Chemotherapy as an adjunct to radiotherapy in locally advanced nasopharyngeal carcinoma
References
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Inclusion period: 1989 to 1995. | |
| Participants | 193 patients. Stages III to IV (AJCC < 1997). Histology WHO 1 to 3. | |
| Interventions | Radiotherapy: Tumor: 70 Gray/7 weeks. N‐ 50 Gray, N+ 66 to 70 Gray | |
| Outcomes | Overall survival, event‐free survival. | |
| Notes | 24% of type 1 histology, concomitant cisplatin every 3 weeks, adjuvant cisplatin + 5‐fluorouracil every 4 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Inclusion period: 1988 to 1991. | |
| Participants | 77 patients. Stage III to IV (Ho classification). Histology WHO 3. | |
| Interventions | Radiotherapy: Tumor: 66 Gray/6.5 weeks. N‐ 58 Gray, N+ 65.5 Gray | |
| Outcomes | Overall survival, event‐free survival. | |
| Notes | 2 cycles of induction and 4 cycles of adjuvant chemotherapy. Radiotherapy : nasopharynx, equivalent of 66 Gy with conventional fractionation + 20 Gy boost if parapharyngeal disease + 18 to 24 Gy using 192Ir if residual disease 4 weeks after radiotherapy; neck: 58 Gy for lower neck, 66 Gy for upper neck. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Inclusion period: 1994 to 1999. | |
| Participants | 350 patients. Stages II to IV (AJCC 1997). Histology WHO 1 to 3. | |
| Interventions | Radiotherapy: Tumor: 66 Gray/6.5 weeks. N‐ 58 Gray, N+ 65.5 Gray | |
| Outcomes | Overall survival, event‐free survival. | |
| Notes | 10 or 20 Gy (depending on the centre) boost if parapharyngeal disease, 21 to 24 Gy using 192Ir if residual local disease after radiotherapy, 7.5 Gy boost if residual nodal disease, radical neck dissection if proven residual neck nodes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Inclusion period: 1994 to 1999. | |
| Participants | 158 patients. Stage IV (AJCC < 1997). Histology WHO 1 to 3. | |
| Interventions | Radiotherapy: Tumor: 70 to 72 Gray/7 to 8 weeks. N‐ 50 Gray lower neck | |
| Outcomes | Overall survival, event‐free survival. | |
| Notes | All drugs provided by continuous injections. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Inclusion period: 1989 to 1993. | |
| Participants | 334 patients. Stages II to IV (AJCC < 1997). Histology WHO 2 to 3. | |
| Interventions | Radiotherapy: Tumor 66 to 74 Gray/6.5 to 7.5 weeks. N‐ 60 to 66 Gray, N+ 66 to 76 Gray. | |
| Outcomes | Overall survival, event‐free survival. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Inclusion period: 1989 to 1993. | |
| Participants | 339 patients. Stages III to IV (AJCC < 1997). Histology WHO 1 to 3. | |
| Interventions | Radiotherapy: Tumor 65 to 70 Gray/6.5 to 7.5 weeks. N‐ 50 Gray, N+ 65 Gray. | |
| Outcomes | Overall survival, event‐free survival. | |
| Notes | 110 patients treated with conventional radiotherapy and 176 patients with hypofractionated radiotherapy, 2.5 Gy x 3/weeks followed by 3.5 Gy x 3/week. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Inclusion period: 1991 to 1998. | |
| Participants | 80 patients. Stages I to IV (AJCC < 1997). Histology WHO 1 to 3. | |
| Interventions | Radiotherapy: Tumor: 66 to 68 Gray/6.5 to 7 weeks. N‐ 50 Gray, N+ 66 to 68 Gray | |
| Outcomes | Overall survival, event‐free survival. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Inclusion period: 1995 to 2000. | |
| Participants | 222 patients. Stages II to IV (AJCC 1997). Histology WHO 1 to 3. | |
| Interventions | Radiotherapy: Tumor: 62.5 to 68 Gray/7 weeks. N 62.5 to 66 Gray/7weeks +/‐ boost 10 Gray | |
| Outcomes | Overall survival, Event‐free survival | |
| Notes | 2 X 2 design, concomitant chemotherapy versus none, adjuvant chemotherapy versus none, concomitant+adjuvant chemotherapy versus adjuvant chemotherapy, concomitant +adjuvant chemotherapy versus concomitant chemotherapy ; for adjuvant chemotherapy, alternating cycles of cisplatin + 5‐fluorouracil and vincristine + bleomycin + methotrexate. +/‐ 10 Gy additional boost in case of parapharyngeal space involvement and/or palpable residual nodes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | See Kwong 2004a | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | See Kwong 2004a | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | See Kwong 2004a | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Kwong 2004a (QMH‐95 conc), Kwong 2004b (QMH‐95adj), Kwong 2004c (QMH‐95adj+), Kwong 2004d (QMH‐95conc+): 4 comparisons issued from QMH‐95 2x2 trial.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Allocation: not properly randomised. | |
| Allocation: not properly randomised. | |
| Allocation: not properly randomised. | |
| Individual data lost by the institution. |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomized phase III trial |
| Participants | 316 (158 per group) locoregionally advanced nasopharyngeal carcinomas |
| Interventions | Radiotherapy (70 Grays in 7 weeks) plus concurrent cisplatin (40 mg/m² weekly) followed by adjuvant cisplatin (80 mg/m² on day 1) + 5 Fluoro‐Uracil (800 mg/m² on days 1 to 5) every 4 weeks for 3 cycles versus same radiotherapy |
| Outcomes | Significant benefit in favour of chemotherapy arm on: |
| Notes | Notes: more toxicity and lower compliance in the chemotherapy arm |
| Methods | Randomized phase II trial |
| Participants | 65 (34 CT + CRT versus 31 CRT) stage III to IVb nasopharyngeal carcinomas |
| Interventions | Induction docetaxel (75 mg/m²) + cisplatin (75 mg/m²) every 3 weeks for 2 cycles followed by radiotherapy (conventional or IMRT) + concurrent cisplatin (40 mg/m²) weekly versus same radiotherapy + same concurrent cisplatin |
| Outcomes | Significant benefit on overall survival in favour of induction chemotherapy arm: |
| Notes | Notes: induction CT followed by concurrent RT + CT was well tolerated |
| Methods | |
| Participants | 335 |
| Interventions | RT versus RT + Concomitant Cisplatin + Adjuvant Cisplatin + 5FU versus RT + Induction CT |
| Outcomes | No significant benefit on overall survival |
| Notes | See footnotes |
| Methods | |
| Participants | 221 |
| Interventions | RT versus RT + Concomitant Cisplatin + Adjuvant Cisplatin + 5FU |
| Outcomes | Significant benefit from CT |
| Notes | See footnotes |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | NPC99‐02 |
| Methods | |
| Participants | 189 |
| Interventions | RT versus accelerated RT versus RT + Concomitant Cisplatin + Adjuvant Cisplatin + 5FU versus accelerated RT + Concomitant Cisplatin + Adjuvant Cisplatin + 5FU |
| Outcomes | Preliminary results in favour of accelerated RT + chemotherapy |
| Starting date | 1999 |
| Contact information | Lee AW, Hong Kong Nasopharyngeal Cancer Study Group and the Princess Margaret Hospital in Canada |
| Notes | See footnotes |
| Trial name or title | VUMCA II |
| Methods | |
| Participants | 509 |
| Interventions | Induction CT+ RT versus Induction CT + RT + concomitant CT |
| Outcomes | Not ready for analysis |
| Starting date | 1996 |
| Contact information | Institut Gustave‐Roussy |
| Notes |
| Trial name or title | Sun Yat‐Sen University |
| Methods | |
| Participants | 77 |
| Interventions | RT versus RT + concomitant oxaliplatin |
| Outcomes | Significant benefit from CT |
| Starting date | 2001 |
| Contact information | Cancer Centre of Sun Yat Sen University |
| Notes | See footnotes |
Recently published as a full paper:
‐ Lee AW, Lau W H, Tung S Y, Chua D, Chappell R, Xu L, Siu L et al. Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally advanced Nasopharyngeal Carcinoma: NPC‐9901 trial by the Hong‐Kong Nasopharyngeal Cancer Study Group. J Clin Oncol 2005; 23: 6966‐75.
‐ Wee J, Tan EH, Tia BC, Wong H B, Leong S S, Tan T, Chua E T, Yang E, Lee K M,.Fong KW et al. Phase III randomized trial of radiotherapy versus concurrent chemo‐radiotherapy followed by adjuvant chemotherapy in patients with AJCC/UICC (1997) stage 3 and 4 nasopharyngeal cancer of the endemic variety. J Clin Oncol 2005; 23: 6730‐38.
‐ Zhang L, Zhao C, Peng PJ, Lu LX, Han F, Wu SX. Phase III study comparing standard radiotherapy with or without weekly oxaliplatin in treatment of locoregionally advanced nasopharyngeal carcinoma: preliminary results. J Clin Oncol 2005; 23: 8461‐68.
‐ Lee AW, Tung SY, Chan AT, Chappell R, Fu YT, Lu TX, Tan T, Chua DT, O'Sullivan B, Xu SL, Pang ES, Sze WM, Leung TW, Kwan WH, Chan PT, Liu XF, Tan EH, Sham JS, Siu L, Lau WH. Preliminary results of a randomized study (NPC‐9902 Trial) on therapeutic gain by concurrent chemotherapy and/or accelerated fractionation for locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2006 ;66:142‐51.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Effect of chemotherapy on overall survival, hazard ratio of death by timing of chemotherapy Show forest plot | 11 | 1975 | Peto Odds Ratio (95% CI) | 0.82 [0.71, 0.95] |
| Analysis 1.1  Comparison 1 Radiotherapy versus radiotherapy + chemotherapy, Outcome 1 Effect of chemotherapy on overall survival, hazard ratio of death by timing of chemotherapy. | ||||
| 1.1 Induction +/‐ adjuvant chemotherapy | 4 | 830 | Peto Odds Ratio (95% CI) | 0.99 [0.80, 1.21] |
| 1.2 Concomitant +/‐ adjuvant chemotherapy | 4 | 765 | Peto Odds Ratio (95% CI) | 0.60 [0.48, 0.76] |
| 1.3 Adjuvant chemotherapy | 3 | 380 | Peto Odds Ratio (95% CI) | 0.97 [0.68, 1.38] |
| 2 Effect of chemotherapy on event‐free survival, hazard ratio of tumour failure or death by timing of chemo. Show forest plot | 11 | 1975 | Peto Odds Ratio (95% CI) | 0.76 [0.67, 0.86] |
| Analysis 1.2  Comparison 1 Radiotherapy versus radiotherapy + chemotherapy, Outcome 2 Effect of chemotherapy on event‐free survival, hazard ratio of tumour failure or death by timing of chemo.. | ||||
| 2.1 Induction +/‐ adjuvant chemotherapy | 4 | 830 | Peto Odds Ratio (95% CI) | 0.82 [0.68, 0.97] |
| 2.2 Concomitant +/‐adjuvant chemotherapy | 4 | 765 | Peto Odds Ratio (95% CI) | 0.63 [0.51, 0.78] |
| 2.3 Adjuvant chemotherapy | 3 | 380 | Peto Odds Ratio (95% CI) | 0.90 [0.67, 1.20] |
| 3 Effect of chemotherapy on loco‐regional control, HR of loco‐regional failure by timing of chemotherapy Show forest plot | 10 | 1782 | Peto Odds Ratio (95% CI) | 0.76 [0.63, 0.91] |
| Analysis 1.3 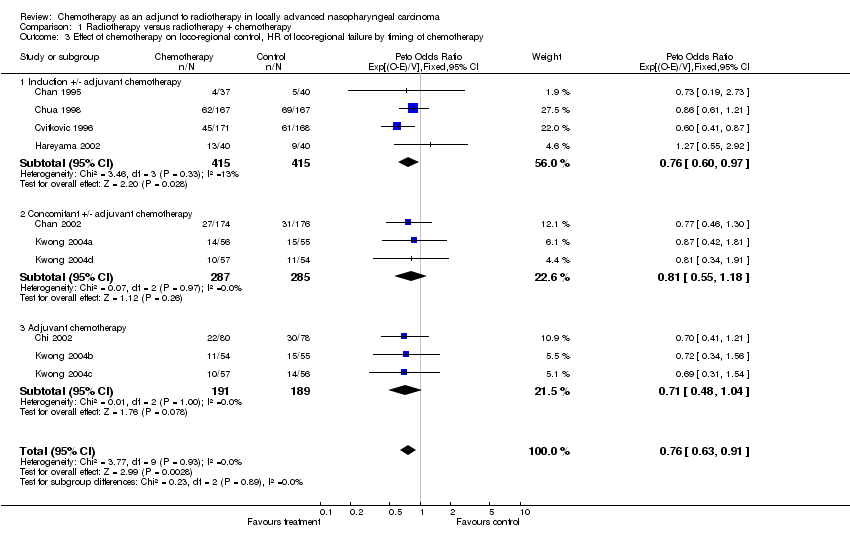 Comparison 1 Radiotherapy versus radiotherapy + chemotherapy, Outcome 3 Effect of chemotherapy on loco‐regional control, HR of loco‐regional failure by timing of chemotherapy. | ||||
| 3.1 Induction +/‐ adjuvant chemotherapy | 4 | 830 | Peto Odds Ratio (95% CI) | 0.76 [0.60, 0.97] |
| 3.2 Concomitant +/‐ adjuvant chemotherapy | 3 | 572 | Peto Odds Ratio (95% CI) | 0.81 [0.55, 1.18] |
| 3.3 Adjuvant chemotherapy | 3 | 380 | Peto Odds Ratio (95% CI) | 0.71 [0.48, 1.04] |
| 4 Effect of chemotherapy on distant control, hazard ratio of distant failure by timing of chemotherapy Show forest plot | 10 | 1782 | Peto Odds Ratio (95% CI) | 0.72 [0.59, 0.87] |
| Analysis 1.4  Comparison 1 Radiotherapy versus radiotherapy + chemotherapy, Outcome 4 Effect of chemotherapy on distant control, hazard ratio of distant failure by timing of chemotherapy. | ||||
| 4.1 Induction +/‐ adjuvant chemotherapy | 4 | 830 | Peto Odds Ratio (95% CI) | 0.65 [0.49, 0.86] |
| 4.2 Concomitant +/‐ adjuvant chemotherapy | 3 | 572 | Peto Odds Ratio (95% CI) | 0.69 [0.49, 0.97] |
| 4.3 Adjuvant chemotherapy | 3 | 380 | Peto Odds Ratio (95% CI) | 1.11 [0.66, 1.85] |

Comparison 1 Radiotherapy versus radiotherapy + chemotherapy, Outcome 1 Effect of chemotherapy on overall survival, hazard ratio of death by timing of chemotherapy.

Comparison 1 Radiotherapy versus radiotherapy + chemotherapy, Outcome 2 Effect of chemotherapy on event‐free survival, hazard ratio of tumour failure or death by timing of chemo..

Comparison 1 Radiotherapy versus radiotherapy + chemotherapy, Outcome 3 Effect of chemotherapy on loco‐regional control, HR of loco‐regional failure by timing of chemotherapy.

Comparison 1 Radiotherapy versus radiotherapy + chemotherapy, Outcome 4 Effect of chemotherapy on distant control, hazard ratio of distant failure by timing of chemotherapy.
| Characteristics | RT + CT | RT |
| n = 1975 | n = 990 | n = 985 |
| Gender: male | 75% | 74% |
| Age: < 40 or equal | 33% | 29% |
| 41 to 50 | 31% | 33% |
| 51+ | 36% | 38% |
| Perform. status (n = 1468)*: 0 | 52% | 50% |
| 1 | 46% | 47% |
| 2 | 2% | 3% |
| Tumour stage: T1 | 46% | 47% |
| T2 | 27% | 28% |
| T3,4 | 27% | 25% |
| Nodal stage (n = 1 898)**: N0 | 10% | 9% |
| N1,2 | 65% | 68% |
| N3 | 25% | 23% |
| Histology (n = 1636)***: WHO 1 | 4% | 3% |
| WHO 2 | 18% | 18% |
| WHO 3 | 78% | 79% |
| * Data missing from three trials. | ||
| Characteristics | n. patients RT+CT/RT | HR of death (95%CI) | P‐value | HR tum. failur/death | P‐value |
| Gender: Male | 742/727 | 0.81 (0.69 to 0.95) | 0.76 (0.66 to 0.87) | ||
| Gender: Female | 248/258 | 0.85 (0.62 to 1.16) | 0.81 | 0.74 (0.58 to 0.96) | 0.89 |
| Age < 40 or equal | 326/285 | 0.85 (0.63 to 1.14) | 0.67 (0.52 to 0.85) | ||
| 41 to 50 | 308/327 | 0.77 (0.59 to 1.01) | 0.80 (0.64 to 1.00) | ||
| > 50 | 356/373 | 0.86 (0.70 to 1.05) | 0.85 (t for trend) | 0.79 (0.66 to 0.95) | 0.31 (t for trend) |
| Performance status: 0 | 380/368 | 0.89 (0.71 to 1.11) | 0.78 (0.64 to 0.94) | ||
| 1 | 342/340 | 0.71 (0.55 to 0.92) | 0.66 (0.53 to 0.83) | ||
| 2 | 17/21 | 1.55 (0.65 to 3.69) | 0.73 (t for trend) | 1.40 (0.65 to 3.02) | 0.92 (t for trend) |
| T stage (AJCC97): T1 | 267/272 | 0.68 (0.51 to 0.90) | 0.69 (0.54 to 0.87) | ||
| T2 | 350/363 | 0.83 (0.64 to 1.07) | 0.82 (0.66 to 1.02) | ||
| T3/T4 | 373/350 | 0.90 (0.73 to 1.12) | 0.12 (t for trend) | 0.73 (0.60 to 0.88) | 0.80 (t for trend) |
| N stage (AJCC97): N0 | 91/83 | 1.02 (0.61 to 1.69) | 0.65 (0.42 to 1.00) | ||
| N1/N2 | 620/643 | 0.82 (0.68 to 0.99) | 0.79 (0.68 to 0.93) | ||
| N3 | 242/219 | 0.68 (0.52 to 0.88) | 0.24 (t for trend) | 0.64 (0.51 to 0.81) | 0.47 (t for trend) |
| WHO type 1 | 29/26 | 0.30 (0.15 to 0.59) | 0.18 (0.09 to 0.36) | ||
| WHO type 2 to 3 | 958/959 | 0.85 (0.73 to 0.98) | 0.003 | 0.78 (0.69 to 0.89) | < 0.0001 |
| Total | 990/985 | 0.82 (0.71 to 0.94) | 0.006 | 0.76 (0.67 to 0.86) | < 0.0001 |
| Trials included | N patients RT+CT/RT | OS. HR. 95%CI | OS. HR. p‐value | OS. heterogeneity I² | OS. Hetero. p‐value | EFS. HR. 95%CI | EFS. HR. p‐value | EFS. hetero. I² | EFS. Hetero. p‐value |
| All trials | 990/985 | 0.82 (0.71 to 0.94) | 0.006 | 50% | 0.03 | 0.76 (0.67 to 0.86) | < 0.0001 | 32% | 0.14 |
| Without Al‐Sarraf 1998 (INT‐0099) | 893/889 | 0.90 (0.77 to 1.05) | 0.17 | 0% | 0.37 | 0.82 (0.72 to 0.93) | 0.002 | 0% | 0.99 |
| Without patients with WHO 1 carcinoma | 958/959 | 0.85 (0.73 to 0.98) | 0.03 | 38% | 0.09 | 0.78 (0.69 to 0.89) | 0.0001 | 0% | 0.58 |
| Without one small trial (Chan 1995 (PWH‐88)) | 953/945 | 0.81 (0.70 to 0.93) | 0.003 | 51% | 0.03 | 0.75 (0.66 to 0.85) | < 0.0001 | 36% | 0.12 |
| Without QMH‐95 combined arms | 876/875 | 0.84 (0.73 to 0.98) | 0.02 | 53% | 0.03 | 0.75 (0.66 to 0.85) | < 0.0001 | 43% | 0.08 |
| Without Chan 1995 (PWH‐88), QMH95: follow up < 5 years | 729/725 | 0.80 (0.68 to 0.93) | 0.004 | 58% | 0.04 | 0.73 (0.64 to 0.84) | < 0.0001 | 32% | 0.03 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Effect of chemotherapy on overall survival, hazard ratio of death by timing of chemotherapy Show forest plot | 11 | 1975 | Peto Odds Ratio (95% CI) | 0.82 [0.71, 0.95] |
| 1.1 Induction +/‐ adjuvant chemotherapy | 4 | 830 | Peto Odds Ratio (95% CI) | 0.99 [0.80, 1.21] |
| 1.2 Concomitant +/‐ adjuvant chemotherapy | 4 | 765 | Peto Odds Ratio (95% CI) | 0.60 [0.48, 0.76] |
| 1.3 Adjuvant chemotherapy | 3 | 380 | Peto Odds Ratio (95% CI) | 0.97 [0.68, 1.38] |
| 2 Effect of chemotherapy on event‐free survival, hazard ratio of tumour failure or death by timing of chemo. Show forest plot | 11 | 1975 | Peto Odds Ratio (95% CI) | 0.76 [0.67, 0.86] |
| 2.1 Induction +/‐ adjuvant chemotherapy | 4 | 830 | Peto Odds Ratio (95% CI) | 0.82 [0.68, 0.97] |
| 2.2 Concomitant +/‐adjuvant chemotherapy | 4 | 765 | Peto Odds Ratio (95% CI) | 0.63 [0.51, 0.78] |
| 2.3 Adjuvant chemotherapy | 3 | 380 | Peto Odds Ratio (95% CI) | 0.90 [0.67, 1.20] |
| 3 Effect of chemotherapy on loco‐regional control, HR of loco‐regional failure by timing of chemotherapy Show forest plot | 10 | 1782 | Peto Odds Ratio (95% CI) | 0.76 [0.63, 0.91] |
| 3.1 Induction +/‐ adjuvant chemotherapy | 4 | 830 | Peto Odds Ratio (95% CI) | 0.76 [0.60, 0.97] |
| 3.2 Concomitant +/‐ adjuvant chemotherapy | 3 | 572 | Peto Odds Ratio (95% CI) | 0.81 [0.55, 1.18] |
| 3.3 Adjuvant chemotherapy | 3 | 380 | Peto Odds Ratio (95% CI) | 0.71 [0.48, 1.04] |
| 4 Effect of chemotherapy on distant control, hazard ratio of distant failure by timing of chemotherapy Show forest plot | 10 | 1782 | Peto Odds Ratio (95% CI) | 0.72 [0.59, 0.87] |
| 4.1 Induction +/‐ adjuvant chemotherapy | 4 | 830 | Peto Odds Ratio (95% CI) | 0.65 [0.49, 0.86] |
| 4.2 Concomitant +/‐ adjuvant chemotherapy | 3 | 572 | Peto Odds Ratio (95% CI) | 0.69 [0.49, 0.97] |
| 4.3 Adjuvant chemotherapy | 3 | 380 | Peto Odds Ratio (95% CI) | 1.11 [0.66, 1.85] |

