18F PET s flutemetamolom u ranoj dijagnostici Alzheimerove demencije i drugih demencija u osoba s umjerenim kognitivnim oštećenjem (MCI)
Appendices
Appendix 1. Glossary
Aetiology: the cause, set of causes, or manner of causation of a disease or condition.
Amyloid beta (Aβ): an amyloid that is derived from a larger precursor protein and is the primary component of plaques characteristic of Alzheimer's disease.
Biomarker: measurable and quantifiable biological parameters (e.g., specific enzyme concentration, specific hormone concentration, presence of biological substances) which serve as indices for health‐ and physiology‐related assessments, such as disease risk, psychiatric disorders, environmental exposure and its effects, disease diagnosis; metabolic processes; etc.
Bolus: a single dose of a drug or other medicinal preparation given all at once.
Cingulate cortex: one of the convolutions on the medial surface of the cerebral hemispheres.
Cortical: the thin layer of grey matter on the surface of the cerebral hemispheres. It reaches its highest development in humans and is responsible for intellectual faculties and higher mental functions.
Epiphenomenon: A secondary effect or by‐product. A secondary symptom or pathology, occurring simultaneously with a disease or condition but not directly related to it.
Frontotemporal: relating to the frontal and the temporal cerebral lobes.
Histopathology: the study of changes in tissues caused by disease.
Hypothyroidism: a syndrome that results from abnormally low secretion of thyroid hormones from the thyroid gland.
Index test: the test under evaluation.
In vivo: (of processes) performed or taking place in a living organism.
Ligand: a molecule that binds to another molecule, used especially to refer to a small molecule that binds specifically to a larger molecule, e.g., an antigen binding to an antibody, a hormone or neurotransmitter binding to a receptor, or a substrate or allosteric effector binding to an enzyme.
Neuritic plaques: accumulations of extracellularly deposited amyloid fibrils within tissues. Is one of the hallmarks of Alzheimer's disease.
Neurofibrillary tangles: abnormal structures located in various parts of the brain and composed of dense arrays of paired helical filaments (neurofilaments and microtubules). Are aggregates of hyperphosphorylated tau protein that are most commonly known as a primary marker of Alzheimer's disease.
Parietal lobe: upper central part of the cerebral hemisphere. It is located anterior to the occipital lobe, and superior to the temporal lobes.
Positron: an extremely small piece of matter with a positive electrical charge, having the same mass as an electron.
Precuneus: is a part of the parietal lobe of the brain, lying on the medial surface of the cerebral hemisphere.
Prodromal: Relating to prodrome; indicating an early stage of a disease.
Radionuclide (sometimes called a radioisotope or isotope): is a chemical which emits a type of radioactivity called gamma rays. The radioactivity can be detected by special scanners.
Reference standard: the best available method for establishing the presence or absence of the target condition.
Sensitivity: a measure of a test’s ability to correctly detect people with the disease. It is the proportion of diseased cases that are correctly identified by the test. It is calculated as follows: Sensitivity = Number with disease who have a positive test/Number with disease.
Specificity: a measure of a test’s ability to correctly identify people who do not have the disease. It is the proportion of people without the target disease who are correctly identified by the test. It is calculated as follows: Specificity = Number without disease who have a negative test/Number without disease.
Stilbene: organic compounds that contain 1,2‐diphenylethylene as a functional group.
Target condition: the disease or condition that the index test is expected to detect.
Temporal lobe: lower lateral part of the cerebral hemisphere responsible for auditory, olfactory, and semantic processing. It is located inferior to the lateral fissure and anterior to the occipital lobe.
Vascular: relating to, affecting, or consisting of a vessel or vessels, especially those which carry blood.
Appendix 2. Search strategy for 18F‐flutemetamol PET ligand
| Source | Search strategy |
| MEDLINE In‐process and other non‐indexed citations and MEDLINE® 1946 to May 2017 (Ovid SP) | 1. Flutemetamol.ti,ab,nm. 2. (VIZAMYL or vizamyl*).ti,ab,nm. 3. "flutemetamol‐fluorine‐18".ti,ab,nm. 4. "18F‐GE067".ti,ab,nm. 5. "[18F]Flutemetamol".ti,ab,nm. 6. "flutemetamol‐PET".ti,ab,nm. 7. or/1‐6 8. Fluorine Radioisotopes/du 19. 7 or 18 |
| Embase 1974 to May 2017 (Ovid SP) | 1. Flutemetamol.ti,ab. 2. (VIZAMYL or vizamyl*).ti,ab. 3. "flutemetamol‐fluorine‐18".ti,ab. 4. "18F‐GE067".ti,ab. 5. "[18F]Flutemetamol".ti,ab. 6. "flutemetamol‐PET".ti,ab. 7. exp flutemetamol f 18/ 8. or/1‐7 9. exp *radioligand/ |
| PsycINFO 1806 to May 2017 (Ovid SP) | 1. Flutemetamol.ti,ab. 2. (VIZAMYL or vizamyl*).ti,ab. 3. "flutemetamol‐fluorine‐18".ti,ab. 4. "18F‐GE067".ti,ab. 5. "[18F]Flutemetamol".ti,ab. 6. "flutemetamol‐PET".ti,ab. 7. or/1‐6 |
| BIOSIS Citation Index (Thomson Reuters Web of Science) (1922 to May 2017) | Topic=(Flutemetamol OR VIZAMYL OR vizamyl* OR "flutemetamol‐fluorine‐18" OR "18F‐GE067" OR "[18F]Flutemetamol" OR "flutemetamol‐PET") Timespan=All years. Databases=BCI |
| Web of Science Core Collection, including the Science Citation Index and the Conference Proceedings Citation Index (Thomson Reuters Web of Science) (1946 to May 2017) | Topic=(Flutemetamol OR VIZAMYL OR vizamyl* OR "flutemetamol‐fluorine‐18" OR "18F‐GE067" OR "[18F]Flutemetamol" OR "flutemetamol‐PET") Timespan=All years. Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, CCR‐EXPANDED, IC. |
| LILACS (BIREME) | Flutemetamol OR VIZAMYL OR vizamyl* OR "flutemetamol‐fluorine‐18" OR "18F‐GE067" OR "[18F]Flutemetamol" OR "flutemetamol‐PET" [Words] |
| CINAHL (EBSCOhost) (1980 to May 2017) | S1 TX Flutemetamol S2 TX VIZAMYL S3 TX vizamyl* S4 TX "flutemetamol‐fluorine‐18" S5 TX "18F‐GE067" S6 TX "[18F]Flutemetamol" S7 TX "flutemetamol‐PET" S8 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 |
| ClinicalTrials.gov (www.clinicaltrials.gov) | Flutemetamol OR VIZAMYL OR vizamyl* OR "flutemetamol‐fluorine‐18" OR "18F‐GE067" OR "[18F]Flutemetamol" OR "flutemetamol‐PET" |
| World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (http://apps.who.int/trialsearch) | Flutemetamol OR VIZAMYL OR vizamyl OR "flutemetamol‐fluorine‐18" OR "18F‐GE067" OR "[18F]Flutemetamol" OR "flutemetamol‐PET" |
| ALOIS, the Cochrane Dementia & Cognitive Improvement Group’s specialized register of dementia studies (http://www.medicine.ox.ac.uk/alois/) | Imaging AND PET |
Appendix 3. Tables (2 × 2) cross‐relating index test results of the reference standards
Table 1. Progression from mild cognitive impairment (MCI) to Alzheimer’s disease dementia (ADD)
| Index test information | Reference standards information | |
| ADD present | ADD absent | |
| Index test‐positive | 18F‐flutemetamol PET ligand for Aβ (+) who progress to ADD (TP) | 18F‐flutemetamol PET ligand for Aβ (+) who remain MCI (FP) and 18F‐flutemetamol PET ligand for Aβ (+) who progress to non‐ADD (FP) |
| Index test‐negative | 18F‐flutemetamol PET ligand for Aβ (‐) who progress to ADD (FN) | 18F‐flutemetamol PET ligand for Aβ (‐) who remain MCI (TN) and 18F‐flutemetamol PET ligand for Aβ (‐) who progress to non‐ADD (TN) |
ADD: Alzheimer's disease dementia
FN: False negative
FP: False positive
MCI: Mild cognitive impairment
PET: Positron emission tomography
TN: True negative
TP: True positive
Table 2. Progression from mild cognitive impairment (MCI) to non‐Alzheimer’s disease dementia (non‐ADD)
| Index test information | Reference standards information | |
| Non‐ADD present | Non‐ADD absent | |
| Index test‐positive | 18F‐flutemetamol PET ligand for Aβ (+) who progress to non‐ADD (TP) | 18F‐flutemetamol PET ligand for Aβ (+) who remain MCI (FP) and 18F‐flutemetamol PET ligand Aβ (+) who progress to ADD (FP) |
| Index test‐negative | 18F‐flutemetamol PET ligand for Aβ (‐) who progress to non‐ADD (FN) | 18F‐flutemetamol PET ligand for Aβ (‐) who remain MCI (TN) and 18F‐flutemetamol PET ligand for Aβ (‐) who progress to ADD (TN) |
ADD: Alzheimer's disease dementia
FN: False negative
FP: False positive
MCI: Mild cognitive impairment
PET: Positron emission tomography
TN: True negative
TP: True positive
Table 3. Progression from mild cognitive impairment (MCI) to any form of dementia
| Index test information | References standard information | |
| Any forms of dementia present | Dementia absent | |
| Index test‐positive | 18F‐flutemetamol PET ligand for Aβ (+) who progress to any form of dementia (TP) | 18F‐flutemetamol PET ligand for Aβ (+) who remain MCI (FP) |
| Index test‐negative | 18F‐flutemetamol PET ligand for Aβ (‐) who progress to any form of dementia (FN) | 18F‐flutemetamol PET ligand for Aβ (‐) who remain MCI (TN) |
FN: False negative
FP: False positive
MCI: Mild cognitive impairment
PET: Positron emission tomography
TN: True negative
TP: True positive
Appendix 4. Assessment of methodological quality table: Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS‐2) tool
| Domain | Patient selection | Index test | Reference standard | Flow and timing |
| Description | Describe methods of patient selection: describe included patients (prior testing, presentation, intended use of index test and setting) | Describe the index test and how it was conducted and interpreted | Describe the reference standard and how it was conducted and interpreted | Describe any patients who did not receive the index test(s) or reference standard, or both, or who were excluded from the 2×2 table (refer to flow diagram): describe the time interval and any interventions |
| Signalling questions (yes/no/unclear) | Was a consecutive or random sample of patients enrolled? | Were the index test results interpreted without knowledge of the results of the reference standard? | Is the reference standard likely to correctly classify the target condition? | Was there an appropriate interval between index test(s) and reference standard? |
| Was a case‐control design avoided? | If a threshold was used, was it prespecified? | Were the reference standard results interpreted without knowledge of the results of the index test? | Did all patients receive a reference standard? | |
| Did the study avoid inappropriate exclusions? | Did all patients receive the same reference standard? | |||
| Were all patients included in the analysis? | ||||
| Risk of bias (high/low/unclear) | Could the selection of patients have introduced bias? | Could the conduct or interpretation of the index test have introduced bias? | Could the reference standard, its conduct, or its interpretation have introduced bias? | Could the patient flow |
| Concerns regarding | Are there concerns that the included patients do not match the review question? | Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Are there concerns that the target condition as defined by the reference standard does not match the review question? |
Appendix 5. Anchoring statements for quality assessment of 18F‐flutemetamol PET scan for Aβ diagnostic studies
Table 4. Review question and inclusion criteria
| Category | Review question | Inclusion criteria |
| Patients | Participants with mild cognitive impairment (MCI), no dementia | Participants that fulfil the criteria for the clinical diagnosis of MCI at baseline |
| Index test | 18F‐flutemetamol PET ligand for Aβ biomarker | 18F‐flutemetamol PET ligand for Aβ biomarker |
| Target condition | Alzheimer’s disease dementia (ADD) (progression from MCI to ADD) | ADD (progression from MCI to ADD) |
| Reference standard | NINCDS‐ADRDA; DSM; ICD; McKeith criteria; Lund criteria; International Behavioural Variant FTD Criteria Consortium; NINDS‐ARIEN criteria | NINCDS‐ADRDA; DSM; ICD; McKeith criteria; Lund criteria; International Behavioural Variant FTD Criteria Consortium; NINDS‐ARIEN criteria |
| Outcome | N/A | Data to construct a 2 × 2 table |
| Study design | N/A | Longitudinal cohort studies and nested case‐control studies if they incorporate a delayed verification design (case‐control nested in cohort studies) |
ADD: Alzheimer's disease dementia
DSM: Diagnostic and Statistical Manual of Mental Disorders
FTD: Frontotemporal dementia
ICD: International Classification of Diseases
MCI: Mild cognitive impairment
NINCDS‐ADRDA: National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association
NINDS‐AIREN: National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherché et l'Enseignement en Neurosciences
PET: Positron emission tomography
Anchoring statements for quality assessment 18F‐flutemetamol PET ligand for Aβ diagnostic studies
We have provided some core anchoring statements for quality assessment in the diagnostic test accuracy (DTA) review of the 18F‐flutemetamol PET ligand for Aβ biomarker in dementia. These statements are designed for use with the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS‐2) tool and are based on the guidance for quality assessment of DTA reviews of Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) in dementia (Quinn 2014). In assessing individual items, the score of unclear should only be given if there is genuine uncertainty. In these situations, we contacted the relevant study teams for additional information. Whenever we scored one question as high risk of bias, we considered the study as having a high risk of bias.
Table 5. Anchoring statements to assist with the 'Risk of bias' assessment
| Question | Response and weighting | Explanation |
| Patient selection | ||
| Was the sampling method appropriate? | No = high risk of bias | Where sampling is used, the designs least likely to cause bias are consecutive sampling or random sampling. Sampling that is based on volunteers or selecting subjects from a clinic or research resource is prone to bias. |
| Was a case‐control or similar design | No = high risk of bias | Designs similar to case‐control that may introduce bias are those designs where the study team deliberately increase or decrease the proportion of subjects with the target condition, which may not be representative. Some case‐control methods may already be excluded if they mix subjects from various settings. |
| Are exclusion criteria described and appropriate? | No = high risk of bias | We automatically graded the study as unclear if the study authors did not detail exclusions (pending contact with study authors). Where a study details exclusions, we graded the study as 'low risk' if we considered exclusions to be appropriate. Certain exclusions common to many studies of dementia are: medical instability; terminal disease; alcohol/substance misuse; concomitant psychiatric diagnosis; other neurodegenerative conditions. Exclusions are not appropriate if they comprise ‘difficult to diagnose’ patients. We labelled post‐hoc and inappropriate exclusions as at 'high risk' of bias. |
| Index test | ||
| Was the 18F‐flutemetamol PET ligand for Aβ biomarker's assessment/interpretation performed without knowledge of clinical dementia diagnosis? | No = high risk of bias | Terms such as 'blinded' or 'independently and without knowledge of' are sufficient and full details of the blinding procedure are not required. Interpretation of the results of the index test may be influenced by knowledge of the results of the reference standard. If the index test is always interpreted prior to the reference standard, then the person interpreting the index test cannot be aware of the results of the reference standard and so this item could be rated as ‘yes’. |
| Was the 18F‐flutemetamol PET ligand for Aβ biomarker's threshold prespecified? | No = high risk of bias | For scales and biomarkers, there is often a reference point (in units or categories) above which subjects are classified as 'test‐positive'; this may be referred to as the threshold, clinical cut‐off, or dichotomisation point. A study is classified at high risk of bias if the study authors define the optimal cut‐off post‐hoc based on their own study data because selecting the threshold to maximise sensitivity and/or specificity may lead to overoptimistic measures of test performance. Certain papers may use an alternative methodology for analysis that does not use thresholds and these papers should be classified as not applicable. |
| Was the 18F‐flutemetamol PET ligand for Aβ scan interpretation done by a trained reader physician? | No = high risk of bias | If a trained reader physician performed the scan interpretation, we scored this item as ’yes’. If no definition of trained reader was done, we scored this item as ’unclear’. If a nontrained reader physician performed the scan interpretation, we scored this item as ’no’. |
| Did the study provide a clear definition of what was considered to be a 18F‐flutemetamol PET ligand for Aβ biomarker’s positive result? | No = high risk of bias | If the study clearly stated the definition of a positive result (e.g. SUV), we scored this item as ’yes’. If the study did not give a definition of what it considered a positive result or the definition of a positive result varied between the participants, we scored this item as ’no’. If the study gave insufficient information to permit judgement, we scored the item as ’unclear’. |
| Reference standard | ||
| Is the assessment used for clinical diagnosis | No = high risk of bias | Commonly used international criteria to assist with clinical diagnosis of dementia included those detailed in DSM‐IV and ICD‐10. |
| Were clinical assessments for dementia performed without knowledge of the 18F‐flutemetamol PET ligand for Aβ biomarker? | No = high risk of bias | Terms such as 'blinded' or 'independently and without knowledge of' were sufficient and full details of the blinding procedure were not required. Interpretation of the results of the reference standard may be influenced by knowledge of the results of the index test. |
| Patient flow | ||
| Was there an appropriate interval between 18F‐flutemetamol PET ligand for Aβ biomarker and clinical dementia assessment? | No = high risk of bias | As we test the accuracy of the 18F‐flutemetamol PET ligand for Aβ biomarker for MCI progression to dementia, there will always be a delay between the index test and the reference standard assessments. The time between the reference standard and the index test will influence the accuracy (Geslani 2005; Okello 2007; Visser 2006), and therefore we noted time as a separate variable (both within and between studies) and will test its influence on the diagnostic accuracy. We have set a minimum mean time to follow‐up assessment of 1 year. If more than 16% of subjects have assessment for MCI progression before nine months, this item was scored ‘no’. |
| Did all subjects get the same assessment for | No = high risk of bias | There may be scenarios where participants who score 'test‐positive' on the index test have a more detailed assessment. Where dementia assessment differs between participants, this should be classified as high risk of bias. |
| Were all patients who received 18F‐flutemetamol PET ligand for Aβ biomarker’s assessment included in the final | No = high risk of bias | If the number of patients enrolled differs from the number of patients included in the 2 × 2 table, then there is the potential for bias. If patients lost to dropouts differ systematically from those who remain, then estimates of test performance may differ. |
| Were missing 18F‐flutemetamol PET ligand for Aβ biomarker's results reported? | No = high risk of bias | Where missing or uninterpretable results are reported, and if there is substantial attrition (we have set an arbitrary value of 50% missing data), we will score this as ‘no’. If the study did not report these results, we scored this as ‘unclear’ and we contacted the study authors. |
| Was the study with 18F‐flutemetamol PET ligand for Aβ biomarker free of commercial funding? | No = high risk of bias | If the funding source is clearly stated and is not commercial, this should be scored as ‘no’. If the funding source is clearly stated and is commercial, this should be scored as ’yes ’. If not enough information is given to assess whether the funding source is commercial, the scored is ’unclear’. |
| Anchoring statements to assist with assessment for applicability | ||
| Question | Explanation | |
| Were included patients representative of | The included patients should match the intended population as described in the review question. The review authors should consider population in terms of symptoms; pretesting; potential disease prevalence; setting. If there is a clear ground for suspecting an unrepresentative spectrum, the item should be rated poor applicability. | |
| Index test | ||
| Were sufficient data on 18F‐flutemetamol PET ligand for Aβ biomarker’s application given for the test to be repeated in an independent study? | Variation in technology, test execution, and test interpretation may affect estimate of accuracy. In addition, the background, and training/expertise of the assessor should be reported and taken in consideration. If 18F‐flutemetamol PET ligand for Aβ biomarker was not performed consistently, this item should be rated poor applicability. | |
| Reference standard | ||
| Was clinical diagnosis of dementia made in a manner similar to current clinical practice? | For many reviews, inclusion criteria and 'Risk of bias' assessments will already have assessed the dementia diagnosis. For certain reviews, an applicability statement relating to the reference standard may not be applicable. There is the possibility that a form of dementia assessment, although valid, may diagnose a far larger proportion of people with disease than usual clinical practice. In this instance, the item should be rated poor applicability. | |
DSM: Diagnostic and Statistical Manual of Mental Disorders
FTD: Frontotemporal dementia
ICD: International Classification of Diseases
MCI: Mild cognitive impairment
NINCDS‐ADRDA: National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association
NINDS‐AIREN: National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherché et l'Enseignement en Neurosciences
PET: Positron emission tomography

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study
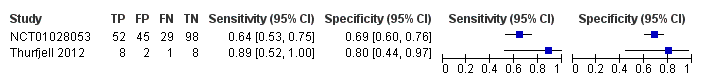
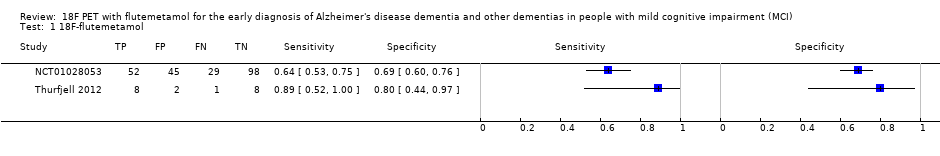
Forest plot of 18F‐flutemetamol.
| What is the diagnostic accuracy of 18F‐flutemetamol PET amyloid biomarker for predict progression to ADD in people with MCI? | |||||||
| Descriptive | |||||||
| Patient population | Participants diagnosed with MCI at the time of performing the test using any of the Petersen criteria or Winblad criteria or CDR = 0.5 or any of the 16 definitions included by Matthews (Matthews 2008). | ||||||
| Sources of referral | Not reported (n = 2) | ||||||
| MCI criteria | Petersen criteria (n = 2) | ||||||
| Sampling procedure | Unclear (n = 2) | ||||||
| Prior testing | The only testing prior to performing the 18F‐flutemetamol PET amyloid biomarker was the application of diagnostic criteria for identifying participants with MCI | ||||||
| Settings | Secondary care (n = 1) Not reported (n = 1) | ||||||
| Index test | 18F‐flutemetamol PET | ||||||
| Threshold pre‐specified at baseline | Yes (n=1) Unclear (n=1) | ||||||
| Threshold interpretation | Visual (n = 1) Quantitative (n = 1) | ||||||
| Thershold | SUVR (Standardised Uptake Volume ratio) of ROI: > 1.5 (n = 1) Not specified: analytical visual approach of ROI: (n = 1) | ||||||
| 18F‐flutemetamol retention region | Global cortex (n = 1) Not reported (n = 1) | ||||||
| Reference Standard | For Alzheimer’s disease dementia: NINCDS‐ADRDA (n = 1) Unclear (n = 1) | ||||||
| Target condition | Progression from MCI to Alzheimer’s disease dementia or any other forms of dementia (non‐ADD) or any form of dementia | ||||||
| Included studies | Prospectively well‐defined cohorts with any accepted definition of MCI (as above). Two studies (N = 252 participants) were included. Number of participants included in analysis: 243 | ||||||
| Quality concerns | Patient selection and index test QUADAS‐2 domain: unclear risk of bias Reference standard domain: low risk of bias Flow and timing domain: high risk of bias There were unclear concerns about applicability in the patient selection and index test domain. Patient selection and index test QUADAS‐2 domain: low risk of bias Reference standard domain: unclear risk of bias Flow and timing domain: high risk of bias. There was unclear concern about applicability in the reference standard domain. | ||||||
| Limitations | Limited investigation of heterogeneity and sensitivity analysis due to an insufficient number of studies. We were unable to evaluate progression from MCI to any other form of dementia (non‐ADD) or any form of dementia due to lack of included studies. | ||||||
| Test | Studies | Cases/Participants | Sensitivity | Specificity | Consequences in a cohort of 100 | ||
| Proportion converting1 | Missed cases2 | Overdiagnosed2 | |||||
| Alzheimer's disease dementia | |||||||
| 18F‐flutemetamol with visual assessment | 1 | 81/224 | 64% (95% CI 53% to 75%) | 69% (95% CI 60% to 76%) | 36 | 13 | 20 |
| 18F‐flutemetamol with SUVR | 1 | 9/19 | 89% (95% CI 52% to 100%) | 80% (95% CI 44% to 97%) | 47 | 5 | 11 |
| Investigation of heterogeneity and sensitivity analysis: The planned investigations were not possible due to the limited number of studies available for each analysis. | |||||||
| Conclusions:18F‐flutemetamol PET scan is not an accurate test for detecting progression from MCI to Alzheimer’s disease dementia. The strength of the evidence was weak because of considerable variation in study methods, unclear methodological quality due to poor reporting, and high risk of bias due to possible conflict of interest. There is a need for conducting studies using standardised 18F‐flutemetamol PET scan methodology in larger populations. | |||||||
| 1. Proportion converting to ADD in each included study 2. Missed and overdiagnosed numbers were computed using the proportion converting to the target condition. ADD: Alzheimer's disease dementia QUADAS‐2: Quality Assessment of Diagnostic Accuracy Studies‐2 | |||||||
| Test | No. of studies | No. of participants |
| 1 18F‐flutemetamol Show forest plot | 2 | 243 |