18F PET s flutemetamolom u ranoj dijagnostici Alzheimerove demencije i drugih demencija u osoba s umjerenim kognitivnim oštećenjem (MCI)
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Study characteristics | |||
| Patient sampling |
| ||
| Patient characteristics and setting |
| ||
| Index tests |
| ||
| Target condition and reference standard(s) |
| ||
| Flow and timing |
| ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the 18F‐flutemetamol PET scan interpretation done by a trained reader physician? | Yes | ||
| Did the study provide a clear definition of what was considered to be a 18F‐flutemetamol positive result? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the study with 18F‐flutemetamol free of commercial funding? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling |
| ||
| Patient characteristics and setting |
| ||
| Index tests |
| ||
| Target condition and reference standard(s) |
| ||
| Flow and timing |
| ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the 18F‐flutemetamol PET scan interpretation done by a trained reader physician? | Unclear | ||
| Did the study provide a clear definition of what was considered to be a 18F‐flutemetamol positive result? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Was the study with 18F‐flutemetamol free of commercial funding? | No | ||
| High | |||
Aβ: Amyloid Beta
APOE ϵ4: Apolipoprotein E4
ADD: Alzheimer's disease dementia
ANC: Anterior cingulate
CAC: Clinical Adjudication Committee
CT: Computed tomography
EUDRACT: European Union Drug Regulating Authorities Clinical Trials
FN: False negative
FP: False positive
FRO: Frontal cortex
LTC: Lateral temporal cortex
MBq: Megabecquerel
mcg: Microgramme
MCI: Mild cognitive impairment
MMSE: Mini‐mental state examination
mSv: Millisievert
NINCDS‐ADRDA: National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association
OCC: Occipital cortex
PAR: Lateral parietal cortex
PET: Positron emission tomography
PON: Pons
ROI: Region of interest
SD: Standard deviation
SUVR: Standardised uptake value ratio
TN: True negative
TP: True positive
VOI: Volume of interest
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Target condition: not looking at progression from MCI to dementia. The focus of the study was the association of Aβ deposition and neuropsychiatric symptoms. | |
| Target condition: not looking at progression from MCI to dementia. The focus of the study was the change in 18F‐flutemetamol PET scan retention over time. | |
| Target condition: not looking at progression from MCI to dementia. The focus of the study was the change in 18F‐flutemetamol PET scan retention over time. | |
| Target condition: not looking at progression from MCI to dementia. The focus of the study was the change in 18F‐flutemetamol retention over time. |
Aβ: Amyloid beta
MCI: Mild cognitive impairment
PET: Positron emission tomography
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Surrogate markers evaluation in pre‐demented Alzheimer’s disease patients and healthy elderly controls |
| Target condition and reference standard(s) | Progression to Alzheimer's disease at the end of the clinical follow‐up period (from 1 to 3 years); no further details were given regarding the target condition(s) and the reference standard. |
| Index and comparator tests | 18F‐flutemetamol |
| Starting date | April 2012 |
| Contact information | Cliniques Universitaires Saint Luc, Nuclear Medicine Department, Dr R.Lhommel, [email protected] |
| Notes |
| Trial name or title | An open‐label study to compare the prognostic value of 18F‐flutemetamol PET scan imaging with longitudinal biomarker data in healthy volunteers and patients with mild cognitive impairment |
| Target condition and reference standard(s) | Progression to Alzheimer's disease and other dementias. No further details were given regarding the target condition and the reference standard(s) used. Included subjects will be followed clinically over at least four years. |
| Index and comparator tests | 18F‐flutemetamol |
| Starting date | February 2012 |
| Contact information | Skånes universitetssjukhus, Minneskliniken, |
| Notes |
| Trial name or title | Study to Identify factors associated with resilience to clinical dementia at old age ‐ 90+ study |
| Target condition and reference standard(s) | Developing ADD in extremely elderly subjects; no further details were given regarding the reference standard(s) used. |
| Index and comparator tests | 18F‐flutemetamol |
| Starting date | July 2016 |
| Contact information | Alzheimer Center, VU Medical Center |
| Notes |
| Trial name or title | The BioFINDER 2 study ‐ improved diagnostics and increased understanding of the pathophysiology of cognitive disorders |
| Target condition and reference standard(s) | Progression from subjective cognitive decline and MCI to ADD or other neurodegenerative disorders; no further details were given regarding the reference standard(s) used. |
| Index and comparator tests | 18F‐flutemetamol |
| Starting date | January 2017 |
| Contact information | Minneskliniken, Skåne University Hospital |
| Notes |
| Trial name or title | Clinical and neuroimaging study on preclinical Alzheimer's disease |
| Target condition and reference standard(s) | Progression rate from asymptomatic preclinical ADD to MCI and further to AD dementia at 36 months of follow‐up; no further details were given regarding the reference standard(s) used. |
| Index and comparator tests | 11C‐PiB, 18F‐florbetapir or 18F‐flutemetamol |
| Starting date | January 2016 |
| Contact information | Graduate School of medicine, Osaka City University, Center for Clinical study on dementia, Hiroshi Mori, [email protected]‐cu.ac.jp |
| Notes |
| Trial name or title | Longitudinal study of brain amyloid imaging in MEMENTO (MEMENTOAmyGing) |
| Target condition and reference standard(s) | Progression to clinical dementia stage according to standardized classifications (DSM‐IV and NINCDS‐ADRDA) at 2 years follow‐up |
| Index and comparator tests | 18F‐flutemetamol and 18F‐florbetapir at baseline |
| Starting date | June 2014 |
| Contact information | University Hospital, Bordeaux Prof. Geneviève Chene: [email protected]‐bordeaux2.fr Carole Dufouil: [email protected]‐bordeaux2.fr |
| Notes |
| Trial name or title | Amyloïd load in elderly population: effect of cognitive reserve (EDUMA) |
| Target condition and reference standard(s) | Prediction of cognitive decline and disease progression; no target condition or reference were prespecified. |
| Index and comparator tests | 18F‐flutemetamol |
| Starting date | July 2014 |
| Contact information | University Hospital, Bordeaux Michele Allard: michele.allard@chu‐bordeaux.fr |
| Notes |
ADD: Alzheimer's disease dementia
DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders (4th ed.)
MCI: Mild cognitive impairment
NINCDS‐ADRDA: National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association
Data
Presented below are all the data for all of the tests entered into the review.
| Test | No. of studies | No. of participants |
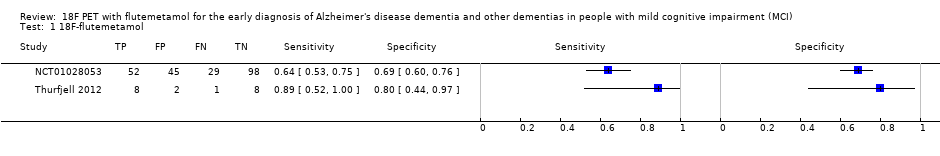
| 1 18F‐flutemetamol Show forest plot | 2 | 243 |
| Test 1  18F‐flutemetamol. | ||

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study
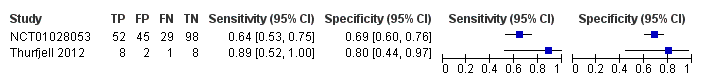
Forest plot of 18F‐flutemetamol.
| What is the diagnostic accuracy of 18F‐flutemetamol PET amyloid biomarker for predict progression to ADD in people with MCI? | |||||||
| Descriptive | |||||||
| Patient population | Participants diagnosed with MCI at the time of performing the test using any of the Petersen criteria or Winblad criteria or CDR = 0.5 or any of the 16 definitions included by Matthews (Matthews 2008). | ||||||
| Sources of referral | Not reported (n = 2) | ||||||
| MCI criteria | Petersen criteria (n = 2) | ||||||
| Sampling procedure | Unclear (n = 2) | ||||||
| Prior testing | The only testing prior to performing the 18F‐flutemetamol PET amyloid biomarker was the application of diagnostic criteria for identifying participants with MCI | ||||||
| Settings | Secondary care (n = 1) Not reported (n = 1) | ||||||
| Index test | 18F‐flutemetamol PET | ||||||
| Threshold pre‐specified at baseline | Yes (n=1) Unclear (n=1) | ||||||
| Threshold interpretation | Visual (n = 1) Quantitative (n = 1) | ||||||
| Thershold | SUVR (Standardised Uptake Volume ratio) of ROI: > 1.5 (n = 1) Not specified: analytical visual approach of ROI: (n = 1) | ||||||
| 18F‐flutemetamol retention region | Global cortex (n = 1) Not reported (n = 1) | ||||||
| Reference Standard | For Alzheimer’s disease dementia: NINCDS‐ADRDA (n = 1) Unclear (n = 1) | ||||||
| Target condition | Progression from MCI to Alzheimer’s disease dementia or any other forms of dementia (non‐ADD) or any form of dementia | ||||||
| Included studies | Prospectively well‐defined cohorts with any accepted definition of MCI (as above). Two studies (N = 252 participants) were included. Number of participants included in analysis: 243 | ||||||
| Quality concerns | Patient selection and index test QUADAS‐2 domain: unclear risk of bias Reference standard domain: low risk of bias Flow and timing domain: high risk of bias There were unclear concerns about applicability in the patient selection and index test domain. Patient selection and index test QUADAS‐2 domain: low risk of bias Reference standard domain: unclear risk of bias Flow and timing domain: high risk of bias. There was unclear concern about applicability in the reference standard domain. | ||||||
| Limitations | Limited investigation of heterogeneity and sensitivity analysis due to an insufficient number of studies. We were unable to evaluate progression from MCI to any other form of dementia (non‐ADD) or any form of dementia due to lack of included studies. | ||||||
| Test | Studies | Cases/Participants | Sensitivity | Specificity | Consequences in a cohort of 100 | ||
| Proportion converting1 | Missed cases2 | Overdiagnosed2 | |||||
| Alzheimer's disease dementia | |||||||
| 18F‐flutemetamol with visual assessment | 1 | 81/224 | 64% (95% CI 53% to 75%) | 69% (95% CI 60% to 76%) | 36 | 13 | 20 |
| 18F‐flutemetamol with SUVR | 1 | 9/19 | 89% (95% CI 52% to 100%) | 80% (95% CI 44% to 97%) | 47 | 5 | 11 |
| Investigation of heterogeneity and sensitivity analysis: The planned investigations were not possible due to the limited number of studies available for each analysis. | |||||||
| Conclusions:18F‐flutemetamol PET scan is not an accurate test for detecting progression from MCI to Alzheimer’s disease dementia. The strength of the evidence was weak because of considerable variation in study methods, unclear methodological quality due to poor reporting, and high risk of bias due to possible conflict of interest. There is a need for conducting studies using standardised 18F‐flutemetamol PET scan methodology in larger populations. | |||||||
| 1. Proportion converting to ADD in each included study 2. Missed and overdiagnosed numbers were computed using the proportion converting to the target condition. ADD: Alzheimer's disease dementia QUADAS‐2: Quality Assessment of Diagnostic Accuracy Studies‐2 | |||||||
| Test | No. of studies | No. of participants |
| 1 18F‐flutemetamol Show forest plot | 2 | 243 |