection criteria
Double blind, randomized, placebo‐controlled parallel or cross‐over trials evaluating the blood pressure (BP) lowering efficacy of coenzyme Q10 for a duration of at least three weeks, in patients with primary hypertension.
Data collection and analysis
The primary author determined trial inclusion, extracted the data and assessed the risk of bias. The second author independently verified trial inclusion and data extraction.
Main results
In this update of the review, one new randomized, controlled cross‐over trial with a total of 30 participants was added, and one trial included in the initial review was excluded. Only two of the three included trials were pooled in the meta‐analysis, as one trial was judged to have an unacceptably high risk of bias. In the meta‐analysis of two RCTs (50 participants), coenzyme Q10 did not significantly change systolic BP: ‐3.68 mm Hg (95% confidence interval (CI) ‐8.86 to 1.49), or diastolic BP: ‐2.03 mm Hg (95% CI ‐4.86 to 0.81] ), based on clinic data.
Authors' conclusions
This review provides moderate‐quality evidence that coenzyme Q10 does not have a clinically significant effect on blood pressure. In one of three trials reporting adverse effects, coenzyme Q10 was well tolerated. Due to the small number of individuals and studies available for analysis, more well‐conducted trials are needed.
PICOs
Plain language summary
Coenzyme Q10 for High Blood Pressure
Background
High blood pressure is a common condition that can increase risk for certain diseases such as heart attacks, strokes, and kidney disease. Coenzyme Q10, a non‐prescription nutritional supplement, has been suggested as a potential drug to lower blood pressure. When this review was completed and published in October 2009, it concluded that "due to the possible unreliability of the three included studies, it is uncertain whether or not coenzyme Q10 reduces blood pressure in the long‐term management of primary hypertension."
Review Question
We asked whether coenzyme Q10 compared to a placebo affected blood pressure in patients with high blood pressure.
Study Characteristics
For this update, databases of registered clinical trials and published trial reports were searched up until 10 November 2015 for any studies that tested the effects of coenzyme Q10 on patients' blood pressure. One new trial was found and one trial in the initial review was excluded. These studies measured the effects of coenzyme Q10 on blood pressure in non‐hospitalized men and women who took the drugs for 8 to 12 weeks. One of the three trials was judged to have an unacceptably high risk of bias and was not included in the pooled analysis. The total number of patients studied in the two pooled trials was 50.
Key Results
Pooled data from two trials showed that coenzyme Q10 did not affect blood pressure compared to placebo. The number of patients stopping the drug due to adverse effects was also an outcome of interest. In one of the three included trials, coenzyme Q10 was well‐tolerated and no adverse effects were reported.
Quality of the Evidence
This review provides moderate‐quality evidence that coenzyme Q10 does not lower blood pressure. However, more well‐conducted studies are needed to be sure.
Authors' conclusions
Summary of findings
| Coenzyme Q10 compared with placebo for primary hypertension | ||||
| Patient population: patients with primary hypertension Settings: primary care in Japan and New Zealand Intervention: coenzyme Q10 100 to 200 mg daily Comparison: placebo | ||||
| Outcomes | Mean difference in BP mmHg [95% CI] | No of Participants | Quality of the evidence | Comments |
| End of treatment SBP (over 12 weeks) | ‐3.7 mm Hg (‐8.9 to 1.5) | 50 (2) | ⊕⊕⊕⊝ | |
| End of treatment DBP (over 12 weeks) | ‐2.0 mm Hg (‐4.8 to 0.8) | 50 (2) | ⊕⊕⊕⊝ | |
| Withdrawals due to adverse effects | 30 (1) | Effect estimate not available; only one study reported this outcome but had no events in either study arm. | ||
| GRADE Working Group grades of evidence | ||||
| 1. Downgraded due to large confidence intervals from a small sample size and small number of included studies. | ||||
Background
Description of the condition
Hypertension is a common medical condition and major risk factor for stroke, myocardial infarction, congestive heart failure, kidney failure, and peripheral vascular disease. Pharmacological interventions have been shown to reduce blood pressure and modestly decrease stroke, myocardial infarction, and mortality (Musini 2009a). However, hypertension remains prevalent in the community and additional treatment options are needed (Burt 1995).
Description of the intervention
Coenzyme Q10 (CoQ10) is a non‐prescription nutritional supplement that is commonly taken daily. Also called ubiquinone, it is a fat soluble molecule that acts as an electron carrier in mitochondria and as a coenzyme for mitochondrial enzymes (Langsjoen 1985). As a bioenergetic molecule, coenzyme Q10 is obtained through both tissue synthesis and diet (Langsjoen 1985). Supplementary oral administration of coenzyme Q10 has been shown to increase coenzyme Q10 levels in plasma, platelets, and white blood cells (Niklowitz 2007).
Studies suggest that CoQ10 deficiency may be associated with a multitude of diseases as diverse as coronary artery disease and congestive heart failure, Parkinson's disease, diabetes, and breast cancer, as well as the risk factor, hypertension (Niklowitz 2007). It has been suggested that CoQ10 has the potential to lower blood pressure without significant adverse events in hypertensive patients (Rosenfeldt 2007).
How the intervention might work
Coenzyme Q10, as an antioxidant, could act directly on vascular endothelium to decrease total peripheral resistance or could act by reducing superoxide synthesis (McCarty 1999). Coenzyme Q10 also has possible anti‐atherogenic effects as a modulator of ß‐integrin levels on the surface of blood monocytes (Quinzii 2007).
Why it is important to do this review
Many people take CoQ10 for hypertension, cardiovascular health, or both. A non‐Cochrane review concluded that CoQ10 reduced blood pressure (Rosenfeldt 2007). However, in that review, there was no reported assessment of the risk of bias in the included trials. The first version of this review was completed and published in October 2009, and concluded that "due to the possible unreliability of the three included studies, it is uncertain whether or not CoQ10 reduces blood pressure in the long‐term management of primary hypertension" (Ho 2009). The present update of this systematic review uses the latest Cochrane methodology to assess the blood pressure lowering effect of different doses of CoQ10. The information derived from this review should assist clinicians in determining whether or not it is worth trying CoQ10 as a therapeutic intervention to lower blood pressure, and to help determine whether further studies that measure cardiovascular outcomes should be conducted.
Objectives
Primary objective:
-
To determine the dose‐related effect of coenzyme Q10 on systolic and diastolic blood pressure in hypertensive patients.
Secondary objectives:
-
To determine the dose‐related effects of coenzyme Q10 on heart rate.
-
To determine the effects of coenzyme Q10 in different doses on withdrawals due to adverse effects.
Methods
Criteria for considering studies for this review
Types of studies
We included study designs that met the following criteria: randomized, placebo‐controlled parallel or cross‐over trial; double blind; treatment duration of at least three weeks; washout period of at least two weeks before the start of trial or between treatment periods; blood pressure measurement at baseline (following washout) and at one or more time points between 3 to 12 weeks after starting treatment. A washout period of at least two weeks is important, to be reasonably sure that the blood pressure is elevated and stable and that there are no longer any effects of antihypertensive drugs that have been stopped.
Types of participants
We included participants with a baseline systolic blood pressure (SBP) of at least 140 mm Hg or a diastolic blood pressure (DBP) of at least 90 mm Hg, measured in a standard way. We excluded patients with significant renal insufficiency and a documented serum creatinine level greater than 1.5 times the normal values. We did not restrict participants by age, gender, baseline risk, or other co‐morbid conditions.
Types of interventions
Intervention: Coenzyme Q10 (CoQ10) in any dose, as either the sole anti‐hypertensive therapy or added to the participants' current anti‐hypertensive medication regimen that remained unchanged throughout the study. Data from trials in which CoQ10 titration to a higher dose was based on blood pressure response were not eligible.
Control: Placebo.
Types of outcome measures
Primary outcomes
Change in systolic and diastolic blood pressure from baseline, or difference in endpoint (end‐of‐treatment) systolic and diastolic blood pressure. Both were considered acceptable outcomes, but if possible, end‐of‐treatment SBP and DBP values were preferred over change in baseline results. If blood pressure measurements were available at more than one time within the acceptable window, the means of blood pressures taken in the 3 to 12 week range were used.
Secondary outcomes
-
Change in heart rate from baseline or endpoint heart rate.
-
Number of patient withdrawals due to adverse effects.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases for primary studies: the Hypertension Group Specialised Register (1946 to November 2015), the Cochrane Central Register of Controlled Trials (The Cochrane Library 2015, Issue 10), MEDLINE (1946 to November 2015), MEDLINE In‐Process (accessed 10 November 2015), EMBASE (1974 to November 2015), Web of Science (1899 to November 2015), CINAHL (1970 to November 2015), and ClinicalTrials.gov (accessed 10 November 2015).
We searched electronic databases using a strategy that combined a variation of the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐maximizing version (2008 revision) with selected MeSH terms and free text terms relating to coenzyme Q10 and hypertension. We used no language restrictions. We adapted the MEDLINE search strategy (Appendix 1) for the Hypertension Group Specialised Register (Appendix 2), CENTRAL (Appendix 3), EMBASE (Appendix 4), CINAHL (Appendix 5), and Web of Science (Appendix 6),using the appropriate controlled vocabulary as indicated.
Searching other resources
a) Reference lists of relevant studies and reviews.
b) Contact authors of trials to ask if they know of any unpublished trial reports.
Data collection and analysis
Selection of studies
During the initial screen for potential relevance, we excluded articles whose titles, abstracts, or both, were clearly irrelevant. We retrieved the full text of the remaining articles, and translated them into English where required. We searched the bibliographies of pertinent articles, reviews, and texts for additional citations. Two review authors independently assessed the eligibility of the trials, using a trial selection form. A third review author resolved discrepancies.
Data extraction and management
Two review authors independently extracted data using a standard form, and then cross‐checked the data. A second person confirmed all numeric calculations and graphic interpolations.
The position of the patient during blood pressure measurement may affect the blood pressure lowering effect. However, in order not to lose valuable data if only one position was reported, we used data from that position. When blood pressure measurement data were available in more than one position, our first preference was the sitting blood pressure. If standing and supine blood pressures were available, we used standing blood pressure.
Assessment of risk of bias in included studies
We assessed all trials using the 'Risk of bias' tool under the categories: adequate sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other biases. For other biases, we considered: patient selection bias, bias specific to the way cross‐over trials were reported, and suspected bias due to unrealistic standard deviations.
Measures of treatment effect
We calculated mean differences, using generic inverse variance for the continuous variables systolic and diastolic blood pressure, and heart rate.
Unit of analysis issues
We only accepted cross‐over trials for data entry if paired analyses from both periods were reported. Data were entered using generic inverse variance and mean difference for continuous data.
Dealing with missing data
In case of missing information in the included studies, we contacted investigators (using email, letter, fax, or a combination) to obtain the missing information.
We calculated standard errors from the standard deviations in the reported data. In the case of a missing standard deviation and standard error in a study, we imputed the standard deviation, based on the information in the same trial or from other trials using the same dose. We used the following hierarchy (listed from high to low preference) to impute standard deviation values (Heran 2008a; Heran 2008b; Heran 2012; Musini 2008):
-
standard deviation of change in blood pressure from a different position than that of the blood pressure data used.
-
standard deviation of blood pressure at the end of treatment.
-
standard deviation of blood pressure at the end of treatment, measured from a different position than that of the blood pressure data used.
-
standard deviation of blood pressure at baseline (unless this measure was used for entry criteria).
-
mean standard deviation of change in blood pressure from other trials using the same drug and dose.
Assessment of heterogeneity
We used Chi² and I² statistics to test for heterogeneity of treatment effect between the trials. In the event of significant heterogeneity, we used the random‐effects model to test for significance. Our preferred method to report the summary statistics of pooled trials was the fixed‐effect model.
Data synthesis
We used the Cochrane Review Manager software, RevMan 5.3.5 for data synthesis and analyses.
We pooled the data for the continuous variables of blood pressure and heart rate, measured at three weeks or more after randomization, using the generic inverse variance fixed‐effect model for mean differences. Using this method allowed us to pool the results of cross‐over and parallel trials. Results from different doses or dosing regimens were pooled due to the paucity of data. The drop‐outs due to adverse effects would have been analyzed using relative risk, risk difference, and number needed to harm. However, there was insufficient information in the included studies for this analysis.
Subgroup analysis and investigation of heterogeneity
It was not possible to do the following planned analyses due to the small number of included trials:
-
Trials that were industry‐sponsored versus non‐industry sponsored trials.
-
Trials with blood pressure data measured in the sitting position versus other measurement positions.
-
Trials with published standard deviations of blood pressure change versus imputed standard deviations.
Sensitivity analysis
The impact of bias on the results was assessed by removing poor quality trials (those with a high risk of bias).
Results
Description of studies
Results of the search
See PRISMA flow diagram (Figure 1) for details.
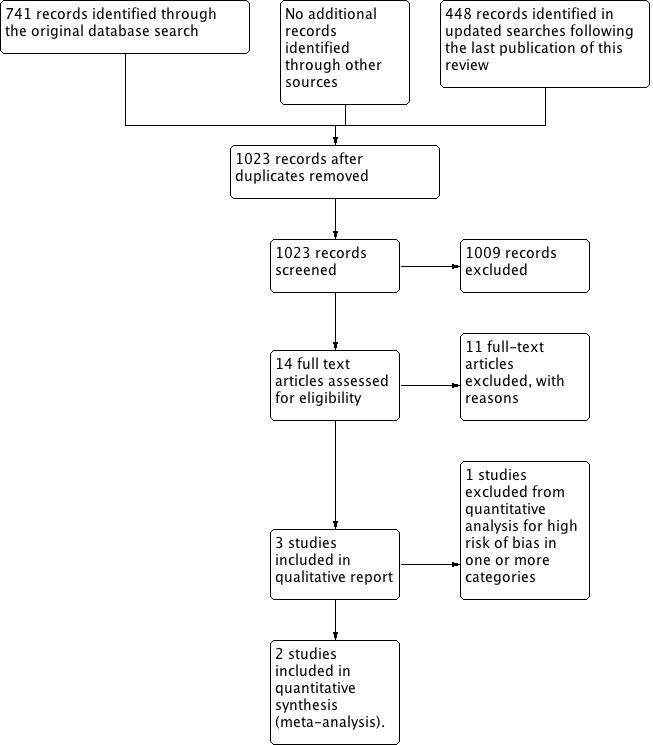
PRISMA Study flow diagram
We identified 741 records in the initial search; 448 more were identified in the updated searches. Of these references, 14 appeared to meet our eligibility criteria; we excluded 11 after screening the full text, one of which had been incorrectly included in the initial review (Singh 1999). Three studies are included in the qualitative report (Digiesi 1990; Yamagami 1986; Young 2012), and two are included in the meta‐analysis (Yamagami 1986; Young 2012). Digiesi 1990 was not included in the meta‐analysis because we judged it to have an unacceptably high overall risk of bias.
Included studies
See Characteristics of included studies for details.
We described three studies in the 'Characteristics of included studies' table. Only two studies, conducted in Japan and New Zealand, respectively, were included in the meta‐analysis (Yamagami 1986; Young 2012). Included participants had an initial blood pressure (BP) higher than 140/90 mm Hg (or at least 130 mm Hg for patients with type 2 diabetes mellitus) in Young 2012, while the participants in Yamagami 1986 had a higher initial BP of at least 150/90 mm Hg. The age of participants ranged from late fifties to early sixties. Young 2012 included participants with metabolic syndrome, while baseline comorbidity data were not available for Yamagami 1986. Yamagami 1986 only included participants with a low CoQ10 level, a low succinate dehydrogenase activity, or both, at baseline. Both trials allowed participants to continue previous anti‐hypertensive therapies, but Young 2012 reported exclusion of participants who were previously taking CoQ10. Young 2012 was a cross‐over trial with a four‐week washout period between two 12‐week treatment periods, whereas Yamagami 1986 compared CoQ10 to placebo in a parallel, double blind, randomized controlled trial after a minimum four‐week washout period. Yamagami 1986 used 100 mg of CoQ10 daily, while Young 2012 used 100 mg twice daily. Clinic BP data were available for both studies, but Young 2012 also reported 24‐hour ambulatory BP monitoring data.
We judged the third included study to have an unacceptably high risk of bias and flaws in study conduct and analysis and thus, we did not include its data in the data analysis for this review (Digiesi 1990). This study was a cross‐over trial with a two‐week washout period before each 10‐week treatment period of 100 mg CoQ10 daily. The problems with the Digiesi 1990 cross‐over study are as follows and are also described in the 'Risk of bias' section below. We decided not to pool the data from this trial because we assessed it to be at high risk of bias for allocation concealment, blinding, smaller than expected BP standard deviations, the lack of BP decrease in the placebo group, and the fact that despite it being a paired comparison, it did not report a paired analysis.
Excluded studies
See Characteristics of excluded studies.
Eleven studies seemed to meet our criteria on abstract screening, but were later excluded after examining the full text. The reasons for exclusion were: lack of randomization (Hata 1977), lack of a placebo control (Digiesi 1994; Drzewoski 1981; Folkers 1981; Langsjoen 1994; Shcherbakova 2010), washout period less than two weeks (Burke 2001), treatment period less than three weeks (Shah 2007), and lack of hypertensive participants in the sample size (Hodgson 2002). We were unable to consider Eghtesadi 2012 further at this time due to the unavailability of a full‐text report.
We included Singh 1999 in the initial review but excluded it here, because after reading it carefully again and assessing it with the new 'Risk of bias' criteria, we felt it did not meet our inclusion criteria. It did not describe the sequence generation or allocation concealment processes; subjects in the two groups met separately, suggesting that the blinding integrity was lost; drop‐outs after randomization were not described; the doses of other anti‐hypertensives were changed during the trial; and BP standard deviations (CoQ10 8.4/4.2 mm Hg, placebo 8.4/4.7 mm Hg) were lower than the average resting BP standard deviation of 14/8 mm Hg seen in other research settings (Musini 2009b; Musini 2014).
Risk of bias in included studies
See risk of bias summary (Figure 2) and Characteristics of included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
One trial described the sequence generation process as using a computer‐generated randomization list in blocks of six, a process done by a statistician not clinically involved in the study (Young 2012). The other two trials did not describe the sequence generation process or how allocation concealment was achieved (Digiesi 1990; Yamagami 1986)
Blinding
All trials reported that personnel were blinded, however, only one described a process that would indicate this was definitely the case, i.e. using identical CoQ10 and placebo capsules in identical numbered bottles that were sequentially dispensed by an independent pharmacist (Young 2012). One study described the process as using numbered capsules and locked key codes, but did not report who performed the blinding or whether the capsules were numbered randomly or sequentially (Yamagami 1986). The third study provided no details of blinding (Digiesi 1990).
Incomplete outcome data
Yamagami 1986 and Digiesi 1990 did not lose any subjects to follow‐up. Young 2012 did not lose any patients to follow‐up either, but one patient in the study had a change in their BP medications in the first treatment phase and was withdrawn from the study.
Selective reporting
Blood pressure data were reported in all trials. Withdrawals due to adverse effects were only reported in Young 2012. They also measured "safety markers, including electrolytes, plasma glucose, renal and liver function, hemoglobin A1c and a full blood count... Urine concentrations of creatinine and sodium were also determined." Neither Yamagami 1986 nor Digiesi 1990 mentioned any drug adverse effects leading to withdrawal.
Other potential sources of bias
Yamagami 1986 did not provide any details about how they measured blood pressures.
Young 2012 reported that they measured clinic BPs using standard sphygmomanometry between 0700 and 1100h "according to current guideline recommendations." They described assessing BP "after five minutes of rest in the sitting position using appropriately sized cuffs. Three BP measurements were obtained at two‐minute intervals and the mean of these recordings were calculated as the final clinic BP." These "serial BP measurements were performed by the same trained operator using the same calibrated sphygmomanometer throughout the study." However, they did not specify which arm was used to measure BP, and whether that same arm was used consistently throughout the study. The American Heart Association 2005 Recommendations (Pickering 2005) that were cited by Young 2012, recommended that "blood pressure should be checked in both arms at the first examination. When there is a consistent interarm difference, the arm with the higher pressure should be used." If there was not a consistent interarm difference at the initial visit, Young 2012 did not specify which arm they chose, and whether the same one was used throughout the study. Their other cited European Recommendations (O'Brien 2005) only specify bilateral measurement on the initial study and referral to a cardiovascular centre if the systolic or diastolic BPs differ by more than 20 or 10 mm Hg respectively.
Digiesi 1990 used the mean of five BP readings, measured successively every minute. Standard deviations (10/5 mm Hg) were lower than the average resting BP standard deviations (14/8 mm Hg) in similar research settings (Musini 2009b; Musini 2014), and there was no BP decrease in the placebo group, which would be expected from similar research studies (Heran 2008a). Digiesi 1990 was a cross‐over trial and did not report the findings as a paired analysis.
Effects of interventions
See: Summary of findings for the main comparison
Change in blood pressure (BP) ‐ clinic data
The pooled results of Young 2012 and Yamagami 1986, showed that CoQ10 did not result in a statistically or clinically significant reduction in systolic BP: ‐3.68 mm Hg (95% confidence interval (CI) ‐8.86 to 1.49) or diastolic BP: ‐2.03 mm Hg (95% CI ‐4.86 to 0.81) compared to placebo (Analysis 1.1; Analysis 1.2; Figure 3; Figure 4).

Forest plot of comparison: 1 Coenzyme Q10 vs Placebo: Clinic data, outcome: 1.1 Mean difference in SBP.

Forest plot of comparison: 1 Coenzyme Q10 vs Placebo: Clinic data, outcome: 1.2 Mean difference in DBP.
Change in heart rate ‐ clinic data
In Young 2012, CoQ10 significantly reduced heart rate by ‐4.80 beats per minute (95% CI ‐9.31 to 0.29) compared to placebo, but this was predominantly due to an increase in heart rate in the placebo group, and is unlikely due to an effect of CoQ10 (Analysis 1.3).
Change in blood pressure (BP) and heart rate ‐ 24‐hour data
We found no effect of CoQ10 on blood pressure or heart rate compared to placebo in 24‐hour ambulatory measurements in Young 2012 (Analysis 2.1; Analysis 2.2; Analysis 2.3).
Withdrawals due to adverse effects
When reported, withdrawals due to adverse effects were not reported by treatment group, so this outcome could not be analyzed in this review.
Discussion
Summary of main results
Please see summary of findings Table for the main comparison for details.
This systematic review of coenzyme Q10 (CoQ10) showed no significant decrease in systolic blood pressure (SBP) or diastolic blood pressure (DBP) after 12 weeks of therapy compared to placebo control, based on two RCTs (Figure 2). This finding contrasts with the meta‐analysis of the initial Cochrane review of this question, which suggested a clinically significant reduction in BP with CoQ10 (Ho 2009). However, in that review, we were sceptical of the findings because of the very low‐quality and high or unclear risk of bias in those RCTs. If we had included Digiesi 1990 in the meta‐analysis for this updated review, a small but statistically significant blood pressure lowering effect of CoQ10 would have been found. Yet it is well established that if randomization, concealment of allocation, and blinding are not performed correctly, the effect size is exaggerated. In addition to the biases identified by the risk of bias tool, Digiesi 1990 also reported lower blood pressure standard deviations than expected, which also shed suspicion on the reliability of the results (see the above paragraph in Included studies describing the problems with the Digiesi 1990 trial). Therefore, our judgement was that the high risk of bias of Digiesi 1990 would make the estimate of treatment effect unreliable, and therefore, we did not include this study in the data analysis.
Singh 1999 was included in the initial review but excluded from this review because changes in other anti‐hypertensive drugs were allowed and occurred during the trial, and we judged it to be highly unlikely to be a blinded trial. The reliability of the data from the Singh 1999 trial was also questionable, given the investigations into scientific misconduct of the first author, Dr. Singh (White 2005). Singh has been investigated by the BMJ and Lancet (Mann 2005), which have published "expressions of concern" about his work.
Given the lack of effect of CoQ10 on blood pressure after pooling the data from the two higher quality trials (Yamagami 1986; Young 2012), we believe that CoQ10 probably does not have a true BP lowering effect. However, this conclusion suffers from imprecision because it is based on two small RCTs (N = 50), which is why we have downgraded the quality of the evidence that CoQ10 does not affect BP to moderate. Data from more well‐conducted RCTs are still needed to address this imprecision. In addition, we would like to be able to add other RCTs addressing this question to our review, which may not have been published in the past because they did not show a significant effect.
The additional findings from Young 2012, showing no effect of CoQ10 on the 24‐hour ambulatory monitoring SBP, DBP, or heart rate compared to placebo provide supporting evidence for the conclusion that CoQ10 has no clinically significant effect on BP. The 24‐hour measurements are done using an automatic machine, and thus are free from observer bias that could occur with clinic measurements done by a physician or nurse.
Coenzyme Q10 appears to be safe, as none of the three included RCTs reported any significant adverse effects associated with it. Young 2012 reported that "coenzyme Q10 was well tolerated and there were no reported serious adverse effects." There were also "no clinically or statistically significant changes in biochemistry and hematology safety parameters." Yamagami 1986 and Digiesi 1990 did not address this outcome.
Overall completeness and applicability of evidence
In this review, the results of only two RCTs with 50 patients were available for meta‐analysis, representing a small number of participants. Young 2012 is the first published RCT of CoQ10 done in hypertensive patients in which there was no trend towards a BP lowering effect. It is probable that there are other RCTs assessing the BP lowering effect of CoQ10 that also showed no effect on blood pressure and were never published. Therefore, it is likely that this review is an incomplete reflection of the totality of available evidence. Hopefully, the publication of the Young 2012 trial and this updated review will encourage individuals who have unpublished negative studies to send them to us or submit them for publication. As evidenced by the Young 2012 publication in the American Journal of Hypertension, publishing trials showing no effect is easier now than it has been in the past.
A possible limitation of this review is that the Young 2012 trial defined hypertension in patients with type 2 diabetes mellitus as SBP at least 130 mm Hg, so that some patients with a SBP lower than 140 mm Hg were likely included. However, this definition of hypertension is consistent with recent practice standards and treatment goals for hypertension in this subset of patients (CHEP 2015), and it is unlikely that inclusion of these patients would affect the results and conclusions.
Quality of the evidence
Interpretation of the findings of this review was difficult because of the substantial bias in some of the available published trials. This problem is a common challenge for many reviews, and was dealt with in this review by not including data for the meta‐analysis from the trial that was judged to have an unacceptably high risk of bias (Digiesi 1990).
Agreements and disagreements with other studies or reviews
The conclusions of this review differ from previous systematic reviews looking at the blood pressure lowering effect of CoQ10 in people with hypertension (Rosendfeldt 2003; Rosenfeldt 2007), as well as the initial version of this Cochrane review (Ho 2009). The Rosenfeldt reviews suffer from all the same limitations of the initial Cochrane review, namely that most of the available trials have a high risk of bias. The Rosenfeldt 2007 review also included open label studies, which suffer from a high risk of bias due to lack of blinding. The initial Cochrane review identified the same three RCTs that were identified in the Rosenfeldt reviews (Ho 2009) .

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Coenzyme Q10 vs Placebo: Clinic data, outcome: 1.1 Mean difference in SBP.

Forest plot of comparison: 1 Coenzyme Q10 vs Placebo: Clinic data, outcome: 1.2 Mean difference in DBP.

Comparison 1 Coenzyme Q10 vs Placebo: Clinic data, Outcome 1 Mean difference in SBP.
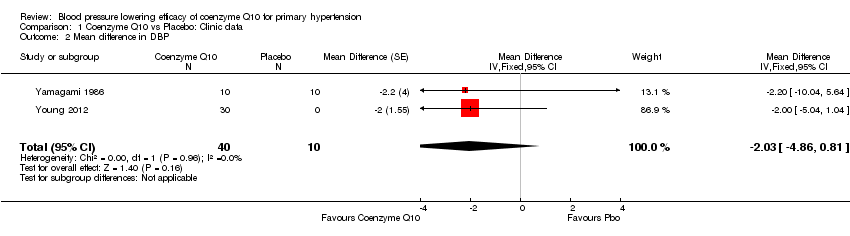
Comparison 1 Coenzyme Q10 vs Placebo: Clinic data, Outcome 2 Mean difference in DBP.

Comparison 1 Coenzyme Q10 vs Placebo: Clinic data, Outcome 3 Mean difference in HR.
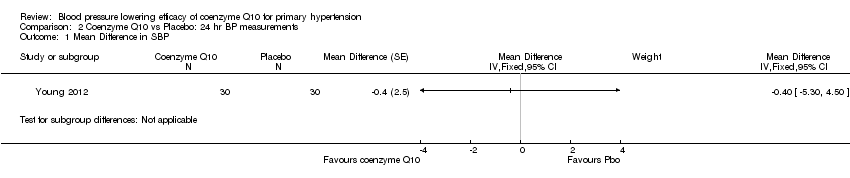
Comparison 2 Coenzyme Q10 vs Placebo: 24 hr BP measurements, Outcome 1 Mean Difference in SBP.
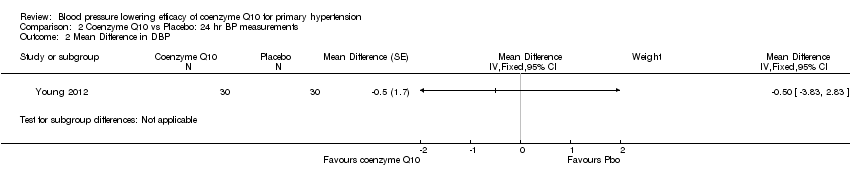
Comparison 2 Coenzyme Q10 vs Placebo: 24 hr BP measurements, Outcome 2 Mean Difference in DBP.

Comparison 2 Coenzyme Q10 vs Placebo: 24 hr BP measurements, Outcome 3 Mean Difference in HR.
| Coenzyme Q10 compared with placebo for primary hypertension | ||||
| Patient population: patients with primary hypertension Settings: primary care in Japan and New Zealand Intervention: coenzyme Q10 100 to 200 mg daily Comparison: placebo | ||||
| Outcomes | Mean difference in BP mmHg [95% CI] | No of Participants | Quality of the evidence | Comments |
| End of treatment SBP (over 12 weeks) | ‐3.7 mm Hg (‐8.9 to 1.5) | 50 (2) | ⊕⊕⊕⊝ | |
| End of treatment DBP (over 12 weeks) | ‐2.0 mm Hg (‐4.8 to 0.8) | 50 (2) | ⊕⊕⊕⊝ | |
| Withdrawals due to adverse effects | 30 (1) | Effect estimate not available; only one study reported this outcome but had no events in either study arm. | ||
| GRADE Working Group grades of evidence | ||||
| 1. Downgraded due to large confidence intervals from a small sample size and small number of included studies. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean difference in SBP Show forest plot | 2 | 50 | Mean Difference (Fixed, 95% CI) | ‐3.68 [‐8.86, 1.49] |
| 2 Mean difference in DBP Show forest plot | 2 | 50 | Mean Difference (Fixed, 95% CI) | ‐2.03 [‐4.86, 0.81] |
| 3 Mean difference in HR Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean Difference in SBP Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 2 Mean Difference in DBP Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 3 Mean Difference in HR Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |

