Biopsia del ganglio centinela para el diagnóstico de la afectación de los ganglios linfáticos en el cáncer de endometrio
Appendices
Appendix 1. MEDLINE (Ovid) search strategy
1. exp Endometrial Neoplasms/
2. ((endometr* or uter*) adj5 (tumor* or tumour* or neoplas* or malignan* or carcinom* or cancer* or adenocarcinoma* or metasta* or micrometasta* or carcinogen* or adenosquamous or growth*)).ti,ab.
3. Neoplasm Invasiveness/ or Lymphatic Metastasis/ or Neoplasm Micrometastasis/ or Neoplasm Recurrence, Local/ or Neoplasm, Residual/
4. Neoplasm Staging/
5. ((lymphovascular or lympho‐vascular) adj4 invasiv*).ti,ab.
6. ((lymph* adj4 metasta*) or (detect* adj4 metasta*)).ti,ab.
7. exp Endometrium/
8. (endometr* or uter*).ti,ab.
9. (3 or 4 or 5 or 6) and (7 or 8)
10. 1 or 2 or 9
11. Lymph Nodes/
12. Sentinel Lymph Node Biopsy/
13. (sentin?l adj3 node*1).ti,ab.
14. ((lymph node*1 or lymph nodal) adj3 (assess* or evaluat* or observ* or involve* or biopsy or biopsies)).ti,ab.
15. (lymph* adj3 (mapping or spread* or staging)).ti,ab.
16. lymphadenectomy.ti,ab.
17. ((pelvi* or para‐aort*) adj3 (nod* or dissect* or status*)).ti,ab.
18. (ultrastaging or ultra staging).ti,ab.
19. Coloring Agents/
20. Technetium Tc 99m Sulfur Colloid/
21. Technetium Tc 99m Aggregated Albumin/
22. Radiopharmaceuticals/
23. Indocyanine Green/
24. Methylene Blue/
25. (technetium or tc 99m* or 99mtc* or blue dye*1 or patent blue or methylene blue or isosulfan or iso sulfan or lymphazurin blue or radiocolloid*).ti,ab,nm.
26. (lymphoscintigraph* or lymphoscintigram*).ti,ab.
27. Lymphography/
28. (scintiphotograph* or scintigraph* or scintigram*).ti,ab.
29. (gamma adj3 (camera*1 or counter*1 or probe*1)).ti,ab.
30. Fluorescent Dyes/
31. Gamma Cameras/
32. (radioisotope* adj scan*).ti,ab.
33. gamma probe.ti,ab.
34. (radioactive adj3 (tracer* or isotope*)).ti,ab.
35. (near infrared adj3 (fluorescen* or imag* or endoscop* or spectroscop*)).ti,ab.
36. (NIR adj3 (fluorescen* or imag* or endoscop* or spectroscop*)).ti,ab.
37. Spectroscopy, Near‐Infrared/
38. 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37
39. 10 and 38
40. exp animals/ not humans.sh.
41. 39 not 40
Key:
mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier
pt=publication type
ab=abstract
sh=subject heading
ti=title
Appendix 2. Embase search strategy
1. exp endometrium tumor/
2. ((endometr* or uter*) adj5 (tumor* or tumour* or neoplas* or malignan* or carcinom* or cancer* or adenocarcinoma* or metasta* or micrometasta* or carcinogen* or adenosquamous or growth*)).ti,ab.
3. tumor invasion/ or lymph node metastatis/ or micrometastatis/ or tumor recurrence/ or minimal residual disease/
4. cancer staging/
5. ((lymphovascular or lympho‐vascular) adj4 invasiv*).ti,ab.
6. ((lymph* adj4 metasta*) or (detect* adj4 metasta*)).ti,ab.
7. exp endometrium/
8. (endometr* or uter*).ti,ab.
9. (3 or 4 or 5 or 6) and (7 or 8)
10. 1 or 2 or 9
11. lymph node/
12. sentinel lymph node biopsy/
13. (sentin?l adj3 node*1).ti,ab.
14. ((lymph node*1 or lymph nodal) adj3 (assess* or evaluat* or observ* or involve* or biopsy or biopsies)).ti,ab.
15. (lymph* adj3 (mapping or spread* or staging)).ti,ab.
16. lymphadenectomy.ti,ab.
17. ((pelvi* or para‐aort*) adj3 (nod* or dissect* or status*)).ti,ab.
18. (ultrastaging or ultra staging).ti,ab.
19. coloring agent/
20. technetium sulfur colloid tc 99m/
21. macrosalb tc 99m/
22. radiopharmaceutical agent/
23. indocyanine green/
24. methylene blue/
25. (technetium or tc 99m* or 99mtc* or blue dye*1 or patent blue or methylene blue or isosulfan or iso sulfan or lymphazurin blue or radiocolloid*).ti,ab.
26. (lymphoscintigraph* or lymphoscintigram*).ti,ab.
27. lymphography/
28. (scintiphotograph* or scintigraph* or scintigram*).ti,ab.
29. (gamma adj3 (camera*1 or counter*1 or probe*1)).ti,ab.
30. fluorescent dye/
31. gamma camera/
32. (radioisotope* adj scan*).ti,ab.
33. gamma probe.ti,ab.
34. (radioactive adj3 (tracer* or isotope*)).ti,ab.
35. (near infrared adj3 (fluorescen* or imag* or endoscop* or spectroscop*)).ti,ab.
36. (NIR adj3 (fluorescen* or imag* or endoscop* or spectroscop*)).ti,ab.
37. near infrared spectroscopy/
38. 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37
39. 10 and 38
40. limit 39 to embase
Key:
mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier
pt=publication type
ab=abstract
sh=subject heading
ti=title
Appendix 3. Stata code
Overall analysis:
meqrlogit true sens, nocons || studyid: sens, nocons cov(ind) binomial(n) refineopts(iterate(3)) intpoints(5) variance
Heterogeneity analysis:
*** Model without covariate ***
meqrlogit true sens, nocons || study: sens, nocons cov(un) ///
binomial(n) refineopts(iterate(3)) intpoints(5) variance nolr
estimates store A
*** Model with covariate terms and equal variances ***
meqrlogit true se1 se2, nocons || study: sens, nocons ///
binomial(n) refineopts(iterate(3)) intpoints(5) variance nolr
estimates store B
*** Model with covariate terms and separate variances ***
meqrlogit true se1 se2, nocons ///
|| study: se1, nocons || study: se2, nocons ///
binomial(n) refineopts(iterate(6)) intpoints(1) variance nolr
estimates store C
*** Perform likelihood ratio tests to compare models ***
lrtest A B
lrtest B C
lrtest A C

Study flow diagram.

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study
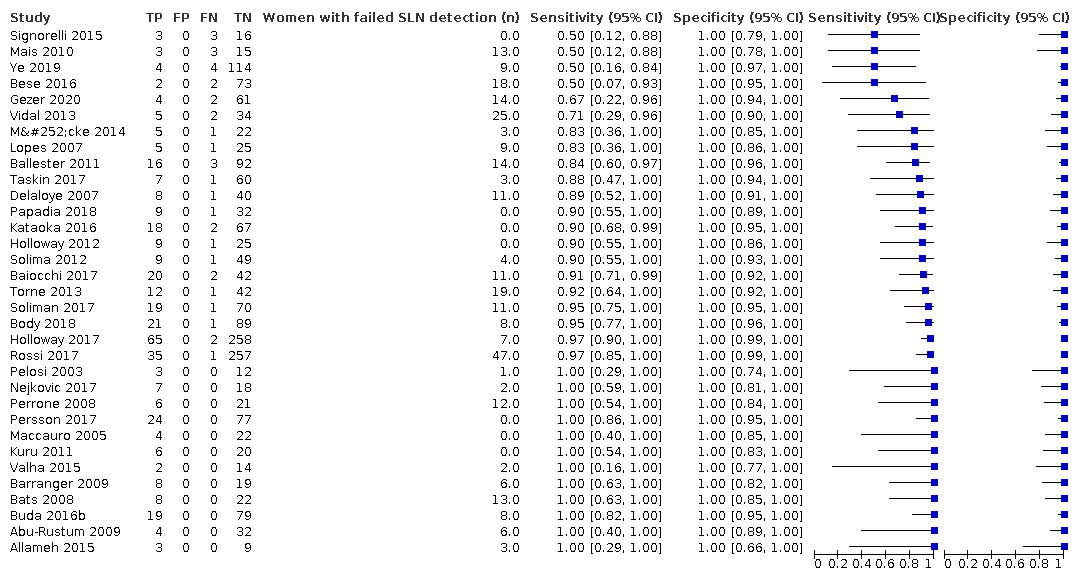
Figure 3 illustrates the (per patient) sensitivity of the included studies ordered by sensitivity.
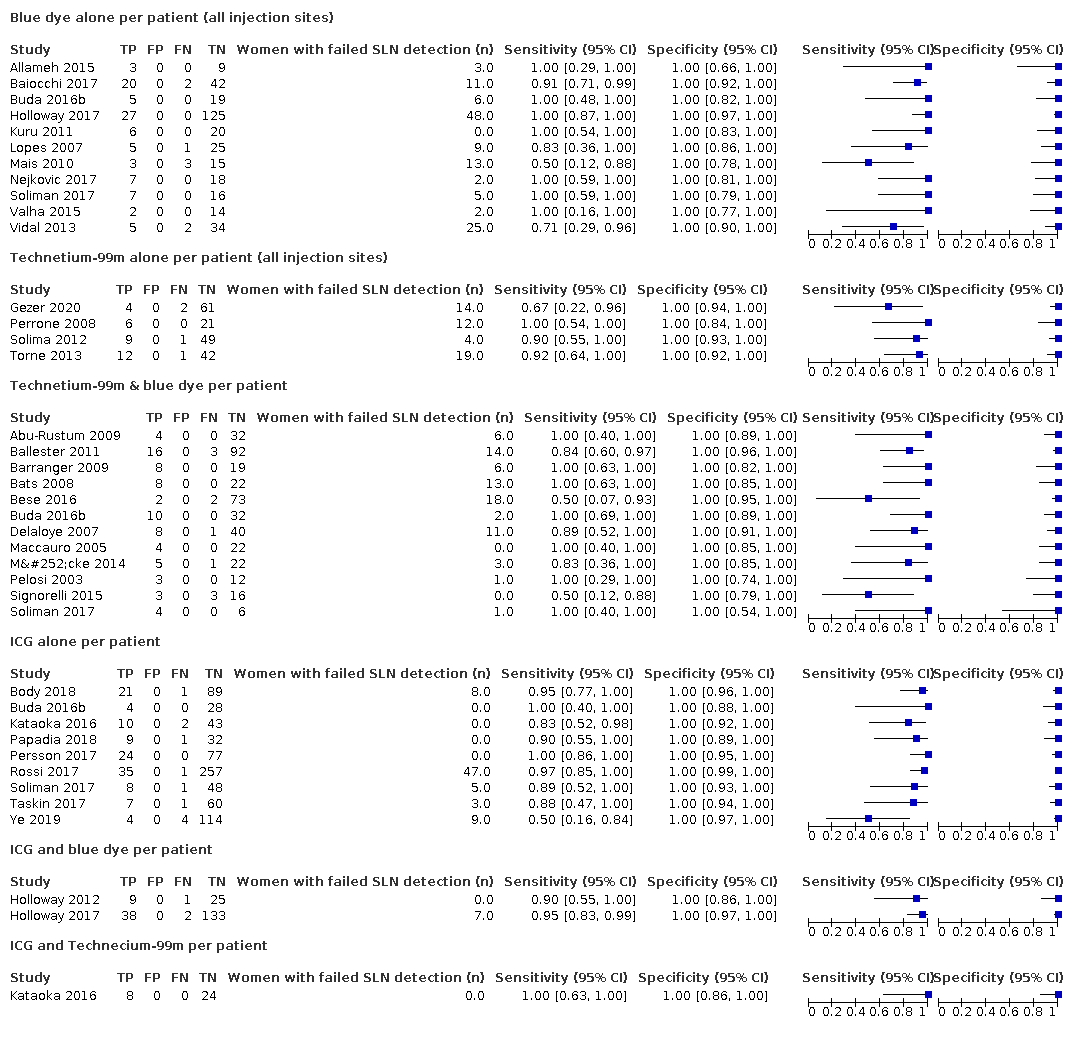
Figure 4 illustrates the (per patient) sensitivity of the included studies ordered by detection method: Blue dye alone, Technetium‐99m alone, Technetium‐99m & blue dye, ICG alone, ICG and blue dye, and ICG and Technecium‐99m.
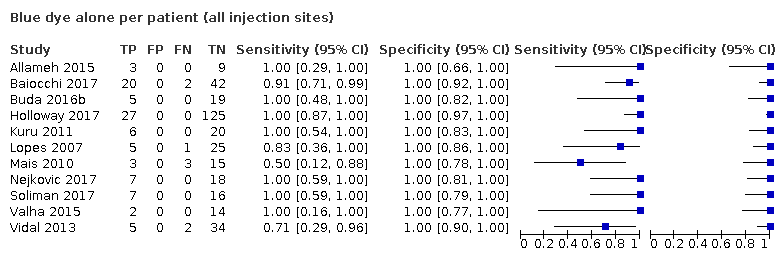
Blue dye alone per patient (all injection sites)
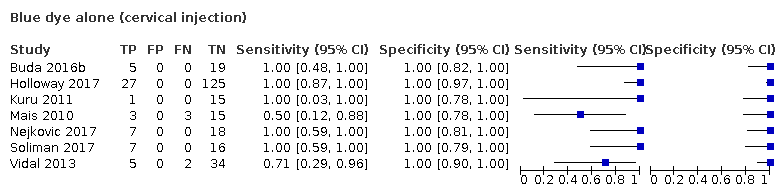
Blue dye alone (cervical injection)
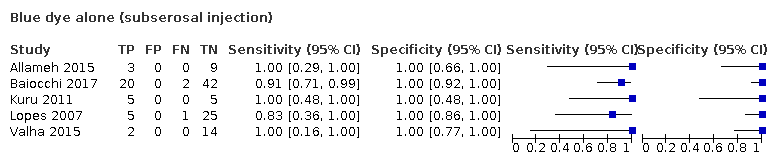
Blue dye alone (subserosal injection)

Technetium‐99m alone per patient (all injection sites)

Technetium‐99m alone per patient (cervical injections)

Technetium‐99m alone per patient (subserosal injection)
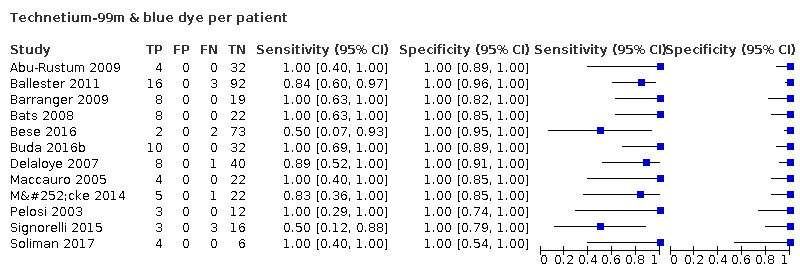
Technetium‐99m & blue dye per patient

ICG alone per patient

ICG and blue dye per patient

ICG and Technecium‐99m per patient

Nanoparticles per patient

SLNB ‐ all tracers per patient
| Review question: what is the diagnostic accuracy of different traces for the detection of sentinel lymph nodes? Patients/population: female adults with presumed early‐stage endometrial (womb) cancer Role: prognostic information for guiding adjuvant therapy after surgery Index tests: sentinel lymph node biopsy (SLNB) after injection of tracer substance/s into the cervix or uterine muscle Threshold for index tests: detection of micrometastases following ultrastaging (ultrasection of sentinel LN and immunohistochemistry). Presence of individual tumour cells (ITCs) excluded as positive result where possible. Reference standards: full pelvic +/‐ para‐aortic lymphadenectomy and standard histological examination Studies: cross‐sectional and cohort studies Setting: secondary/tertiary inpatient care at time of surgery for endometrial cancer | |||||||
| Index test | Number of patients (number of studies) | Mean SLN detection rate (95% CI)1 | Pooled sensitivity results per woman (95% CI)2 | Consequences in a cohort of 1000 women undergoing SLNB, assuming the prevalence of LN metastases to be 20% | Certainty of evidence | ||
|---|---|---|---|---|---|---|---|
| Women with no SLN detected (i.e., failed test; 95% CI)3 | Women with metastatic nodes diagnosed by index test (TP; 95% CI)4 | Women with metastatic nodes missed by index test (FN; 95% CI)5 | |||||
| 1. All tracers | 2237 (33 studies) | 86.9% (82.9% to 90.8%) | 91.8% (86.5% to 95.1% | 131 (92 to 171) | 160 (150 to 162) | 14 (9 to 23) | ⊕⊕⊕⊖ moderate 6 |
| 2. Blue dye alone | 559 women (11 studies) | 77.8% (70.0% to 85.6%) | 95.2% (77.2% to 99.2%) | 222 (144 to 300) | 148 (120 to 154) | 7 (1 to 35) | ⊕⊕⊖⊖ low 7 |
| 3. Technetium‐99m alone | 257 women (4 studies) | 80.9% (63.9 to 97.9) | 90.5% (67.7% to 97.7%) | 191 (21 to 361) | 146 (109 to 158) | 15 (4 to 52) | ⊕⊕⊖⊖ low 7 |
| 4. Technetium‐99m and blue dye | 548 women (12 studies) | 86.3% (80.7 to 91.9) | 91.9% (74.4% to 97.8%) | 137 (81 to 193) | 159 (128 to 169) | 14 (4 to 44) | ⊕⊕⊖⊖ low 7 |
| 5. ICG alone | 953 women (9 studies) | 92.4% (88.7 to 96.2) | 92.5% (81.8% to 97.1%) | 76 (38 to 113) | 171 (151 to 179) | 14 (6 to 34) | ⊕⊕⊕⊖ moderate 6 |
| 6. ICG and blue dye | 215 women (2 studies) | 96.7% (92.7 to 100) | 90.5% (63.2% to 98.1%) | 33 (0 to 73) | 175 (122 to 190) | 18 (4 to 71) | ⊕⊕⊖⊖ low 7 |
| 7. ICG and Technetium‐99m | 32 women (1 study) | 100% | 100% (63% to 100%) | 0 | 200 (126 to 200) | 0 (0 to 74) | ⊕⊖⊖⊖ very low 8 |
| * Prevalence of positive LN rate 20% chosen to represent those with higher risk of LN metastasis (as per Creasman 1987). A false‐positive result cannot occur, as the histological examination of the SLN is unchanged by the results from any additional nodes removed at systematic lymphadenectomy. Abbreviations SLN: sentinel lymph node SLNB: sentinel lymph node biopsy LN: lymph node ICG:indolcyanine green dye (visualised with near infra‐red fluorescence) TP: true positive FN: false negative | |||||||
| GRADE certainty of the evidence High: we are very confident that the true effect lies close to that of the estimate of the effect. | |||||||
| 1 Calculated as the arithmetic mean of the total number of SLNs detected out of the total number of women included in the included studies with the woman as the unit of analysis. 2 Calculated as the pooled estimate of sensitivity, using a univariate random‐effects logistic regression model (Takwoingi 2017) with the woman as the unit of analysis. 3 Calculated by subtracting the mean SLN detection rate estimates from 1000. 4 Calculated by subtracting the number of women with no SLN detected (i.e., with a failed test) from 1000 and then multiplying that with the prevalence and the pooled sensitivity estimate. 5 Calculated by subtracting the number of women with no SLN detected (i.e., with a failed test) from 1000 and then multiplying that with the prevalence and the false negative rate estimates. The false negative rate estimates were calculated by subtracting the sensitivity estimates from 100, 6 Downgraded by 1 level for risk of bias (for a combination of unclear patient selection and unclear risk of publication bias) 7 Downgraded by 2 levels; 1 for risk of bias (1 level for a combination of unclear patient selection and unclear risk of publication bias) and imprecision (1 level for wide confidence intervals). 8 Downgraded by 3 levels: 1 level for risk of bias (unclear patient selection and unclear risk of publication bias) and 2 levels for imprecision (1 small study with few true positive nodes with wide confidence intervals). | |||||||
| Review question: what is the diagnostic accuracy of different traces for the detection of sentinel lymph nodes? Patients/population: female adults with presumed early‐stage endometrial (womb) cancer Role: prognostic information for guiding adjuvant therapy after surgery Index tests: sentinel lymph node biopsy (SLNB) after injection of tracer substance/s into the cervix or uterine muscle Threshold for index tests: detection of micrometastases following ultrastaging (ultrasection of sentinel LN and immunohistochemistry). Presence of individual tumour cells (ITCs) excluded as positive result where possible. Reference standards: full pelvic +/‐ para‐aortic lymphadenectomy and standard histological examination Studies: cross‐sectional and cohort studies Setting: secondary/tertiary inpatient care at time of surgery for endometrial cancer | |||||||
| Index test | Number of patients (number of studies) | Mean SLN detection rate (95% CI)1 | Pooled sensitivity results per woman (95% CI)2 | Consequences in a cohort of 1000 women undergoing SLNB, assuming the prevalence of LN metastases to be 5% | Certainty of evidence | ||
|---|---|---|---|---|---|---|---|
| Women with no SLN detected (i.e., failed test; 95% CI)3 | Women with metastatic nodes diagnosed by index test (TP; 95% CI)4 | Women with metastatic nodes missed by index test (FN; 95% CI)5 | |||||
| 1. All tracers | 2237 (33 studies) | 86.9% (82.9% to 90.8%) | 91.8% (86.5% to 95.1%) | 131 (92 to 171) | 40 (38 to 41) | 4 (2 to 6) | ⊕⊕⊕⊖ moderate 6 |
| 2. Blue dye alone | 559 women (11 studies) | 77.8% (70.0% to 85.6%) | 95.2% (77.2% to 99.2%) | 222 (144 to 300) | 37 (30 to 39) | 2 (0 to 9) | ⊕⊕⊖⊖ low 7 |
| 3. Technetium‐99m alone | 257 women (4 studies) | 80.9% (63.9 to 97.9) | 90.5% (67.7% to 97.7%) | 191 (21 to 361) | 37 (27 to 40) | 4 (1 to 13) | ⊕⊕⊖⊖ low 7 |
| 4. Technetium‐99m and blue dye | 548 women (12 studies) | 86.3% (80.7 to 91.9) | 91.9% (74.4% to 97.8%) | 137 (81 to 193) | 40 (32 to 42) | 3 (1 to 11) | ⊕⊕⊖⊖ low 7 |
| 5. ICG alone | 953 women (9 studies) | 92.4% (88.7 to 96.2) | 92.5% (81.8% to 97.1%) | 76 (38 to 113) | 43 (38 to 45) | 3 (1 to 8) | ⊕⊕⊕⊖ moderate 6 |
| 6. ICG and blue dye | 215 women (2 studies) | 96.7% (92.7 to 100) | 90.5% (63.2% to 98.1%) | 33 (0 to 73) | 44 (31 to 47) | 5 (1 to 18) | ⊕⊕⊖⊖ low 7 |
| 7. ICG and Technetium‐99m | 32 women (1 study) | 100% | 100% (63% to 100%) | 0 | 50 (32 to 50) | 0 (0 to 19) | ⊕⊖⊖⊖ very low 8 |
| * Prevalence of positive LN rate 5% chosen to represent those with lower risk of LN metastasis (as per Creasman 1987). A false‐positive result cannot occur, as the histological examination of the SLN is unchanged by the results from any additional nodes removed at systematic lymphadenectomy. Abbreviations SLN: sentinel lymph node SLNB: sentinel lymph node biopsy LN: lymph node ICG:indolcyanine green dye (visualised with near infra‐red fluorescence) TP: true positive FN: false negative | |||||||
| GRADE certainty of the evidence High: we are very confident that the true effect lies close to that of the estimate of the effect. | |||||||
| 1 Calculated as the arithmetic mean of the total number of SLNs detected out of the total number of women included in the included studies with the woman as the unit of analysis. 2 Calculated as the pooled estimate of sensitivity, using a univariate random‐effects logistic regression model (Takwoingi 2017) with the woman as the unit of analysis. 3 Calculated by subtracting the mean SLN detection rate estimates from 1000. 4 Calculated by subtracting the number of women with no SLN detected (i.e., with a failed test) from 1000 and then multiplying that with the prevalence and the pooled sensitivity estimate. 5 Calculated by subtracting the number of women with no SLN detected (i.e., with a failed test) from 1000 and then multiplying that with the prevalence and the false negative rate estimates. The false negative rate estimates were calculated by subtracting the sensitivity estimates from 100, 6 Downgraded by 1 level for risk of bias (for a combination of unclear patient selection and unclear risk of publication bias) 7 Downgraded by 2 levels; 1 for risk of bias (1 level for a combination of unclear patient selection and unclear risk of publication bias) and imprecision (1 level for wide confidence intervals). 8 Downgraded by 3 levels: 1 level for risk of bias (unclear patient selection and unclear risk of publication bias) and 2 levels for imprecision (1 small study with few true positive nodes with wide confidence intervals). | |||||||
| Stage I: | Cancer confined to the uterus (womb), which has not spread to other parts of the body |
|---|---|
| Stage IA: | Cancer confined to the endometrium, or less than one‐half of the myometrium |
| Stage IB: | Cancer spread to the outer half of the myometrium |
| Stage II: | Cancer spread from the uterus to the cervical stroma, but not to other parts of the body |
| Stage III: | Cancer spread beyond the uterus, but it is still only in the pelvic area |
| Stage IIIA: | Cancer spread to serosa of the uterus, fallopian tubes and ovaries, or a combination, but not to other parts of the body |
| Stage IIIB: | Cancer spread to the vagina or parametria |
| Stage IIIC1: | Cancer spread to the regional pelvic lymph nodes |
| Stage IIIC2: | Cancer spread to the para‐aortic lymph nodes with or without spread to the regional pelvic lymph nodes |
| Stage IV: | Direct invasion into adjacent organs or distant spread |
| Stage IVA: | Cancer spread to the mucosa of the rectum or bladder |
| Stage IVB: | Cancer spread to lymph nodes in the groin area, or it has spread to distant organs, such as the bones or lungs |
| Item | Description |
|---|---|
| Domain 1: Patient selection | |
| A. Risk of bias | |
| Was a consecutive or random sample of participants enrolled? | Yes, if a consecutive or random sample of participants were enrolled No, if a consecutive or random sample of participants were not enrolled Unclear, if the study did not describe the method of participants enrolment |
| Was a case‐control design avoided? | The answer to this item will be 'yes' for all the included studies, as one of the exclusion criteria is case‐control studies |
| Did the study avoid inappropriate exclusions? | Yes, if the characteristics of the participants were well described and probably typical of a secondary healthcare setting No, if the sample was unrepresentative of people with apparent early‐stage endometrial cancer (i.e. a unexpectedly high proportion with rarer high grade histopathological types of endometrial cancer) Unclear, if the source or characteristics of participants was not adequately described |
| Could the selection of participants have introduced bias? | A judgement of low, high, or unclear risk of bias will be made, based on a balanced assessment of the responses to the above signalling questions. |
| B. Concerns about applicability | |
| Are there concerns that the included participants and setting do not match the review question? | A judgement of low, high, or unclear concerns about applicability will be made based on a balanced assessment of the response to the third signalling question above, and on how closely the sample matched the target population of interest |
| Domain 2: Index test | |
| A. Risk of bias | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes, if the report stated that the person undertaking the index test did not know the results of the reference tests No, if the report stated that the same person performed both tests, or that the results of the index tests were known to the person undertaking the reference tests Unclear, if insufficient information was provided |
| Did the study provide a clear definition of what was considered to be a positive result? | Yes, if the definition of a diagnosis of lymph node metastasis was clearly stated, including ultrastaging of the sentinel node No, if no definition of what was considered a positive result of lymph node metastasis was stated, or the definition of a positive result varied between the participants Unclear, if not enough information was given to permit judgement |
| If a threshold was used, was it prespecified? | This item is not applicable, as the test is not subject to a threshold. |
| Could the conduct or interpretation of the index test have introduced bias? | A judgement of low, high, or unclear risk of bias will be made based on a balanced assessment of the responses to the above signalling questions. |
| B. Concerns about applicability | |
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | A judgement of low, high, or unclear concerns about applicability will be made, based on a balanced assessment of the information detailed under ’Index test’ in the 'Characteristics of included studies' tables. |
| Domain 3: Reference standard | |
| A. Risk of bias | |
| Is the reference standards likely to correctly classify the target condition? | Yes, if a full pelvic or aortic node dissection (or both) was carried out adequately to correctly classify the target condition. A pelvic node dissection includes bilateral removal of nodal tissue from the distal one‐half of each common iliac artery, the anterior and medial aspect of the proximal half of the external iliac artery and vein, and the distal half of the obturator fat pad anterior to the obturator nerve. A para‐aortic node includes resection of nodal tissue over the distal vena cava, from the level of the inferior mesenteric artery (or left renal vein) to the mid‐right common iliac artery, and between the aorta and the left ureter, from the inferior mesenteric artery to the left mid‐common iliac artery. No, if a full pelvic or aortic node dissection (or both) has not been carried out adequately to correctly classify the target condition. Unclear, if insufficient information was provided to assess whether the reference standard had been carried out adequately (e.g. no mention of site or number of lymph node yield). |
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes, if the report stated that for SLNB, the histological examination from the lymphadenectomy was done without knowledge of the sentinel lymph node status. No, if the report stated that histological examination of the pelvic lymphadenectomy was carried out with the knowledge of the sentinel lymph node status. Unclear, if insufficient details given as to whether the histological examination was carried out with or without knowledge of sentinel lymph node status. |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | A judgement of low, high, or unclear risk of bias will be made, based on a balanced assessment of the responses to the above signalling questions. |
| B. Concerns about applicability | |
| Are there concerns that the target condition, as defined by the reference standard, does not match the question? | The answer to this question will always be low, because the target condition that the reference standard defines will always be the target condition of the review, i.e. adenocarcinoma of the endometrium of endometrioid, serous, clear cell, or mixed types, with apparent cancer confined to the uterus. Otherwise, the study will not be included. |
| Domain 3: Flow and timing | |
| A. Risk of bias | |
| Was there an appropriate interval between index test and reference | Yes, if the time period between the index test and the reference standard was no more than one month No, if the time period between the index test and the reference standard was longer than one month Unclear, if insufficient information was provided |
| Did all participants receive the same reference standard? | Yes, if the same reference test was used, regardless of the index test results No, if different reference tests were used, depending on the results of the index test Unclear, if insufficient information was provided Report if any participants received a different reference test, what the reasons stated for this were, and how many participants were involved. |
| Were all participants included in the analysis? | Yes, if there were no participants excluded from the analysis, or if exclusions were adequately described No, if there were participants excluded from the analysis and there was no explanation given Unclear, if not enough information was given to assess whether any participants were excluded from the analysis Report how many participants were excluded from the analysis for reasons other than uninterpretable results Report how many results were uninterpretable (of the total) |
| Could the patient flow have introduced bias? | A judgement of low, high, or unclear risk of bias will be made, based on a balanced assessment of the responses to the above signalling questions. |
| Tracer (studies) | Number detected/total number (%; 95% CI) |
| Blue dye alone (11) | 435/559 (77.8; 95%CI 70.0 to 85.6) |
| Technetium‐99m alone (4) | 208/257 (80.9; 95% CI 63.9 to 97.9) |
| Technetium‐99m and blue dye (12) | 473/548 (86.3; 95% CI 80.7 to 91.9) |
| ICG alone (9) | 881/953 (92.4; 95% CI 88.7 to 96.2) |
| ICG and blue dye (2) | 208/215 (96.7; 95% CI 92.7 to 100) |
| ICG and technetium‐99m (1) | 32/32 (100%) |
| CI: confidence interval | |
| Covariate | Studies (women) | Sensitivity % (95% CI) | Statistical significance |
| FIGO stage | |||
| Majority of women with 1a | 16 (1252) | 91.1 (80.7 to 96.2) | Equal variances assumed: Chi2 (1) = 0.13; P = 0.72 Separate variances assumed: Chi2 (2) = 1.21; P = 0.55 |
| Majority of women with 1b or above | 3 (95) | 91.3 (71.1 to 97.8) | |
| Sentinel lymph node identification method | |||
| Blue dye alone | 11 (435) | 95.2 (77.2 to 99.2) | 6 levels (all tracers and tracer combinations): Equal variances assumed: Chi2 (5) = 2.29; P = 0.81 3 levels (blue dye alone, ICG alone, technetium‐99m and blue dye): Equal variances assumed: Chi2 (2) = 0.22; P = 0.90 Separate variances assumed: Chi2 (4) = ‐0.55; P = 1 |
| ICG alone | 9 (881) | 92.5 (81.8 to 97.1) | |
| Technetium‐99m alone | 4 (208) | 90.5 (67.7 to 97.7) | |
| ICG and blue dye | 2 (208) | 90.5 (63.2 to 98.1) | |
| Technetium‐99m and blue dye | 12 (473) | 91.9 (74.4 to 97.8) | |
| Technetium‐99m and ICG | 1 (32) | 100 | |
| Injection site | |||
| Subserosal | 11 (445) | 90.4 (82.6 to 94.9) | 3 levels (subserosal, cervical and combined/mixed): Equal variances assumed: Chi2 (2) = 1.85; P = 0.4 2 levels (subserosal, cervical): Equal variances assumed: Chi2 (1) = 0.07; P = 0.79 Separate variances assumed: Chi2 (2) = 4.39; P = 0.11 |
| Cervical | 23 (1746) | 91.7 (84.0 to 95.9) | |
| Combined subserosal and cervical | 1 (10) | 100 | |
| Cervical with/without subserosal | 1 (36) | 100 | |
| Lymph node basin | |||
| Pelvic | 23 (1405) | 90.5 (81.9 to 95.3) | Equal variances assumed: Chi2 (1) = 1.48; P = 0.22 Separate variances assumed: Chi2 (2) = 4.4; P = 0.11 |
| Pelvic and para‐aortic | 10 (779) | 94.0 (88.8 to 96.8) | |
| CI: confidence interval | |||
| Test | No. of studies | No. of participants |
| 1 Blue dye alone per patient (all injection sites) Show forest plot | 11 | 435 |
| 2 Blue dye alone (cervical injection) Show forest plot | 7 | 302 |
| 3 Blue dye alone (subserosal injection) Show forest plot | 5 | 133 |
| 4 Technetium‐99m alone per patient (all injection sites) Show forest plot | 4 | 208 |
| 5 Technetium‐99m alone per patient (cervical injections) Show forest plot | 2 | 48 |
| 6 Technetium‐99m alone per patient (subserosal injection) Show forest plot | 4 | 160 |
| 7 Technetium‐99m & blue dye per patient Show forest plot | 12 | 473 |
| 8 ICG alone per patient Show forest plot | 9 | 881 |
| 9 ICG and blue dye per patient Show forest plot | 2 | 208 |
| 10 ICG and Technecium‐99m per patient Show forest plot | 1 | 32 |
| 11 Nanoparticles per patient Show forest plot | 0 | 0 |
| 18 SLNB ‐ all tracers per patient Show forest plot | 33 | 2237 |

