Uso de TEP con flutemetamol F 18 para el diagnóstico temprano de la demencia de la enfermedad de Alzheimer y otras demencias en pacientes con deterioro cognitivo leve (DCL)
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012884Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 22 noviembre 2017see what's new
- Tipo:
-
- Diagnostic
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Demencia y trastornos cognitivos
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
-
Gabriel Martínez, Robin WM Vernooij, and Paulina Fuentes Padilla: contributed to conception, design, and draft of the protocol; overall responsibility of study selection; data extraction; contacted the authors; draft of discussion; and authors’ conclusion sections.
-
Leon Flicker: contributed to conception, and designed and reviewed the draft protocol and final manuscript.
-
Xavier Bonfill Cosp: reviewed the draft protocol and final manuscript.
-
Javier Zamora: designed and drafted the protocol, performed statistical analyses, updated the statistical methods section and finalised the manuscript.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
This protocol was supported by the NIHR, via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed therein are those of the protocol authors and do not necessarily reflect those of the Systematic Reviews Programme, the NIHR, the NHS, or the Department of Health
Declarations of interest
-
Gabriel Martínez has no known conflicts of interest.
-
Leon Flicker has no known conflicts of interest.
-
Robin WM Vernooij has no known conflicts of interest.
-
Paulina Fuentes Padilla has no known conflicts of interest.
-
Javier Zamora has no known conflicts of interest.
-
Xavier Bonfill Cosp has no known conflicts of interest.
Acknowledgements
Gabriel Martínez is a PhD candidate in Methodology of Biomedical Research and Public Health at the Department of Paediatrics, Obstetrics and Gynaecology and Preventive Medicine, Universitat Autònoma de Barcelona, Barcelona, Spain.
We are grateful to the authors of included and excluded studies who responded to our requests for additional information.
We thank the Cochrane Dementia and Cognitive Improvement Group (CDCIG) for strong support, especially Sue Marcus in finalizing the review.
We thank Anna Noel‐Storr, Information Specialist of the CDCIG, for her assistance with the design of the search strategy.
We thank Gerard Urrútia and Marta Roqué i Figuls for their contribution in the preparation of the protocol for the review (Martínez 2016)
We thank the peer reviewers for their many helpful suggestions.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Nov 22 | 18F PET with flutemetamol for the early diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI) | Review | Gabriel Martínez, Robin WM Vernooij, Paulina Fuentes Padilla, Javier Zamora, Leon Flicker, Xavier Bonfill Cosp | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- *Positron‐Emission Tomography;
- Alzheimer Disease [*diagnostic imaging, metabolism];
- Amyloid;
- Aniline Compounds [economics, *pharmacokinetics];
- Benzothiazoles [economics, *pharmacokinetics];
- Biomarkers;
- Cognitive Dysfunction [complications, *diagnostic imaging, metabolism];
- Confidence Intervals;
- Disease Progression;
- Early Diagnosis;
- False Negative Reactions;
- False Positive Reactions;
- Follow‐Up Studies;
- Radiopharmaceuticals [economics, *pharmacokinetics];
- Reference Standards;
- Sensitivity and Specificity;
- Time Factors;
Medical Subject Headings Check Words
Aged; Female; Humans; Male;

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study
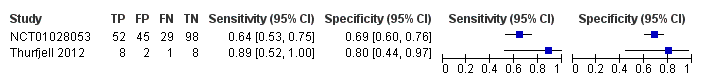
Forest plot of 18F‐flutemetamol.
| What is the diagnostic accuracy of 18F‐flutemetamol PET amyloid biomarker for predict progression to ADD in people with MCI? | |||||||
| Descriptive | |||||||
| Patient population | Participants diagnosed with MCI at the time of performing the test using any of the Petersen criteria or Winblad criteria or CDR = 0.5 or any of the 16 definitions included by Matthews (Matthews 2008). | ||||||
| Sources of referral | Not reported (n = 2) | ||||||
| MCI criteria | Petersen criteria (n = 2) | ||||||
| Sampling procedure | Unclear (n = 2) | ||||||
| Prior testing | The only testing prior to performing the 18F‐flutemetamol PET amyloid biomarker was the application of diagnostic criteria for identifying participants with MCI | ||||||
| Settings | Secondary care (n = 1) Not reported (n = 1) | ||||||
| Index test | 18F‐flutemetamol PET | ||||||
| Threshold pre‐specified at baseline | Yes (n=1) Unclear (n=1) | ||||||
| Threshold interpretation | Visual (n = 1) Quantitative (n = 1) | ||||||
| Thershold | SUVR (Standardised Uptake Volume ratio) of ROI: > 1.5 (n = 1) Not specified: analytical visual approach of ROI: (n = 1) | ||||||
| 18F‐flutemetamol retention region | Global cortex (n = 1) Not reported (n = 1) | ||||||
| Reference Standard | For Alzheimer’s disease dementia: NINCDS‐ADRDA (n = 1) Unclear (n = 1) | ||||||
| Target condition | Progression from MCI to Alzheimer’s disease dementia or any other forms of dementia (non‐ADD) or any form of dementia | ||||||
| Included studies | Prospectively well‐defined cohorts with any accepted definition of MCI (as above). Two studies (N = 252 participants) were included. Number of participants included in analysis: 243 | ||||||
| Quality concerns | Patient selection and index test QUADAS‐2 domain: unclear risk of bias Reference standard domain: low risk of bias Flow and timing domain: high risk of bias There were unclear concerns about applicability in the patient selection and index test domain. Patient selection and index test QUADAS‐2 domain: low risk of bias Reference standard domain: unclear risk of bias Flow and timing domain: high risk of bias. There was unclear concern about applicability in the reference standard domain. | ||||||
| Limitations | Limited investigation of heterogeneity and sensitivity analysis due to an insufficient number of studies. We were unable to evaluate progression from MCI to any other form of dementia (non‐ADD) or any form of dementia due to lack of included studies. | ||||||
| Test | Studies | Cases/Participants | Sensitivity | Specificity | Consequences in a cohort of 100 | ||
| Proportion converting1 | Missed cases2 | Overdiagnosed2 | |||||
| Alzheimer's disease dementia | |||||||
| 18F‐flutemetamol with visual assessment | 1 | 81/224 | 64% (95% CI 53% to 75%) | 69% (95% CI 60% to 76%) | 36 | 13 | 20 |
| 18F‐flutemetamol with SUVR | 1 | 9/19 | 89% (95% CI 52% to 100%) | 80% (95% CI 44% to 97%) | 47 | 5 | 11 |
| Investigation of heterogeneity and sensitivity analysis: The planned investigations were not possible due to the limited number of studies available for each analysis. | |||||||
| Conclusions:18F‐flutemetamol PET scan is not an accurate test for detecting progression from MCI to Alzheimer’s disease dementia. The strength of the evidence was weak because of considerable variation in study methods, unclear methodological quality due to poor reporting, and high risk of bias due to possible conflict of interest. There is a need for conducting studies using standardised 18F‐flutemetamol PET scan methodology in larger populations. | |||||||
| 1. Proportion converting to ADD in each included study 2. Missed and overdiagnosed numbers were computed using the proportion converting to the target condition. ADD: Alzheimer's disease dementia QUADAS‐2: Quality Assessment of Diagnostic Accuracy Studies‐2 | |||||||
| Test | No. of studies | No. of participants |
| 1 18F‐flutemetamol Show forest plot | 2 | 243 |