Certolizumab pegol para inducir la remisión de la enfermedad de Crohn
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomized, double‐blind, placebo‐controlled, multicenter trial | |
| Participants | Patients (16‐65 years) with active Crohn's disease (CDAI: 220‐450) and C‐reactive protein value ≥ 10 mg/L (N = 94) | |
| Interventions | Subcutaneous administration at week 0, 2, and 4 Group 1: Placebo (n = 32) | |
| Outcomes | Primary outcome: Clinical response (> 100 points CDAI decrease) or remission (CDAI ≤ 150) at week 6 | |
| Notes | This study was conducted between March 2006 and November 2007 The follow‐up period was 6 weeks. Adverse events were followed for 28 weeks Funding source was UCB Inc, which manufactures Certolizumab pegol All authors were from Japan Conflict of interest was reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralized randomization schemes with randomization code were used |
| Allocation concealment (selection bias) | Low risk | Centralized randomization schemes with randomization code were used |
| Blinding of participants and personnel (performance bias) | Low risk | Masking: Triple (Participant, Care Provider, Investigator) |
| Blinding of outcome assessment (detection bias) | Low risk | Masking: Triple (Participant, Care Provider, Investigator) |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomized, double‐blind, placebo‐controlled, multicenter trial | |
| Participants | Adult patients (≥ 18 years) with active Crohn's disease (CDAI: 220‐450) (N = 660) | |
| Interventions | Subcutaneous administration at week 0, 2, 4 and then every 4 weeks Group 1: Placebo (n = 329) | |
| Outcomes | Primary outcome: Clinical response (> 100 points CDAI decrease) at week 6 and at both weeks 6 and 26 in patients with a baseline serum CRP ≥10 mg/L Secondary outcomes: In the patients with a baseline serum CRP ≥10 mg/L 1. Clinical remission (CDAI ≤ 150) at week 6 2. Clinical remission at both week 6 and week 26 3. IBDQ response (≥ 16 points total score increase) at week 6 4. IBDQ response at both week 6 and 26 In all of the patients 1. Clinical response at week 6 2. Clinical remission at week 6 3. Clinical response at both week 6 and 26 4. Clinical remission at both week 6 and 26 This study reported both clinical remission and response at week 2, 4, 6, 8, 12, 16, 20, 24, and 26 | |
| Notes | This study was conducted between December 2003 and May 2005 The follow‐up period was 26 weeks Funding source was UCB Inc which manufactures certolizumab pegol. Other funding sources were the National Center for Research Resources, a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research; and by a grant for infrastructure from the German Federal Ministry of Education and Research’s competence network for chronic inflammatory bowel disease Authors were from 7 countries: USA, Canada, Bulgaria, South Africa, Belgium, UK, and Germany Conflict of interest was reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralized randomization schemes with randomization code were used |
| Allocation concealment (selection bias) | Low risk | Centralized randomization schemes with randomization code were used |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Data were collected from diaries kept by patients who were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) | Low risk | The number of patients lost to follow‐up was only two in the certolizumab pegol group |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomized, double‐blind, placebo‐controlled, multicenter trial | |
| Participants | Adult patients (18‐75 years) with active Crohn's disease (CDAI: 220‐450) (N = 439) No previous treatment with TNF‐α inhibitors | |
| Interventions | Subcutaneous administration at week 0, 2, and 4 Group 1: Placebo (n = 216) | |
| Outcomes | Primary outcome: Clinical remission (CDAI ≤ 150) at week 6 In all of the patients 2. IBDQ remission (total score ≥ 170) at weeks 2, 4, and 6 3. Change in total CDAI score from week 0 to weeks 2, 4, and 6 4. Change in HBI score from week 0 to week 6 5. Clinical remission at weeks 2 and 4 In the patients with a baseline serum CRP ≥10 mg/L In the patients with a baseline serum CRP <10 mg/L | |
| Notes | This study was conducted between March 2008 and June 2009 The follow‐up period was 6 weeks Funding source was UCB Inc, which manufactures certolizumab pegol Authors were from 6 countries: USA, Germany, Canada, UK, Belgium, and Netherlands Conflict of interest was reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralized randomization schemes with randomization code were used |
| Allocation concealment (selection bias) | Low risk | Centralized randomization schemes with randomization code were used |
| Blinding of participants and personnel (performance bias) | Low risk | Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor) |
| Blinding of outcome assessment (detection bias) | Low risk | Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor) |
| Incomplete outcome data (attrition bias) | Low risk | The number of patients lost to follow‐up was only one in the certolizumab pegol group |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomized, double‐blind, placebo‐controlled, multicenter trial | |
| Participants | Adult patients (18‐75 years) with active Crohn's disease (CDAI: 220‐450) (N = 292) | |
| Interventions | Subcutaneous administration at week 0, 4, and 8 Group 1: Placebo (n = 73) Group 2: 100 mg of certolizumab pegol (n = 74) | |
| Outcomes | Primary outcome: Clinical response (> 100 points CDAI decrease) or remission (CDAI ≤ 150) at week 12 Secondary outcomes: 2. Remission at weeks 2, 4, 6, 8, 10, and 12 | |
| Notes | This study was conducted between February 2001 and March 2002 The follow‐up period was 20 weeks Funding source was Celltech R&D, Ltd (now UCB Inc). Additional support was provided by the German Federal Ministry for Education and Research Competence Network “Inflammatory Bowel Disease" Authors were from 5 countries: Germany, Belgium, Canada, UK, and Denmark Conflict of interest was reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralized randomization schemes with randomization code were used |
| Allocation concealment (selection bias) | Low risk | Centralized randomization schemes with randomization code were used |
| Blinding of participants and personnel (performance bias) | High risk | The color or viscosity was different between Certolizumab pegol and placebo although patients received the treatment from independent healthcare workers |
| Blinding of outcome assessment (detection bias) | High risk | Full blinding to the patients were not possible, and outcomes were based on a daily diary of their symptoms |
| Incomplete outcome data (attrition bias) | Low risk | The number of patients with lost to follow‐up was only one in each group |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| This study was not a RCT | |
| Maintenance of remission study The study participants were the patients responded to CZP | |
| This study was not a RCT | |
| Maintenance of remission study The study participants were the patients in remission and receiving corticosteroids | |
| This study was not conducted | |
| This study was not a RCT | |
| This study was not a RCT | |
| This study was not a RCT | |
| This study was not a RCT | |
| This study was not a RCT | |
| Maintenance of remission study The study participants were the patients who responded to CZP. Moreover, the participants were allocated to two different dosing schedules of CZP (every two weeks or every four weeks) | |
| CZP was administered intravenously rather than subcutaneously |
CZP: certolizumab pegol; RCT: randomized controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical remission at week 8 Show forest plot | 4 | 1485 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [1.11, 1.66] |
| Analysis 1.1  Comparison 1 Certolizumab pegol versus placebo, Outcome 1 Clinical remission at week 8. | ||||
| 2 Clinical response at week 8 Show forest plot | 4 | 1485 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [1.09, 1.53] |
| Analysis 1.2  Comparison 1 Certolizumab pegol versus placebo, Outcome 2 Clinical response at week 8. | ||||
| 3 CRP at week 8 (log‐scales of geometric mean CRP ratio between baseline and week 8) Show forest plot | 4 | 1271 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.49, ‐0.24] |
| Analysis 1.3  Comparison 1 Certolizumab pegol versus placebo, Outcome 3 CRP at week 8 (log‐scales of geometric mean CRP ratio between baseline and week 8). | ||||
| 4 IBDQ total score at week 8 (mean change from baseline) Show forest plot | 4 | 1315 | Mean Difference (IV, Random, 95% CI) | 2.12 [‐1.27, 5.50] |
| Analysis 1.4  Comparison 1 Certolizumab pegol versus placebo, Outcome 4 IBDQ total score at week 8 (mean change from baseline). | ||||
| 5 Adverse events Show forest plot | 4 | 1485 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.97, 1.10] |
| Analysis 1.5  Comparison 1 Certolizumab pegol versus placebo, Outcome 5 Adverse events. | ||||
| 6 Serious adverse events Show forest plot | 4 | 1485 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.93, 1.97] |
| Analysis 1.6  Comparison 1 Certolizumab pegol versus placebo, Outcome 6 Serious adverse events. | ||||
| 7 Withdrawals due to adverse events Show forest plot | 4 | 1485 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.57, 1.78] |
| Analysis 1.7  Comparison 1 Certolizumab pegol versus placebo, Outcome 7 Withdrawals due to adverse events. | ||||
| 8 Clinical remission at week 8 (Subgroup analysis based on CZP doses) Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.8  Comparison 1 Certolizumab pegol versus placebo, Outcome 8 Clinical remission at week 8 (Subgroup analysis based on CZP doses). | ||||
| 8.1 Certolizumab pegol 100mg | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 2.48 [0.81, 7.58] |
| 8.2 Certolizumab pegol 200mg | 2 | 142 | Risk Ratio (M‐H, Random, 95% CI) | 1.84 [0.75, 4.50] |
| 8.3 Certolizumab pegol 400mg | 4 | 1244 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.06, 1.60] |
| 9 Clinical remission at week 8 (Subgroup analysis of no previous treatment with TNF‐α inhibitors) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.9  Comparison 1 Certolizumab pegol versus placebo, Outcome 9 Clinical remission at week 8 (Subgroup analysis of no previous treatment with TNF‐α inhibitors). | ||||
| 10 Clinical remission at week 8 (Subgroup analysis of CRP levels at baseline) Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.10  Comparison 1 Certolizumab pegol versus placebo, Outcome 10 Clinical remission at week 8 (Subgroup analysis of CRP levels at baseline). | ||||
| 10.1 CRP ≥ 10 mg/L | 4 | 702 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [1.17, 2.16] |
| 10.2 CRP < 10 mg/L | 3 | 762 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.88, 1.50] |
| 11 Clinical remission at week 8 (Sensitivity analysis of excluding studies with high risk of bias) Show forest plot | 3 | 1193 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [1.05, 1.59] |
| Analysis 1.11  Comparison 1 Certolizumab pegol versus placebo, Outcome 11 Clinical remission at week 8 (Sensitivity analysis of excluding studies with high risk of bias). | ||||
| 12 Clinical remission at week 8 (sensitivity analysis of using available case data) Show forest plot | 4 | 1463 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [1.11, 1.67] |
| Analysis 1.12  Comparison 1 Certolizumab pegol versus placebo, Outcome 12 Clinical remission at week 8 (sensitivity analysis of using available case data). | ||||
| 13 Clinical remission at week 8 (Sensitivity analysis of studies with the approval dosing regimen) Show forest plot | 3 | 1147 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.03, 1.57] |
| Analysis 1.13  Comparison 1 Certolizumab pegol versus placebo, Outcome 13 Clinical remission at week 8 (Sensitivity analysis of studies with the approval dosing regimen). | ||||

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
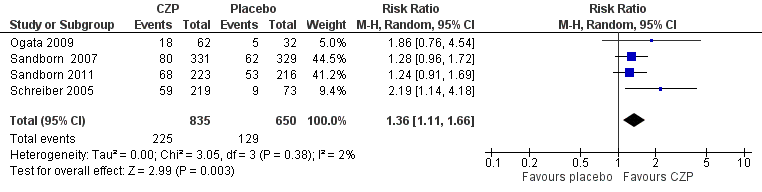
Forest plot of comparison: 1 Certolizumab pegol versus placebo, outcome: 1.1 Clinical remission at week 8.

Forest plot of comparison: 1 Certolizumab pegol versus placebo, outcome: 1.2 Clinical response at week 8.

Forest plot of comparison: 1 Certolizumab pegol versus placebo, outcome: 1.6 Serious adverse events.

Comparison 1 Certolizumab pegol versus placebo, Outcome 1 Clinical remission at week 8.

Comparison 1 Certolizumab pegol versus placebo, Outcome 2 Clinical response at week 8.
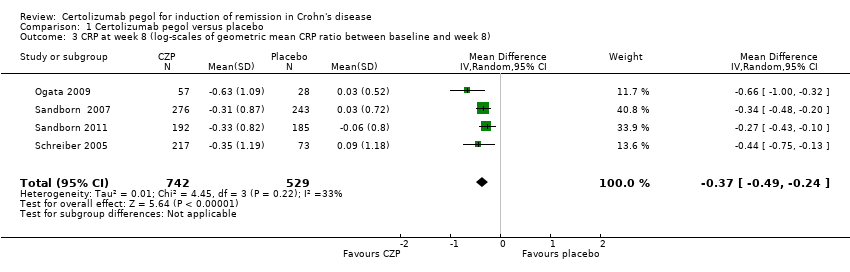
Comparison 1 Certolizumab pegol versus placebo, Outcome 3 CRP at week 8 (log‐scales of geometric mean CRP ratio between baseline and week 8).

Comparison 1 Certolizumab pegol versus placebo, Outcome 4 IBDQ total score at week 8 (mean change from baseline).
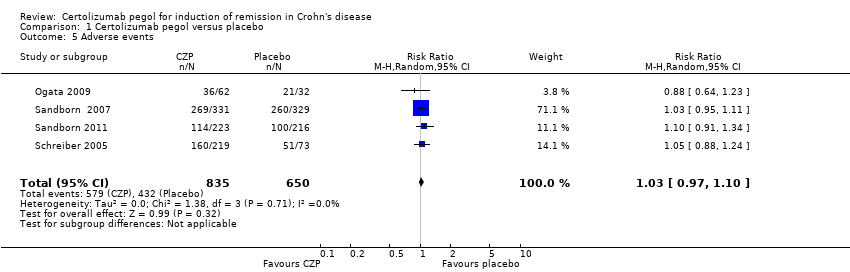
Comparison 1 Certolizumab pegol versus placebo, Outcome 5 Adverse events.
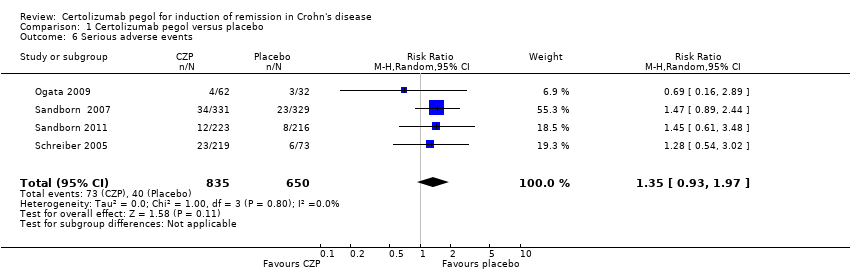
Comparison 1 Certolizumab pegol versus placebo, Outcome 6 Serious adverse events.

Comparison 1 Certolizumab pegol versus placebo, Outcome 7 Withdrawals due to adverse events.

Comparison 1 Certolizumab pegol versus placebo, Outcome 8 Clinical remission at week 8 (Subgroup analysis based on CZP doses).

Comparison 1 Certolizumab pegol versus placebo, Outcome 9 Clinical remission at week 8 (Subgroup analysis of no previous treatment with TNF‐α inhibitors).
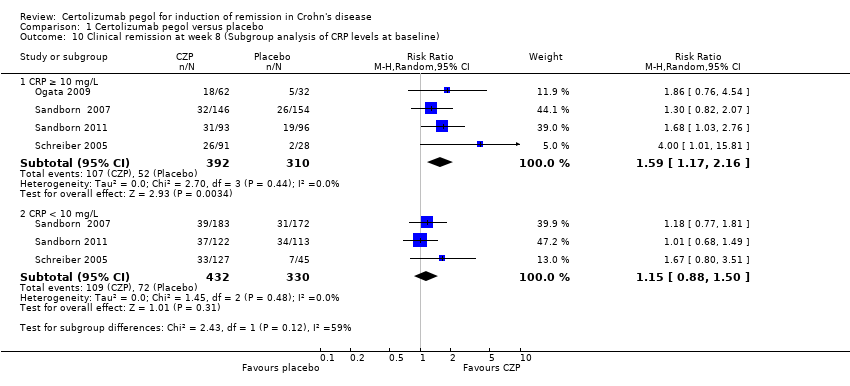
Comparison 1 Certolizumab pegol versus placebo, Outcome 10 Clinical remission at week 8 (Subgroup analysis of CRP levels at baseline).
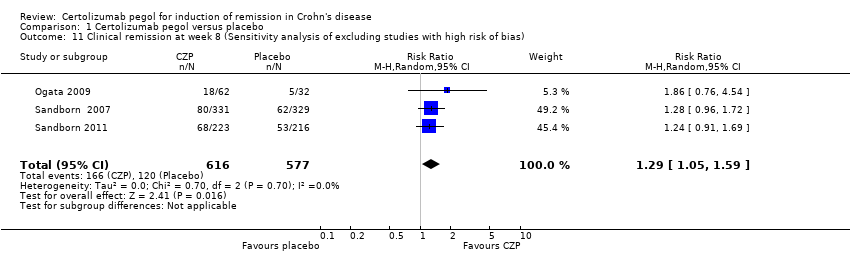
Comparison 1 Certolizumab pegol versus placebo, Outcome 11 Clinical remission at week 8 (Sensitivity analysis of excluding studies with high risk of bias).

Comparison 1 Certolizumab pegol versus placebo, Outcome 12 Clinical remission at week 8 (sensitivity analysis of using available case data).
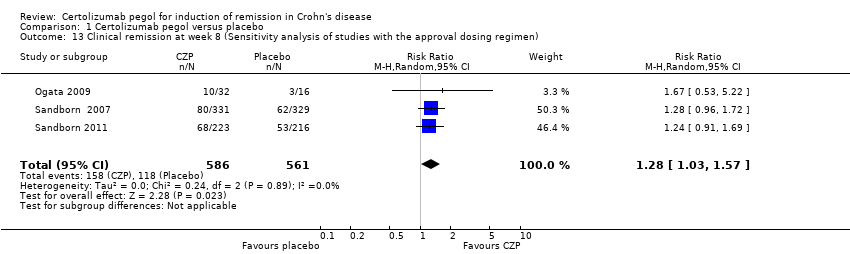
Comparison 1 Certolizumab pegol versus placebo, Outcome 13 Clinical remission at week 8 (Sensitivity analysis of studies with the approval dosing regimen).
| Certolizumab pegol compared to placebo for induction of remission in Crohn's disease | ||||||
| Patient or population: Patients with active Crohn's disease Settings: Outpatient Intervention: Certolizumab pegol Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Certolizummab pegol | |||||
| Clinical remission Follow‐up: 8 weeks | 198 per 1000 | 270 per 1000 (220 to 329) | RR 1.36 (1.11 to 1.66) | 1485 | ⊕⊕⊕⊝ | Certolizumab pegol was shown to be superior to placebo regarding clinical remission at week 8 Clinical remission was defined as a CDAI < 150 |
| Clinical response Follow‐up: 8 weeks | 309 per 1000 | 399 per 1000 (337 to 473) | RR 1.29 (1.09 to 1.53) | 1485 (4 studies) | ⊕⊕⊕⊝ | Clinical response was defined as CDAI reduction ≥ 100 from baseline |
| Serious adverse events Follow‐up: 8 weeks | 62 per 1000 | 83 per 1000 (57 to 121) | RR 1.35 (0.93 to 1.97) | 1485 (4 studies) | ⊕⊕⊕⊝ | Reported serious adverse events included worsening Crohn's disease, infections, and malignancy |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to high risk of bias in one study in the pooled analysis 2 Downgraded one level due to imprecision (113 events) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical remission at week 8 Show forest plot | 4 | 1485 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [1.11, 1.66] |
| 2 Clinical response at week 8 Show forest plot | 4 | 1485 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [1.09, 1.53] |
| 3 CRP at week 8 (log‐scales of geometric mean CRP ratio between baseline and week 8) Show forest plot | 4 | 1271 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.49, ‐0.24] |
| 4 IBDQ total score at week 8 (mean change from baseline) Show forest plot | 4 | 1315 | Mean Difference (IV, Random, 95% CI) | 2.12 [‐1.27, 5.50] |
| 5 Adverse events Show forest plot | 4 | 1485 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.97, 1.10] |
| 6 Serious adverse events Show forest plot | 4 | 1485 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.93, 1.97] |
| 7 Withdrawals due to adverse events Show forest plot | 4 | 1485 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.57, 1.78] |
| 8 Clinical remission at week 8 (Subgroup analysis based on CZP doses) Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Certolizumab pegol 100mg | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 2.48 [0.81, 7.58] |
| 8.2 Certolizumab pegol 200mg | 2 | 142 | Risk Ratio (M‐H, Random, 95% CI) | 1.84 [0.75, 4.50] |
| 8.3 Certolizumab pegol 400mg | 4 | 1244 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.06, 1.60] |
| 9 Clinical remission at week 8 (Subgroup analysis of no previous treatment with TNF‐α inhibitors) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10 Clinical remission at week 8 (Subgroup analysis of CRP levels at baseline) Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 CRP ≥ 10 mg/L | 4 | 702 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [1.17, 2.16] |
| 10.2 CRP < 10 mg/L | 3 | 762 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.88, 1.50] |
| 11 Clinical remission at week 8 (Sensitivity analysis of excluding studies with high risk of bias) Show forest plot | 3 | 1193 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [1.05, 1.59] |
| 12 Clinical remission at week 8 (sensitivity analysis of using available case data) Show forest plot | 4 | 1463 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [1.11, 1.67] |
| 13 Clinical remission at week 8 (Sensitivity analysis of studies with the approval dosing regimen) Show forest plot | 3 | 1147 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.03, 1.57] |

