Biopsia del ganglio centinela para el diagnóstico de la afectación de los ganglios linfáticos en el cáncer de endometrio
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Study characteristics | |||
| Patient Sampling | Country: USA Study design: a prospective, non‐randomised study of women presenting with a preoperative diagnosis of endometrial cancer with clinical stage I disease conducted between March 2006 and August 2008. Inclusion criteria: preoperative diagnosis of endometrial cancer with clinical stage I disease; performance status of 0, 1, 2, or 3 by the Gynecologic Oncology group criteria; patients agreed to undergo total hysterectomy, removal of both adnexae, and bilateral regional lymphadenectomy via laparotomy or laparoscopy. Exclusion criteria: not reported | ||
| Patient characteristics and setting | Number of patients: 42 Median age: 60.5 years (range, 34‐82 years) Median body mass index: 29.3 kg/m2 (range, 19‐61 kg/m2) Histopathological cell type: endometrial endometrioid carcinoma FIGO stage: IA = 24 (57%), IB = 9 (21%), IC = 1 (2%), IIA = 1 (2%), IIIA = 3 (7%), IIIC = 3 (7%), IVB = 1 (2%)‐ old FIGO staging system used. Grade on hysterectomy specimen: grade 1 = 32 (76%), grade 2 = 9 (21%), grade 3 = 1 (2%) Lymphovascular space involvement: present in 7 cases (17%), absent in 35 cases (83%) Setting: single tertiary centre, namely Memorial Sloan‐Kettering Cancer Centre in New York, USA | ||
| Index tests | Type of endometrial sampling: office endometrial biopsy in 17 cases (40%), dilatation and curettage in 25 cases (60%) Experience of operator: 3 failed cases were performed by surgeons with beginner's experience (1‐2 cases) in SLN mapping early on in the study Tracer used and amount: 0.1 ‐ 0.5 mci Tc99 in 0.1 ‐ 0.5 mL volume; and 2 cc (n = 21) or 4 cc (n = 21) of isosulfan or methylene blue dye. Method and timing of application: Tc99 was injected using a 27‐gauge Potocky needle into the stroma of the cervix at 3 and 9 o'clock either on the morning of (n = 14; 33%), or day prior to surgery (n = 28; 67%) and a preoperative lymphoscintigram was taken. Blue dye was injected into the cervix at the beginning of the operation using a spinal needle. In the first half (n = 21) of the cohort study the injection of blue dye was into the cervical stroma at the 3 and 9 o'clock positions; for patients enrolled in the second half of the study cohort, 2 additional blue dye injections into were added that were injected into the anterior mid fundus and posterior mid fundus using a 22‐gauge 36‐cm Nezhat‐Dorsey aspiration/injection laparoscopic needle. Method of detection: from each paraffin block lacking metastatic carcinoma appreciable in a routine section stained with a haematoxylin and eosin (H&E), 2 adjacent 5‐micron sections were cut at each of two levels 50 microns apart. At each level, one slide was stained with H&E and the other with immunohistochemistry (IHC) using the anti‐cytokeratin AE1:AE3 (Ventana Medical Systems, Inc., Tucson, AZ) for a total of 4 slides per block. | ||
| Target condition and reference standard(s) | Type: patients with G1 endometrial cancer all of whom had pelvic and para‐aortic dissection performed to the level of the right ovarian vein/IMA. Twenty‐five cases (60%) were performed by laparoscopic surgery, and 17 (40%) were treated by laparotomy. Lymph node number and site: total of 145 SLNs were identified; 58 SLNs (40%) located in the right pelvis; 55 SLNs (38%) located in the left pelvis; 4 SLNs (3%) were in right para‐aortic nodes; 1 SLN (0.6%) was in left para‐aortic nodes. The most common anatomical sites were: internal iliac nodes = 52 (36%), external iliac = 43 (30%), obturator = 34 (23%), common iliac = 11 (8%), para‐aortic = 5 (3%). | ||
| Flow and timing | All patients received the index and reference standard within 1 month. All patients received the same reference standard of pelvic and para‐aortic dissection performed to the level of the right ovarian vein/IMA. All patients were included in the analysis. | ||
| Comparative | |||
| Notes | Study results: preoperative lymphoscintigraphy visualised SLNs in 30 patients (71%); intraoperative localisation of the SLN was possible in 36 (86%). A median of 3 SLNs (range, 1–14) and 14.5 non‐SLNs (range, 4–55) were examined. Six patients (14%) had a failed mapping, with no SLN identified. In all, 4/36 (11%) had positive SLNs—3 seen on H&E and 1 as cytokeratin‐positive cells on IHC. All node‐positive cases were picked up by the SLN; there were no false‐negative cases. The sensitivity of the SLN procedure in the 36 patients who had an SLN identified was 100%. TP rate was 4. The FN rate was 6. Of the 42 patients, 38 had negative nodes following full dissection, although only 32 were detected by SLNB (TN) and 4 patients had positive nodes (TP). There were no FP nodes. TP = 4; FP = 0; FN = 0; TN = 32; failed = 6. Type 1 tumours; FIGO Stage IA = 24; Stage Ib+ =18. Cervical injections; bilateral detection rate = not reported. Adverse reaction from index or reference test: Not reported. Operating time: not reported. Other intraoperative complications: not reported. Other postoperative complications: not reported. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| Study characteristics | |||
| Patient Sampling | Country: Iran Study design: cross‐sectional pilot study for patients undergoing staging surgery for stage I and II endometrial or cervical cancer (15 endometrial, 8 cervical) between November 2012 and February 2014 Inclusion criteria: patients with stage I and II endometrial or cervical cancer who were candidate for systematic lymph node dissection Exclusion criteria: patients with prior radiotherapy | ||
| Patient characteristics and setting | Number of patients: 23 (15 endometrial, 8 cervical) Mean age: 63.47 +/‐ 1.09 years Mean body mass index: 29.13 +/‐ 0.5 kg/m2 Histopathological cell type: endometrioid carcinoma = 13 (86.7%), clear cell carcinoma = 1 (6.7%), papillary serous carcinoma = 1 (6.7%) FIGO stage (pre 2009): IA = 7 (46.7%), IB = 3 (20%), IC= 1 (6.7%), IIA = 1 (6.7%), IIB = 2 (13.3%), IIIA = 1 (6.7%). Estimated FIGO stage (post 2009) = IA=10; IB+=5. Grade on hysterectomy specimen: not reported. Lymphovascular space involvement: not reported. Setting: two centres in Isfahan, Iran, namely Shahid Beheshti and Sadoughi Hospitals. | ||
| Index tests | Type of endometrial sampling: not reported. Experience of operator: mention of limitations for access to trained surgeons, though no explicit report of experience of operators. Tracer used and amount: 4 mL of 1% methylene blue Method and timing of application: injection into the fundus of the uterus after clamping the fallopian tubes using a 25‐gauge needle. To prevent spillage of dye, fundal gentle pressure on the sites of injection was used. Method of detection: sections from nodal tissue, including SLN were first stained with haematoxylin and eosin (H&E) and studied for metastatic involvement. SLNs showing metastatic involvement on H&E stained sections were considered as positive for metastatic disease. One extra section from the deeper levels of SLN was then prepared and stained with immunohistochemistry (IHC) technique using anti‐cytokeratin (AE1/AE3) antibody if results from the initial study of SLN for metastatic disease were negative. If the SLN section stained with IHC technique was positive for keratin immunoreactivity, SLN was considered to have metastatic disease. | ||
| Target condition and reference standard(s) | Type: patients with stage I or II endometrial cancer all underwent systematic lymph node dissection. All patients were treated by laparotomy The level of lymph node dissection is not reported. Lymph node number and site: a total of 15 SLNs were identified. The most common anatomical sites were: obturator = 8 (53.3%), internal iliac = 6 (40%), para‐aortic = 1 (6.7%). | ||
| Flow and timing | All patients received the index and reference standard within 1 month. It is unclear whether all patients received the same reference standard, as the level of the lymph node dissection is not reported. All patients were included in the analysis. | ||
| Comparative | |||
| Notes | Study results: the median SLN count was 3 (range, 1‐4). Sentinel LN detection rate was 80%. Sensitivity was 100%, specificity was 25%; PPV was 25%; NPV was 100%. There were no FP nodes. Adverse reaction from index or reference test: uneventful light blue urine was made in most patients that resolved by 24 hours postoperatively. TP = 3; FP = O; FN = 0 ; TN = 9; failed = 3. Type 1 tumours. FIGO Stage IA = 10; FIGO stage IB+ = 5. Subserosal uterine injections; bilateral detection rate = not reported. Operating time: not reported. Other intraoperative complications: no perioperative complication was observed. Other postoperative complications: no perioperative complication was observed. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Country: Brazil Study design: a study of patients treated for endometrial cancer from June 2007 to February 2017 at AC Camargo Cancer Center, Brazil, by the same gynaecologic oncology team. Patients were grouped into the non‐sentinel lymph node group (N‐SLN) if they had only undergone systematic lymphadenectomy as part of the surgical staging procedure without SLN‐mapping, or the sentinel lymph node group (SLN) if they had undergone both systematic lymphadenectomy and SLN‐mapping. The SLN group was recruited prospectively, while the N‐SLN group was retrospectively analysed. Inclusion criteria: patients with high risk endometrial cancers (defined as requiring one of the following: high‐grade tumour (endometrioid grade 3 and non‐endometrioid histologies: serous, clear cell, or carcinosarcoma), deep myometrial invasion (greater than or equal to 50%, or the presence of LVSI). Exclusion criteria: suspicious lymph node involvement on preoperative imaging or found during surgery; patients who did not undergo lymph node dissection together with SLN‐mapping; low‐risk endometrial cancers were excluded (endometrioid grades 1 or 2, < 50% myometrial invasion, absence of LVSI) | ||
| Patient characteristics and setting | Number of patients: N‐SLN group = 161 patients, SLN group = 75 patients. Median age: 61 years (range, 41‐83 years) Median body mass index: 27.2 kg/m2 (range, 17.9 ‐ 43.7 kg/m2) Histopathological cell type: endometrioid carcinoma = 107 (66.5%) in N‐SLN group & 48 (64%) in SLN group, serous carcinoma = 22 (13.6%) in N‐SLN group & 10 (13.3%) in SLN group, clear cell carcinoma = 13 (8.1%) in N‐SLN group & 8 (10.7%) in SLN group, carcinosarcoma = 14 (8.7%) in N‐SLN group & 7 (9.3%) in SLN group, serous + clear cell carcinoma = 3 (1.9%) in N‐SLN group & 2 (2.7%) in SLN group, undifferentiated = 2 (1.2%) in N‐SLN group & 0 in SLN group. FIGO stage: not reported. Grade on hysterectomy specimen: grade I = 21 (13%) in N‐SLN group & 7 (9.3%) in SLN group, grade II = 19 (11.8%) in N‐SLN group & 23 (30.7%) in SLN group, grade IIIa = 121 (75.2%) in N‐SLN group & 45 (60%) in SLN group. Lymphovascular space involvement: presence of LVSI in 25 cases (15.5%) in N‐SLN group & 32 cases (42.7%) in SLN group. Setting: single centre in Brazil, namely AC Camargo Cancer Center. | ||
| Index tests | Type of endometrial sampling: not reported. Experience of operator: not reported. Tracer used and amount: 4 mL of patent blue dye Method and timing of application: a total of 4 mL of patent blue dye (1 mL superficial and 1 mL at 1 cm depth) was injected into the cervix at 3 and 9 o'clock. Method of detection: the SLNs were examined by IHC when the haematoxylin and eosin (H&E) stain was negative. The SLNs were serially sectioned every 2 mm and stained with H&E at three levels of the tissue block. If the sample was negative, a pan‐cytokeratin stain was performed at each of the three levels. The SLNs were classified as macrometastasis (tumour > 2.0 mm), micrometastases (tumour cell aggregates between 0.2 mm and 2 mm), isolated tumour cells (ITCs) (individual tumour cells or aggregates </= 0.2 mm), or negative. All lymph nodes with macroscopic, microscopic, and isolated tumour cells were considered to be positive. Non‐ sentinel lymph nodes were reported as positive or negative for metastases based on routine sectioning and examination of a single H&E‐stained slide per a standard protocol. | ||
| Target condition and reference standard(s) | Type: patients with high‐risk endometrial cancer all underwent pelvic +/‐ para‐aortic lymph node dissection. In the N‐SLN group, 19 patients (11.8%) had pelvic lymph node dissection alone and 142 patients (88.2%) had pelvic and para‐aortic lymph node dissection. In the SLN group, 23 patients (30.7%) had pelvic lymph node dissection alone and 52 patients (69.3%) had pelvic and para‐aortic lymph node dissection. In the N‐SLN group 5 patients (3.1%) had minimally invasive surgery; in the SLN group 51 patients (68%) had minimally invasive surgery, of which 35 (46.7%) were conventional laparoscopies and 16 (21.3%) were robotic assisted laparoscopies. Lymph node number and site: median SLN detected was 2 (range, 1‐5). Of all lymph node metastases, 20 (31.3%) were pelvic and 6 (12%) were para‐aortic. | ||
| Flow and timing | All patients received the index and reference standard within 1 month. The patients did not all receive the same reference standard, as only 88.2% and 69.3% received full pelvic and para‐aortic lymph node dissection in the N‐SLN and SLN groups, respectively. The remaining 11.8% and 30.7% received only pelvic lymph node dissections in the N‐SLN and SLN groups, respectively. All patients were included in the analysis. | ||
| Comparative | |||
| Notes | Study results: the median SLN count was 2 (range, 1‐5). The median positive SLN count was 1.5 (range, 1‐4). The SLN detection rate was 85.3%, and bilateral SLNs were observed in 60% of patients. Sensitivity was 90%, NPV was 95.7%, false‐negative predictive value was 4.3%, and the false negative rate was 10%. Of 20 positive SLNs, 8 (40%) were detected only after immunohistochemistry (IHC). Blue dye alone: TP = 20; FN = 0; FN = 2; TN= 42; failed = 11 (2 ITCs moved from TP to TN group c.f. published paper as defined as TN for the review if ITC only). Type 1 tumours; FIGO stage = not clear; Subserosal uterine injections; 45 bilateral LN detection. Adverse reaction from index or reference test: not reported. Operating time: not reported. Other intraoperative complications: not reported. Other postoperative complications: not reported. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Country: France Study design: a retrospective study of 133 women with early stage endometrial cancer who were consecutively enrolled in nine gynaecological oncology centres in France from July 2007 and August 2009. Inclusion criteria: invasive endometrial carcinoma (FIGO stages I and II according to the 1988 FIGO classification) confirmed by biopsy, patients older than age 18 years affiliated with the French Health Care System and able to speak and read French, and intention of surgical staging. Exclusion criteria: preoperative FIGO stage III and IV, previous lymphadenectomy or surgery that could change the uterine lymphatic drainage (conisation, myomectomy), and pregnancy. | ||
| Patient characteristics and setting | Number of patients: 133; 8 were excluded for protocol deviations. Median age: 63 years (range, 38‐100) Median body mass index: 27 kg/m2 (range, 18 ‐ 54 kg/m2) Histopathological cell type: type 1 tumours = 94 (84.7%), type 2 tumours = 17 (15.3%) Assumed FIGO 2009 by preoperative MRI for 125 included patients: Stage 1A = 82; 1B+ =43. Stage and grade on hysterectomy specimen: not reported. Lymphovascular space involvement: 6 of 9 (66.7%) true‐positive cases had LVSI reported as present; 3 of 7 false‐negative cases had LVSI reported as present. Setting: nine specialist gynaecological oncology centres in France, namely Tenon University Hospital (Paris), Croix Rousse University Hospital (Lyon), Georges Pompidou University Hospital (Paris), Poissy‐Saint Germain en Laye University Hospital (Poissy), Edouard Herriot University Hospital (Lyon), Bretonneau University Hospital (Tours), Claudius Regaud Comprehensive Cancer Center (Toulouse), Centre Hospitalier Lyon Sud (Lyon), and Centre Oscar Labret (Lille). | ||
| Index tests | Type of endometrial sampling: diagnosis confirmed by biopsy. Experience of operator: not reported. Tracer used and amount: 0.2 mL of unfiltered technetium sulphur colloid, 2 mL of patent blue dye Method and timing of application: four cervical injections of 0.2 mL of unfiltered technetium sulphur colloid were given with a 25‐gauge spinal needle, at 3, 6, 9, and 12 o'clock positions the day of or morning before surgery. Patent blue was injected intracervically through a 25‐gauge spinal needle at 3 and 9 o'clock positions. Method of detection: ultrastaging was done for SLNs only. Normal‐appearing SLNs were cut perpendicular to the long axis. Air‐dried cytological smears were prepared by scraping the cut surfaces and staining with a rapid May‐Grünwald‐Giemsa method. Each half‐SLN was sectioned at 3‐mm intervals. Each 3‐mm section was analysed at four additional levels of 200 µm and four parallel sections: one was used for H&E staining, and H&E‐negative sections were examined by IHC with an anticytokeratin antibody cocktail (cytokeratins AE1‐AE3; Dako Corporation, Glostrup, Denmark). Non‐SLNs were submitted totally and blocked individually after 3‐mm sectioning and H&E staining. Isolated tumour cells (ITCs) were defined as cells or masses of cells with a size </= 0.2 mm, micrometastases as tumours with a size >0.2 mm but </=2 mm, and macrometastases as tumours of a size >2 mm. SLNs were recorded as positive when they contained macrometastases, micrometastases, or ITCs. | ||
| Target condition and reference standard(s) | Type: patients with stage I‐II endometrial cancer had pelvic +/‐ para‐aortic lymph node dissection. Patients had surgery performed either by laparoscopy of by an open approach, but the ratios are not reported. Lymph node number and site: sentinel lymph nodes were detected in 111 patients. 87 of these 111 patients (78.4%) had an intraoperative examination: 30 by frozen section (34.5%) and 57 by imprint cytology (65.5%). The anatomical location was not reported. | ||
| Flow and timing | All patients received the index and reference standard within 1 month. It is unclear whether all received the same reference standard (pelvic +/‐ para‐aortic lymph node dissection) as the ratios are not reported. All patients in whom SLNs were detected were included in the analysis, but not all patients who were recruited were included in the analysis. | ||
| Comparative | |||
| Notes | Study results: SN detection rate was 111/125 (89%). NPV 100% for hemipelvis, sensitivity 100% for hemipelvis. For per patient analysis NPV 97%, sensitivity 84%. 3 false negative cases occurred. TP = 16; FP = 0; FN = 3; TN = 92; failed = 14. FIGO stage from preoperative MRI: IA = 82; Stage IB+ = 43. Type 1 and 2 tumours. Cervical injections; bilateral detection 77 patients. Adverse reaction from index or reference test: not reported Operating time: not reported. Other intraoperative complications: not reported. Other postoperative complications: not reported. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Unclear | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Country: France Study design: a prospective study of 33 consecutive patients with endometrial cancer from July 2002 to October 2005. Inclusion criteria: biopsy‐confirmed endometrial cancer of apparent stage I or II according to the preoperative criteria of the FIGO. Exclusion criteria: not reported. | ||
| Patient characteristics and setting | Number of patients: 33 Median age: 66.1 years (range, 46‐84 years) Median body mass index: 25.8 kg/m2 (range, 17.8 ‐ 36.9 kg/m2) Histopathological cell type: endometrioid adenocarcinoma = 29 (87.9%), adenosquamous carcinoma = 1 (3%), clear cell carcinoma = 2 (6.1%), malignant mixed Muellerian tumour = 1 (3%) 1988 FIGO stage: Ia = 4 (12.1%), Ib = 13 (39.4%), Ic = 13 (39.4%), II = 3 (9.1%). Grade on hysterectomy specimen: for endometrial adenocarcinoma, grade 1 = 18 (54.5%), grade 2 = 9 (27.3%), and grade 3 = 2 (6.1%) Lymphovascular space involvement: not reported. | ||
| Index tests | Type of endometrial sampling: diagnosis confirmed by biopsy. Experience of operator: not reported Tracer used and amount: 0.2 mL of unfiltered technetium sulfur colloid, 2 mL of patent blue Method and timing of application: all patients received four pericervical injections (1.5 cm depth) of 0.2 mL (10 MBq each) of unfiltered technetium sulfur colloid (Nanocis; CIS Bio International, Saclay, France) administered with a 25‐G spinal needle on the day before surgery. All patients received Patent blue dye injected pericervically (1mL per injection, 1.5 cm deep) with a 25‐G spinal needle at 3 and 9 o’ clock. Grossly metastatic nodes were sectioned. Normal‐appearing SNs were cut perpendicular to the long axis. All SNs were examined intraoperatively by imprint cytology for the pathologist learning curve. Air‐dried cytologic smears were prepared by scraping the cut surfaces and were stained by using a rapid May‐Grünwald‐Giemsa method. Each half‐SN was sectioned at 3‐mm intervals. Each 3‐mm section was analysed by 4 additional levels of 150 µm and 4 parallel sections; 1 section was used for haematoxylin and eosin (H&E) staining, and H&E‐negative sections were then examined by immunohistochemistry with an anticytokeratin antibody cocktail (Cytokeratin AE1‐AE3; Dako Corporation, Glostrup, Denmark). Non‐SNs were totally submitted and blocked individually following 3‐mm distances and H&E staining. | ||
| Target condition and reference standard(s) | Type: patients with stage I‐II endometrial cancer all underwent pelvic +/‐ para‐aortic lymphadenectomy performed to the level of the aortic bifurcation. Patients with clinical stage I disease underwent laparoscopic treatment, including peritoneal washing, bilateral salpingo‐oophorectomy, the SN procedure, systematic pelvic lymphadenectomy, and laparoscopically assisted vaginal hysterectomy. Patients with apparent stage II disease underwent a peritoneal washing, bilateral salpingo‐oophorectomy, SN procedure followed by systematic pelvic lymphadenectomy and laparoscopic radical hysterectomy. Lymph node number and site: At least 1 SN was identified in only 27 patients (81.8%). The mean number of SNs was 2.5 per patient (range, 1‐5). A total of 71 SNs was removed. 18 patients (54.5%) had an identified bilateral SN. The most common site of the SNs was the medial external iliac region (67.6%). Other sites included intermediate external iliac region (2.8%), obturator fossa (5.6%), interiliac area (19.7%), common iliac region (2.8%), and aortic bifurcation (1.4%). | ||
| Flow and timing | All patients received the index and reference standard within 1 month. Patients did not all receive the same reference standard, as 90.9% received laparoscopy‐assisted vaginal hysterectomy and 9.1% received laparoscopic radical hysterectomy. All patients underwent systemic pelvic lymph node dissection. Para‐aortic lymph node dissection was only performed if a para‐aortic SN was detected. None of the patients required para‐aortic lymph node dissection, as no para‐aortic SN was detected. All patients were included in the analysis. | ||
| Comparative | |||
| Notes | Study results: Total number of SNs removed was 71. SN were identified in only 27 patients (81.8%). Mean number of SNs was 2.5/patient. 54.5% had bilateral SNs detected. No false‐negative SLNs were reported. Fourteen SNs (19.7%) from 8 patients were found to be metastatic at the final histological assessment. Sensitivity, specificity and true positives are not reported. TP = 8; FP = 0; FN = 0; TN ‐ 19; Failed = 6. FIGO Stage (converted to 2009 classification) Ia = 17; IB+ = 16. Type 1 & 2 tumours. Cervical injections; 18 patients with bilateral detection. Adverse reaction from index or reference test: no anaphylactic reactions to patent blue occurred. Operating time: median of 160.2 minutes (range, 120‐280). Other intraoperative complications: No intraoperative complications occurred. Other postoperative complications: not reported. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Country: France Study design: a prospective study of patients with clinical stage I endometrial cancer of any histological type from January 2002 to March 2006. Inclusion criteria: clinical stage I endometrial cancer of any histological type scheduled for primary surgery Exclusion criteria: not reported. | ||
| Patient characteristics and setting | Number of patients: 43 Mean age: 67.8 +/‐ 10.4 years (range, 40‐90 years) Median/mean body mass index: not reported. Histopathological cell type: endometrioid adenocarcinoma = 35 (81.4%), papillary serous carcinoma = 4 (9.3%), clear cell carcinoma = 1 (2.3%), carcinosarcoma = 3 (7.5%) 1988 FIGO stage: IA = 3 (7.0%), IB = 14 (32.6%), IC = 9 (20.9%), IIA =3 (7.0%), IIB = 3 (7.0%), IIIA = 1 (2.3%), and IIIC = 10 (23.2%). FIGO 2009 conversion: stage IA = 17; Stage 1B+ = 26; Type 1 & 2 tumours. Grade on hysterectomy specimen: not reported. Lymphovascular space involvement: not reported. Setting: single centre in France, namely The Hôpital Européen Georges‐Pompidou, Paris | ||
| Index tests | Type of endometrial sampling: not reported. Experience of operator: not reported, though mention that SLN detection rate was not influenced by team experience (P = 0.1). Tracer used and amount: 120 MBq of Colloidal rhenium sulfate labelled with technetium‐99m (Nanocis, Schering, CIS BIO International, 91192, Gif‐sur‐Yvette, France), 2 mL of 50% patent blue dye Method and timing of application: colloidal rhenium sulfate labelled with technetium‐99m was injected into the cervix in the nuclear medicine department, on the day before surgery. A 25‐gauge needle was used to inject a total dose of 120 MBq, 30 MBq at each of four sites, at the 12, 3, 6, and 9 o’clock positions on the cervix. Lymphoscintigraphy was performed 12 hours later using a dual‐head camera (Axis 2000, Philips Medical Systems, Cleveland, OH). During surgery, 2 mL of 50% patent blue dye was injected into the cervix at the 12, 3, 6 and 9 o'clock positions. SLNs were formalin‐fixed, mostly bisected and paraffin embedded. Both parts of SLNs were sampled by five step sectioning at 250 µm intervals. Four sections were stained with haematoxylin‐eosin‐saffron (HES) and one section was used for immunohistochemistry with the broad‐spectrum monoclonal antibody AE1/AE3 (Dako, Trappes, France). Non‐sentinel lymph nodes were assessed according to routine HES staining. | ||
| Target condition and reference standard(s) | Type: patients with stage I endometrial cancer who had pelvic +/‐ para‐aortic lymphadenectomy performed. Laparoscopy was used in 36 (83.7%) patients. The remaining 7 (16.3%) patients required laparotomy either because of marked uterine enlargement or anaesthesia‐related contraindications to laparoscopy. Para‐aortic lymph node dissection was performed in 9 (20.9%) patients. 34 patients (79.1%) had pelvic lymph node dissection only. Lymph node number and site: 86 SLNs were detected in total from 30 patients. The mean number of SLNs per patient was 2.9 overall, 1.4 on the right side, and 1.3 on the left side, with the remaining SLNs being located on or near the midline. Bilateral SLNs were detected in 16 (53.3%) of the 30 patients. The interiliac area was the main site of SLN detection, with 28 (93.3%) patients and 71 of the total 86 SLNs (82.6%). Of these 37 (43%) were right‐sided and 34 (39.5%) were left‐sided. 5 (5.8%) were located in the common iliac area on the right side, 4 (4.7%) in the common iliac area on the left side, and 6 (7.0%) were found at the promontery. None of the patients had inguinal or para‐aortic SLNs without interiliac SLNs. | ||
| Flow and timing | All patients received the index and reference standard within 1 month. The patients did not all receive the same reference standard, as only 20.9% received full pelvic and para‐aortic lymph node dissection; the remaining 79.1% received pelvic lymph node dissection only. All patients were included in the analysis. | ||
| Comparative | |||
| Notes | Study results: 86 SLNs were identified in 30 patients (69.8%). The prevalence of node involvement was 23.2% (10/43 patients). Two of these 10 patients had a failure of the SLN detection, and 8 had involvement of the SLNs; thus the prevalence of SLN involvement was 27% (8/30). In six of the eight patients with SLN involvement, six had negative nonsentinel nodes. All the metastatic SLNs were located in the interiliac area. There were no false‐negative results; therefore, the negative predictive value of SLN biopsy was 100%. The sensitivity for detecting lymph nodes disease was 100%. Para‐aortic node involve‐ ment was found in a single patient, who also had positive pelvic nodes; in this patient, no SLNs were detected. TP = 8; FP = 0; FN = 0; TN = 22; failed =13. FIGO 2009 (converted) stage IA = 17; Stage 1B+ = 26; Type 1 & 2 tumours. Cervical injections; bilateral detection = 16 patients. Adverse reaction from index or reference test: no adverse events related to SLN detection and biopsy were recorded. More specifically, no cases of blue dye sensitisation or blue dye‐related desaturation during surgery occurred. There were no port‐site metastases (follow‐up = 15 months). Operating time: not reported. Other intraoperative complications: not reported. Other postoperative complications: not reported. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Country: Istanbul Study design: a retrospective study of patients with a preoperative histopathologically‐proven endometrioid endometrial cancer from September 2012 to December 2015. Inclusion criteria: preoperative histopathologically‐proven endometrioid endometrial cancer Exclusion criteria: inability to perform the optimal procedure (defined as abdominal or laparoscopic total hysterectomy, bilateral salpingo‐oophorectomy, and SLN mapping with PLA, with or without para‐aortic lymphadenectomy (PALA) (because of morbidly obese patients, patients with serious co‐morbidities limiting the time of surgery); PET/CT findings from another clinic; history of neoadjuvant treatment; diagnosis of any other malignancy | ||
| Patient characteristics and setting | Number of patients: 95 Median age: 58.9 years (range, 33‐82 years) Median body mass index: not reported Histopathological cell type: endometrioid endometrial cancer FIGO stage: IA = 61 (64.2%), IB = 16 (16.8%), II = 11 (11.6%), IIIA = 1 (1.1%), IIIC1 = 4 (4.2%), IVB = 2 (2.1%) Grade on hysterectomy specimen: grade 1 = 39 (41.1%), grade 2 = 49 (51.6%), grade 3 = 7 (7.3%) Lymphovascular space involvement: present in 27 (28.4%), absent in 68 (71.6%) Setting: single centre in Turkey, namely the Cerrahpasa Medical Faculty, Division of Gynecologic Oncology, Department of Gynecology and Obstetrics, Istanbul University | ||
| Index tests | Type of endometrial sampling: not reported. Experience of operator: study performed by only 3 surgeons (TB, V, and NT); experience of operators not reported explicitly Tracer used and amount: 4 mL of 1% methylene blue dye Method and timing of application: methylene blue dye (1%) was injected to the cervix at 3‐ and 9‐o’clock positions for a total of 4 mL (1 mL to the superficial surface [depth of 2‐3 mm] and 1 mL deep in the tissue [depth of 1 cm ‐ 2 cm]). All the SLNs were sectioned into 2‐mm sections, and in the first step, the SLNs were examined by imprint cytology and frozen sections. Afterwards, the lymph nodes were completely embedded in paraffin; then, the lymph nodes were first examined by a routine histological technique, staining with haematoxylin and eosin (H&E). If the SLNs were negative upon the initial H&E staining, the ultrastaging pathology protocol was applied for the SLNs. Ultrastaging consists of 2 adjacent 5 µm sections cut from each paraffin block at 2 levels, 50 µm apart, for a total of 4 slides per block. At each level, 1 slide was stained with H&E, and the other was stained with immunohistochemistry, using anticytokeratin AE1:AE3 (Ventana Medical Systems, Inc, Tucson, AZ). | ||
| Target condition and reference standard(s) | Type: patients with grade I, II, or III endometrial cancer who had pelvic +/‐ para‐aortic dissection performed to the level of the left renal artery. Thirty‐nine cases (41.1%) were performed by laparoscopic surgery, and 56 (58.9%) were treated by laparotomy. Pelvic lymph node dissection only was performed in 68 patients (71.6%); pelvic and para‐aortic lymph node dissection was performed in 27 patients (28.4%). Lymph node number and site: the total number of SLNs removed was 227. The mean number of SLNs removed per patient was 2.95 (range, 1‐9). A SLN was detected in 77 patients (81.1%) and was absent in 18 patients (18.9%). 53% of patients had bilateral SLNs removed; 47% of patients had unilateral SLNs removed. The most common anatomical site for SLNs was: right obturator region 25.1%, right external iliac 17.6%, right common iliac 5.7%, vena cava 2.2%, right internal iliac 0.4%, left obturator region 22.5%, left external iliac 19.4%, left common iliac 4.9%, left internal iliac 2.2%. | ||
| Flow and timing | All patients receive the index and reference standard within 1 month. The patients did not all receive the same reference standard (pelvic +/‐ para‐aortic lymphadenectomy), as only 28.4% received full pelvic and para‐aortic lymph node dissection, the remaining 71.6% received pelvic lymph node dissection only. All patients were included in the analysis. | ||
| Comparative | |||
| Notes | Study results: FDG PET/CT study was performed preoperatively in all patients. 227 SLNs were identified in 77 patients (81.1%). The mean number of SLNs removed was 2.95 per patient (range, 1‐9). Lymph node metastases were found in 4 (4.2%) of 95 patients. Results were analysed on a per‐patient basis and as a combination of preoperative PET/CT and SLN frozen section results, and showed a sensitivity of 33%, specificity of 100%, PPV of 100%, and NPV of 97.1%. TP = 2; FP = 0; FN = 2; TN = 73; failed = 18. Type 1 tumours. FIGO stage IA = 61; Stage 1B= = 34. Cervical injection; bilateral detection 41 patients. Adverse reaction from index or reference test: not reported. Operating time: not reported. Other intraoperative complications: not reported. Other postoperative complications: not reported. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Country: Canada Study design: a prospective study of patients with biopsy‐proven endometrial cancer from February 2014 to December 2015. Inclusion criteria: biopsy‐proven endometrial cancer Exclusion criteria: not reported. | ||
| Patient characteristics and setting | Number of patients: 119 Median age: 65.5 years (range, 49‐91 years) Median body mass index: 31.0 kg/m2 (range, 18‐56 kg/m2) Histopathological cell type: endometrioid = 101 (85%), serous = 8 (7%), dedifferentiated = 2 (2%), clear cell = 4 (3%), carcinosarcoma = 4 (3%) FIGO stage: IA = 69 (58%), IB = 30 (25%), II = 3 (3%), IIIA = 4 (3%), IIIC1 = 8 (7%), IIIC2 = 4 (3%), IVB = 1 (1%) Grade on hysterectomy specimen: grade 1 = 1 (51%), grade 2 = 33 (28%), grade 3 = 25 (21%) Lymphovascular space involvement: present in 38 (32%), absent in 81 (68%) Setting: single centre in Canada, namely L'Hotel‐Dieu de Québec hospital | ||
| Index tests | Type of endometrial sampling: not reported. Experience of operator: mention of the effect of the surgeon's learning curve on SLN detection, but experience of operator not explicitly defined. Tracer used and amount: 4 mL of ICG. Method and timing of application: ICG was injected by intracervical injection with a 25‐gauge spinal needle. 1 mL of ICG was injected superficially and 1 mL was injected deeper into the cervical stroma at the 3 and 9 o'clock position immediately before the surgery. SLNs were not routinely submitted for frozen section unless there was a suspicion of metastatic disease on gross inspection. For ultrastaging, SLNs were cut perpendicular to the long axis at 3 mm intervals and embedded in paraffin. Six 4 μm sections at 40 μm intervals were cut from the paraffin blocks and stained with haematoxylin and eosin (H&E). An additional section was taken adjacent to the 3rd H&E cut and used for immunohistochemistry using anti‐cytokeratin AE1/ AE3 (DAKO, Agilent, CA). The non‐sentinel lymph nodes (non‐SLNs) were bisected parallel to the long axis and examined with routine H&E on one level. | ||
| Target condition and reference standard(s) | Type: 119 patients with grade I, II, or III endometrial cancer all of whom had pelvic +/‐ para‐aortic dissection at the surgeon's discretion. Thirty‐one (26%) cases were performed by laparoscopic surgery, 59 (50%) were performed by robotic surgery, and 29 (24%) were treated by laparotomy. Pelvic lymph node dissection only was performed in 100 patients (84%); pelvic and para‐aortic lymph node dissection was performed in 19 patients (16%). Lymph node number and site: the total number of SLNs removed was 267. The median number of SLNs identified per patient was 2 (range, 0‐7). SLNs were located primarily in the external iliac node basin (70%), usually on or just under the medial aspect of the external iliac vein near the bifurcation, followed by the obturator area (23%). Unusual locations (6%) were the common iliac area, the parametrium, the para‐aortic or the presacral area. | ||
| Flow and timing | All patients receive the index and reference standard within 1 month. The patients did not all receive the same reference standard (pelvic +/‐ para‐aortic lymphadenectomy) as only 16% receive full pelvic and para‐aortic lymph node dissection, the remaining 84% received pelvic lymph node dissection only. All patients were included in the analysis. | ||
| Comparative | |||
| Notes | Study results: SLNs were detected bilaterally in 89 patients (74%) and unilaterally in 22 patients (19%). No SLNs were identified in 8 patients (6%). The overall SLN detection rate was 93%. In terms of hemipelvis, sentinel lymph nodes were detected in 84% (200/238) of hemipelvis, and not detected in 16% (38/238). When calculated on a per‐patient basis, sensitivity and negative predictive value were both 100% in patients with bilateral detection; 95% and 99% respectively in patients with at least unilateral detection. The side‐specific sensitivity and negative predictive value were respectively, 92.5% and 98.8%. Failed SLN detection rate 37%. 1 false negative at unilateral level, so the patient was not counted to be a false negative case at a patient level. Per patient results: TP = 21; FP = 0; FN = 1; TN = 89; failed = 8. FIGO stage IA= 69; Stage IB+ = 50. Type 1 and 2 tumours. Cervical injections; bilateral detection =89 patients. Per hemi‐pelvis results: 230 hemi‐pelvices total. TP = 25; FP = 0 ; FN = 2; TP = 173; failed = 30 (8 bilateral/ 22 unilateral) Adverse reaction from index or reference test: not reported. Operating time: not reported. Other intraoperative complications: not reported. Other postoperative complications: not reported. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Country: Italy Study design: a retrospective study of patients with preoperative stage I endometrial cancer and stage I (1A2‐1B1) cervical cancer from October 2010 to May 2015. Patients were split into three groups: Group 1 received technetium‐99 and blue dye (n = 77); Group 2 received blue dye alone (n = 38); Group 3 received ICG (n = 48). Inclusion criteria: Patients with preoperative stage I endometrial cancer and stage I (1A2‐1B1) cervical cancer Exclusion criteria: not reported. | ||
| Patient characteristics and setting | Number of patients: 163; 118 (72.4%) patients with endometrial cancer and 45 (27.6%) patients with cervical cancer. Median age: group 1 = 61 +/‐ 13.4 years (range, 26‐86), group 2 = 62 +/‐ 13.2 years (range, 29‐86), group 3 = 59 +/‐ 14.5 years (range, 29‐86) Median body mass index: group 1 = 23 +/‐ 4.9 kg/m2 (range, 18‐50), group 2 = 26 +/‐ 5.3 kg/m2 (range, 18‐40), group 3 = 25 +/‐ 6.6 kg/m2 (range, 15‐50) Histopathological cell type: EIN = 2 (5.1%), endometrioid = 101 (85.6%), serous papillary = 6 (5.1%), other = 9 (7.6%) FIGO stage: EIN = 2 (5.1%), IA = 67 (56.8%), IB = 21 (17.8%), II = 5 (4.2%), IIIA = 1 (0.8%), IIIC1 = 19 (16.1%), IIIC2 = 1 (0.8%), IV = 2 (1.7%) Grade on hysterectomy specimen: for cervical and endometrial cancer combined grade 1 = 44 (27%), grade 2 = 67 (41.1%), grade 3 = 48 (29.4%), NA = 4 (2.5%) Lymphovascular space involvement: for cervical and endometrial cancer combined present in 55 (33%) of patients, absent in 108 (66.3%) of patients Setting: single centre in Italy, namely the Gynecology Oncology Surgical Unit of the San Gerardo Hospital, Italy | ||
| Index tests | Type of endometrial sampling: not reported. Experience of operator: authors state that they began SLN mapping in 2010. After the completion of the learning curve and initial experience, [they] moved in the direction of an injection of blue dye alone into the cervix before ICG injection, which started in 2014. Tracer used and amount: 4 mL of blue dye, 4 mL to 5 mL of ICG solution (1.25 mg/mL), technetium‐99 (amount not reported) Method and timing of application: a total of 4 mL of blue dye (2 mL per injection for each side) was injected at the 3‐ and 9 o’clock positions. The ICG concentration used was 1.25 mg/mL. For each patient, a 25 mg vial with ICG powder was diluted in 20 mL of aqueous sterile water. A total of 4 mL to 5 mL of this ICG solution were injected into the cervix alone, divided into the 3‐ and 9‐ o’clock positions. One millilitre of ICG solution was injected with penetration to a depth of 1 cm into the stroma, and 1 mL was injected into the submucosal layer on the right and the left of the cervix, usually after initial laparoscopic exploration to evaluate the feasibility of the surgical procedure. For open surgical cases performed with the Vitom II exoscope, the cervical injection was performed once the laparotomy was completed. LNs with macroscopic metastases were sectioned, and SLNs that appeared normal were cut perpendicular to the long axis. Two adjacent 5 µm sections were cut at each of 2 levels 50 µm apart from each block lacking metastatic carcinoma, detected by means of a section routinely stained with haematoxylin and eosin (H&E). At each level, one slide was stained with H&E and the other with immunohistochemistry using AE1/AE3, an anti‐cytokeratin antibody (Dako, Glostrup, Denmark), as well as one other negative control slide, for a total of five slides per block. All other non‐SLNs were examined only by routine H&E. Micrometastasis was defined as a metastatic deposit within the LNs ranging from 0.2 mm to no more than 2 mm in size. Isolated tumour cells were defined as single tumour cells or as clusters of malignant epithelial cells less than 0.2 mm in size. | ||
| Target condition and reference standard(s) | Type: 118 patients with endometrial cancer all of whom had pelvic +/‐ para‐aortic lymph node dissection performed. In the women with endometrial cancer, aortic lymphadenectomy was performed in 14 % of cases (17 of 118). For cervical and endometrial cancer combined, in 70 % of the cases (115 of 163), the surgical procedure was laparoscopy. Lymph node number and site: for cervical and endometrial cancer combined, the total number of SLNs was 384 and the median number of SLNs per hemipelvis was 2. SLNs were most frequently located in the external iliac region (71 %), internal iliac (2 %), obturator fossa (10 %), common iliac (12 %) and para‐aortic (2 %), sacral (2 %), and parametrial (1 %). | ||
| Flow and timing | All patients received the index and reference standard within 1 month. The patients did not all receive the same reference standard. Para‐aortic lymph node dissection was only performed in patients with endometrial cancer with a positive preoperative positron emission tomography/computed tomography (PET/CT) scan and in the absence of SLN mapping or unilateral mapping. In the women with endometrial cancer, aortic lymphadenectomy was performed in 14% of cases (17 of 118), while the remaining 101 (86%) had only pelvic lymph node dissection. All patients were included in the analysis. | ||
| Comparative | |||
| Notes | Tc99 & blue dye three arms to study: (A) TC 99 & Blue dye: TP=10; FP=0; FN=0; TN=32; failed=2. Type 1 tumours. FIGO stage IA=23; Stage IB+ = 26. Cervicalx injections; bilateral detection = 22. (B) Blue dye only: TP=5; FP=0; FN=0; TN=19; failed =6. Type 1 tumours. FIGO stage IA= 17; Stage IB+ =26. Cervical injections; bilateral detection = 14. (C) ICG only TP=4; FP=0; FN=0; TN=28; failed=0. Type 1 tumours. FIGO stage IA = 29; Stage IB+ =10. Cervical injections; bilateral detection = 32 | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Country: Switzerland Study design: a prospective study of patients with histologically‐proven early endometrial carcinoma from July 2001 to June 2005. Inclusion criteria: histologically‐proven early endometrial cancer Exclusion criteria: not reported. | ||
| Patient characteristics and setting | Number of patients: 60 (note although stated 60 patient in study, staging given for 61 patients) Median age: 65 years (range, 43‐87 years) Median body mass index: 27 kg/m2 (range, 21 ‐ 45 kg/m2) Histopathological cell type: endometrioid = 54 (90%), serous papillary = 4 (7%), carcinosarcoma = 2 (3%) 1988 FIGO stage: IA = 12 (20%), IB = 22 (36%), IC = 5 (8%), IIA = 2 (3%), IIB = 5 (8%), IIIA = 6 (10%), IIIC = 9 (15%) Based on Table 1 FIGO 1A = 34 and FIGO 1B+ is 28 (note amounts to 601, not 60), Grade on hysterectomy specimen: grade 1 = 22 (37%), grade 2 = 25 (42%), grade 3 = 13 (21%) Lymphovascular space involvement: not reported. Setting: single centre in Italy, namely the Department of Gynecology and Obstetrics, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland | ||
| Index tests | Type of endometrial sampling: not reported. Experience of operator: states that the staging accuracy of the SN procedure depends on the surgeon's proficiency, but the experience of the operator is not explicitly reported. Tracer used and amount: 2 mL of patent blue dye and technetium‐99 m‐colloidal albumin Method and timing of application: In all patients but one [polyallergic], 2 mL of patent blue dye and then technetium‐99 m‐colloidal albumin (Nanocoll®, Amershaw Health AG, 8869 Wädenswil, Switzerland) were injected into the sub‐endometrial layer beneath the tumour using a Cook needle. The lymph nodes were analysed without freezing. SN were fixed in neutral buffered formaldehyde for 24 to 72 hours, then cut into 0.2 cm or less thick slices, and embedded in a paraffin block per node. Multiple sections were prepared from each block. A set of three 4 μm thick sections was cut every 250 μm. One section was stained by haematoxylin–eosin (H&E). When negative, then immunohistochemistry (IHC) was performed, using the cytokeratin C‐11 antibody (Readysysteme, AG, Bad Zurzach, CH). Detection of tumour cells defined a positive SN. Nonsentinel nodes were totally processed without serial sections or immunohistochemistry. | ||
| Target condition and reference standard(s) | Type: 60 patients with grade I, II, or III endometrial cancer all of whom had pelvic and para‐aortic lymphadenectomy to the level of the left renal vein performed. Thirteen patients (21.7%) had laparoscopic surgery, and 47 (78.3%) were treated by laparotomy. All patients had pelvic and para‐aortic lymph node dissections. Lymph node number and site: Sentinel nodes were identified in 49 of 60 patients (82%). The mean number of SN retrieved was 3.7 per patient (range, 1 to 8). Sixteen patients (33%) had SN in both pelvic and para‐aortic areas. No patient had SN only at the para‐aortic level. The anatomical sites were as follows: internal iliac = 81 (12%), external iliac = 229 (34%), obturator = 187 (28%), common iliac = 111 (17%), para‐aortic = 63 (9%). | ||
| Flow and timing | All patients receive the index and reference standard within 1 month. The patients all received the same reference standard. All patients were included in the analysis. | ||
| Comparative | |||
| Notes | Study results: sentinel nodes were identified by radiotracer alone in 15 patients, by blue dye alone in 1 patient, and by both tracers in 33 patients, for a total of 49 patients (82%). No SN was identified in 11 patients (18.3%). Twenty‐seven patients had unilateral SN only and 22 patients had bilateral SN. More than one SN was identified in 30 of 49 patients (61%). Mean number of nodes was 3.7 per patient. Metastases were found in nine patients (15%), and in SN of eight of them. In seven of these eight patients, metastases were confined to the SN. Adverse reaction from index or reference test: no SN was identified in 11 patients, because of hazardous injection of tracers due to insufficient intrauterine pressure (4 patients), or injection into the bloodstream (5 patients), through a damaged endometrium (1 patient) or through the myometrium (1 patient). This 18% failure rate corresponds to the main author's learning curve. TP = 8; FN = 0; FN = 1; TN = 40; failed = 11. re‐calculated 2009 FIGO stage IA = 34; stage IB+ = 28; Type 1 and type 2; subserosal uterine injections; bilateral node detection = 22 patients. Operating time: not reported. Other intraoperative complications: no morbidity was related to the procedure. Other postoperative complications: no morbidity was related to the procedure. Of note, the stage numbers for Delaloye et al add up to 61 patients in table 1, but only 60 included in the study. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| Study characteristics | |||
| Patient Sampling | Country: Turkey Study design: a prospective, randomised‐controlled trial of patients with histologically‐confirmed endometrial cancer from July 2018 to June 2019. Patients were randomised to two groups: the cervical group (n = 40) and thee endometrial group (n = 41) based on the site of tracer injection. Inclusion criteria: histologically‐confirmed endometrial cancer Exclusion criteria: patients with suspected lymph node disease and metastatic disease in preoperative imaging, those who were treated with radiotherapy or chemotherapy, those who underwent surgery without lymph node dissection, patients who had uterine sarcoma histology, co‐existent ovarian malignancies. | ||
| Patient characteristics and setting | Number of patients: 81; cervical group = 40, endometrial group = 41 Median age: cervical group = 61 years (range, 53.2‐68.7 years), endometrial group = 62 years (range, 55‐65 years) Median body mass index: cervical group = 32 kg/m2 (range, 31.2‐32.6 kg/m2), endometrial group = 32.7 kg/m2 (range, 32‐33.4 kg/m2) Histopathological cell type: Cervical group: endometrioid = 34 (85%), serous = 2 (5%), endometrioid + serous = 1 (2.5%), clear cell = 1 (2.5%), endometrioid + clear cell = 1 (2.5%), undifferentiated = 1 (2.5%) Endometrial group: endometrioid = 33 (80.5%), serous = 5 (12.2%), endometrioid + serous = 1 (2.4%), clear cell = 1 (2.4%), mucinous = 1 (2.4%) FIGO stage: Cervical group: IA = 21 (52.5%), IB = 9 (22.5%), II = 3 (7.5%), III = 5 (12.5%), IV = 2 (5%) Endometrial group: IA = 27 (65.9%), IB = 4 (9.8%), II = 3 (7.3%), III = 4 (9.8%), IV = 3 (7.3%) Grade on hysterectomy specimen: Cervical group: Grade 1 = 19 (51.4%), grade II = 11 (29.7%), grade III = 7 (18.9%) Endometrial group: Grade 1 = 18 (51.4%), grade II = 14 (40%), grade III = 3 (8.6%) Lymphovascular space involvement: Cervical group: present in 4 (10%), absent in 36 (90%) Endometrial group: present in 7 (17.1%), absent in 34 (82.9%) Setting: Single centre in Turkey, namely the Kocaeli University Hospital, Kocaeli, Turkey | ||
| Index tests | Type of endometrial sampling: not reported. Experience of operator: not reported. Tracer used and amount: 4 mL (1 Mci) of 99m‐technetium labelled with nano colloid albumin particles Method and timing of application: In the cervical group, injections of 99mTc (4 mL, 1 Mci) were performed at the 3 and 9 o’clock positions of the uterine cervix using a 25‐gauge hypodermic needle. In the endometrial group, 99mTc (4 mL, 1 Mci) was injected into the fundal endometrium using a 21 gauge, 20 cm sheathed transcervical catheter. Both tracer injections were performed 3 hours before surgery. Thirty minutes after the tracer injection the patients were evaluated using lymphoscintigraphy. The histopathologic examination for lymph nodes consisted of haematoxylin and eosin (H&E) staining after being fixed in 10% buffered formalin and embedded in paraffin wax. All SLNs went through a pathologic ultrastaging process. For ultrastaging, the SLNs were sectioned along the longitudinal axis into 2 mm thick sections and stained with H&E. In the event that the first H&E stained section was negative for metastasis, three additional sections at 50 μm intervals were obtained for H&E. Anti‐cytokeratin antibody immunohistochemistry (AE1/AE3 and PCK26, Ventana Medical Systems, Tucson, Arizona, USA) was used to detect low‐volume metastasis on one ultra‐section specimen. After ultrastaging, metastases of ≤0.2 mm were identified as isolated tumour cells, those >0.2 mm and ≤ 2 were micro‐metastases, and those >2 mm were considered as macro‐metastases. | ||
| Target condition and reference standard(s) | Type: 81 patients with grade I, II, or III endometrial cancer all of whom had pelvic and para‐aortic lymphadenectomy. All surgeries were performed by laparotomy. Lymph node number and site: the median number of pelvic SLNs for each group was 2. The median number of para‐aortic SLNs for each group was 0. The bilateral detection rate was higher in the endometrial group than in the cervical group, but the difference was not statistically significant (69% vs 43%, P = 0.085). | ||
| Flow and timing | All patients receive the index and reference standard within 1 month. The patients all received the same reference standard. All patients were included in the analysis. | ||
| Comparative | |||
| Notes | Study results: The rate of detection of at least one SLN, sensitivity, and negative predictive value were 80%, 66.6%, 96.6% for the cervical group and 85%, 66.6%, and 96.9% for the endometrial group, respectively. The false‐negative rate for both groups was 33%. The pelvic bilaterality rates with cervical and endometrial injections were 43% and 69%, respectively. Arm A‐ subserosal TP = 2, FP = 0, FN = 1, TN = 32; failed SLN = 6. FIG0 stage 1A = 27; Stage Ib+ = 14; Type 1 tumours. Subserosal uterine injection site; bilateral detection = 20. Arm B ‐ cervical injection TP = 2, FP = 0, FN = 1, TN = 29; failed SLN = 8. FIG0 stage 1A = 21; Stage Ib+ = 19; Type 1 tumours. Cervical injection site; bilateral detection = 14. Adverse reaction from index or reference test: none. Operating time: not reported. Other intraoperative complications: none. Other postoperative complications: none. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| Study characteristics | |||
| Patient Sampling | Country: USA Study design: a retrospective study of patients with endometrial cancer from May 2011 to September 2011. Inclusion criteria: not reported. Exclusion criteria: not reported. | ||
| Patient characteristics and setting | Number of patients: 35 Mean age: 63.4 +/‐ 10.4 years (range, 35‐88) Mean body mass index: 33.1 +/‐ 9.3 (range, 18‐56) Histopathological cell type: Mayo Clinic criteria "Low‐risk" = 9 (25.7%), Mayo Clinic criteria "High‐risk" = 26 (74.3%) FIGO stage: not reported. Grade on hysterectomy specimen: grade 1 = 13 (37.1%), grade 2 = 14 (40%), grade 3 = 8 (22.9%) Lymphovascular space involvement: present in 13 (37%), absent in 22 (63%) Setting: single centre in USA, namely Florida Hospital Cancer Institute and the Global Robotics Institute, Florida, USA | ||
| Index tests | Type of endometrial sampling: not reported. Experience of operator: not reported. Tracer used and amount: 4 mL of isosulfan blue, 2 mL of diluted ICG dye (25mg of ICG in 20ml normal saline [1.25 mg/mL]) Method and timing of application: immediately prior to insertion of the uterine manipulator, 1 mL of ISB was injected sub‐mucosally in four quadrants of the cervix (1 to 2 mm with no blood return prior to injection). Immediately prior to docking, 0.5 mL of diluted ICG dye (25 mg of ICG in 20 mL normal saline [1.25 mg/mL]) was injected in each cervical quadrant immediately prior to the placement of a uterine manipulator. The pelvic dissections were initiated within approximately 10 minute of the cervical injections. Pathologists recorded initial H&E impressions of ultra‐sectioned SLN (at least 6 serial sections 4 μm thick at 40 μm intervals) prior to IHC analysis, and then amended their reports after IHC if groups of malignant cells were subsequently identified in H&E slides with the aid of IHC. An additional 4 μm section was cut between the third and fourth levels and immuno‐stained with mouse monoclonal anti‐AE1/AE‐3 cytokeratin (Dako, Carpentaria, CA). “Isolated cytokeratin tumour cells” were less than 0.2 mm; and micro‐metastases were defined as 0.2 mm to 2 mm of tumour. Non‐sentinel lymph nodes underwent one section with routine H&E staining. | ||
| Target condition and reference standard(s) | Type: 35 patients with grade I, II, or III endometrial cancer all of whom received pelvic +/‐ para‐aortic lymphadenectomy. All surgeries were robotic‐assisted laparoscopic surgeries. 13 patients (37%) had pelvic lymph node dissection only, and 22 (63%) had pelvic and para‐aortic lymph node dissection. Lymph node number and site: Bilateral SLNs were detected in 34 (97%) patients. Two cases had sentinel nodes identified in the pre‐sacral and lower aortic areas by fluorescent imaging that were not identified by ISB. Three cases had common iliac SLN, and all remaining SLN were identified in the true pelvis (obturator or external iliac chains). | ||
| Flow and timing | All patients receive the index and reference standard within 1 month. The patients did not all receive the same reference standard. Sixty‐three per cent of cases received comprehensive pelvic and aortic lymphadenectomy with a single central‐docking procedure, and 14 (63.4%) of these had infra‐renal nodes dissected. The remaining 13 patients (37.1%) underwent pelvic lymph node dissection only. All patients were included in the analysis. | ||
| Comparative | |||
| Notes | Study results: all positive SLN biopsies were pelvic nodes, correctly identifying nine cases (90% sensitivity). The one false negative SLN biopsy failed to detect the metastasis in an immediately adjacent external iliac lymph node. Twenty‐five patients had normal lymph nodes on final assessment, and SLN biopsies correctly predicted all cases (100% specificity). The PPV was reported as 100% and the NPV was reported as 96%. TP = 9; FP = 0; FN = 1; TN = 25; failed = 0; FIGO stage not reported; Type 1 and 2 tumours. Cervical injections; bilateral detection = 34 patients. Adverse reaction from index or reference test: 0% transfusion rate, 0% conversion to laparotomy, estimated blood loss 118 +/‐ 47ml (range, 50‐250) Operating time: 169 +/‐ 37 minutes (range, 103‐236) Other intraoperative complications: not reported. Other postoperative complications: not reported. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Country: USA Study design: a prospective cohort study of patients with clinical stage I endometrial cancer from September 2012 to January 2015. The aim of the study was to assess two SLN detection methods by comparing ISB‐dye determinations and subsequent ISB + ICG with NIR imaging determinations for the same 180 study participants, with an additional 20 control participants who had ISB dye alone. Inclusion criteria: clinical stage 1 endometrial cancer (type 1 and 2 histologies) Exclusion criteria: suspected allergies to blue dyes, iodine, or ICG dye, inability to undergo robotically‐assisted hysterectomy for any reason | ||
| Patient characteristics and setting | Number of patients: 200 Mean age: 64.5 +/‐ 8.4 years Mean body mass index: 33 +/‐ 7.6 kg/m2 Histopathological cell type: endometrioid = 162 (81%), papillary serous = 23 (11.5%), MMMT = 9 (4.5%), clear cell = 5 (2.5%), other = 1 (0.5%) Grade on hysterectomy specimen: grade 1 = 86 (43%), grade 2 = 60 (30%), grade 3 = 14 (7%), type 2 = 40 (20%) Lymphovascular space involvement: present in 63 (31.5%), absent in 137 (68.5%) Setting: single centre in USA, namely Florida Hospital Cancer Institute, Florida, USA | ||
| Index tests | Type of endometrial sampling:not reported. Experience of operator: not reported. Tracer used and amount: 2 mL of ISB, 2 mL of ICG Method and timing of application: 2 mL of ISB (0.5 mL per quadrant) and 2 mL of ICG (1 mg/mL) were injected in the cervix submucosa 0.3 mm ‐ to 0.5 mm deep, and all the participants underwent SLN mapping in white light. After the blue dye results were recorded, the randomisation envelopes were opened, and 180 participants were randomised to NIR imaging. Mapping of SLN with NIR was performed, results were recorded, and SLNs were harvested. A two‐quadrant (3 and 9 o’clock) cervical submucosal injection technique was used for cases 1–100, and a four‐ quadrant injection (2, 4, 8, and 10 o’clock) technique was used for cases 101–200. The absolute volume of dye was identical (2 mL of each dye) for each injection method. Sentinel lymph nodes were processed in blocks, and haematoxylin and eosin (H&E) slides were evaluated. The H&E‐negative blocks were further micro‐sectioned 50 µm apart to obtain three H&E slides and one immunohistochemical (IHC) slide using anticytokeratin AE1:AE3. Non‐ SLNs were examined only by routine H&E. Lymph node metastases were described as macro‐metastasis (>2 mm), micro‐metastasis (0.2mm to 2 mm), or isolated tumour cell (ITC)‐positive. | ||
| Target condition and reference standard(s) | Type: 200 patients with grade I, II, III, or type 2 endometrial cancer, all of whom had pelvic +/‐ para‐aortic lymphadenectomy performed. All surgeries were robotic‐assisted laparoscopic surgeries. 106 (53%) of patients had pelvic lymph node dissection only, and 94 (47%) had pelvic and para‐aortic lymph node dissection. Lymph node number and site: The median SLN count was 2 (range, 0 to 4). The distribution of total SLNs (n = 567) by anatomic location in the 200‐case study group was as follows: external iliac (n = 297, 52.4%), obturator (n = 225, 39.7%), internal iliac (n = 28, 4.9%), common iliac (n = 10, 1.8%), aortic (n = 5, 0.9%), pre‐ sacral (n = 1, 0.2%), and parametrial (n = 1, 0.2%). | ||
| Flow and timing | All patients received the index and reference standard within 1 month. The patients did not all receive the same reference standard. 106 (53%) of patients had pelvic lymph node dissection only, and 94 (47%) had pelvic and para‐aortic lymph node dissection. All patients were included in the analysis. | ||
| Comparative | |||
| Notes | Study results: the detection rate for ISB + ICG was reported as 83.9%, and for ISB alone it was reported as 40%. The median number of SLNs removed was 2 (range, 0 to 4). The false‐negative rate for SLN biopsy was 2.5%. The sensitivity was 97.5%, NPV was 99.3%, and FNR was 2.5%. ISB + ICG and NIR detected more SLNs and more LN metastases than ISB alone. Blue dye + ICG (180 patients) TP =38; FP = 0; FN = 1; TN = 157; failed = 7. FIGO stage I breakdown not given; Type 1 and 2 tumours. Cervical injection; bilateral LN detection = 151 patients. TP include those with ITC only. Blue dye only (20 + 180 patients ‐ detection rate after blue dye only in IGC+blue dye group) TP = 27; FP = 0; FN = 0: TN = 125; failed = 48. FIGO stage I breakdown not given; Type 1 and 2 tumours. Cervical injection; bilateral LN detection = 80 patients. TP include those with ITC only. Adverse reaction from index or reference test: None. Estimated blood loss was 86 +/‐ 57ml. Operating time: 136 +/‐ 35 minutes Other intraoperative complications: none. Other postoperative complications: none. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Country: Japan Study design: a prospective cohort study of patients with histologically‐diagnosed endometrial cancer clinical stage IA‐II scheduled for surgery, between April 2009 and December 2012. Patients received either Tc99 labelled phytate (Tc99) method), or Tc99 + indocyanine green (ICG) (Tc99+ICG). Inclusion criteria: patients with endometrial cancer verified by pathological diagnosis scheduled to receive hysterectomy with retroperitoneal lymphadenectomy, clinical stage IA–II (FIGO2009) and confined to uterus by MRI and CT imaging, over 20 years old at the time of signature of informed consent. Exclusion criteria: patients with grade 1 endometrioid adenocarcinoma without muscular invasion at preoperative evaluation with MRI; obvious extrauterine spread at their preoperative evaluation with MRI and CT; other malignancy within previous 5 years; perioperative findings of bulky lymph nodes or extrauterine dissemination; concomitant cancer elsewhere. | ||
| Patient characteristics and setting | Number of patients: 55 (Tc99+ICG 32; ICG alone 23) Median age years (range):Tc99 + ICG = 54 (30‐77); ICG alone = 54 (27‐76) Mean/median BMI: not reported Histopathological cell type: Tc99+ICG: endometrioid = 27 (84.3%); serous = 2 (6.3%); mixed = 3 (9.4%) ICG alone: endometrioid = 22 (95.7%); serous = 0 (0%); mixed = 1 (4.3%) FIGO stage: Tc99+ICG: IA = 15 (46.9%), IB = 2 (6.3%), II = 3 (9.4%), IIIA = 3 (9.4%), IIIB = 1 (3.1%), IIIC1 = 1 (3.1%), IIIC2 = 7 (21.9%) ICG alone: IA = 14 (60.9%), IB = 3 (13.0%), II = 1 (4.3%), IIIA = 0 (0%), IIIB = 0 (0%), IIIC1 = 2 (8.7%), IIIC2 = 3 (13.0%) Grade on hysterectomy specimen: Tc99 + ICG: grade 1 = 4 (12.5%), grade 2 = 20 (62.5%) , grade 3 = 8 (25%) ICG alone: grade 1 = 7 (30.5%), grade 2 = 13 (56.5%), grade 3 = 3 (13%) Lymphovascular space involvement: Tc99+ICG: present = 18 (56.3), absent = 14 (43.7%) ICG alone: present = 10 (43.5%), absent = 13 (56.5%) Setting: single centre in Japan, namely Department of Obstetrics and Gynecology, Keio University School of Medicine, Shinjuku‐ku, Tokyo, Japan. | ||
| Index tests | Type of endometrial sampling: not reported Experience of operator: not reported Tracer used and amount: ICG: Indocyanine green (ICG) (volume not specified) Tc99+ICG: As above + 99m‐Technetium (99mTc)‐labelled phytate (0.2 mL at 5 points, total 2 mCi). Method and timing of application: ICG: Intraoperatively, ICG was injected sub‐serosally at 5 points of the uterine corpus, and dye uptake was detected by macroscopic observation (observation by the naked eye). Six minutes after injection, retroperitoneal spaces were then opened and retroperitoneal sentinel dye‐containing nodes were detected macroscopically. When identified, these nodes were marked with ligation approximately within 30 minutes after the injection. Tc99+ICG: As above, as well as: 16 hours preoperatively, 99m‐Technetium (99mTc)‐labelled phytate was injected into the sub‐endometrium at 5 points (0.2 mL × 5, total 2 mCi) just around the tumour using hysteroscopy of a 6.5 mm operative hysteroscope after cervical dilation under anaesthesia. Preoperative lymphoscintigraphy was obtained 15 hours after the tracer injection. Intraoperative radio‐labelled SN sampling was performed using a handheld gamma probe Method of detection (histopathological assessment, including ultrastaging): intraoperative frozen section diagnosis of the SNs was performed in every case. Pathologic ultrastaging was performed, with multiple serial sectioning of the entire sentinel lymph node at 200 μm to 300 μm, with 3 consecutive HE levels with one slide for immunostaining per level. Immunostaining for cytokeratin (clone AE1/AE3, Milipore Inc., dilution 1:150) was performed in SNs after HE routine histological examination. Non‐SNs were processed using entire node examination with HE staining. Macrometastases were considered as tumour foci larger than 2 mm, whereas micrometastases were considered as tumour foci between 0.2 mm and 2 mm. Isolated tumour cells (ITC) were defined as deposits less than 0.2 mm. | ||
| Target condition and reference standard(s) | Type: 55 patients with a preoperative histological diagnosis of grade I, II, or III endometrial cancer, all of whom received pelvic +/‐ para‐aortic (38.2%) lymphadenectomy. All surgeries were carried out by laparotomy. Lymph node number and site: SN detection rate 100%. Mean SNs removed per patient: Tc99+ICG: 6.0 (range 1‐10) ICG: 4.1 (range 1‐9). Anatomical sites: Tc99+ICG: pelvic 3.7 (range 1‐7), para‐aortic 2.3 (range 0‐4) ICG: pelvic 2.9 (range 0‐5), para‐aortic 1.1 (range 0‐5) | ||
| Flow and timing | All patients received the index and reference standard within 1 month. The patients did not all receive the same reference standard. Only 21/55 (38%) patients received pelvic and para‐aortic lymphadenectomy. The remaining 62% received pelvic lymphadenectomy alone. All patients were included in the analysis. | ||
| Comparative | |||
| Notes | Study results: the sentinel node detection rate was 100% for both RI + Dye and Dye alone methods. Based on the final diagnosis with cytokeratin staining: Tc99+ICG: sensitivity 100% (8/8), negative predictive value (NPV) 100% (24/24), ICG alone (includes patients in Tc99 + ICG group): sensitivity 83.3% (10/12), negative predictive value (NPV) 95.3% (10/12). Tc99 + ICG TP = 8; FP = 0; FN = 0; TN = 24; failed = 0. FIGO stage IA = 15; Stage Ib+ = 17; Type 1 and 2 tumours. Subserosal injection; bilateral detection rate not given. ICG only (includes patients in Tc99+ICG group) TP = 10; FP = 0; FN = 2; TN = 43; failed = 0. FIGO stage IA = 29 (including 15 from Tc99+ICG group); FIGO IB+ = 26 (including 17 fro Tc99+ICG group); Type 1 and 2 tumours. Subserosal injection; bilateral detection rate not given. Operating time: not given Intraoperative complications: no allergic reactions. Nothing else specified. Postoperative complications: nothing specified. There appear to be 13 patients with positive nodes in table 4, although only 12 accounted for in description of FP/FN rates. The additional patient is in the IGC only group. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Country: Turkey Study design: a prospective study of patients with histologically confirmed endometrial cancer between 2010 and 2011 (specific dates not provided). Inclusion criteria: the presence of the histological diagnosis of primary endometrial cancer, signing of informed consent form. Exclusion criteria: distant metastases; extra‐uterine disease on preoperative imaging, recurrent disease, quote: "medical diseases which contraindicated general anaesthesia or conditions that makes it difficult to perform lymphadenectomy such as morbid obesity and congenital anomalies". | ||
| Patient characteristics and setting | Number of patients: 26 Mean age (SD, range): 57.73 (+‐6.36, range 42‐79) Mean BMI (SD, range): 35.25 (+‐ 5.58 years, range 20.3‐45.7) Histopathological cell type: eEndometrioid 24 (92%), serous 1 (4%), clear cell 1 (4%). FIGO stage: I = 24 (92%), II = 2 (8%). Grade on hysterectomy specimen: grade I = 20 (77%), grade II = 6 (23%). Lymphovascular space involvement: present 6 (23%), absent 20 (77%). Setting: a single centre in Turkey, namely the Gynecologic Oncology department of the Istanbul Medical Facility, Instanbul University, Istanbul, Turkey. | ||
| Index tests | Type of endometrial sampling: not reported. Experience of operator: not reported. Tracer used and amount: first 16 patients: 5cc of methylene blue. Second 10 patients: 3 cc isosulphane blue dye. Method and timing of application: first 16 patients: 5ml of methylene blue injected into the uterine subserosal myometrium 5 minutes before surgery commenced. Second 10 patients: bilateral paratubal, posterior and anterior isthmic uterine wall injections of 0.5 mL isosulphane blue dye in each of the areas 10 minutes before clamping the broad ligaments. Method of detection (histopathological assessment, including ultrastaging): pathologists evaluated the sentinel node and other nodes macroscopically. They were divided into those with gross metastatic nodes and normal‐looking ones and were cut perpendicular to the axis. Each lymph node was cut perpendicular to the axis and then parallel at intervals of up to 5 mm across, and haematoxylin‐eosin (HE) staining was performed. After the parallel sections of 3 mm were obtained, sentinel nodes were cut at 150 μm intervals and at least 4 primary sections for HE staining, followed by 4 secondary cross‐section were taken for immunohistochemistry (IHC). For IHC staining, the anti‐cytokeratin antibody (Cytokeratin AE1‐AE3, Dako Corporation, Glostrup, Denmark) was used. | ||
| Target condition and reference standard(s) | Type: 26 Patients with grade I or II endometrial cancer, all of whom received pelvic +/‐ para‐aortic (7.6%) lymphadenectomy. All surgeries were performed by laparotomy. Lymph node number and site: a total of 8 SLNs were removed. The mean number of SLNs per patient was 0.3. The only provided anatomical site for SLNs was the obturator area (62.5% of positive SLNs). Location of SLNs not specified. | ||
| Flow and timing | All patients receive the index and reference standard within 1 month. Not all patients received the same reference standard. Only 2 of 26 patients (7.7%) received pelvic and para‐aortic lymphadenectomy. The remaining 92.3% received pelvic lymphadenectomy alone. All patients were included in the analysis. | ||
| Comparative | |||
| Notes | Study results: Total number of nodes removed 8. Overall SLN detection rate 23% (6 patients). SLN detection rate of methylene blue 6% (1/16), SLN detection rate of isosulphane blue method 50% (5/10). Overall sensitivity 23%, specificity 0%, positive predictive value 100%, negative predictive value 43%. False negative rate 0%. Unilateral/bilateral detection rates not provided. Arm A ‐ Subserosal and cervical injection (n= 10) TP = 5; FP = 0; FN=0; TN = 5; failed 0. FIGO stage IA = 7; Stage 1B+ = 3; type 1 and 2 tumours. Subserosal uterine plus cervix combined injections; bilateral detection rate not given. Arm B ‐ Cervical injection only (n=16) TP = 1; FP = 0; FN = 0; TN = 15; failed = 0. FIGO Atage IA = 12; Stage IB+ =4; Type 1 and 2 tumours; Cervical injection: bilateral detection rate not given. Adverse reaction from index or reference test: nil reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Country: Brazil. Study design: A prospective cohort study of patients with clinical stage I/II endometrial cancer between February 2002 and December 2005. Inclusion criteria: Clinical FIGO stage I/II endometrial cancer. Nil else reported. Exclusion criteria: Not reported. | ||
| Patient characteristics and setting | Number of patients: 40 Mean age (SD, range): 64.5 (+‐7.7, range 48‐80). Mean/median BMI: not reported. (Mean weight 72kg +‐ 14.3, range 36‐110). Histopathological cell type: Endometrioid = 31 (77.5%), carcinosarcoma = 3 (7.5%), papillary serous = 3 (7.5%), adenosquamous = 2 (5%), clear cell = 1 (2.5%). 1988 FIGO stage: I/II (100%) ‐ breakdown not provided. Grade on hysterectomy specimen: Grade 1 = 6 (17.1%), Grade 2 = 27 (77.1%), Grade 3 = 2 (5.8%). Lymphovascular space involvement: Not reported. Setting: A single centre in Brazil, namely the Department of Gynecology, Hospital do Servidor Publico Estadual de Sao Paulo, Sao Paulo, Brazil. | ||
| Index tests | Type of endometrial sampling: Not reported. Experience of operator: Not reported. Tracer used and amount: Patent blue dye V, 3ml. Method and timing of application: 3 mL of patent blue V was injected using an insulin needle; 1 mL into the subserosal myometrium in an equidistant point to the uterine horns and 2 cm below; 1 mL into the anterior wall and 1mL into the posterior wall. Injections were after clamping the terminal uterine tubes and 10 minutes prior to opening the retroperitoneum. Method of detection (histopathological assessment, including ultrastaging): Following the main lymphatic path, the sentinel lymph node was identified, observing its topographic localization. The sentinel node was excised and submitted to frozen section examination of specimen, stained with hematoxylin and eosin (H&E). All lymph nodes excised were subsequently examined by means of paraffin‐embedded slices stained with H&E and imunohistochemistry with antikeratin antibody AE1/AE3. | ||
| Target condition and reference standard(s) | Type: 40 patients with a clinical stage I/II endometrial cancer all of whom had pelvic and para‐aortic lymphadenectomy to the level of the renal veins. All surgeries were carried out by laparotomy. Lymph node number and site: A total of 63 SLNs were removed. Mean SLNs per patient 1.6 (range 1‐6). 22 SLNs were resected in the para‐aortic region, 41 resected in the pelvic region. | ||
| Flow and timing | All patients receive the index and reference standard within 1 month. The patients all received the same reference standard. All patients were included in the analysis. | ||
| Comparative | |||
| Notes | Study results: Sensitivity = 75%, specificity = 92.3%, positive predictive value = 60%, negative predictive value = 96%, and accuracy = 90%. False negative rate = 1/25 (4%). Unilateral/bilateral detection rates not reported. TP = FP = 0; FN = 1; TN =25; failed = 9. FIGO stages I and II by 1988 criteria, breakdown not given; Type 1 and 2 tumours In 5 patients with failed SLN detection a positive LN was found on lymphadenectomy. Subserosal uterine injection; bilateral detection rate not reported. Adverse reaction from index or reference test: Nil reported. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| Study characteristics | |||
| Patient Sampling | Country: Italy Study design: a prospective cohort study of patients with a histological diagnosis of endometrial cancer between 2002 and 2004. Inclusion criteria: endometrial cancer. Exclusion criteria: clinical evidence of lymphatic metastases; concurrent and/or previous cancers; preoperative histological diagnosis of clear cell carcinoma or serous papillary adenocarcinoma; risks related to anaesthesia; preoperative and intraoperative evidence of distant metastasis. However, when the histological diagnosis of clear cell carcinoma or serous papillary adenocarcinoma was made at surgery, as occurred in 7 of the 26 patients examined, data were retained and are reported. | ||
| Patient characteristics and setting | Number of patients: 26 Mean age (range): 54 years (46‐67) Mean/median BMI: not reported Histopathological cell type: endometrioid = 18 (69.2%), clear cell = 3 (11.5%), serous = 4 (15.4%), unclassified = 1 (3.9%) FIGO stage (1988 criteria): Ia = 6 (23.1%), Ib = 13 (50%), IIIa = 2 (76.9%), IIIc = 5 (19.2%). Converted to 2009 FIGO IA = 19 and IB+ 7 Grade on hysterectomy specimen: not reported Lymphovascular space involvement: not reported Setting: a single centre in Italy, namely the Istituto Nazionale Tumori, Milan, Italy. | ||
| Index tests | Type of endometrial sampling: not reported Experience of operator: not reported Tracer used and amount: approximately 111 MBq of 99m Tc‐Nanocoll in 5 mL of saline and 8 mL of blue dye (Monico S.p.A., Venezia/Mestre, Italy). Method and timing of application: the radiopharmaceutical and blue dye (Monico S.p.A., Venezia/Mestre, Italy) were injected by hysteroscopy (Solima‐ Zupi hysteroscope; Wolf, Knitlingen, Germany) in the subendometrial layer of the uterine wall within 2–3 mm of the visible edge of the tumour. The radiopharmaceutical consisted of albumin nanocolloid particles ranging in size from 5 nm to 80 nm, labelled with 99mTc (Nanocoll; Nycomed Amersham Sorin S.r.l., Saluggia, Italy). The number of injections was three or four depending on the lesion site and shape, and the injections were placed around the cancer at approximately 90° or 120° to each other. After the administration of radiocolloid, a total volume of 8 mL of blue dye was injected in the peritumoural area at the same site as the radiopharmaceutical injection. Radiopharmaceutical and blue dye were injected concurrently, by means of separate injections and volumes, because the two tracers have a different kinetic behaviour and because transitory impairment of visibility at hysteroscopy may be observed following injection of blue dye owing to leakage into the uterus. No anaesthesia was used during the entire procedure. Method of detection (histopathological assessment, including ultrastaging): following hysterectomy and bilateral salpingo‐oophorectomy, but prior to lymphadenectomy, a crystal scintillator (CsI) gamma probe (C‐Track System; Care‐Wise, CA, USA)was used intraoperatively to confirm the location of the SLN as seen on scintigraphy and using the images and skin mark as guides. The lymph node emitting the highest signal was recorded and identified as an SLN and then isolated for pathological examination. Following dissection, all surgical specimens were scanned by the probe to identify residual hot spots in the abdomen or in the lymphadenectomy specimen. In the event of focal accumulations of radioactivity, specimens were sent in separately as SLNs with progressive identification numbers. The threshold level was set at 80% of the radioactivity of the hottest SLN if the lymph node was close to the hottest node, and at 30% if the lymph node was far from the hottest node, in another lymphatic basin. All surgically‐removed lymph nodes were re‐examined with the gamma probe ex vivo. All lymph nodes were removed from the fatty tissue without freezing or preservation and were examined using haematoxylin and eosin staining; in the case of serous papillary and clear cell tumours, the nodes were also examined immunohistochemically using anti‐cytokeratin AE1/AE3 monoclonal antibody of the acidic and basic subfamilies. Nodes of at least 0.5 cm in diameter were dissected, and those smaller than 0.5 cm were fixed and embedded and cut. Three sections were obtained from each node (100–500 μm apart). The tumour was histologically‐classified according to the FIGO classification of endometrial cancer | ||
| Target condition and reference standard(s) | Type: 26 patients with a histological diagnosis of endometrial cancer all of whom had pelvic lymphadenectomy (from the common iliac vessels to the deep obturator fossae, and para‐aortic lymphadenectomy in 7/26 patients). All cases were performed by laparotomy. Lymph node number and site: a total of 65 SLNs were detected. In all patients, drainage to more than one lymphatic basin was observed, and the mean number of SLNs detected was 3 (range 2 to 4). Unilateral SLNs were identified in the common iliac region (7, 10.8%), external iliac (12, 18.5%), internal iliac (2, 3.1%), obturator (6, 9.2%) and para‐aortic region (14, 21.5%). Bilateral SLNs were identified in the common iliac (5, 7.7%), external iliac (4, 6.2%) and obturator regions (3, 4.6%). | ||
| Flow and timing | All patients received the index and reference standard within 1 month. Not all patients received the same reference standard. 7 of 26 (27%) received pelvic and para‐aortic dissection; the remaining 73% received pelvic lymphadenectomy alone. All patients were included in the analysis. | ||
| Comparative | |||
| Notes | Study results: Sensitvity 100%. SN detection rate 100%. Bilateral detection rate not specified. Mean number of nodes detected was 3. Specificity, FNR, NPV, and PPV not provided. TP = 4; FP = 0 FN = 0; TN = 22; failed = 0. FIGO IA = 19 and IB+ 7; type 1 and 2 tumours; subserosal uterine injection; bilateral detection rate not reported. Two patients had transient vagal symptoms with index test. nil else reported. Operating time: not provided. Other intraoperative complications: nil reported Other postoperative complications: nil reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Country: Italy Study design: prospective quasi‐randomised trial comparing laparotomy to laparoscopy for SLN detection rates. Thirty‐four patients with a preoperative diagnosis of grade I (50%), II (32.3%), or III (17.7%) endometrial cancer treated at a single institution in Italy. Histologicaly types were recorded as endometrioid (82.4%), clear cell (8.8%), serous papillary (2.9%), clear cell/serous papillary (2.9%), and undifferentiated (2.9%). Inclusion criteria: patients with clinical stage I‐II endometrial cancer due to undergo surgical staging through laparotomy or laparoscopy, with written informed consent obtained. Exclusion criteria: not reported. | ||
| Patient characteristics and setting | Number of patients: 34 Mean age (SD): 62.2 (+/‐ 8.7) years Mean/median BMI: not reported Histopathological cell type: endometrioid = 28 (82.4%), clear cell = 3 (8.8%), serous papillary = 1 (2.9%), clear cell/serous papillary = 1 (2.9%), and undifferentiated = 1(2.9%). FIGO stage (1988): Stage I = 31 (91.2%), Stage II = 3 (8.8%) (Converted to 2009 FIGO Stage 1A; 25; Stage IB+ = 9) Grade on hysterectomy specimen: grade I = 17 (50%), grade II = 11 (32.3%), grade III = 6 (17.7%). Lymphovascular space involvement: not reported | ||
| Index tests | Type of endometrial sampling: direct biopsy Experience of operator: not reported Tracer used and amount: 4 mL of Patent Blue Violet Method and timing of application: 4 mL of Patent Blue Violet injected directly into the substance of the cervix at 3, 6, 9, and 12 O’clock (depth of injection 0.5 – 1 cm) using a 22‐gauge spinal needle after the induction of general anaesthesia Method of detection (histopathological assessment, including ultrastaging): histopathological examination of both SLNs and non‐SLNs was performed by haematoxylin and eosin (H&E) staining followed by immunohistochemical (IHC) staining. Nodes were examined by sections at reduced intervals to identify micrometastases. Blue‐ dyed specimens sent for pathology as SLNs where defined ‘‘false’’ SLNs when they did not contain lymphatic tissue but only retro‐ peritoneal fat tissue. | ||
| Target condition and reference standard(s) | Type: 34 patients with clinical stage I or II endometrial cancer, all of whom received systematic pelvic lymphadenectomy. Patients were quasi‐randomised to either laparoscopy (17) or open surgery (17). Lymph node number and site: SLN detection rate 82% in patients undergoing LAVH and 41% TAH, with a global detection rate of 62%. Despite this, the study reported that the SLNs detected per patient ranged from 1‐4. Total SLNs and average SLN per patient not provided. Number of patients with "false" SLN was 3 overall. | ||
| Flow and timing | All patients received the index and reference standard within 1 month. Not all patients received the same reference standard (quasi‐randomised to receive either laparoscopy (17) or laparotomy (17)). All patients were included in the analysis. | ||
| Comparative | |||
| Notes | Study results: the SLN detection rate for laparoscopy was 82%, laparotomy 41%. Global detection rate of 62%. SLN detected per patient 1‐4. No of patients with "false" SLN was 3 overall. Specificity, sensitivity, NPV, and PPV omitted. The mean number of pelvic lymph nodes dissected per patient at systematic dissection was 15.8 ranging from 4 to 37. Conclusion is that SLN detection rate is significantly higher through laparoscopy than laparotomy. TP = 3; FP = 0; FN = 3; TN = 15; failed = 13. FIGO Stage 1A; 25; Stage IB+ = 9; type I and 2 tumours. Cervical injections; bilateral detection rate not recorded. Adverse reaction from index or reference test: not reported Other intraoperative complications: not reported Other postoperative complications: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Country: Germany Study design: a prospective cohort study of 31 patients with histologically‐proven endometrial cancer between August 2008 and March 2012, comparing SLN detection rates by single photon emission computed tomography with CT (SPECT/CT) with planar lymphoscintigraphy. Inclusion criteria: histologically‐proven endometrial cancer. Nil else reported. Exclusion criteria: all patients were excluded who were expected to have low‐risk tumours (G1/G2 histology, expected tumour size < 2 cm or expected < 50% myometrial infiltration in preoperative ultrasound). | ||
| Patient characteristics and setting | Number of patients: 31 Mean age years (range): 62 (41–77) Mean BMI (range): 32 (18–68) Histopathological cell type: adenocarcinoma = 28 (90%), clear cell adenocarcinoma = 1 (3%), and papillary = 2 (6%). FIGO stage: pT1a = 8 (26%), pT1b = 16 (52%), pT2a = 1 (3%), pT2b = 1 (3%), pT3a = 5 (16%). 2009 FIGO 1a= 24 and Ib+ is 7 Grade on hysterectomy specimen: grade 1 = 6 (19%), grade 2 = 16 (52%), grade 3 = 9 (29%) Lymphovascular space involvement: not reported Setting: single centre in Germany, namely the Department of Obstetrics and Gynecology, Hannover Medical School, Germany. | ||
| Index tests | Type of endometrial sampling: not reported Experience of operator: not reported Tracer used and amount: Tc‐99m nanocolloid (2 × 2.5 MBq; 0.5 (range 0.25–0.85) mL each). 4 mL patent blue. Method and timing of application: transcervical subepithelial injection of Tc‐99m nanocolloid (2 × 2.5 MBq; 0.5 (range 0.25 to 0.85) mL each) into the isthmocervical myometrium. Marker application, preoperative SLN detection and surgery were carried out same day. After a mean time of 40 minutes (range 30 to 60) minutes, lymphoscintigraphy and SPECT/CT were performed. Additionally, all patients received an injection of 4 mL patent blue into the same injection side directly before surgery. Method of detection (histopathological assessment, including ultrastaging): all sentinel nodes were sectioned perpendicularly to their long axis at 2 mm intervals and submitted totally for routine paraffin embedding. 4 step sections (3 μm thick) were taken at 250 μm intervals from each block and HE stained. All SLN negative on routine examination were examined immunohistochemically with antibody against cytokeratin AE1/AE3. Immunohistochemical examination was performed using the avidin–biotin complex method. Macrometastases were defined as tumour deposits >2.0 mm in size; micrometastases were defined as deposits of 0.2 mm to 2 mm in size; and ITC were defined as deposits no larger than 0.2 mm, including the presence of single non‐cohesive cytokeratin‐positive tumour cells. | ||
| Target condition and reference standard(s) | Type: 31 patients with grade I, II, or III endometrial cancer, all of whom received pelvic lymphadenectomy (100%) +/‐ para‐aortic lymphadenectomy (93.5%) up to the level of the renal vessels. 21 (68%) cases were treated by laparoscopy and 10 (32%) cases were treated by laparotomy. Lymph node number and site: In 28/31 patients (90.3%) at least one SLN was detected intraoperatively. A mean of 3.7 (range 1–11, SD 2.68) SLN were detected during surgery. Bilateral pelvic SLN detection succeeded in 16/28 (57%) patients. Para‐aortic SLN were detected in 7/28 (25%) patients. NB: In all patients, lymphoscintigraphy and SPECT/CT were carried out preoperatively. Using lymphoscintigraphy, at least one SLN was detected in 21/31 patients (68%). On average, 1.3 SLN (range 1–7, SD 1.46) were detected. Bilateral pelvic SLN identification succeeded in 7/21 (33%) patients and para‐aortic SLN were found in 3/21 (14%) patients. In contrast, SPECT/CT identified significantly more SLN compared to planar lymphoscintigraphy (p b 0.01). SPECT/CT showed at least one SLN in 28/31 patients (90.3% (CI95% = 79.5–100%)) and detected a mean of 2.2 SLN (range 1–8, SD 1.83). In 11/28 (39%) patients, SLN were bilaterally identified and para‐aortic SLN were found in 5/28 (18%) patients. | ||
| Flow and timing | All patients received the index and reference standard within 1 month. All patients received the same index test, however not all received the same reference standard (88% laparoscopy, 12% laparotomy). Pelvic + para‐aortic lymphadenectomy was performed in 93.5%; pelvic alone in 6.5%. All patients were included in the analysis. | ||
| Comparative | |||
| Notes | Study results: On average, 26 (range 10–46) pelvic LN and 12 (range 2–25) para‐aortic LN were removed. Evaluating the SLN method per patient, in 27/28 (96%) patients, regional LN status was correctly predicted by SLN. In this group of patients, the following values resulted in application of the SLN concept in EC: sensitivity 83%, NPV 96% and false‐negative rate (1/6) 17%. SPECT/CT significantly identified more SLN than lymphoscintigraphy (mean 2.2 (1–8) to 1.3 (1–7)) in more patients (29/31 (93.5%) to 21/31 (68%), P <0.01). If SLN were identified in one hemi‐pelvis, the histological evaluation of the SLN correctly predicted lymph node (LN) metastases for this basin which led to sensitivity 100%, negative predictive value (NPV) 100%, and false negative results 0%. TP = 5; FP = 0; FN = 1; TN = 22; failed = 3. FIGO stage IA = 8; Stage IB+ = 23; type 1 and 2 tumours. Cervical injection; bilateral detection 16 patients (using SPECT‐CT) Adverse reaction from index or reference test: nil reported Other intraoperative complications: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Country: Serbia Study design: prospective study of patients with histologically confirmed endometrial cancer between March 2015 and May 2016 at a single centre in Serbia. Inclusion criteria: endometrial cancer of medium (grade 2) and high risk (grade 3); FIGO Stage I; written informed consent. Exclusion criteria: preoperative FIGO Stages II‐IV; previous surgery that could change the uterine lymphatic drainage (conisation, ceasarean section, metroplasty, myomectomy); history of congenital uterus anomalies; duplex malignancies; deep vein thrombosis in lower extremities; allergies to the contrast agent. | ||
| Patient characteristics and setting | Number of patients: 27 Mean age (range): 65 (47‐79) years Mean BMI (range): 28.7 kg/m2 (21.9‐41.5 kg/m2) Histopathological cell type: endometrioid = 19 (70.4%), serous papillary = 4 (14.8%), clear cell = 1 (3.7%), endometrioid + serous papillary = 1 (3.7%), and other = 2 (7.4%). FIGO stage (postoperatively): IA = 9 (33.3%); IB = 11 (40.7%); II = 5 (18.5%); IIIC1 = 2 (7.4%) Grade on hysterectomy specimen: not reported Lymphovascular space involvement: present = 7 (25.9%); absent = 20 (74.1%) Setting: single centre in Belgrade, namely the University of Belgrade. | ||
| Index tests | Type of endometrial sampling: not reported Experience of operator: not reported Tracer used and amount: isosulfan blue (4‐6 cm3) Method and timing of application: intracervical injection (at three and nine o’clock positions) of isosulfan blue, with the superficial and deep infiltration in the quantity from 2 mL to 3 mL per position. This procedure was performed in the period of ten minutes prior to the commencement of the surgery, and after placing the female patient under anaesthesia. Method of detection (histopathological assessment, including ultrastaging): all nodes were stained by haematoxylin and eosin (H&E), followed by immunohistochemistry for pancytokeratin AE1:AE3 for the purpose of verification of the existence of macro and micrometastases (> 0.2 mm and ≤ 2 mm) including isolated tumour cells (≤ 0.2 mm). | ||
| Target condition and reference standard(s) | Type: 27 patients with early‐stage endometrial cancer, all of whom received systematic pelvic lymphadenectomy alone. All surgeries were performed by laparotomy. Lymph node number and site: a total of 163 SLNs were removed. Median SLNs per patient = 6. Anatomical location of SLNs not provided. | ||
| Flow and timing | All patients receive the index and reference standard within 1 month. The patients all received the same reference standard (no patients received para‐aortic lymphadenectomy). All patients were included in the analysis. | ||
| Comparative | |||
| Notes | Study results: a total of 319 non‐ SLNs were removed, with median number of non‐SLNs per patient of 10. Overall, SLN detection rate 92.6%; bilateral SLN detection rate 81.5%; FNR 0%; NPV 100%; PPV 57%; Sensitivity 100%; Specificity 86.9%. quote: "Of the 7 females with positive SLNs, at definitive histology, pelvic non‐SLNs were metastatic in four (57.1%) cases and negative in three (42.9%) cases. The false‐negative rate of sentinel procedure was 0%." TP = 7; FP = 0; FN = 0; TN = 22; failed = 3. FIGO stage IA = 9; STage IB+ = 18. Type 1 and 2 tumours. Cervical injection; bilateral SLN detection = 22. Adverse reaction from index or reference test: Not reported. Operating time: not reported Other intraoperative complications: not reported Other postoperative complications: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| Study characteristics | |||
| Patient Sampling | Country: Switzerland Study design: a retrospective analysis of patients with endometrial cancer between December 2012 and January 2017. Inclusion criteria: patients with histologically‐confirmed poorly differentiated endometrioid endometrial carcinoma, uterine papillary serous carcinoma (UPSC), clear cell carcinoma of the endometrium, neuroendocrine carcinoma, and carcinosarcoma, who underwent undergoing a laparoscopic NIR‐ICG SLN mapping followed by a total hysterectomy with bilateral salpingo‐oophorectomy and a full pelvic and para‐aortic lymphadenectomy up to the renal vessels were included in the study. Exclusion criteria: patients in whom only a pelvic lymphadenectomy or a para‐aortic lymphadenectomy that did not reach the renal vessels. | ||
| Patient characteristics and setting | Number of patients: 42 Median age (range): 65 years (43‐83) Median BMI (range): 26.8 kg/m2 (19‐46.3 kg/m2) Histopathological cell type: endometrioid = 24 (57.1%), papillary serous = 9 (21.4%), clear cell = 3 (7.1%), carcinosarcoma = 5 (11.9%), and neuroendocrine = 1(2.4%). FIGO stage: IA = 17 (40.5%); IB = 11 (26.2%); II = 1 (2.4%); IIIA = 3 (7.1%); IIIC1 = 2 (4.8%); IIIC2 = 8 (19%). Grade on hysterectomy specimen: grade 3 = 100% Lymphovascular space involvement: present = 10 (23.8%); absent = 32 (76.2%) Setting: single centre in Switzerland, namely the University Hospital of Bern, Bern, Switzerland. | ||
| Index tests | Type of endometrial sampling: not reported. Experience of operator: not reported Tracer used and amount: 8 mL of ICG. Method and timing of application: following a diagnostic laparoscopy, the cervix was injected submucosally, 1 cm deep in the stroma at the four cardinal points, with a total of 8 mL of ICG. Method of detection (histopathological assessment, including ultrastaging): at the final histopathological analysis, an ultrastaging of the SLNs was performed (three slides of haematoxylin and eosin (H&E) 200 μm). | ||
| Target condition and reference standard(s) | Type: 42 patients with grade 3 endometrial cancer, all of whom underwent pelvic and para‐aortic lymphadenectomy to the level of the renal vessels. Procedures were performed by laparoscopy. Lymph node number and site: the median number of retrieved lymph nodes retrieved per patient was 54. Median SLNs per patient was 3 (range 1‐18). Anatomical sites not reported. | ||
| Flow and timing | All patients received the index and reference standard within 1 month. The patients all received the same reference standard (pelvic and para‐aortic lymphadenectom). All patients were included in the analysis. | ||
| Comparative | |||
| Notes | Study results: overall detection rate 100%, bilateral detection rate 90.5%. Sensitivity 90% (95% CI 76–96%); NPV 97.1% (95% CI 85–99%); FN rate was 10%. TP = 9; FP = 0; FN = 1; TN = 32; failed = 0. FIGO stage 1A = 17; stage IB+ = 25; type 2 tumours only. cervical injection; bilateral SN detection in 38 patients. Adverse reaction from index or reference test: Not reported Other intraoperative complications: not reported Other postoperative complications: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| Study characteristics | |||
| Patient Sampling | Country: Italy Study design: prospective study of patients with early stage endometrial cancer between February 2001 and April 2002 Inclusion criteria: written informed consent signed. Exclusion criteria: not reported. | ||
| Patient characteristics and setting | Number of patients: 16 Mean age (standard deviation (SD)): 63 +/‐ 9.4 years (range, 50‐83) Mean/median BMI: not provided Histopathological cell type: adenocarcinoma = 16 (100%). No further information given. Unclear if any restriction on subtype. 1988 FIGO stage: Ib = 16 (100%). Converted to 2009 stage IA = 16. Grade on hysterectomy specimen: not reported Lymphovascular space involvement: not reported Setting: single centre in Italy. | ||
| Index tests | Type of endometrial sampling: not reported Experience of operator: not reported Tracer used and amount: 0.4 mL of 99mTc‐labelled albumin colloidal particles + 4 mL of patent blue dye. Method and timing of application: the day before surgery, 37 MBq of 99mTc‐labelled albumin colloidal particles were injected into the cervix, at 3, 6, 9 and 12 o'clock. The injection depth was 0.5 ±1 cm. Immediately before the surgical procedure (0 ± 20 minutes), in order to visualise SLNs, 4 mL of PBV was injected into the cervixat 3, 6, 9 and 12 o'clock (1 mL for each injection site). The injection depth was 0. 5 ±1 cm. Method of detection (histopathological assessment, including ultrastaging): SLNs were positively identified if they were blue, had in vivo radioactive counts at least three‐fold above background radioactivity, or both. Lymph nodes were marked as sentinel or non‐sentinel. The status of the dissected SLNs was examined to identify micrometastases by sections at reduced intervals (mean lymph node sections=24). Lymph nodes were formalin fixed, paraffin embedded and evaluated with haematoxylin and eosin and cytokeratin antibody (AE1/3, monoclonal antibody,1:250;Boehringer Mannheim, Indianapolis, IN, USA). A negative control was used. All non‐SLNs were evaluated with standard haematoxylin and eosin‐stained sections. | ||
| Target condition and reference standard(s) | Type: 16 patients with early stage endometrial cancer, all of whom received systematic pelvic lymphadenectomy alone. All surgeries were performed by laparoscopy. Lymph node number and site: a total of 24 SLNs was detected in 15 patients: six monolateral and 18 bilateral. All detected SLNs were internal iliac lymph nodes. | ||
| Flow and timing | All patients received the index and reference standard within 1 month. The patients all received the same reference standard (no patients received para‐aortic lymphadenectomy). All patients were included in the analysis. | ||
| Comparative | |||
| Notes | Study results: a total of 24 SLNs were identified and removed. In patients with a positive scan, an average of 1.6 SLNs were visualised. In one patient, SLNs were not localised with any technique. The sensitivity and negative predictive value of lymphoscintigraphy were both 100%. At histological analysis, three of the 24 were positive for micrometastases, whereas the remaining 21 were negative. No other surgically dissected lymph nodes presented metastases. TP = 3; FP = 0; FN = 0; TN = 12; failed = 1. 2009 FIGO stage IA = 16; IB+ = 0. Unclear if any restriction on tumour type (therefore assumed both). Cervical injection; bilateral detection in 9 patients. Adverse reaction from index or reference test: not reported Other intraoperative complications: not reported Other postoperative complications: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| Study characteristics | |||
| Patient Sampling | Country: Italy | ||
| Patient characteristics and setting | Number of patients: 54 | ||
| Index tests | Type of endometrial sampling suction device, curettage or direct biopsy: not reported | ||
| Target condition and reference standard(s) | Type: 54 patients with endometrial cancer all of whom had pelvic +/‐ para‐aortic lymphadenectomy performed. Four women in cervical group excluded due to operative difficulties. 10 women in hysteroscopic group excluded (8 due to patient refusal, and 2 due to operative difficulties ‐ tracer injected into tumour). All had laparoscopic lymphadenectomy. | ||
| Flow and timing | All patients received the index and reference standard within 1 month. The patients did not all receive the same reference standard, as only 2 patients (both in hysteroscopic injection group) received full pelvic and para‐aortic lymph node dissection. The remaining patients received pelvic lymph node dissection only. All patients were included in the analysis. | ||
| Comparative | |||
| Notes | 54 women were recruited prospectively between January 2001 and March 2007 with stage I and II endometrial cancer. For the mapping procedure the patients were divided into two groups: the cervical injection group and hysteroscopic injection group with Tch‐99. Intraoperative detection rate of SLN was 70% in cervical group and 65% in the hysteroscopic group. Mean SLN removed was 18 for cervical group and 20 for hysteroscopic group. No false negatives were found for either injection site group. Cervical injection TP = 4, FN = 0, FP=0, TN = 12, failed SLN = 7. FIGO stage ‐ not recorded; Type 1 and 2 tumours. Cervical injection; bilateral detection in 6 patients. Subserosal injection TP = 2 FP = 0 FN = 0 TN = 9; failed SLN = 6. FIGO stage ‐ not recorded; type 1 and 2 tumours. Subserosal uterine injection; bilateral detection in 3 patients. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Country: Sweden | ||
| Patient characteristics and setting | Number of patients:102 | ||
| Index tests | Type of endometrial sampling: not reported | ||
| Target condition and reference standard(s) | Type: 102 patients with high risk endometrial cancer scheduled for robotic‐assisted surgery. All had robotic‐assisted infrarenal paraaortic (unless in cases of severe comorbidity or advanced age) and full pelvic lymph‐node dissection in addition to a hysterectomy and bilateral salpingo‐oophorectomy and in case of a non‐endometrioid histology, even an infra‐colic omentectomy. I patient excluded from analysis due to intraoperative complications (see below). | ||
| Flow and timing | All patients received the index and reference standard within 1 month. The patients all received the same reference standard. Not all patients were included in the analysis ‐ 102 high‐risk endometrial cancer patients were recruited, but only 101 patients included in the analysis. One patient was excluded because her surgery was converted to open surgery due to extensive intraabdominal adhesions, and no ICG was injected. A further six patients were not included in the statistical analysis regarding displaying of the lower paracervical pathway as a result of impaired exposure due to obesity or adhesions, however, this does not impact on the TP, TN, sensitivity, or specificity analyses. | ||
| Comparative | |||
| Notes | Study results (sentinel node detection rate for unilateral and bilateral pelvic nodes, sentinel node detection for para‐aortic nodes (for false negative cases, are reference nodes on same side or is there a failure to detect the sentinel node: [MS1] Unilateral pelvic detection rate: 100% after re‐injection. TP = 24; FP = 0; FN = 0; TN = 77; failed = 0. FIGO stage IA = 60; Stage IB+ = 41 (breakdown only by uterine factors ‐ final IA/IB= not given). Type 1 and 2 tumours. Cervical injection (and re‐injection if failed detection bilaterally); bilateral detection in 96 patients. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Country:USA | ||
| Patient characteristics and setting | Number of patients:385 | ||
| Index tests | Type of endometrial sampling: not reported | ||
| Target condition and reference standard(s) | Type: 385 patients ‐ 356 had study intervention (29 had no intervention; 16 had dye without lymphadenectomy and were staged by biopsy of extra‐uterine disease). All were performed robotically. | ||
| Flow and timing | All patients received index test and reference standard within 1 month. Pelvic lymphadenectomy was done in all patients. Para‐aortic dissection was done in 74 (74%) of 100 patients with high‐grade tumours. All patients were included in the analysis | ||
| Comparative | |||
| Notes | Study results (sentinel node detection rate for unilateral and bilateral pelvic nodes, sentinel node detection for para‐aortic nodes (for false negative cases, are reference nodes on same side or is there a failure to detect the sentinel node: [MS1] Unilateral detection rate: unclear TP = 35; FP = 0; FN = 1; TN = 257; failed = 47. FIGO stage (of those included in final analysis) IA = 226; IB+ = 114. Type 1 and 2 tumours. Cervical injection; bilateral detection in 177 patients. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Country: Italy Study design: a prospective, non‐randomised study of women presenting with a preoperative diagnosis of endometrial cancer conducted between January 2006 and December 2012. The aim of this study was to evaluate the role of PET/CT and sentinel lymph node (SLN) biopsy in staging high‐risk endometrial cancer patients in early clinical stage. All patients underwent PET/CT followed by surgery, but the SLN procedure was only introduced from December 2010 onwards, therefore only a subset of patients underwent both PET/CT and SLN mapping. Inclusion criteria: Preoperative diagnosis of histologically proven grade 2 endometrioid tumours with myometrial invasion of more than 50% at preoperative imaging examination or any grade 3 endometrioid carcinoma, carcinosarcoma, serous carcinoma, or clear cell carcinoma. Exclusion criteria: not reported | ||
| Patient characteristics and setting | Number of patients: 93; of these only 22 underwent SLN mapping and PET/CT Median age: 63 years (range, 27‐86 years) Median BMI: 28.2 kg/m2 (range, 20.8‐43.1 kg/m2) Histopathological cell type: endometrioid = 80 (86%), clear cell/serous = 5 (5.4%), MMMT = 6 (6.5%), mixed = 2 (2.1%) FIGO stage: IA = 36 (38.7%), IB = 17 (18.3%), II = 7 (7.5%), IIIA = 7 (7.5%), IIIB = 4 (4.3%), IIIC1 = 10 (10.8%), IIIC2 = 7 (7.5%), IVB = 5 (5.4%)‐ this is for all 93 patients and not the 22 included in the study. Grade on hysterectomy specimen: grade 1 = 2 (2.1%), grade 2 = 37 (39.9%), grade 3 = 54 (58%) Lymphovascular space involvement: present in 38 cases (40.9%), absent in 55 cases (59.1%) Setting: single centre, namely the San Gerardo Hospital, Monza, Italy | ||
| Index tests | Type of endometrial sampling: not reported Experience of operator: not reported Tracer used and amount: 200‐300 µCi radiolabeled filtered Tc99‐albumin nanocolloid in 0.2‐0.3 mL volume; and 1 mLof methylene blue (1%) dye Method and timing of application: preoperative lymphoscintigraphy was performed (within 20 hours to surgery) with 4 submucosal cervical injections (3‐, 6‐, 9‐, and 12‐o'clock positions) of 200 to 300 μCi radiolabeled filtered 99mTc‐albumin nanocolloid in 0.2 to 0.3 mL volume. Second, in the operating theatre, 2 submucosal cervical injections of 1 mL of methylene blue dye (methylene blue 1%; Bioindustria LIM, Novi Ligure, Italy) were performed using 22‐gauge spinal needles at 3‐ and 9‐o'clock positions. Method of detection: SLNs were assessed in a standardised manner by ultrastaging and examined by immunohistochemistry. Further detail is not reported. | ||
| Target condition and reference standard(s) | Type: patients with G1‐3 endometrial cancer, all of whom underwent surgical treatment including peritoneal cytology total extrafascial hysterectomy, bilateral salpingo‐oophorectomy, and systematic pelvic LN dissection, including the superficial and deep obturator, external, and superficial LNs, and deep common iliac LNs. Aortic lymphadenectomy was performed only in patients with positive PET/CT findings in the pelvic and/or aortic area or suspicious aortic nodes during surgery. Thirty‐six cases (39%) were performed by open surgery, and 57 (61%) were treated by laparoscopic surgery. Lymph node number and site: the median number of pelvic LNs removed was 28 (range, 14‐56). A total of 2572 pelvic LNs were removed and analysed. In the 22 patients who underwent the SLN procedure, 54 SLNs were identified; 52 were in the pelvic region and 2 in the aortic area. The median number of SLNs was 2 (range, 1‐5). | ||
| Flow and timing | All patients received the index and reference standard within 1 month. Patients did not all receive the same reference standard. Aortic lymphadenectomy was performed only in patients with positive PET/CT findings in the pelvic and/or aortic area or suspicious aortic nodes during surgery (3/22 patients in the SLN group). Thirty‐six cases (39%) were performed by open surgery, and 57 (61%) were treated by laparoscopic surgery. It is unclear whether all patients were included in the analysis. | ||
| Comparative | |||
| Notes | Study results: an optimal bilateral SLN detection was achieved in 16 (72.7%) of 22 women, whereas a monolateral detection was found in the remaining 6. Six (27.3%) of 22 women had pelvic LN metastases—3 of 6 correctly identified by both PET/CT and SLN biopsy and 3 of 6 identified only by SLN mapping and ultrastaging. A total of 12 positive SLNs were diagnosed, of which 7 only by ultrastaging (63.6%), with a median diameter of tumour deposits of 0.6 mm (range, 0.2–2 mm) and 5 by a standard hematoxylin‐eosin histological examination. Ten positive SLNs were harvested in the pelvic area (external or superficial obturator LNs), one in the presacral region, and one in the aortic area under the inframesenteric artery. For patients undergoing both PET/CT and SLNs mapping the sensitivity is reported as 45.5% (CI 16‐74.9), the specificity as 100%, the PPV as 100%, the NPV as 94.8% (CI 90.6‐98.8), and the accuracy as 95% (CI 91‐98.9) by pelvic nodal chains‐based analysis. There were no FP nodes. By patient‐based analysis. TP = 3; FP =0; FN = 0; TN = 16; failed = 0. FIGO staging not reported separately for SLN sub‐group; type 1 and 2 tumours. Cervical injection; bilateral detection in 16 patients. Adverse reaction from index or reference test: not reported. Operating time: not reported. Other intraoperative complications: not reported. Other postoperative complications: not reported. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Country: Italy | ||
| Patient characteristics and setting | Number of patients: 59 Age: mean (range): 57.7 (29.3 – 75.0) Preoperative: endometrioid – 57, serous papillary ‐ 2 ‐ 2009: IA ‐ 34 (57.6%), IB ‐ 9 (15.3%), II – 3 (5.1%), IIIA ‐ 2 (3.3%), IIIC1 – 7 (11.9%), IIIC2 ‐ 3 (5.1%), IVB ‐ 1 (1.7%), | ||
| Index tests | Type of endometrial sampling: not reported. ‐ Preoperative: 15‐min dynamic planar acquisition was performed in the anterior view with a gamma equipped with a low‐energy general‐purpose collimator. Initial acquisition followed by sequential static imaging every 5 minutes for up to 1 hour or until at least one SLN was identified. | ||
| Target condition and reference standard(s) | Type: the extent of lymphadenectomy was defined according to preoperative and intraoperative pathological findings assessed by frozen section. Patients undergoing open or laparoscopic surgery with one of 1) endometrioid adenocarcinoma with intraoperative staging equal to or higher than IBG2 (FIGO 1988) or 2) clear cell or serous carcinoma had full pelvic and para‐aortic lymphadenectomy. Lymphadenectomy was considered adequate when more than 20 nodes were harvested. | ||
| Flow and timing | All patients received the index and reference standard within 1 month. 49 underwent open surgery, 10 underwent laparoscopic surgery – parameters for deciding this not reported. 2 different types of hysterectomy performed (type A in 48, type B1 in 11) ‐ parameters for deciding this not reported. All patients were included in the analysis. | ||
| Comparative | |||
| Notes | Study results: Bilateral detection rate: not reported Lymph node number and site: ‐ SLN detection: both para‐aortic and pelvic ‐ 31 (52.5%), Isolated para‐aortic ‐ 2 (3.4%), exclusive pelvic ‐ 26 (44.1%). NPV was 98.0% (95% CI 89.4–100.0) and sensitivity 90.0% (95%CI: 55.5–99.8). No uninterpretable results were reported as the SLN identification rate was 100%. TP = 9; FP = 0; FN = 1; TN = 49; failed = 4. FIGO stage IA = 34; Stage IB = 25; type 1 and 2 tumours. Cervical injection; bilateral detection rate not given. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Country: USA | ||
| Patient characteristics and setting | Number of patients: 101 BMI: Median (range): 30.8 (15.8 – 64.3). | ||
| Index tests | Type of endometrial sampling suction device: not reported (cervical lesions directly biopsied). Robotic: Method and timing of application: see above, all cervical injections superficial (submucosal) and deep (1 cm into the stroma), at 3 and 9 o’ clock. SLN detection: not reported | ||
| Target condition and reference standard(s) | Type: patients with high‐risk endometrial cancer all of whom had full surgical staging, including pelvic and para‐aortic lymph node dissection to the level of the renal vessels. Forty‐four cases (44%) were performed by laparoscopic surgery, 37 (37%) by robotic surgery, 17 (17%) by laparotomy, and 3 (3%) by combined laparoscopy and robotic surgery. Lymph node number and site: Total (n = 101): | ||
| Flow and timing | All patients received the index and reference standard within 1 month. Surgical approach: Laparoscopy 44 (44%), Robotic 37 (37%), Laparotomy 17 (17%), Combined laparoscopy and robotic 3 (3%) – left to surgeon’s preference. All patients were included in the analysis. | ||
| Comparative | |||
| Notes | Prospective study of 101 patients with high‐risk endometrial cancer at a single centre in the USA. Patients received cervical injection of either ICG (61%), blue dye (28%), or Tc‐99 (11%). SLN detection rate was 89%; bilateral detection rate was 58%. Overall sensitivity was 95% per patient. False negative rate was 5% by per patient analysis. NPV 98.6% per patient. Per hemi‐pelvis, sensitivity was 92.9%, NPV 98%, false negative rate 7.1%. Study results: Any detection: 90 (89%) Tc99 and blue dye (n = 11) TP = 4; FP = 0; FN = 0; TN = 6; failed =1. FIGO stage breakdown not given per group; type 1 and 2 tumours. Cervical injection; bilateral detection 9 patients. Blue dye alone (n = 28) TP = 8; FP = 0; FN = 0; TN = 16; failed = 5. FIGO stage breakdown not given per group; type 1 and 2 tumours. Cervical injection; bilateral detection 11 patients. ICG (n = 62) TP = 8; FP = 0; FN = 1; TN = 48; failed = 5. FIGO stage breakdown not given per group; type 1 and 2 tumours. Cervical injection; bilateral detection 32 patients. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Country: Turkey Study design: patients with clinically early‐stage endometrial cancer were included in this prospective study between July 1 2016 and February 28 2017. | ||
| Patient characteristics and setting | Number of patients: 71 | ||
| Index tests | Type of endometrial sampling suction device: not reported | ||
| Target condition and reference standard(s) | Type: laparoscopy and laparotomy (preferred when contraindications for Trendelenburg position present or increased intraabdominal pressure due to medical comorbidities.) | ||
| Flow and timing | All patients received the index and reference standard within 1 month. The patients did not all receive the same reference standard, with 46 patients (64.7%) receiving pelvic lymph node dissection only and 25 patients (35.2%) receiving pelvic and para‐aorticl lymph node dissection. All patients were included in the analysis. | ||
| Comparative | |||
| Notes | Study results: Bilateral detection rate: 55 (77.4%) TP = 7; FP = 0; FN = 1; TN = 60; failed = 3. FIGO stage IA = 40; Stage IB+ = 31; type 1 and 2 tumours. Cervical injection; bilateral detection in 55 patients. Adverse reaction from index or reference tests: none related to SLN mapping | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Country: Spain | ||
| Patient characteristics and setting | Number of patients:74 | ||
| Index tests | Type of endometrial sampling suction device, curettage or direct biopsy: curettage or hysteroscopic direct biopsy | ||
| Target condition and reference standard(s) | Type: all patients underwent laparoscopic surgery, included pelvic and para‐aortic lymph node dissection to the level of the left renal vein. Area of SLN drainage (n = 55); pelvic (exclusive) 30 (54.5%), paraaortic (exclusive) 7 (12.8%) Pelvic and paraaortic 18 (32.7%) Side of pelvic node detection (n = 48): ‐ Left pelvic 21 (43.7%) ‐ Right pelvic 13 (27.1%) ‐ Bilateral 14 (29.2%) Site of paraaortic node detection (n = 25): ‐ Supramesenteric 4 (16.0%) ‐ Inframesenteric 15 (60.0%) ‐ Supramesenteric ‐ Inframesenteric 6 (24.0%). | ||
| Flow and timing | All patients received the index and reference standard within 1 month. All patients received the same reference standard and index tests. The initial 9 patients in the pilot received a different amount of tracer and were not included in the analysis; this initial subgroup served to evaluate the volume of radiotracer required for adequate SLN visualisation. All patients that were not part of this pilot were subsequently included in the analysis. | ||
| Comparative | |||
| Notes | Prospective study of 74 patients with high‐risk endometrial cancer recruited between March 2006 and March 2011 at a single centre in Barcelona. All patients received myometrial injection of Tch‐99. SLN detection rate was 74.3%. Sensitivity 92.3%. NPV 97.7%. Study results: Bilateral detection: 14 (29%) (NB unclear TP = 12; FP = 0; FN = 1; TN = 42; failed = 19. FIGO stage IA = 30; Stage IB+ = 44; type 1 and 2 tumours. Subserosal uterine injection (transvaginal USS guidance); bilateral detection = 14 patients. Adverse reaction from index or reference test: none | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| Study characteristics | |||
| Patient Sampling | Country: Czech Republic
Exclusion criteria: 1. FIGO III‐ IV endometrial carcinomas and other histological subtypes of adenocarcinoma with the exception of endometrial 2. Distant metastases 3. Contraindications to surgery | ||
| Patient characteristics and setting | Number of patients:18 | ||
| Index tests | Type of endometrial sampling suction device, curettage or direct biopsy: curettage Experience of operator: not reported Tracer used and amount: 4 mL of Patent Bleu. Method and timing of application: subserosally injection from the dorsal side of uterus body in the level of ligamentum ovarii proprium and uterine vascular bundles in the isthmic part of the uterus. Injection depth was approximately 1 mm. Method of detection (histopathological assessment, including ultrastaging): following dissection of pelvic peritoneum to inspect the retroperitoneal space, blue nodes were removed and identified as sentinel describing the anatomical area of location and laterality. A pathologist evaluated SLNs at first with H&E staining and then all negative sentinel nodes were processed by sentinel node ultra‐section technique. Six sections in one node at 200 μm intervals were performed. An additional cut was amended between the third and fourth section upon which immunohistochemistry examinations were applied; it was namely a mouse monoclonal antibody anti AE1/AE3 cytokeratine. Non SLNs were stained only by H&E. | ||
| Target condition and reference standard(s) | Type: 18 patients with a histological diagnosis of stage I‐II endometrial cancer all of whom had pelvic and para‐aortic lymphadenectomy. All cases were performed by laparotomy. Lymph node number and site: Total of 773 lymph nodes were removed in 18 patients: pelvic 420 (54%) and para‐aortic 353 (46%). SLNs were detected in 16 of 18 patients totaling 59 nodes (7.6% of all nodes). Forty‐eight were identified in the pelvic area (81%) and 11 nodes (19%) in the para‐aortic area. Three metastatic SLNs were found in two patients (11%). No false‐negative nodes were demonstrated. | ||
| Flow and timing | All patients received the index test and reference standard within 1 month. All patients received the same reference standard. All patients were included in the analysis. | ||
| Comparative | |||
| Notes | Study results: FNR 0. Sensitivity and specificity not reported. SLN detection rate 88%. TP = 2; FP = 0; FN = 0; TN = 14; failed = 2. FIGO stage breakdown not give; type 1 and 2 tumours in inclusion criteria. Subserosal endometrial injection; bilateral detection rate not given. Adverse reaction from index or reference test: not reported. Other intraoperative complications: not reported. Other postoperative complications: not reported. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| Study characteristics | |||
| Patient Sampling | Country: France Inclusion criteria: biopsy‐confirmed endometrial cancer, apparent stage I after clinical and radiologic work‐up. Exclusion criteria: not reported. | ||
| Patient characteristics and setting | Number of patients: 66 Preoperative: IA47 (71.3%), IB 16 (24.2%), unknown 3 (4.5%) Postoperative: IA 38 (57.6%), IB 13 (19.7%), II 7 (10.6%), IIIA 1 (1.5%), IIIC 7 (10.6%) | ||
| Index tests | Type of endometrial sampling suction device, curettage or direct biopsy: not reported Experience of operator: local surgeons had to be experienced Tracer used and amount: 2 mL Patent Blue in 2 mL NaCl Method and timing of application: The solution was injected into the cervix through a 25‐gauge spinal needle at the 12‐, 3‐, 6‐, and 9‐o’clock positions (1 mL per injection). Surgery was started immediately after the injection Method of detection (histopathological assessment, including ultrastaging): the pelvic and para‐aortic regions were carefully inspected transperitoneally for blue lymph | ||
| Target condition and reference standard(s) | Type: 66 patients with a histological diagnosis of stage I endometrial cancer all of whom had peritoneal washing, SLN procedure, bilateral salpingo‐oophorectomy, systematic bilateral pelvic lymphadenectomy, and extrafascial hysterectomy. Para‐aortic node dissection performed only when perioperative analysis of the SLN showed an involvement or when a para‐aortic SLN was identified. Performed laparoscopically (55 cases) or by laparotomy (11 cases) Lymph node number and site: a total of 926 lymph nodes were removed: 453 in the right pelvis (48.9%), 433 in the left pelvis (46.8%), and 40 in para‐aortic area (4.3%). SLN detection rate 62.1% (at least 1 in 41 patients). Thirty‐eight sentinel nodes (51.3%) were located in the right pelvis, and 36 sentinel nodes (48.7%) were located in the left pelvis. None was identified in the para‐aortic area. False negative rate of 40%. | ||
| Flow and timing | All patients received the index test and reference standard within 1 month. Not all patients received the same reference standard. Para‐aortic node dissection was only performed when perioperative analysis of the SLN showed an involvement or when a para‐aortic SLN was identified (ratios not reported). In addition, the procedures were performed laparoscopically (55 cases) or by laparotomy (11 cases). All patients were included in the analysis. | ||
| Comparative | |||
| Notes | Study results: FNR 40%. Sensitivity 60%. SLN 62.1%. NPV 94.7 %. Adverse reaction from index or reference test: no anaphylactic reaction to patent blue occurred. Other intra‐perative complications: not reported. Other postoperative complications: not reported. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| Study characteristics | |||
| Patient Sampling | Country: China Study Design: a prospective study of 131 consecutive patients attending Shanghai First Maternity and Infant Hospital with endometrial cancer were enrolled between July 2016 and July 2018. Inclusion Criteria: age of 18+ years, diagnosed with stage I or II endometrial cancer defined as normal preoperative CT abdomen and pelvis or magnetic resonance imaging findings and normal pulmonary imaging (chest x‐ray or CT scan) findings Exclusion Criteria: evidence of extrauterine disease; previous treatment for endometrial cancer; or contraindications for receiving the ICG tracer, including a history of hepatic impairment or iodine allergy. | ||
| Patient characteristics and setting | Age: median (range): 55.8 (35‐76) years BMI: median (range): 24.8 (18.5 ‐ 37.8) Histopathological cell type: endometrioid 112 (85.5%), serous 12 (9.2%), clear cell 4 (3.1%), carcinosarcoma 3 (2.3%) FIGO Stage: IA 96 (73.3%), IB 18 (13.7%), II 4 (3.1%), IIIa 5 (3.8%), IIIc1 4 (3.1%), IIIc2 4 (3.1%) Grade on hysterectomy specimen: 1 ‐ 98 (74.8%), 2 ‐ 8 (6.1%), 3 ‐ 25 (19.1%) Lymphcovascular space involvement: not reported Setting: single centre in Shanghai, China (Shanghai First Maternity and Infant Hospital) | ||
| Index tests | Type of endometrial sampling suction device, curettage or direct biopsy: not reported | ||
| Target condition and reference standard(s) | Type: all patients underwent laparoscopic surgery, but only high‐risk patients had complete para‐aortic lymphadenectomy (n = 25). ‐ External iliac (155, 53.3%) ‐ Obturator (76, 26.1%) ‐ Internal iliac (47, 16.2%) ‐ Common iliac (8, 2.8%) ‐ Para‐aortic (4, 1.3%). | ||
| Flow and timing | All patients received the index test and reference standard within 1 month. Patients did not all receive the same reference standard. All patients had pelvic lymphadenectomy, but only 25 (19%) had para‐aortic lymphadenectomy. All patients were included in the analysis. | ||
| Comparative | |||
| Notes | Study results: Bilateral detection: 81 (61.8%) FN rate: 50% | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
CI: confidence interval;CT: computed tomography; H&E; haemotxylin and eosin; ; IHC: mmunohistochemistry; ICG: indocyanine green; IHC: mmunohistochemistry; ITC: individual tumour cells; LVSI: lymphovascular space invasion; MMMT: malignant mixed Müllerian tumour; MRI: magnetic resonance imaging;NaCl: sodium chloride; NIR: Near‐infrared; NPV: negative predictive value; PPV: positive predictive value; ; SLN: sentinel lymph node.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| No comparison of SLN versus lymphadenectomy. This paper investigates the diagnosis of pelvic lymph node invasion in cancers of the vulva and uterus by combined lymphography and phlebography. | |
| Systematic review (references screened for inclusion) | |
| Letter/commentary | |
| Review article (references screened for inclusion) | |
| Review article (references screened for inclusion) | |
| Letter/commentary | |
| No ultra‐section of SLN | |
| Wrong study design ‐ study of IHC for SLN. It investigates how immunohistochemical workup of sentinel nodes in endometrial cancer improves diagnostic accuracy. | |
| Letter/commentary | |
| Meeting abstract only | |
| Systematic review (references screened for inclusion) | |
| Review article (references screened for inclusion) | |
| Wrong intervention ‐ this study investigates indirect radionuclide lymphography using ln‐113m in patients with uterine cancer. | |
| Did not have to perform full pelvic LND | |
| Meeting abstract describing surgical technique. | |
| No ultra‐section | |
| Meeting abstract only; no evidence of ultrasection of SLN. | |
| No ultra‐section | |
| No ultra‐section | |
| histological study looking at node processing. Not all patients had systematic LND. | |
| Systematic review (references screened for inclusion) | |
| Letter/commentary | |
| Letter/commentary | |
| Description of algorithm of SLN used in clinical practice | |
| Review article (article in French) | |
| Study looking at value of adding SPECT/CT to aid detection rate of SLN rather than diagnostic test accuracy study | |
| <10 cases | |
| No evidence of ultra‐section/ultrastaging | |
| Comparison of SLN techniques ‐ no requirement for full PLN unless high risk patient. | |
| Meeting abstract only ‐ no evidence of ultrasection of SLN | |
| Review article (references screened for inclusion) | |
| <10 cases | |
| Did not have to perform full pelvic LND | |
| Review article (references screened for inclusion) | |
| No ultra‐section | |
| Systematic review (references screened for inclusion) | |
| Systematic review (references screened for inclusion) | |
| Meeting abstract only ‐ no requirement for systematic lymphadenectomy ‐ experience of single surgeon. | |
| Wrong study design. This study reports on the long‐term results of the SENTI‐ENDO study, which has already been included. It looks at the impact of SLN biopsy on management and survival in patients with early stages of endometrial cancer. This study is a secondary endpoint reporting the long‐term recurrence‐free survival (RFS) and the impact of the SLN procedure on adjuvant therapies. | |
| Systematic review (references screened for inclusion) | |
| Review article (not systematic) | |
| Did not have to perform full pelvic LND | |
| Meeting abstract only ‐ audit of adherence to protocol and standardised reporting of histology | |
| Letter/commentary | |
| Comparison of cohorts with and without SLN detection. | |
| Letter/commentary | |
| Did not have to perform full pelvic LND | |
| Wrong study design ‐ this is a retrospective study reporting on the initial experience of robotic blue‐dye SLN mapping and examining factors predicting successful SLN mapping. | |
| Review article (references screened for inclusion) | |
| No ultra‐section | |
| Wrong study design ‐ this study investigates the impact of obesity on SLN mapping in patients with newly diagnosed uterine cancer undergoing robotic surgery. | |
| Wrong study design ‐ this study compares the detection of SLN using ICG versus blue dye during robotic surgery in uterine cancer, but it does not do a full lymphadenectomy for each patient. | |
| Did not have to perform full pelvic LND | |
| No evidence of ultrastaging of SLN (quote: "Multiple sections were prepared from each block. A set of three 4‐mm‐thick sections was cut every 250 nm and stained by hematoxylin‐eosin. Detection of tumor cells defined a positive SLN." ) No IHC so excluded as although ultrasection, no ultrastaging as less sensitive without IHC. | |
| Letter/commentary | |
| <10 cases | |
| No evidence of ultrasection of SLN | |
| Wrong study design ‐ this paper reports on results of the SENTI‐ENDO study, specifically looking at the role of pre‐operative lymphoscintigraphy for SLN mapping in women with early stage endometrial cancer. | |
| No ultra‐section | |
| Letter/commentary | |
| Letter/commentary | |
| No ultra‐section | |
| Meeting abstract only ‐ wrong study design ‐ retrospective review to identify factors associated with successful bilateral SLN mapping | |
| Did not have to perform full pelvic LND | |
| Wrong outcomes ‐ this is a study of the uterine lymphatic anatomy and it's role in standardisation of pelvic SLN detection in endometrial cancer. | |
| No ultra‐section | |
| Letter/commentary | |
| Wrong study design ‐ this is a study of using the Memorial Sloan Kettering Cancer Center (MSKCC) surgical algorithm to aid in the detection of SLNs by ICG and near‐infrared (NIR) fluorescence mapping. | |
| Wrong study design ‐ this study compares the SLN detected via injection to the uterine corpus or the cervix. It shows that lymphatic drainage to the pelvic area from both these anatomic locations is identical, and that both methods of injection are valid for detection of SLNs. Patients do not subsequently receive full lymphadenectomy. | |
| <10 cases | |
| No ultra‐section | |
| Only 50% had ultrastaging performed | |
| Wrong outcomes ‐ this study evaluates the anatomical location of SLNs following cervical injection of tracers in endometrial cancer. | |
| Systematic review (full text not freely available for screening references) | |
| Review article (references screened for inclusion) | |
| Did not have to perform full pelvic LND | |
| No ultra‐section | |
| No ultra‐section | |
| Systematic review (references screened for inclusion) | |
| Meeting abstract only ‐ single institution experience ‐ standard H&E pathology ‐ no evidence of ultrasection.. | |
| Did not have to perform full pelvic LND. Proportion of patients included in Abu‐Rustum 2009. Author emailed for further clarification to define 2x2 table for unique patients. | |
| Wrong study design ‐ this study evaluates the role of ultra‐staging in a low‐risk group of patients with endometrioid adenocarcinoma. | |
| Did not have to perform full pelvic LND | |
| Wrong indication ‐ this study evaluates the efficency of SLN mapping with ICG in cervical cancer. | |
| Wrong study design ‐ this study looks at SLNs, planar scintigraphy and SPECT/CT in various types of tumours, and evaluates factors influencing detection success. | |
| Meeting abstract only. Meting abstract of on‐going RCT study (details limited) and no evidence of ultrasection of SLN. | |
| Abstract only. Feasibility study of introducing SLN mapping for endometrial cancer patients at robotic and open surgery. Appear to look at SLN detection rates but 2x2 data not presented. | |
| No evidence of ultrasection of SLN | |
| No evidence of ultrasection, although IHC used if H&E negative. Same population as Lelievre 2004 (with 3 additional patients), which stated quote: "The sentinel nodes were then examined on two levels per half with standard H&E staining, and in case of negativity, an immunohistochemical analysis with an anticytokeratin anti‐ body was realized (monoclonal antikeratin antibody large spectrum, KL1, Immunotech, Marseille, France)" | |
| Review article (references screened for inclusion) | |
| Systematic review (references screened for inclusion) | |
| No evidence of ultra‐section | |
| Letter/commentary | |
| <10 cases | |
| Review article (references screened for inclusion) | |
| Wrong study design ‐ the aim of this study was to evaluate the freehand SPECT to detect the SLN in patients with uterine or cervical cancers. Patients do not all receive full pelvic lymphadenectomy. | |
| Did not have to perform full pelvic LND | |
| Did not have to perform full pelvic LND | |
| Letter/commentary | |
| Meeting abstract only ‐ SLN detection rates as part of prospective cohort study ‐ no requirement for PLND | |
| No ultra‐section | |
| Meeting abstract only ‐ initial experience of technique and SLN detection rate reported. Does not appear to have been requirement for full PLND. | |
| No ultra‐section | |
| Did not have to perform full pelvic LND | |
| Description of technique of SLN. | |
| Wrong study design ‐ comparison of SLN detection methods and unable to extract 2x2 table data | |
| Review article | |
| No ultra‐section | |
| Letter/commentary | |
| Did not have to perform full pelvic LND | |
| Prospective study of 40 patients with endometrial cancer (dates not provided), comparing SPECT‐CT to planar lymphoscintigraphy to localise SLNs. Wrong study design. | |
| Wrong indication ‐ this is a retrospective study to evaluate factors predicting a higher SLN removal count in cervical and endometrial cancer by univariate and multivariate analysis. | |
| 75 patients of whom only 42 underwent systematic pelvic or pelvic and para‐aortic lymph node dissection | |
| Letter/commentary | |
| Did not have to perform full pelvic LND | |
| Meeting abstract only ‐ no evidence of ultrasection, but likely same patients as in included study (Papadia 2018). | |
| Meeting abstract only ‐ unable to confirm if ultrasection performed and if full LND in all cases from data available. | |
| No ultra‐section | |
| Wrong outcomes ‐ this study evaluates the use of SPECT/CT for improved SLN localisation in endometrial cancer. | |
| Letter/commentary | |
| Wrong outcomes ‐ this study evaluates the outcome and the role of adjuvant treatment in the management of patients with endometrial cancer and isolated tumour cells identified by SLN mapping. | |
| Feasibility study of SLN detection in 17 patients. No evidence of ultrasection. | |
| No ultra‐section | |
| No ultra‐section | |
| No ultra‐section | |
| Systematic review (references screened for inclusion) | |
| Although ultra‐section was performed, immunohistochemistry evaluation of negative SLNs was not performed in this study, so does not meet our criteria for ultra‐staging in the review. | |
| Did not have to perform full pelvic LND | |
| Systematic review (references screened for inclusion) | |
| No ultra‐section | |
| Did not have to perform full pelvic LND | |
| Did not have to perform full pelvic LND | |
| Did not have to perform full pelvic LND | |
| Not all patients had full LND and unable to separate those who did from those who did not. Unable to extract 2x2 table of patients who met inclusion criteria. | |
| Systematic review (references screened for inclusion) | |
| Letter/Commentary re SENTI‐ENDO study (Ballester 2011) | |
| Meeting abstract only ‐ comparison of Tc99 and blue due versus IGC techniques for successful SLN mapping | |
| Wrong study design ‐ this is a study that presents a video on the technique of SLN mapping in 2 cases (1 endometrial and 1 cervical cancer) using ICG and the near‐infrared technology provided by the newest Da Vinci Xi robotic system. | |
| Wrong indication ‐ this study investigates sentinel node detection with (99m)Tc‐phytate in cervical cancer. | |
| Meeting abstract only ‐ routine H&E and imprint. No evidence of ultrasection. | |
| Did not have to perform full pelvic LND | |
| Wrong study design ‐ the aim of this study was to characterise treatment patterns and oncologic outcomes in patients with low‐volume lymph node metastasis (isolated tumour cells and micrometastasis) discovered during SLN mapping for endometrial carcinoma. | |
| Wrong study design. Implementation of a sentinel lymph node mapping algorithm for endometrial cancer: surgical outcomes and hospital charges. No requirement for full PLND. | |
| Wrong study design. Implementation of a sentinel lymph node mapping algorithm for endometrial cancer: surgical outcomes and hospital charges. No requirement for full PLND. | |
| Did not have to perform full pelvic LND | |
| No ultra‐section | |
| Meeting abstract only ‐ ultrasection performed and all appear to have had full PLND but unable to extract data for 2x2 table from abstract. | |
| Review article (references screened for inclusion) | |
| Wrong patient population ‐ this study investigates the utility of SLN mapping in the management of endometrial atypical hyperplasia. | |
| Study of learning curves for SLN technique | |
| Meeting abstract only ‐ evaluation of learning curve of SLN technique | |
| Meeting abstract only ‐ comparison of positive LN rates comparing SLNB and full lymphadenectomy in different patient cohorts. Unable to extract 2x2 data or determine if ultrasection performed. | |
| Did not have to perform full pelvic LND | |
| Not all patients required full PLND. Quote: "Systematic lymphadenectomy was completed patients in with high grade histology or deep myometrial invasion" | |
| Systematic review of sentinel node detection in various cancer types (references screened for inclusion) | |
| No ultra‐section | |
| Did not have to perform full pelvic LND | |
| No ultra‐section | |
| No evidence of ultrastaging of SLN (quote: "SLNs were sectioned perpendicular to their longitudinal axis into at least 2 sections and submitted for routine hematoxylin‐eosin staining. If 1 section was suspected to be positive for metastasis, serial sections were obtained and submitted to immunohistochemistry staining with an anticytokeratin antibody (AE1/AE3; MXB Biotechnologies, Fuzhou, China) to detect cytokeratin. One section of each non‐SLN was submitted to routine hematoxylin‐eosin staining.") |
H&E; haemotxylin and eosin; ICG: indocyanine green; IHC: mmunohistochemistry; PLN: pelvic lymph node; PLND: pelvic lymph node; PLND: pelvic lymph node dissection; RCT: randomised controlled trial; ; SLN: sentinel lymph node; SLNB: sentinel lymph node biopsy; SPECT/CT: single photon emission computed tomography.
Characteristics of studies awaiting classification [ordered by study ID]
| Patient Sampling | Unclear |
| Patient characteristics and setting | 127 women with gynaecological malignancies (39 vulval, 52 cervical, 36 endometrial). Single‐centre hospital inpatient setting in Poland. |
| Index tests | Sentinel LN detection: 23 had patent blue; 13 had radiotracer + patent blue by subserosal injection at time of operation |
| Target condition and reference standard(s) | systematic lymphadenectomy for endometrial cancer (36 patients) |
| Flow and timing | SN detection followed by systematic lymphadenectomy at time of same operation. |
| Comparative | 32/36 (88.9%) sentinel node detection rate. Sensitivity 87.9%, NPV 100%. FNR not given. |
| Notes | Authors contacted for clarification re whether LN examined by ultrasection and IHC. Response awaited. |
| Patient Sampling | Consecutive sampling |
| Patient characteristics and setting | 10 patients with clinically stage IA2 to IB1 cervical cancer and 25 patients with stage I endometrial cancer were enrolled between July 2010 and August 2011 at a single centre in Monza, Italy. |
| Index tests | Technetium Tc 99m albumin nanocolloid was injected submucosally at 4 points of the cervix. Patients underwent SPECT/CT emission‐transmission study at least 3 hours after standard planar images. Methylene blue was injected into the cervix just before surgery under general anaesthesia. |
| Target condition and reference standard(s) | Patients with endometrial cancer all underwent pelvic lymphadenectomy. Para‐aortic lymphadenectomy was done in selected cases. |
| Flow and timing | SN detection followed by systematic lymphadenectomy at time of same operation. |
| Comparative | Sensitivity of Tc99: 87.5%. Sensitivity of methylene blue = 66.2%. Detection rate 91%. NPV = 100%. |
| Notes |
| Patient Sampling | Country: Italy Study design: a prospective study of patient with early‐stage cervical or apparent confined endometrial cancer from October 2016 to May 2017. The main objective of the study was to explore the possible intraoperative differences between 2 laparoscopic platforms in terms of duration of SLN mapping and detection rate of SLNs. The participants were split into group A (Storz system, n = 14) and group B (Novadaq system, n = 10) Inclusion criteria: not reported. Exclusion criteria: not reported. |
| Patient characteristics and setting | Number of patients: 34; 10 (29.4%) patients with cervical cancer and 24 (70.6%) patients with endometrial cancer. Median age: 60 years (range, 34‐69 years) in group A and 50.9 years (range, 24–78) in group B Median BMI = 23.5 kg/m2 (range, 20‐50 kg/m2) in Group A and 26 kg/m2 (range, 17.5–45) in Group B Histopathological cell type: endometrioid = 80% in group A and 93% in group B FIGO stage: I (100%) preoperatively Grade on hysterectomy specimen: not reported. Lymphovascular space involvement: not reported. Setting: Single centre in Italy, namely the Gynecology Oncology Surgical Unit of the San Gerardo Hospital, Italy |
| Index tests | Type of endometrial sampling: not reported. Experience of operator: All SLN mapping procedures performed by the same senior surgeon. Tracer used and amount: 4 mL of ICG solution Method and timing of application: 4 mL of the ICG solution (1.25 mg/mL) was injected into the cervix in the operating theatre, after the induction of general anaesthesia and once the operative trocars were inserted. The routine sectioning, staining with haematoxylin and eosin, and followed by the ultrastaging protocol with multiple sectioning and immunohistochemistry, using the AE1/ AE3 anticytokeratin antibody (Dako Company, Glostrup, Denmark), was performed on all SLNs removed. Macrometastases contain a metastatic deposit greater than 2 mm. Micrometastasis was defined as a metastatic deposit ranging from 0.2 mm to no more than 2 mm in size. Isolated tumour cells were defined as single tumour cells or a cluster of malignant epithelial cells less than 0.2 mm, as seen on corresponding haematoxylin and eosin sections and not just immunohistochemical staining. |
| Target condition and reference standard(s) | Type: 24 patients with apparent stage I endometrial cancer all of whom had pelvic +/‐ para‐aortic lymph node dissection performed. All patients underwent a minimally invasive laparoscopic approach. The proportion of patients with endometrial cancer who underwent pelvic +/‐ para‐aortic lymph node dissection is unclear. Lymph node number and site: the median number of SLNs removed for patients with endometrial cancer or cervical cancer was 2 (range, 0‐5) for group A and 2 (range, 1‐3) for group B. It is unclear what proportion of these were from only patients with endometrial cancer. The site from which lymph nodes were removed is not reported. |
| Flow and timing | All patients receive the index and reference standard within 1 month. The patients did not all receive the same reference standard. The proportions of patients receiving pelvic lymph node dissection alone, pelvic and para‐aortic lymph node dissection, or no lymph node dissection is not reported. All patients were included in the analysis. |
| Comparative | Study results: the results are reported for patients with cervical or endometrial cancer together. A total of 216 lymph nodes were removed, of which 77 were SLNs (53 in Group A and 24 in Group B). Median numbers of SLNs removed were 2 each for both groups. Detection rates of SLNs were 90.9% in Group A and 100% in Group B. Bilateral mapping of the SLNs was 77.3% in Group A and 83.3% in Group B. Adverse reaction from index or reference test: not reported. Operating time: not reported. Other intraoperative complications: none. Other postoperative complications: none. |
| Notes | Authour contacted for further details, so we could separate those with cervical cancer from those with endometrial cancer. As yet no response so unable to extract data for a 2x2 table for patients with endometrial cancer. |
| Patient Sampling | Unclear. |
| Patient characteristics and setting | 33 patients with endometrial cancer were enrolled between April 2002 and March 2005 at a single centre in Olomouc, Czech Republic. |
| Index tests | All patients received preoperative Tc‐99 nanocolloid (50 MBq), administered by a 25 Gauge needle transcervically into the myometrium. |
| Target condition and reference standard(s) | All patients received pelvic lymphadenectomy. Para‐aortic lymphadenectomy was performed in 11 of 33 patients. |
| Flow and timing | SN detection followed by systematic lymphadenectomy at time of same operation. |
| Comparative | SN detection rate = 79%. The mean number of SNs detected was 2.9 (range, 1‐10) per patient. No false negative lymph nodes were observed. |
| Notes | Paper in Czech ‐ awaiting translation. |
| Patient Sampling | Unclear |
| Patient characteristics and setting | 36 patients with high‐risk uterine cancer (grade 3 endometrioid, clear cell, serous, or carcinosarcoma) were enrolled between 2012 and 2015 in the USA. |
| Index tests | 4 mL of methylene blue or ICG dye was injected into the cervix at 3 and 9 o'clock. |
| Target condition and reference standard(s) | All patients underwent full pelvic lymph node dissection. Para‐aortic lymph node dissection was performed at the discretion of the surgeon. |
| Flow and timing | SN detection followed by systematic lymphadenectomy at time of same operation. |
| Comparative | SN detection rate = 83%. Bilateral SN detection rate = 56%, unilateral SN detection rate = 25%, aortic nodes only in 3%. False‐negative rate = 7.7%. NPV = 92.3%. |
| Notes |
| Patient Sampling | Prospective, randomised study |
| Patient characteristics and setting | 120 patients with early‐stage endometrial cancer with low risk for lymph node metastasis enrolled between June 2016 and June 2017 in Alexandria, Egypt. |
| Index tests | Methylene blue dye injected using 4 different methods: hysteroscopic guided methylene blue injection, transcervical injection, subserosal uterine injection, and combined transcervical and subserosal injection |
| Target condition and reference standard(s) | All patients underwent full pelvic lymph node dissection. |
| Flow and timing | SN detection followed by systematic lymphadenectomy at time of same operation. |
| Comparative | Clinical and pathological SLN detection were more with hysteroscopic technique than others and pathological detection was lower than clinical detection in all techniques. Sensitivity and specificity were not reported. |
| Notes | Author emailed to clarify whether ultra‐section was used and to ascertain the sensitivity/specificity of each of the 4 injection groups. |
| Patient Sampling | Unclear |
| Patient characteristics and setting | 40 patients with stage I endometrial cancer enrolled between July 2010 and July 2014 from a single centre in Italy. The aim of this study was to compare preoperative SPECT/CT with gamma‐probe and methylene blue‐dye (by cervical injection) in the identification of SLN in early‐stage endometrial cancer. |
| Index tests | 12 MBq of radiolabeled filtered 99mTc albumin nanocolloid in 0.2‐0.3 mL volume was injected within 20 hours before surgery with 4 submucosal cervical injections (3, 6, 9, and 12 o'clock). Immediatley prior to surgery, 2 mL of blue dye (1 mL per injection) was injected into the cervix through a 20‐gauge spinal needle at 3 and 9 o'clock positions. |
| Target condition and reference standard(s) | All patients underwent full pelvic lymphadenectomy. Para‐aortic lymphadenectomy was recommended in case of pathological pelvic nodal uptake at preoperative PET/CT or in case of suspicious enlarged nodes at surgery. |
| Flow and timing | SN detection followed by systematic lymphadenectomy at time of same operation. |
| Comparative | Detection rate with SPECT/CT 90%, with gamma‐probe intraoperative 88%, and with methylene blue 80%. Median number of nodes removed was 2. False‐negative rate 0%. SPECT/CT had the highest detection rate and achieved the highest rate of bilateral mapping, compared to gamma‐probe and MDB. |
| Notes |
| Patient Sampling | Unclear |
| Patient characteristics and setting | 21 patients with endometrial cancer were enrolled between May 2006 and January 2009. |
| Index tests | 99mTc‐labelled nanocolloid (100 MBq) was administered preoperatively. On the day of surgery, radiocolloid together with blue dye was injected via a 20‐gauge needle under the endometrium using hysteroscopy. |
| Target condition and reference standard(s) | Patients received pelvic lymphadenectomy at the time of surgery. In patients with high‐risk prognostic factors, para‐aortic lymphadenectomy was also performed. |
| Flow and timing | SN detection followed by systematic lymphadenectomy at time of same operation. |
| Comparative | At least one SLN was detected in 81%. The mean number of detected SLNs was 2 (range, 1‐5). Sensitivity and specificity were 100%. |
| Notes | Paper in Czech ‐ awaiting translation. |
| Patient Sampling | Unclear how patients were selected |
| Patient characteristics and setting | 25 cases of endometrial cancer, laparoscopically‐assisted vaginal hysterectomy and pelvic lymphadenectomy were completed successfully in 23 women out of 24 laparoscopically‐staged patients (95.8%). One patient with FIGO stage IIa had radical abdominal surgery. |
| Index tests | Patent blue‐V was injected into the subserosal myometrium (13 cases, SM group) or cervico‐subserosal myometrium (12 cases, CSM group) during a surgical staging procedure. |
| Target condition and reference standard(s) | Endometrial cancer. Unclear if all women had full lymphadenectomy following SLNB, but would appear so. |
| Flow and timing | Unclear whether SLN taken separately or deposition of dye noted ex vivo on examination of nodes. |
| Comparative | Comparing SM and CSM groups the statistical significant difference was found in the DCLN/LN rate and mean number of sentinel lymph nodes (P = 0.03, P = 0.05, respectively). |
| Notes | Abstract only. appears to compare detection rate of SLN with different injection sites (cervical and subserosal (CSM) versus subserosal (SM) and no data in abstract to enable 2x2 table to be produced. Not been able to obtain the full text paper, but likely this would be an excluded study. |
| Patient Sampling | Country: Canada Study design: a prospective study of patients with clinical stage I endometrial cancer from April 2013 to August 2014. Inclusion criteria: clinical stage I endometrial cancer of any histological type scheduled for primary surgery. Exclusion criteria: patients with apparent metastatic disease prior to surgical staging. |
| Patient characteristics and setting | Mean age was 64 +/‐ 11.5 years. Mean BMI was 31.5 +/‐ 8 kg/m2. All patients were treated by robot‐assisted laparoscopic surgery. Number of patients: 100 |
| Index tests | Type of endometrial sampling: not reported. Experience of operator: states that prior to the initiation of this study, the three gynaecologic oncologists performing the robotic surgical procedures had performed more than 500 robotic surgeries and had performed sentinel node sampling ob more than 180 endometrial cancer cases in addition to their experience with SLN mapping in cervical and vulvar cancers since 2003 (Study commenced in 2013). Tracer used and amount: all enrolled patients received a mixture of the following tracers: a) Blue dye either methylene blue (methylene blue injection USP, Omega laboratories, Montreal, Canada) or patent blue (patent blue sodium injection Guebert product imported by Methapharm Inc., Brantford, ON, Canada). b) Indocyanine green (IC‐Green, Akorn Pharmaceuticals, Lake Forest, USA) Method and timing of application: per patient, four 1 mL syringes, each containing a mixture of blue dye (0.8 mL), ICG (0.1 mL, 0.25 mg/mL), and 99mTc‐SC (0.1 mL) were prepared to inject directly into the cervix at the three and nine o'clock position. At each of these positions, a 1 mL syringe was injected superficially (2–3 mm) into the cervical submucosa and another 1 mL was injected deep (3–4 cm) into the stroma of the cervix to reach the lower uterine segment in order to allow for diffusion of the dye into the surrounding uterine area. Injection was performed following prepping of the patient under anaesthesia and just prior to skin incision. Detection of SLNs was accomplished through any of the three following methods: 1) direct visualisation of either blue coloured lymphatics/nodes 2) visualisation of green coloured lymphatics/nodes via the immunofluorescent imaging mode on the da Vinci surgical platform 3) detection of radioactive nodes by a handheld gamma probe. Method of detection (histopathological assessment, including ultrastaging): Intraoperative frozen section analysis of the SLNs was performed in all cases. Furthermore, the LNs were first bisected and stained with haematoxylin & eosin (H&E). Following the first staining, pathologic ultrastaging was performed with multiple serial sectioning of the entire SLN at 200 μm, to 300 μm, with three consecutive H&E levels with one slide for immunostaining per level. Immunostaining for cytokeratin (clone AE1/AE3, Milipore Inc., dilution 1:150) was performed in SLNs after H&E routine histological examination. Non‐SLNs were processed using entire node examination with H&E staining. Note: This study injected a mixture of various overlapping patients with combinations of Blue/ICG and Tc99. We have not been able to separate out cohorts for inclusion of data in the review. The authors have been contacted for further information. |
| Target condition and reference standard(s) | Type: 100 patients with grade I, II, or III endometrial cancer, all of whom had pelvic +/‐ para‐aortic (32%) lymphadenectomy performed. All patients had robot assisted laparoscopic surgery. Lymph node number and site: sentinel nodes were identified in 92 of 100 patients (92%). 76 patients had sentinel nodes detected on both sides thus bilateral detection rate 76%. The mean number of SN retrieved was 2.9 per patient. The anatomical sites were as follows: obturator = 148 SNs (52%), external iliac = 69 SNs (24%), common iliac 3.5%, para‐aortic 4.5%, iliac bifurcation 9.5%. |
| Flow and timing | All patients receive the index and reference standard within 1 month. The patients did not all receive the same reference standard. The proportions of pelvic +/‐ para‐aortic lymphadenectomy are not given. All patients were included in the analysis. |
| Comparative | |
| Notes | Study results: ICG detection rate 87%. Blue detection rate 71%. Technetium detection rate 87%. ICG and 99mTc‐SC had similar SLN detection rates in both overall (87% vs 88%, respectively; P = 0.83) and bilateral (71% vs 65%, respectively; P =0.36). ICG had a significantly higher SLN detection rate when compared to blue dye in both overall (87% vs 71%, respectively; P = 0.005) and bilateral (65% vs 43%, respectively; P = 0.002) detection. To evaluate the diagnostic accuracy of the SLN biopsy, eight patients without SLN detection were excluded, resulting in remaining total of 92 cases. Overall, there were 10 cases with metastatic lymph node spread and all had SLN detection. In nine of these 10 patients, the SLN was positive for metastatic disease resulting in a sensitivity of 90% (95% CI 54% to 99%). There were no false‐positive results, yielding a specificity of 100% (95% CI 94–100) and a positive predictive value of 100% (95% CI 60–100). Eighty‐two of 83 cases were true negatives, yielding a negative predictive value of 99% (95% CI 93%–100%). Adverse reaction from index or reference test: no complications, allergic reactions or toxicities occurred. Operating time: not given. Other intraoperative complications: no complications reported. Other postoperative complications: no complications reported. |
| Patient Sampling | Unclear |
| Patient characteristics and setting | 115 patients with endometrial cancer recruited between September 2005 and March 2009 at a single centre in New York, USA. |
| Index tests | 2cm3 of methylene blue or isosulfan injected into the cervical stroma (1 cm3 at each of the 3 and 9 o'clock positions). In addition, as part of an early pilot protocol in our service, some patients in the early part of the study also received two additional blue dye injections into the fundus: 1 cm3 in the anterior mid fundus and 1 cm3 in the mid posterior fundus. Preoperative lymphoscintigraphy, performed the day prior to or the morning of surgery, was obtained following cervical injection of radiolabeled Tc99 microsulfur colloid at the 3 and 9 o'clock positions. |
| Target condition and reference standard(s) | Following identification and removal of the SLN, a regional lymphadenectomy was performed including bilateral pelvic and bilateral inframesenteric paraaortic basins, when feasible. Paraaortic infrarenal dissection was performed if disease was suspected in that region. |
| Flow and timing | SN detection followed by systematic lymphadenectomy at time of same operation. |
| Comparative | Overall SLN detection was achieved in 85% of cases. 2 false negative cases. In 74 cases both blue dye and Tc99m were used, in 41 cases only blue dye was used. The cervix was the only site of injection in 82 cases (71%), while a combined cervical and fundal injection was performed in 33 cases (29%). The median number of SLN detected was 3. No specificity or sensitivity provided for either method. Concluded that increasing surgical volume (30 cases) is associated with significantly increased detection rates (they split analysis by time periods). |
| Notes |
| Patient Sampling | Unclear |
| Patient characteristics and setting | 49 patients (28 with endometrial cancer, 11 vulval cancer, 10 cervical cancer) with early‐stage vulval, cervical, or endometrial cancer enrolled between October 2012 and September 2014 at a single centre in Oxford, UK. The aim of the study was to assess the feasibility of a novel, custom‐made, optical imaging system for SLN detection that can detect multiple fluorescence dyes and allow simultaneous bright‐field imaging during open or laparoscopic surgery. |
| Index tests | 1 mL ICG (5 mg/mL) was injected immediately before surgery. ICG was injected using a 27‐gauge needle into the 3 and 9 o'clock positions, initially submucosally and then deep into the stroma, on both sides of there cervix. In two cases, a further injection of 4 mL of methylene blue ("Proveblue") was performed. |
| Target condition and reference standard(s) | The proportion of women receiving full pelvic lymph node dissection or pelvic and para‐aortic lymph node dissection is not reported. |
| Flow and timing | SN detection followed by systematic lymphadenectomy at time of same operation. |
| Comparative | SLN detection rate for endometrial cancer 68%; optimised dose group (n = 16) SLN detection rate 100%. Average number of SLN detected 0.9 for endometrial cancer; 1.5 in the optimised dose group. Overall FNR 8%, overall sensitivity 12%. |
| Notes | There was a dose optimisation and learning phase. |
| Patient Sampling | Retrospective study |
| Patient characteristics and setting | 76 patients (39 cervical, 37 endometrial ca) with cervical or endometrial cancer enrolled at the Peking University People's Hospital in China. |
| Index tests | All patients underwent SLN biopsy with ICG and/or carbon nanoparticles. |
| Target condition and reference standard(s) | 37 patients with endometrial cancer were included. It is unclear what proportion of patients received systematic pelvic lymphadenectomy (only 55 of 76). |
| Flow and timing | SN detection followed by systematic lymphadenectomy at time of same operation. |
| Comparative | The overall detection rate was 95% (72/76), with 74% (56/76) positive bilaterally. The difference of SLN detection rate between cervical and endometrial cancer patients were not significant (P > 0.05). Among 55 patients underwent systematic pelvic lymphadenectomy, the sensitivity of SLN detection was 75% and the negative predictive value was 96%. The sensitivity and negative predictive value were both 100% in patients with successfully bilateral mapped of SLN. |
| Notes | Paper in Chinese ‐ awaiting translation. |
| Patient Sampling | |
| Patient characteristics and setting | |
| Index tests | |
| Target condition and reference standard(s) | |
| Flow and timing | |
| Comparative | |
| Notes | Unable to find full text paper. |
| Patient Sampling | |
| Patient characteristics and setting | |
| Index tests | |
| Target condition and reference standard(s) | |
| Flow and timing | |
| Comparative | |
| Notes | Translation requested |
| Patient Sampling | |
| Patient characteristics and setting | |
| Index tests | |
| Target condition and reference standard(s) | |
| Flow and timing | |
| Comparative | |
| Notes | Unable to find full text paper. |
| Patient Sampling | Country:Canada |
| Patient characteristics and setting | Number of patients:50 BMI: median (range): 29 (19 to 56) |
| Index tests | Type of endometrial sampling: not reported |
| Target condition and reference standard(s) | Type: 50 patients (42 endometrial cancer, 8 cervical cancer). Following SLN mapping, the majority of patients (72%) either had robotic (46%), laparoscopic (18%) or laparoscopically assisted vaginal surgery (8%). All patients underwent pelvic dissection, only 22% underwent para‐aortic dissection. |
| Flow and timing | All patients received the index and reference standard within 1 month. Patients did not receive the same reference standard, some underwent robotic‐assisted, some laparoscopic and some laparoscopic assisted. Paraaortic node dissection was performed in 22% of cases. All patients were included in the analysis. |
| Comparative | |
| Notes | Study results Note we were unable to separate data for patients with cervical and endometrial cancer and so have not been able to include in the study. The authors have been contacted for further information. |
| Patient Sampling | Unclear |
| Patient characteristics and setting | 29 patients with endometrial cancer were enrolled between November 2011 and September 2012 in the USA. |
| Index tests | The first 17 study participants received cervical stromal injections of 1 mg ICG (0.5 mg/mL) using a 21‐gauge spinal needle at 1cm depth at the 3 and 9 o'clock positions. The following 12 study participants received hysteroscopically‐guided endometrial injections of 0.5 mg ICG (0.5 mg/mL) using an 18‐gauge 500 mm oocyte recovery set needle with attached tubing. |
| Target condition and reference standard(s) | All patients underwent complete pelvic lymphadenectomy. A para‐aortic lymph node dissection was performed in 83% of patients. |
| Flow and timing | SN detection followed by systematic lymphadenectomy at time of same operation. |
| Comparative | The detection rate for at least 1 SLN was 82% for the cervical injection cohort and 33% for the endometrial injection cohort. The median number of SLNs mapped was 5 (range, 1‐9) for the cervical cohort and 2 (range, 1‐3) for the endometrial cohort. Sentinel lymph nodes were seen bilaterally in 57% (8/14) of the cervical injection group and 50% (2/4) of the hysteroscopic group. There was 1 false‐negative SLN in the cervical injection group. |
| Notes |
| Patient Sampling | Country: Japan |
| Patient characteristics and setting | Number of patients: 57 |
| Index tests | Type of endometrial sampling: not reported |
| Target condition and reference standard(s) | Type: brief description of reference standard including whether open or laparoscopicMethod and extent of dissection decided by surgeon. Inter‐iliac and obturator lymph nodes bilaterally was a mandated component of surgical staging. |
| Flow and timing | Unclear whether patients received index standard within 1 month. 43 Open, 14 laparoscopic – decided by surgeon. 41 had pelvic lymphadenectomy, 16 had pelvic and para‐aortic lymphadenectomy ‐ decidd by surgeon. All patients were included in the analysis. |
| Comparative | Retrospective study of 57 patients with FIGO stage I endometrial cancer at a single centre in Taiwan. All patients received cervical injection of Technetium‐99 and/or ICG. Unilateral detection rate was 94.7%, ilateral detection rate was 80.7%. Median number of SLNs removed = 2. Sensitivity 100%, NPV 100%. No specificity or PPV given. Study results: successful mapping on at least one side, successful bilateral mapping, sensitivity for LNMs, and negative predictive values for LNM: 95%, 81%, 100%, and 100%, respectively. The sensitivity and negative predictive value for detecting lymph node metastasis were both 100% |
| Notes | Methods and reporting unclear and unable to extract 2x2 data for different injection sites for inclusion in the review. |
| Patient Sampling | |
| Patient characteristics and setting | |
| Index tests | |
| Target condition and reference standard(s) | |
| Flow and timing | |
| Comparative | |
| Notes | Most of patient cohort appear to be included in Holloway 2017 and unable to separate to ensure patients entered only once in data analysis. The number receiving ICG alone is not given. |
| Patient Sampling | Unclear |
| Patient characteristics and setting | 211 patients with endometrial cancer enrolled between September 2012 and June 2017 at a single centre in Japan. |
| Index tests | 2 mL of fluid containing 110 MBq 99m‐Technetium labelled tin colloids was injected submucosally in the four quadrants of the cervix (at 12, 3, 6, and 9 o'clock) at a depth of 5 mm on the day prior to surgery. On the day of the operation, 5 mL of indigo carmine (IDC) (2 mg/mL) and/or indocyanine green (ICG) (50 μg/mL) was injected into the cervix in the same way as the technetium at the start of surgery. The same quantity of IDC and/or ICG was also injected into the uterine fundus upon reaching the intra‐abdominal cavity. |
| Target condition and reference standard(s) | Only 206 of 211 (97.6%) patients received pelvic lymph node dissection. 5 patients (2.4%) received no systematic pelvic lymph node dissection. 49 of 211 (23.2%) additionally receive para‐aortic lymph node dissections. |
| Flow and timing | SN detection followed by systematic lymphadenectomy at time of same operation. |
| Comparative | Detection rate for Tch‐99 was 77.9%; ICG 73.4%; and indigo carmine 17%. Overall detection rate was 81%. Overall sensitivity 80%. Overall false negative rate was 3.2%. |
| Notes |
| Patient Sampling | Unclear |
| Patient characteristics and setting | 52 patients with high grade endometrial cancer were enrolled between December 2012 and December 2015 at a single centre in the USA. |
| Index tests | The cervical stroma was injected at 3 and 9 o'clock positions with either isosulfan blue (during examination under anaesthesia) or ICG (during placement of uterine manipulator). |
| Target condition and reference standard(s) | All patients receive full pelvic and para‐aortic lymph node dissection to the level of the renal vessels. |
| Flow and timing | SN detection followed by systematic lymphadenectomy at time of same operation. |
| Comparative | False negative rate was 22%. Detection rate per hemi‐pelvis was 73.1%. Failed detection rate was 13.5%. |
| Notes |
| Patient Sampling | Unclear |
| Patient characteristics and setting | 268 with endometrial cancer were enrolled between November 2010 and November 2013 at a single centre in Quebec, Canada. |
| Index tests | A superficial cervical injection (2–3 mm deep) of 0.2 mL (37 MBq solution) of Antimony Trisulfide Colloid (ATC) Technetium‐99 (Health Sciences Centre, Winnipeg, Manitoba, Canada), was performed with a 25‐gauge spinal needle, at the 3 and 9 o'clock positions, 2 hours before surgery, followed by a lymphoscintigram 15 to 20 minutes after the injection. Just before surgery, after examination under anaesthesia, 1 cc of Patent Blue (Metapharm Inc, ON, Canada) was also injected intracervically with a 25‐gauge spinal needle at the 3 and 9 o'clock positions. |
| Target condition and reference standard(s) | All patients underwent SLN biopsy followed by complete pelvic lymphadenectomy, total hysterectomy and bilateral salpingo‐oophorectomy, either by laparoscopy, robotic surgery or laparotomy. A para‐aortic lymphadenectomy was performed in type 2 endometrial cancer histology, if intraoperative histology of SLN (frozen section) revealed pelvic node metastasis or if the radiologic preoperative assessment of para‐aortic nodes was suspicious. |
| Flow and timing | SN detection followed by systematic lymphadenectomy at time of same operation. |
| Comparative | The median number of SLN removed by patient was 2 (range, 0–5). At least one SLN was detected in 252/268 patients (94%). Bilateral SLN was identified in 197/268 patients (73.5%). In those cases, sensitivity and negative predictive value of the sentinel lymph node mapping were 97.2% and 99.4%, respectively. |
| Notes |
| Patient Sampling | Unclear |
| Patient characteristics and setting | 128 patients with high‐risk endometrial cancer were enrolled between November 2010 and November 2016 at a single centre in Quebec, Canada. |
| Index tests | All patients received either blue dye alone (39.1%), Tch‐99 alone (15.5%), ICG alone (22.7%), blue dye plus Tch‐99 (21.8%), ICG plus Tch‐99 (0.9%). All tracers were injected in the cervix at 3 and 9 o'clock with a 25‐gauge spinal needle. A total of 4 cm3 was used for the intracervical injection for either the blue dye or the ICG (2.5 mg/mL) and a total of 2 cm3 for the Tc‐ 99. The blue dye and ICG were injected after induction of anaesthesia and theTc‐99 was injected in the nuclear medicine suite the morning of the surgery followed by a lymphoscintigram. An intracervical injection of 1 mL (1 mCi) of Tc‐99 was injected. |
| Target condition and reference standard(s) | All patients underwent pelvic lymph node dissection. Additional para‐aortic lymph dissection was performed at the discretion of the surgeon. |
| Flow and timing | SN detection followed by systematic lymphadenectomy at time of same operation. |
| Comparative | SLN detection rate was 89.8% overall. Bilateral detection rate was 63.2% overall. When SLNs were detected bilaterally, 1 false negative occurred, sensitivity was 95.8%, and NPV was 98.2%. |
| Notes |
| Patient Sampling | Unclear |
| Patient characteristics and setting | 92 patients with endometrial cancer were enrolled between 2009 and 2014 at a single centre in Japan. |
| Index tests | Patients underwent either the dye method (prior to December 2011; n = 52) or the fluorescence method (from December 2011 onward; fluorescence A n = 17, fluorescence B n = 23). The dye method involved 10 mL of ICG (2.5 mg/mL) to be injected at 5 locations in the uterine subserosa (anterior wall, posterior wall, left wall, right wall, and fundus). The SN was identified as a node that was macroscopically stained green. The fluorescence method involved 10 mL of diluted ICG (fluorescence A = 0.05 mg/mL; fluorescence B = 0.025 mg/mL) injected into the 5 same locations in the uterine subserosa. Sixteen hours before surgery, 0.2 mL of 99mTc‐phytate was injected in 5 specified locations (a total of 2 mCi) in the endocervix or endometrium. |
| Target condition and reference standard(s) | All patients received pelvic lymph node dissection. Para‐aortic lymph node dissection was performed if any of the following were present: (1) invasion of more than half of the myometrium, (2) G3 endometrioid adenocarcinoma or nonendometrioid adenocarcinoma, (3) metastases to PLNs, or and (4) metastases to the adnexa. |
| Flow and timing | SN detection followed by systematic lymphadenectomy at time of same operation. All surgeries were performed by laparotomy. |
| Comparative | The SN detection rate was 100% for the dye method; 100% for the fluorescence method, and 96% for the radioisotope method. Sensitivity was 92% for the dye method, 100% for the fluorescence method, and 100% for the radioisotope method. The NPV was 98%, 100%, and 100%, respectively, for the 3 methods. |
| Notes |
| Patient Sampling | |
| Patient characteristics and setting | |
| Index tests | |
| Target condition and reference standard(s) | |
| Flow and timing | |
| Comparative | |
| Notes | Translation requested. |
| Patient Sampling | |
| Patient characteristics and setting | |
| Index tests | |
| Target condition and reference standard(s) | |
| Flow and timing | |
| Comparative | |
| Notes | Article not accessible |
BMI: body mass index; CSM: cervico‐subserosal myometrium; IHC: mmunohistochemistryICG: indocyanine green ;LN: lymph node; NPV: negative predictive value; PPV: positive predictive value; SD: standard deviation; SLN: sentinel lymph node; SM: subserosal myometrium;SN: sentinel node; ; SPECT/CT: single photon emission computed tomography.
Data
Presented below are all the data for all of the tests entered into the review.
| Test | No. of studies | No. of participants |
| 1 Blue dye alone per patient (all injection sites) Show forest plot | 11 | 435 |
| Test 1  Blue dye alone per patient (all injection sites) | ||
| 2 Blue dye alone (cervical injection) Show forest plot | 7 | 302 |
| Test 2  Blue dye alone (cervical injection) | ||
| 3 Blue dye alone (subserosal injection) Show forest plot | 5 | 133 |
| Test 3  Blue dye alone (subserosal injection) | ||
| 4 Technetium‐99m alone per patient (all injection sites) Show forest plot | 4 | 208 |
| Test 4  Technetium‐99m alone per patient (all injection sites) | ||
| 5 Technetium‐99m alone per patient (cervical injections) Show forest plot | 2 | 48 |
| Test 5  Technetium‐99m alone per patient (cervical injections) | ||
| 6 Technetium‐99m alone per patient (subserosal injection) Show forest plot | 4 | 160 |
| Test 6  Technetium‐99m alone per patient (subserosal injection) | ||
| 7 Technetium‐99m & blue dye per patient Show forest plot | 12 | 473 |
| Test 7  Technetium‐99m & blue dye per patient | ||
| 8 ICG alone per patient Show forest plot | 9 | 881 |
| Test 8  ICG alone per patient | ||
| 9 ICG and blue dye per patient Show forest plot | 2 | 208 |
| Test 9  ICG and blue dye per patient | ||
| 10 ICG and Technecium‐99m per patient Show forest plot | 1 | 32 |
| Test 10  ICG and Technecium‐99m per patient | ||
| 11 Nanoparticles per patient Show forest plot | 0 | 0 |
| Test 11  Nanoparticles per patient | ||
| 18 SLNB ‐ all tracers per patient Show forest plot | 33 | 2237 |
| Test 18  SLNB ‐ all tracers per patient | ||

Study flow diagram.

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study
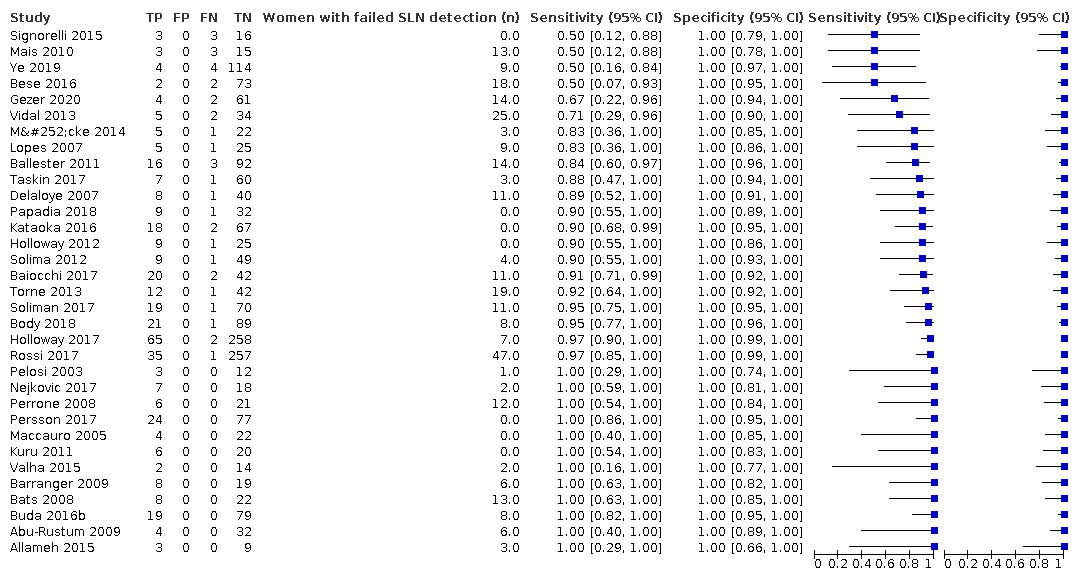
Figure 3 illustrates the (per patient) sensitivity of the included studies ordered by sensitivity.
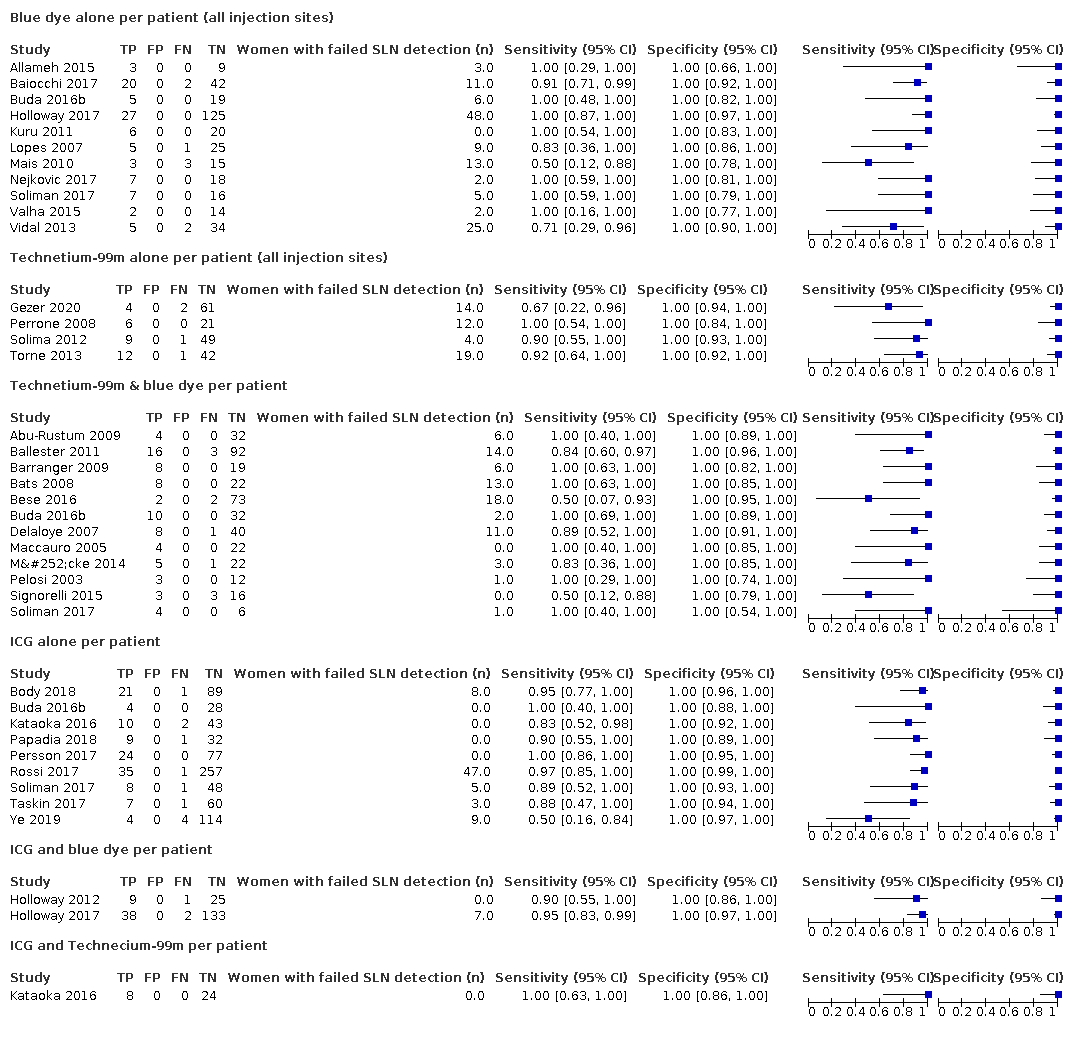
Figure 4 illustrates the (per patient) sensitivity of the included studies ordered by detection method: Blue dye alone, Technetium‐99m alone, Technetium‐99m & blue dye, ICG alone, ICG and blue dye, and ICG and Technecium‐99m.
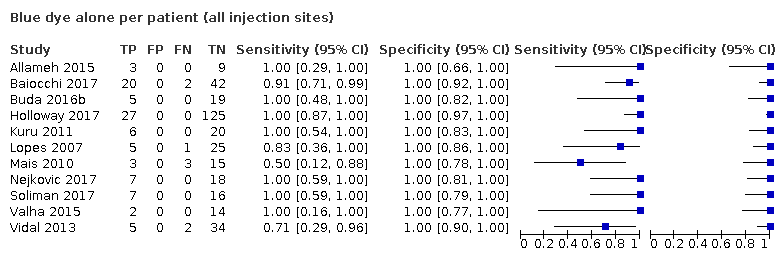
Blue dye alone per patient (all injection sites)
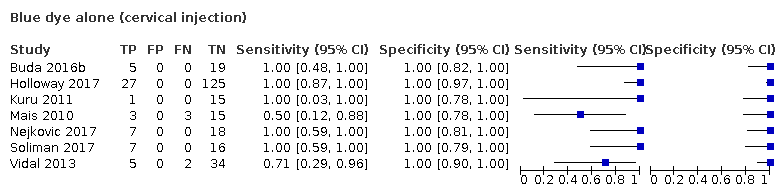
Blue dye alone (cervical injection)
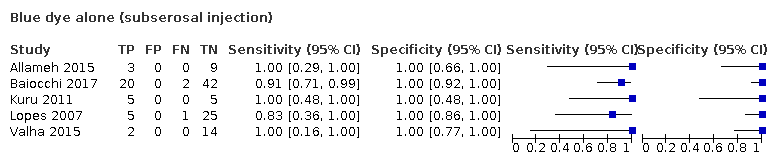
Blue dye alone (subserosal injection)

Technetium‐99m alone per patient (all injection sites)

Technetium‐99m alone per patient (cervical injections)

Technetium‐99m alone per patient (subserosal injection)
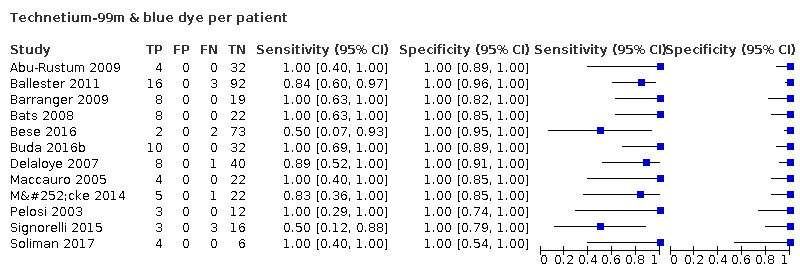
Technetium‐99m & blue dye per patient

ICG alone per patient

ICG and blue dye per patient

ICG and Technecium‐99m per patient

Nanoparticles per patient

SLNB ‐ all tracers per patient
| Review question: what is the diagnostic accuracy of different traces for the detection of sentinel lymph nodes? Patients/population: female adults with presumed early‐stage endometrial (womb) cancer Role: prognostic information for guiding adjuvant therapy after surgery Index tests: sentinel lymph node biopsy (SLNB) after injection of tracer substance/s into the cervix or uterine muscle Threshold for index tests: detection of micrometastases following ultrastaging (ultrasection of sentinel LN and immunohistochemistry). Presence of individual tumour cells (ITCs) excluded as positive result where possible. Reference standards: full pelvic +/‐ para‐aortic lymphadenectomy and standard histological examination Studies: cross‐sectional and cohort studies Setting: secondary/tertiary inpatient care at time of surgery for endometrial cancer | |||||||
| Index test | Number of patients (number of studies) | Mean SLN detection rate (95% CI)1 | Pooled sensitivity results per woman (95% CI)2 | Consequences in a cohort of 1000 women undergoing SLNB, assuming the prevalence of LN metastases to be 20% | Certainty of evidence | ||
|---|---|---|---|---|---|---|---|
| Women with no SLN detected (i.e., failed test; 95% CI)3 | Women with metastatic nodes diagnosed by index test (TP; 95% CI)4 | Women with metastatic nodes missed by index test (FN; 95% CI)5 | |||||
| 1. All tracers | 2237 (33 studies) | 86.9% (82.9% to 90.8%) | 91.8% (86.5% to 95.1% | 131 (92 to 171) | 160 (150 to 162) | 14 (9 to 23) | ⊕⊕⊕⊖ moderate 6 |
| 2. Blue dye alone | 559 women (11 studies) | 77.8% (70.0% to 85.6%) | 95.2% (77.2% to 99.2%) | 222 (144 to 300) | 148 (120 to 154) | 7 (1 to 35) | ⊕⊕⊖⊖ low 7 |
| 3. Technetium‐99m alone | 257 women (4 studies) | 80.9% (63.9 to 97.9) | 90.5% (67.7% to 97.7%) | 191 (21 to 361) | 146 (109 to 158) | 15 (4 to 52) | ⊕⊕⊖⊖ low 7 |
| 4. Technetium‐99m and blue dye | 548 women (12 studies) | 86.3% (80.7 to 91.9) | 91.9% (74.4% to 97.8%) | 137 (81 to 193) | 159 (128 to 169) | 14 (4 to 44) | ⊕⊕⊖⊖ low 7 |
| 5. ICG alone | 953 women (9 studies) | 92.4% (88.7 to 96.2) | 92.5% (81.8% to 97.1%) | 76 (38 to 113) | 171 (151 to 179) | 14 (6 to 34) | ⊕⊕⊕⊖ moderate 6 |
| 6. ICG and blue dye | 215 women (2 studies) | 96.7% (92.7 to 100) | 90.5% (63.2% to 98.1%) | 33 (0 to 73) | 175 (122 to 190) | 18 (4 to 71) | ⊕⊕⊖⊖ low 7 |
| 7. ICG and Technetium‐99m | 32 women (1 study) | 100% | 100% (63% to 100%) | 0 | 200 (126 to 200) | 0 (0 to 74) | ⊕⊖⊖⊖ very low 8 |
| * Prevalence of positive LN rate 20% chosen to represent those with higher risk of LN metastasis (as per Creasman 1987). A false‐positive result cannot occur, as the histological examination of the SLN is unchanged by the results from any additional nodes removed at systematic lymphadenectomy. Abbreviations SLN: sentinel lymph node SLNB: sentinel lymph node biopsy LN: lymph node ICG:indolcyanine green dye (visualised with near infra‐red fluorescence) TP: true positive FN: false negative | |||||||
| GRADE certainty of the evidence High: we are very confident that the true effect lies close to that of the estimate of the effect. | |||||||
| 1 Calculated as the arithmetic mean of the total number of SLNs detected out of the total number of women included in the included studies with the woman as the unit of analysis. 2 Calculated as the pooled estimate of sensitivity, using a univariate random‐effects logistic regression model (Takwoingi 2017) with the woman as the unit of analysis. 3 Calculated by subtracting the mean SLN detection rate estimates from 1000. 4 Calculated by subtracting the number of women with no SLN detected (i.e., with a failed test) from 1000 and then multiplying that with the prevalence and the pooled sensitivity estimate. 5 Calculated by subtracting the number of women with no SLN detected (i.e., with a failed test) from 1000 and then multiplying that with the prevalence and the false negative rate estimates. The false negative rate estimates were calculated by subtracting the sensitivity estimates from 100, 6 Downgraded by 1 level for risk of bias (for a combination of unclear patient selection and unclear risk of publication bias) 7 Downgraded by 2 levels; 1 for risk of bias (1 level for a combination of unclear patient selection and unclear risk of publication bias) and imprecision (1 level for wide confidence intervals). 8 Downgraded by 3 levels: 1 level for risk of bias (unclear patient selection and unclear risk of publication bias) and 2 levels for imprecision (1 small study with few true positive nodes with wide confidence intervals). | |||||||
| Review question: what is the diagnostic accuracy of different traces for the detection of sentinel lymph nodes? Patients/population: female adults with presumed early‐stage endometrial (womb) cancer Role: prognostic information for guiding adjuvant therapy after surgery Index tests: sentinel lymph node biopsy (SLNB) after injection of tracer substance/s into the cervix or uterine muscle Threshold for index tests: detection of micrometastases following ultrastaging (ultrasection of sentinel LN and immunohistochemistry). Presence of individual tumour cells (ITCs) excluded as positive result where possible. Reference standards: full pelvic +/‐ para‐aortic lymphadenectomy and standard histological examination Studies: cross‐sectional and cohort studies Setting: secondary/tertiary inpatient care at time of surgery for endometrial cancer | |||||||
| Index test | Number of patients (number of studies) | Mean SLN detection rate (95% CI)1 | Pooled sensitivity results per woman (95% CI)2 | Consequences in a cohort of 1000 women undergoing SLNB, assuming the prevalence of LN metastases to be 5% | Certainty of evidence | ||
|---|---|---|---|---|---|---|---|
| Women with no SLN detected (i.e., failed test; 95% CI)3 | Women with metastatic nodes diagnosed by index test (TP; 95% CI)4 | Women with metastatic nodes missed by index test (FN; 95% CI)5 | |||||
| 1. All tracers | 2237 (33 studies) | 86.9% (82.9% to 90.8%) | 91.8% (86.5% to 95.1%) | 131 (92 to 171) | 40 (38 to 41) | 4 (2 to 6) | ⊕⊕⊕⊖ moderate 6 |
| 2. Blue dye alone | 559 women (11 studies) | 77.8% (70.0% to 85.6%) | 95.2% (77.2% to 99.2%) | 222 (144 to 300) | 37 (30 to 39) | 2 (0 to 9) | ⊕⊕⊖⊖ low 7 |
| 3. Technetium‐99m alone | 257 women (4 studies) | 80.9% (63.9 to 97.9) | 90.5% (67.7% to 97.7%) | 191 (21 to 361) | 37 (27 to 40) | 4 (1 to 13) | ⊕⊕⊖⊖ low 7 |
| 4. Technetium‐99m and blue dye | 548 women (12 studies) | 86.3% (80.7 to 91.9) | 91.9% (74.4% to 97.8%) | 137 (81 to 193) | 40 (32 to 42) | 3 (1 to 11) | ⊕⊕⊖⊖ low 7 |
| 5. ICG alone | 953 women (9 studies) | 92.4% (88.7 to 96.2) | 92.5% (81.8% to 97.1%) | 76 (38 to 113) | 43 (38 to 45) | 3 (1 to 8) | ⊕⊕⊕⊖ moderate 6 |
| 6. ICG and blue dye | 215 women (2 studies) | 96.7% (92.7 to 100) | 90.5% (63.2% to 98.1%) | 33 (0 to 73) | 44 (31 to 47) | 5 (1 to 18) | ⊕⊕⊖⊖ low 7 |
| 7. ICG and Technetium‐99m | 32 women (1 study) | 100% | 100% (63% to 100%) | 0 | 50 (32 to 50) | 0 (0 to 19) | ⊕⊖⊖⊖ very low 8 |
| * Prevalence of positive LN rate 5% chosen to represent those with lower risk of LN metastasis (as per Creasman 1987). A false‐positive result cannot occur, as the histological examination of the SLN is unchanged by the results from any additional nodes removed at systematic lymphadenectomy. Abbreviations SLN: sentinel lymph node SLNB: sentinel lymph node biopsy LN: lymph node ICG:indolcyanine green dye (visualised with near infra‐red fluorescence) TP: true positive FN: false negative | |||||||
| GRADE certainty of the evidence High: we are very confident that the true effect lies close to that of the estimate of the effect. | |||||||
| 1 Calculated as the arithmetic mean of the total number of SLNs detected out of the total number of women included in the included studies with the woman as the unit of analysis. 2 Calculated as the pooled estimate of sensitivity, using a univariate random‐effects logistic regression model (Takwoingi 2017) with the woman as the unit of analysis. 3 Calculated by subtracting the mean SLN detection rate estimates from 1000. 4 Calculated by subtracting the number of women with no SLN detected (i.e., with a failed test) from 1000 and then multiplying that with the prevalence and the pooled sensitivity estimate. 5 Calculated by subtracting the number of women with no SLN detected (i.e., with a failed test) from 1000 and then multiplying that with the prevalence and the false negative rate estimates. The false negative rate estimates were calculated by subtracting the sensitivity estimates from 100, 6 Downgraded by 1 level for risk of bias (for a combination of unclear patient selection and unclear risk of publication bias) 7 Downgraded by 2 levels; 1 for risk of bias (1 level for a combination of unclear patient selection and unclear risk of publication bias) and imprecision (1 level for wide confidence intervals). 8 Downgraded by 3 levels: 1 level for risk of bias (unclear patient selection and unclear risk of publication bias) and 2 levels for imprecision (1 small study with few true positive nodes with wide confidence intervals). | |||||||
| Stage I: | Cancer confined to the uterus (womb), which has not spread to other parts of the body |
|---|---|
| Stage IA: | Cancer confined to the endometrium, or less than one‐half of the myometrium |
| Stage IB: | Cancer spread to the outer half of the myometrium |
| Stage II: | Cancer spread from the uterus to the cervical stroma, but not to other parts of the body |
| Stage III: | Cancer spread beyond the uterus, but it is still only in the pelvic area |
| Stage IIIA: | Cancer spread to serosa of the uterus, fallopian tubes and ovaries, or a combination, but not to other parts of the body |
| Stage IIIB: | Cancer spread to the vagina or parametria |
| Stage IIIC1: | Cancer spread to the regional pelvic lymph nodes |
| Stage IIIC2: | Cancer spread to the para‐aortic lymph nodes with or without spread to the regional pelvic lymph nodes |
| Stage IV: | Direct invasion into adjacent organs or distant spread |
| Stage IVA: | Cancer spread to the mucosa of the rectum or bladder |
| Stage IVB: | Cancer spread to lymph nodes in the groin area, or it has spread to distant organs, such as the bones or lungs |
| Item | Description |
|---|---|
| Domain 1: Patient selection | |
| A. Risk of bias | |
| Was a consecutive or random sample of participants enrolled? | Yes, if a consecutive or random sample of participants were enrolled No, if a consecutive or random sample of participants were not enrolled Unclear, if the study did not describe the method of participants enrolment |
| Was a case‐control design avoided? | The answer to this item will be 'yes' for all the included studies, as one of the exclusion criteria is case‐control studies |
| Did the study avoid inappropriate exclusions? | Yes, if the characteristics of the participants were well described and probably typical of a secondary healthcare setting No, if the sample was unrepresentative of people with apparent early‐stage endometrial cancer (i.e. a unexpectedly high proportion with rarer high grade histopathological types of endometrial cancer) Unclear, if the source or characteristics of participants was not adequately described |
| Could the selection of participants have introduced bias? | A judgement of low, high, or unclear risk of bias will be made, based on a balanced assessment of the responses to the above signalling questions. |
| B. Concerns about applicability | |
| Are there concerns that the included participants and setting do not match the review question? | A judgement of low, high, or unclear concerns about applicability will be made based on a balanced assessment of the response to the third signalling question above, and on how closely the sample matched the target population of interest |
| Domain 2: Index test | |
| A. Risk of bias | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes, if the report stated that the person undertaking the index test did not know the results of the reference tests No, if the report stated that the same person performed both tests, or that the results of the index tests were known to the person undertaking the reference tests Unclear, if insufficient information was provided |
| Did the study provide a clear definition of what was considered to be a positive result? | Yes, if the definition of a diagnosis of lymph node metastasis was clearly stated, including ultrastaging of the sentinel node No, if no definition of what was considered a positive result of lymph node metastasis was stated, or the definition of a positive result varied between the participants Unclear, if not enough information was given to permit judgement |
| If a threshold was used, was it prespecified? | This item is not applicable, as the test is not subject to a threshold. |
| Could the conduct or interpretation of the index test have introduced bias? | A judgement of low, high, or unclear risk of bias will be made based on a balanced assessment of the responses to the above signalling questions. |
| B. Concerns about applicability | |
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | A judgement of low, high, or unclear concerns about applicability will be made, based on a balanced assessment of the information detailed under ’Index test’ in the 'Characteristics of included studies' tables. |
| Domain 3: Reference standard | |
| A. Risk of bias | |
| Is the reference standards likely to correctly classify the target condition? | Yes, if a full pelvic or aortic node dissection (or both) was carried out adequately to correctly classify the target condition. A pelvic node dissection includes bilateral removal of nodal tissue from the distal one‐half of each common iliac artery, the anterior and medial aspect of the proximal half of the external iliac artery and vein, and the distal half of the obturator fat pad anterior to the obturator nerve. A para‐aortic node includes resection of nodal tissue over the distal vena cava, from the level of the inferior mesenteric artery (or left renal vein) to the mid‐right common iliac artery, and between the aorta and the left ureter, from the inferior mesenteric artery to the left mid‐common iliac artery. No, if a full pelvic or aortic node dissection (or both) has not been carried out adequately to correctly classify the target condition. Unclear, if insufficient information was provided to assess whether the reference standard had been carried out adequately (e.g. no mention of site or number of lymph node yield). |
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes, if the report stated that for SLNB, the histological examination from the lymphadenectomy was done without knowledge of the sentinel lymph node status. No, if the report stated that histological examination of the pelvic lymphadenectomy was carried out with the knowledge of the sentinel lymph node status. Unclear, if insufficient details given as to whether the histological examination was carried out with or without knowledge of sentinel lymph node status. |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | A judgement of low, high, or unclear risk of bias will be made, based on a balanced assessment of the responses to the above signalling questions. |
| B. Concerns about applicability | |
| Are there concerns that the target condition, as defined by the reference standard, does not match the question? | The answer to this question will always be low, because the target condition that the reference standard defines will always be the target condition of the review, i.e. adenocarcinoma of the endometrium of endometrioid, serous, clear cell, or mixed types, with apparent cancer confined to the uterus. Otherwise, the study will not be included. |
| Domain 3: Flow and timing | |
| A. Risk of bias | |
| Was there an appropriate interval between index test and reference | Yes, if the time period between the index test and the reference standard was no more than one month No, if the time period between the index test and the reference standard was longer than one month Unclear, if insufficient information was provided |
| Did all participants receive the same reference standard? | Yes, if the same reference test was used, regardless of the index test results No, if different reference tests were used, depending on the results of the index test Unclear, if insufficient information was provided Report if any participants received a different reference test, what the reasons stated for this were, and how many participants were involved. |
| Were all participants included in the analysis? | Yes, if there were no participants excluded from the analysis, or if exclusions were adequately described No, if there were participants excluded from the analysis and there was no explanation given Unclear, if not enough information was given to assess whether any participants were excluded from the analysis Report how many participants were excluded from the analysis for reasons other than uninterpretable results Report how many results were uninterpretable (of the total) |
| Could the patient flow have introduced bias? | A judgement of low, high, or unclear risk of bias will be made, based on a balanced assessment of the responses to the above signalling questions. |
| Tracer (studies) | Number detected/total number (%; 95% CI) |
| Blue dye alone (11) | 435/559 (77.8; 95%CI 70.0 to 85.6) |
| Technetium‐99m alone (4) | 208/257 (80.9; 95% CI 63.9 to 97.9) |
| Technetium‐99m and blue dye (12) | 473/548 (86.3; 95% CI 80.7 to 91.9) |
| ICG alone (9) | 881/953 (92.4; 95% CI 88.7 to 96.2) |
| ICG and blue dye (2) | 208/215 (96.7; 95% CI 92.7 to 100) |
| ICG and technetium‐99m (1) | 32/32 (100%) |
| CI: confidence interval | |
| Covariate | Studies (women) | Sensitivity % (95% CI) | Statistical significance |
| FIGO stage | |||
| Majority of women with 1a | 16 (1252) | 91.1 (80.7 to 96.2) | Equal variances assumed: Chi2 (1) = 0.13; P = 0.72 Separate variances assumed: Chi2 (2) = 1.21; P = 0.55 |
| Majority of women with 1b or above | 3 (95) | 91.3 (71.1 to 97.8) | |
| Sentinel lymph node identification method | |||
| Blue dye alone | 11 (435) | 95.2 (77.2 to 99.2) | 6 levels (all tracers and tracer combinations): Equal variances assumed: Chi2 (5) = 2.29; P = 0.81 3 levels (blue dye alone, ICG alone, technetium‐99m and blue dye): Equal variances assumed: Chi2 (2) = 0.22; P = 0.90 Separate variances assumed: Chi2 (4) = ‐0.55; P = 1 |
| ICG alone | 9 (881) | 92.5 (81.8 to 97.1) | |
| Technetium‐99m alone | 4 (208) | 90.5 (67.7 to 97.7) | |
| ICG and blue dye | 2 (208) | 90.5 (63.2 to 98.1) | |
| Technetium‐99m and blue dye | 12 (473) | 91.9 (74.4 to 97.8) | |
| Technetium‐99m and ICG | 1 (32) | 100 | |
| Injection site | |||
| Subserosal | 11 (445) | 90.4 (82.6 to 94.9) | 3 levels (subserosal, cervical and combined/mixed): Equal variances assumed: Chi2 (2) = 1.85; P = 0.4 2 levels (subserosal, cervical): Equal variances assumed: Chi2 (1) = 0.07; P = 0.79 Separate variances assumed: Chi2 (2) = 4.39; P = 0.11 |
| Cervical | 23 (1746) | 91.7 (84.0 to 95.9) | |
| Combined subserosal and cervical | 1 (10) | 100 | |
| Cervical with/without subserosal | 1 (36) | 100 | |
| Lymph node basin | |||
| Pelvic | 23 (1405) | 90.5 (81.9 to 95.3) | Equal variances assumed: Chi2 (1) = 1.48; P = 0.22 Separate variances assumed: Chi2 (2) = 4.4; P = 0.11 |
| Pelvic and para‐aortic | 10 (779) | 94.0 (88.8 to 96.8) | |
| CI: confidence interval | |||
| Test | No. of studies | No. of participants |
| 1 Blue dye alone per patient (all injection sites) Show forest plot | 11 | 435 |
| 2 Blue dye alone (cervical injection) Show forest plot | 7 | 302 |
| 3 Blue dye alone (subserosal injection) Show forest plot | 5 | 133 |
| 4 Technetium‐99m alone per patient (all injection sites) Show forest plot | 4 | 208 |
| 5 Technetium‐99m alone per patient (cervical injections) Show forest plot | 2 | 48 |
| 6 Technetium‐99m alone per patient (subserosal injection) Show forest plot | 4 | 160 |
| 7 Technetium‐99m & blue dye per patient Show forest plot | 12 | 473 |
| 8 ICG alone per patient Show forest plot | 9 | 881 |
| 9 ICG and blue dye per patient Show forest plot | 2 | 208 |
| 10 ICG and Technecium‐99m per patient Show forest plot | 1 | 32 |
| 11 Nanoparticles per patient Show forest plot | 0 | 0 |
| 18 SLNB ‐ all tracers per patient Show forest plot | 33 | 2237 |

