Blood pressure lowering efficacy of coenzyme Q10 for primary hypertension
References
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Randomized, placebo‐controlled, cross‐over trial. 2 week washout period, 10 week treatment period, 2 week treatment suspension, 10 week cross‐over treatment. | |
| Participants | 18 patients (4 women, 14 men) with essential hypertension, WHO stages 1 and 2, average age 55.9 years (range 42 to 66 years). Patients older than 70 years, renal failure or body weight > 90 kg excluded. | |
| Interventions | Intervention: Monotherapy with 100 mg oral coenzyme Q10 daily for 10 weeks Control: Placebo | |
| Outcomes | Resting supine SBP and DBP at 10 weeks | |
| Notes | Sources of funding not stated. Small study size. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "...patients were randomly assigned... then each patient in group A crossed over to placebo treatment and each patient in group B crossed over to CoQ treatment..." Does not describe the sequence generation process. |
| Allocation concealment (selection bias) | High risk | Insufficient information provided. Does not describe how allocation concealment was ensured. |
| Blinding (performance bias and detection bias) | High risk | No information provided as to whether blinding was achieved. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | High risk | BP reported was stated as end of treatment. BPs at other times were not reported. |
| Other bias | High risk | No indication as to how patients were selected, "eighteen subjects were selected from among those presenting with essential arterial hypertension," so they could have been selected based on previous response to CoQ10. BP standard deviations are lower and not as variable as would be expected. |
| Methods | Randomized, placebo‐controlled, double blind trial. | |
| Participants | 52 patients with essential hypertension (BP > 150/90 mm Hg) were selected at random from the outpatient clinic of The Center for Adult Diseases in Osaka, Japan. 20 patients (8 men and 12 women, mean age 60 years) with low CoQ10 and low SDH‐Q reductase activity were accepted. Conventional hypertension therapies were continued without change. | |
| Interventions | Intervention: Monotherapy with 33.3 mg CoQ10 3x daily (100 mg/day) Control: Placebo | |
| Outcomes | SBP and DBP at 2 week intervals (no description of position of patient or method of BP measurement). | |
| Notes | Sources of funding not stated. Limited to patients with low CoQ10 levels, who could be particularly responsive to BP lowering effect of intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A total of 20 patients was randomized..." Does not describe the sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | "The capsules were numbered and the code was kept... until all trial had been over." Not clear whether numbers were random or in sequence. |
| Blinding (performance bias and detection bias) | Unclear risk | Quote [direct quotation, note typographical errors]: "The capsules were numbered and the code was kept... until all trial had been over... After all data were fixed in each case, key code was opened and the change of blood pressure was compared between coQ group and placebo group." Comment: insufficient information about how key codes were assigned. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | BP data at all time points was provided. |
| Other bias | Low risk | SD data is as would be expected. |
| Methods | Randomized, placebo‐controlled, double blind cross‐over trial. | |
| Participants | 30 patients (15 men, 15 women) with metabolic syndrome and inadequate BP control (SBP ≥ 140 or ≥ 130 for patients with type 2 diabetes) on an unchanged, anti‐hypertensive regimen. Patients with significant comorbidities (cardiovascular, renal, hepatic, metabolic, etc.) were excluded. | |
| Interventions | Cross‐over with CoQ10 (100 mg BID) and placebo added to current, unchanged, conventional hypertensive regimen. Patients taking antioxidant vitamin supplementation, including CoQ10, before the trial were excluded (no washout period). A washout period of 4 weeks was present between treatment periods. | |
| Outcomes | Mean 24‐hour ambulatory SBP and DBP, MAP, pulse pressure, HR, mean daytime and nighttime BP and HR, clinic BP (sitting, mean of 3 measurements after 5 minutes of rest) and HR, plasma CoQ10 levels. | |
| Notes | Compliance, safety data and adverse events also documented. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed in permutation blocks of six from a computer‐generated randomization list by a statistician with no clinical involvement in the study." |
| Allocation concealment (selection bias) | Low risk | "The study treatments were dispensed by an independent pharmacist in identical numbered bottles with the lowest available number allocated to each sequential participant." |
| Blinding (performance bias and detection bias) | Low risk | "Participants and investigators administering the treatment and assessing outcomes were blinded to treatment assignment and to plasma coenzyme Q10 levels." "Both Q‐Gel and placebo were supplied by Tishcon (Salisbury, MD), and were identical in appearance and taste." |
| Incomplete outcome data (attrition bias) | Low risk | The authors performed a modified intention‐to‐treat analysis: "Of 60 potential participants screened, 31 entered and 30 completed the study and were included in the analysis." The reason for withdrawal from the study of the 1 study participant was obtained by electronic communication from the study authors ‐ the patient had an increase in BP medication during the study. |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes were reported. |
| Other bias | Low risk | Research was supported by a grant from the National Heart Foundation of New Zealand (Grant Number 1155). Coenzyme Q10 (Q‐Gel) and placebo capsules were supplied by Tishcon, Salisbury, MD. Authors declared no conflicts of interest. |
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Washout period was only 10 days. | |
| No placebo control. | |
| No placebo control. | |
| Only an abstract available | |
| No placebo control. | |
| Trial is not randomized, no parallel placebo group. Washout period is only 1 to 2 weeks. | |
| Included mostly patients with normal blood pressure, baseline BP ranged from 127/75 to 136/80 mm Hg and therefore, did not meet hypertension criteria. | |
| No placebo control. | |
| Treatment period was less than 3 weeks (longest post‐dose period was 8 hours). | |
| Not a placebo controlled trial | |
| Not likely a double blinded trial as different treatment groups met separately. Changes in antihypertensive medications were allowed and occurred during the trial. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean difference in SBP Show forest plot | 2 | 50 | Mean Difference (Fixed, 95% CI) | ‐3.68 [‐8.86, 1.49] |
| Analysis 1.1  Comparison 1 Coenzyme Q10 vs Placebo: Clinic data, Outcome 1 Mean difference in SBP. | ||||
| 2 Mean difference in DBP Show forest plot | 2 | 50 | Mean Difference (Fixed, 95% CI) | ‐2.03 [‐4.86, 0.81] |
| Analysis 1.2 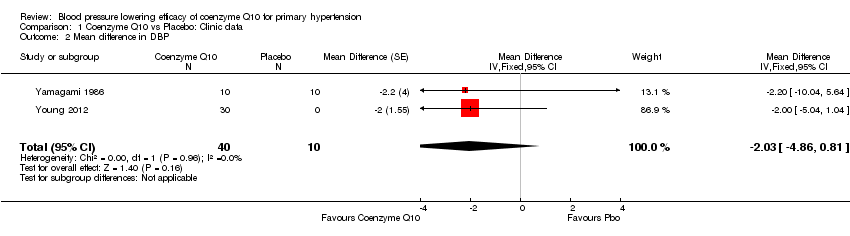 Comparison 1 Coenzyme Q10 vs Placebo: Clinic data, Outcome 2 Mean difference in DBP. | ||||
| 3 Mean difference in HR Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Coenzyme Q10 vs Placebo: Clinic data, Outcome 3 Mean difference in HR. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean Difference in SBP Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1 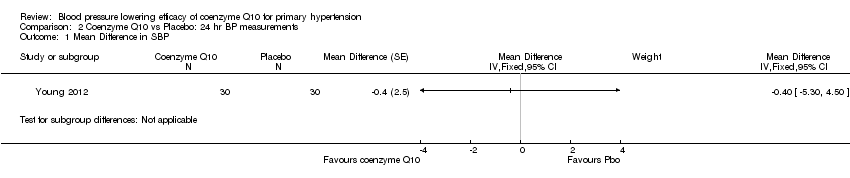 Comparison 2 Coenzyme Q10 vs Placebo: 24 hr BP measurements, Outcome 1 Mean Difference in SBP. | ||||
| 2 Mean Difference in DBP Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| Analysis 2.2 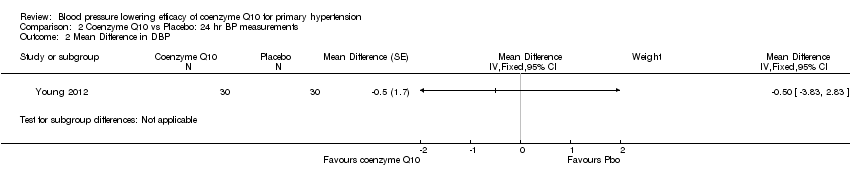 Comparison 2 Coenzyme Q10 vs Placebo: 24 hr BP measurements, Outcome 2 Mean Difference in DBP. | ||||
| 3 Mean Difference in HR Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 Coenzyme Q10 vs Placebo: 24 hr BP measurements, Outcome 3 Mean Difference in HR. | ||||

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Coenzyme Q10 vs Placebo: Clinic data, outcome: 1.1 Mean difference in SBP.

Forest plot of comparison: 1 Coenzyme Q10 vs Placebo: Clinic data, outcome: 1.2 Mean difference in DBP.

Comparison 1 Coenzyme Q10 vs Placebo: Clinic data, Outcome 1 Mean difference in SBP.

Comparison 1 Coenzyme Q10 vs Placebo: Clinic data, Outcome 2 Mean difference in DBP.

Comparison 1 Coenzyme Q10 vs Placebo: Clinic data, Outcome 3 Mean difference in HR.

Comparison 2 Coenzyme Q10 vs Placebo: 24 hr BP measurements, Outcome 1 Mean Difference in SBP.

Comparison 2 Coenzyme Q10 vs Placebo: 24 hr BP measurements, Outcome 2 Mean Difference in DBP.

Comparison 2 Coenzyme Q10 vs Placebo: 24 hr BP measurements, Outcome 3 Mean Difference in HR.
| Coenzyme Q10 compared with placebo for primary hypertension | ||||
| Patient population: patients with primary hypertension Settings: primary care in Japan and New Zealand Intervention: coenzyme Q10 100 to 200 mg daily Comparison: placebo | ||||
| Outcomes | Mean difference in BP mmHg [95% CI] | No of Participants | Quality of the evidence | Comments |
| End of treatment SBP (over 12 weeks) | ‐3.7 mm Hg (‐8.9 to 1.5) | 50 (2) | ⊕⊕⊕⊝ | |
| End of treatment DBP (over 12 weeks) | ‐2.0 mm Hg (‐4.8 to 0.8) | 50 (2) | ⊕⊕⊕⊝ | |
| Withdrawals due to adverse effects | 30 (1) | Effect estimate not available; only one study reported this outcome but had no events in either study arm. | ||
| GRADE Working Group grades of evidence | ||||
| 1. Downgraded due to large confidence intervals from a small sample size and small number of included studies. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean difference in SBP Show forest plot | 2 | 50 | Mean Difference (Fixed, 95% CI) | ‐3.68 [‐8.86, 1.49] |
| 2 Mean difference in DBP Show forest plot | 2 | 50 | Mean Difference (Fixed, 95% CI) | ‐2.03 [‐4.86, 0.81] |
| 3 Mean difference in HR Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean Difference in SBP Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 2 Mean Difference in DBP Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 3 Mean Difference in HR Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |