Efectividad comparativa del tratamiento continuado y de mantenimiento para el trastorno depresivo persistente en adultos
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Design: RCT Phases: acute (12 weeks), continuation (16 weeks), maintenance (52 weeks) Comparison groups: nefazodone vs placebo Funded by: Bristol‐Myers Squibb | |
| Participants | Number of participants randomized (NRCT: number of participants included): 160 Criteria for relapse/recurrence: "If depressive symptoms began to emerge, as evidenced by a HAM‐D‐24 score of 16 or greater, another evaluation was scheduled within 2 weeks. Evaluations continued every 2 weeks until either the symptoms subsided or recurrence criteria were met. Recurrence was defined as a HAM‐D‐24 score of 16 or greater, together with a diagnosis of MDD as determined from a DSM‐IV MDD checklist administered by the independent evaluator, on two consecutive visits. At the second of these visits, the recurrence also needed confirmation by each site's senior investigator based on a clinical interview. In addition, because some patients had elevated HAM‐D‐24 scores but did not meet MDD criteria, or discontinued before the confirmatory visit, a committee of senior investigators conducted a blinded review of all patient data at the end of the study. Recurrence was declared if there was consensus among the committee that an episode of MDD had occurred. The committee also indicated the date of onset of the recurrence. The final definition of time‐to‐recurrence was based on the first recurrence declared by either one of the two methods to define recurrence." (p. 809) Age distribution in sample (mean): nefazodone: 44.4 (SD 11.1), placebo: 44.1 (SD 8.4) Sex distribution in sample (% women): nefazodone 69.7; placebo 65.5 Diagnoses in sample: nefazodone: 34.2% chronic major depressive disorder, 36.8% double depression, 29.0% recurrent depressive disorder without complete remission between episodes; placebo: 28.6% chronic major depressive disorder, 42.9% double depression, 28.6% recurrent depressive disorder without complete remission between episodes Depression severity at continuation/maintenance baseline (mean): HAM‐D‐24 nefazodone: 5.9 (SD 4.4); placebo: 5.6 (SD 4.0) Age of onset (mean): nefazodone: 24.1 (SD 13.3) years; placebo: 27.7 (SD 12.7) years Length current/last major episode (mean): nefazodone: 100.8 (SD 129.6) months; placebo: 87.6 (SD 90.0) months | |
| Interventions | Maintenance treatment (52 weeks) Nefazodone (participants = 76) Name (class and type): nefazodone (SNDRI) Planned dosage of drug: 300–600 mg/day Dosage of drug (mean): 485.9 (SD 115.6) mg/day Placebo (participants = 84) Name (class and type): placebo Planned dosage of placebo: NR Dosage of placebo (mean): 504.0 (SD 115.9) mg/day Notes: for all medication visits, any formal psychotherapeutic interventions were proscribed. Participants in the placebo arm received identical (but inactive) tablets without any tapering down between continuation and maintenance phase. | |
| Outcomes | Relapse/recurrence HAMD‐24 mean Dropout any Dropout due to adverse events | |
| Notes | Probably conflict of interest because of funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information. |
| Allocation concealment (selection bias) | Unclear risk | No information. |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind comparison: participants assigned to placebo received apparently identical, inactive tablets. |
| Blinding of outcome assessment (detection bias) | Low risk | Primary dependent measure was the 24‐item HAM‐D, which was rated by trained, independent evaluators blind to treatment assignment. |
| Incomplete outcome data (attrition bias) | Low risk | Low number of missing data, main outcomes seem to be reported for all participants, see statistical methods. (p. 809) |
| Selective reporting (reporting bias) | Unclear risk | Unclear, no information on the measures in the maintenance phase, but relevant outcomes (HAM‐D, recurrence, adverse events) were reported, no study protocol. |
| Other bias | High risk | Insufficient treatment adherence: the medication doses were within the planned range, no laboratory tests are reported. Allegiance bias/conflict of interest: supported by grants from Bristol‐Myers Squibb. Attention bias: medication visits were equal in both groups. |
| Methods | Design: RCT Phases: continuation treatment (26.1 weeks) after response to phenelzine treatment Comparison groups: phenelzine vs placebo Funded by: probably internal funding by the authors' institution, no information given | |
| Participants | Number of participants randomized (NRCT: number of participants included): 12 Criteria for relapse/recurrence: "Patients were considered to have relapsed and were withdrawn from the protocol if they scored 3 or more on the CGI for 2 consecutive weeks. Patients received a score of 3 on the CGI only if they had a clear recurrence of depressive symptoms." (p. 347) Age distribution in sample: unclear Sex distribution in sample (% women): 83.3 Diagnoses in sample: phenelzine: 20.0% dysthymia, 80.0% double depression; placebo: 58.0% dysthymia, 42.0% double depression Depression severity at continuation/maintenance baseline (mean): HAM‐D phenelzine: 1.8 (SD 1.3); placebo: 4.4 (SD 3.9) Age of onset: unclear Length current/last major episode in months: unclear | |
| Interventions | Continuation treatment (26.1 weeks) Phenelzine (participants = 5) Name (class and type): phenelzine (MAOI) Planned dosage of drug: unclear Dosage of drug (mean): 51.0 (SD 7.4) mg/day Placebo (participants = 7) Name (class and type): tablet placebo Planned dosage of placebo: NR Dosage of placebo: NR Notes: the placebo group discontinued phenelzine treatment over 14 days by tapering the daily dose by 15 mg every 2–3 days according to a predetermined schedule. No information about concomitant treatments. | |
| Outcomes | Relapse/recurrence HAM‐D mean Dropout any Dropout due to adverse event Experiencing any adverse event (no data available for the placebo group) Serious adverse events (no data available for the placebo group) | |
| Notes | After relapse, participants were treated openly as clinically indicated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The double blind condition was maintained by providing individual daily medication packets in which the number of tablets was kept constant by substituting matching placebo." (pp. 346–7) |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | No study protocol |
| Other bias | Unclear risk | Insufficient treatment adherence: no information on treatment adherence. Allegiance bias/conflict of interest: no information about funding/possible conflict of interest. Attention bias: no indication for attention bias, all participants in the placebo group also saw the physician. |
| Methods | Design: RCT Phases: acute (8 weeks), continuation (16 weeks) Comparison groups: fluoxetine vs fluoxetine + group psychotherapy Funded by: grant from Eli Lilly Company. | |
| Participants | Number of participants randomized (NRCT: number of participants included): 40 Criteria for relapse/recurrence: not available Age distribution in sample (mean): 45.1 (SD 9.8) years Sex distribution in sample (% women): 50 Diagnoses in sample: 100% dysthymia Depression severity at continuation/maintenance baseline (mean): HAM‐D 21: fluoxetine: 7.8 (SD 4.7); combination: 6.2 (SD 4.9) Age of onset: unclear Length current/last major episode in months: unclear | |
| Interventions | Continuation treatment (16 weeks) Fluoxetine (participants = 18) Name (class and type): fluoxetine (SSRI) Planned dosage of drug: 20–80 mg/day Dosage of drug (mean): 38.8 (SD 18.9) mg/day Combination (participants = 19) Name (class and type): fluoxetine (SSRI) + group psychotherapy (CT/IPT) Planned number of sessions + dosage of drug: 16 sessions + 20–80 mg/day Dosage of drug (mean): 37.4 (SD 17.3) mg/day Notes: participants were not allowed to currently undergo another psychotherapy. In the medication group, psychiatrists were instructed not to engage in psychotherapy, counselling, or supportive interventions. | |
| Outcomes | HAM‐D‐21 mean (end of intervention and follow‐up) Dropout any SWLS (end of intervention and follow‐up) | |
| Notes | Possibly conflict of interest (funded by Eli Lilly); discrepant information given in text vs tables; sometimes also unclear/discrepant: information given in text itself; treatment/group therapy = CIGP‐CD manual, which is not classified by Cochrane. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | High risk | Psychotherapy trial, no blinding possible. |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Unblinded raters" (p. 101) |
| Incomplete outcome data (attrition bias) | High risk | LOCF method for physician rated scales: 7/35 (20%) dropout at follow‐up (36 weeks), these scales are main outcomes; no comment why participants dropped out. |
| Selective reporting (reporting bias) | Unclear risk | No existing study protocol. |
| Other bias | High risk | Quote: "Insufficient treatment adherence: Sessions were audiotaped and reviewed in weekly supervision meetings for adherence to the manual." (pp. 96–7) Allegiance bias/conflict of interest: financed by pharmaceutical company, but unclear/no further information. Attention bias: more attention in the combination group as this group also received psychotherapy. Other: very likely that randomization was before acute treatment, but it was not described clearly. Discrepant information in the text. |
| Methods | Design: RCT Phases: acute (12 weeks), continuation (16 weeks), maintenance (76 weeks) Comparison groups: sertraline vs placebo Funded by: grant from Pfizer (NY) | |
| Participants | Number of participants randomized (NRCT: number of participants included): 161 Criteria for relapse/recurrence: recurrence: DSM‐III‐R criteria for major depression for ≥ 3 weeks; CGI severity score of ≥ 4 (at least moderate severity); CGI improvement score ≥ 3 (minimally improved or less); and an increase in HAM‐D score ≥ 4 points higher than the maintenance baseline; next visit 1 week later in total ≥ 4 weeks of clinical worsening; additionally: senior investigator supporting diagnosis/recurrence. (pp. 1666–7) Age distribution in sample (mean): sertraline: 40.8 (SD 9.0) years; placebo: 42.4 (SD 9.7) years Sex distribution in sample (% women): sertraline: 62.3; placebo: 69.0 Diagnoses in sample: sertraline: 52.0% chronic major depressive disorder, 48.0% double depression; placebo: 43.0% chronic major depressive disorder, 57.0% double depression Depression severity at continuation/maintenance baseline (mean): sertraline: 5.5 (SD 4.2); placebo: 6.3 (SD 3.7) Age of onset (mean): sertraline: 24.9 (SD 11.2) years; placebo: 25.7 (SD 12.5) years Length current/last major episode (mean): sertraline: 88.2 (SD 121.7) months; placebo: 54.9 (SD 80.8) months | |
| Interventions | Maintenance treatment (76 weeks) Sertraline (participants = 77) Name (class and type): sertraline (SSRI) Planned dosage of drug: 50–200 mg/day Dosage of drug (mean): 146.1 mg/day Placebo (participants = 84) Name (class and type): placebo tablets Planned dosage of placebo: unclear Dosage of placebo (mean): 3.4 tablets/day Notes: participants in the placebo arm tapered sertraline by 50 mg reduction per week as placebo substitution. No information about concomitant treatments. | |
| Outcomes | Relapse/recurrence HAM‐D‐24 mean Dropout any SF‐36 Dropout due to adverse event Experiencing any adverse event | |
| Notes | Probably conflict of interest because of funding. They used 2 different criteria for relapse/recurrence, we extracted the stricter one; therefore, maybe less relapse observed than actual happened, in combination with numerous of dropouts with possible bias of results. "Patients meeting recurrence criteria could continue in the study if both patient and study physician agreed that no change in the study medication was indicated at that time. Instead, an increase in daily dose was undertaken at a rate of 50mg/week up to the maximum daily dose of 200mg of sertraline hydrochloride. A similar double‐blind titration was also used for patients receiving placebo treatment." (further details see p. 1667) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind Quote: "To maintain blinding, this group of patients continued (as a parallel but non‐randomised group) receiving imipramine during subsequent continuation and maintenance phases… The integrity of the study's double‐blind component was not compromised." (p. 1666) |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | High risk | 70% dropout in the placebo group. The data were replaced by the LOCF ‐method (i.e. 70% of data replaced by last observation point, the participant's condition was probably better at this earlier time). |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | High risk | Insufficient treatment adherence: no information. Allegiance bias/conflict of interest: whole study financed by Pfizer. Attention bias: most likely, each treatment group gained same attention (as both groups received tablets). |
| Methods | Design: RCT Phases: acute (12 weeks), continuation (16 weeks), maintenance (52 weeks) Comparison groups: CBASP vs assessment only Funded by: Bristol‐Myers Squibb | |
| Participants | Number of participants randomized (NRCT: number of participants included): 82 Criteria for relapse/recurrence: "Recurrence was defined in the protocol as a HRSD‐24 [HAM‐D] score of 16 or greater on two consecutive visits and a diagnosis of MDD as determined from a DSM–IV MDD checklist administered by the independent evaluator. At the second of these visits, the recurrence also needed confirmation by the site's senior investigator on the basis of a clinical interview." (p. 683) Age distribution in sample (mean): CBASP: 44.2 (SD 11.7) years; assessment only: 46.0 (SD 11.1) years Sex distribution in sample (% women): CBASP: 81.0; assessment only: 52.5 Diagnoses in sample: CBASP: 50.0% chronic major depressive disorder, 26.2% double depression, 23.8% recurrent depressive disorder with incomplete remission between episodes; assessment only: 60.0% chronic major depressive disorder, 20.0% double depression, 20.0% recurrent depressive disorder with incomplete remission between episodes Depression severity at continuation/maintenance baseline (mean): HAM‐D‐24: CBASP: 6.6 (SD 3.8); assessment only: 6.2 (SD 4.4) Age of onset (mean): CBASP: 27.0 (SD 12.4) years; assessment only: 29.5 (SD 13.5) years Length current/last major episode in months (mean): CBASP: 92.4 (SD 115.2); assessment only: 85.2 (SD 122.4) | |
| Interventions | Maintenance treatment (52 weeks) Name (class and type): CBASP Planned number of sessions: 13 Number of sessions (mean): 11.1 (SD 3.8) Name (class and type): assessment only Planned number of sessions: 13 Number of sessions: unclear Notes: "In both conditions, all psychotropic medication and non‐protocol psychotherapy were prohibited." (p. 683) | |
| Outcomes | Relapse/recurrence HRSD‐24 (HAM‐D) mean Dropout any | |
| Notes | Probably conflict of interest because of funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | High risk | As CBASP was compared to assessment only, blinding of participants was not possible. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The primary outcome measure throughout all phases of the study was the HRSD‐24, which was administered by certified rates who were unaware of patient's treatment condition. Patients in the CBASP condition were also seen by the independent evaluator every 4 weeks but did not receive an honorarium. All patients were reminded at each visit not to mention anything that might reveal their treatment condition to the independent evaluator. If patients had questions or concerns about the study, they were instructed to raise them with the project coordinator rather than the independent evaluator. In the rare instances that the blind was broken, the patient was seen by a different independent evaluator at subsequent visits. In both conditions, all psychotropic medication and non‐protocol psychotherapy were prohibited." (p. 683) |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "We compared time to recurrence between the CBASP and assessment only groups using survival analysis. Patients who failed to complete the maintenance phase were included in these analyses using all available data up to the time of exiting the study." (p. 684) |
| Selective reporting (reporting bias) | Unclear risk | Study protocol only described outcome measurements for the acute phase. |
| Other bias | Low risk | Insufficient treatment adherence. Quote: "Sessions were videotaped and reviewed weekly–biweekly by the site supervisor or James P. McCullough to assess adherence to the treatment procedures. Adherence was assessed using a rating scale described in McCullough (2000). When non‐adherence was identified, it was immediately discussed with the therapist and efforts at remediation were provided." (p. 683) Allegiance bias/conflict of interest: some authors were well‐known CBASP therapists (e.g. J McCullough), but there were also other authors; interests were balanced across authors. Attention bias: in both conditions, participants saw the therapist or project co‐ordinator every 4 weeks. The project co‐ordinator provided them with some attention but no active treatment. |
| Methods | Design: NRCT Phases: acute (6–10 weeks), continuation (16–20 weeks), maintenance (104.4 weeks) Comparison groups: imipramine vs desipramine Funded by: no information | |
| Participants | Number of participants randomized (NRCT: number of participants included): 73 Criteria for relapse/recurrence: no information; this outcome was not addressed. Age distribution in sample (mean): 36.0 (SD 10.0) years Sex distribution in sample (% women): 64.1 Diagnoses in sample: 37.0% dysthymia, 63.0% double depression Depression severity at continuation/maintenance baseline: unclear Age of onset: unclear Length current/last major episode in months: unclear | |
| Interventions | Continuation treatment (16–20 weeks) Imipramine (participants = 23) Name (class and type): imipramine (TCA) Planned dosage of drug: 300 mg/day Dosage of drug: unclear Sertraline (participants = 50) Name (class and type): desipramine (TCA) Planned dosage of drug: 200 mg/day Dosage of drug (mean): 232 (SD 72) mg/day Notes: "Patients were allowed to remain in stable long‐term psychotherapy during the study but were not allowed to enter into new psychotherapy arrangements." (p. 214) No data provided about the percentage of participants receiving parallel psychotherapy. "Concomitant psychotropic medications were proscribed." (p. 214) | |
| Outcomes | Dropout any Dropout due to adverse event | |
| Notes | There were 3 different treatment arms in the acute treatment, but it was unclear how participants were allocated to the different treatment arms, e.g. if there were randomized. Additionally, the rationale of the acute treatment was unclear (e.g. some participants received medication on a double blind and some on an open basis). | |
| Methods | Design: RCT Phases: acute (10 weeks), continuation (16 weeks), maintenance (104.4 weeks) Comparison groups: desipramine vs placebo Funded by: grant from the National Institute of Mental Health | |
| Participants | Number of participants randomized (NRCT: number of participants included): 53 Criteria for relapse/recurrence: "Suspected relapse occurred when a HAM‐D score rose above 12 during the maintenance phase. Clinicians discussed and encouraged compliance and obtained a plasma drug level, which was reviewed by a non blind observer who was not involved in the treatment. The non‐blind observer gave instructions or dummy instructions for dosage adjustments. Relapse was defined as HAM‐D scores greater than 12 and GAS scores below 60 on three successive ratings over a period of 4 weeks or at least one rating meeting these criteria and an urgent need for alternative treatment for a depressive syndrome." (p. 771) Age distribution in sample (mean): 36.9 (SD 9.6) years Sex distribution in sample (% women): 57.4 Diagnoses in sample: 10.9% chronic major depressive disorder, 39.5% dysthymia, 49.6% double depression Depression severity at continuation/maintenance baseline: unclear Age of onset (mean): 12.6 (SD 6.9) years Length current/last major episode in months: unclear | |
| Interventions | Maintenance treatment (104.4 weeks) Desipramine (participants = 28) Name (class and type): desipramine (TCA) Planned dosage of drug: 75–350 mg/day Dosage of drug: unclear Placebo (participants = 25) Name (class and type): placebo Planned dosage of drug: participants in the placebo group were tapered by approximately 25% per week over the month and then received identical placebo at the same dose equivalent for the next 23 months or until relapse. Dosage of drug: unclear Notes: participants in the placebo arm were tapered down by 25% per week during the first month of maintenance treatment followed by receiving identical placebo tablets. Stable psychotherapeutic treatment was allowed during the study, 39% of participants from the desipramine group and 40% of participants from the placebo group were in stable psychotherapeutic treatment during the study. | |
| Outcomes | Relapse/recurrence Dropout any | |
| Notes | Desipramine (norpramine) and matching placebo were provided by Marion Merrill Dow Inc., Kansas City, MO. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "A third possible limitation in the present study was the absence of independent raters. Ratings were done by study clinicians who may have been able to guess the maintenance treatment based on side effects." (p. 773). |
| Incomplete outcome data (attrition bias) | Low risk | 50/53 (5.7% dropout during maintenance treatment) participants completed ≥ 1 month of maintenance treatment, outcome data were provided for this sample. |
| Selective reporting (reporting bias) | High risk | No study protocol available. Quote: "A self‐rated measure of social impairment and function, the Social Adjustment Scale‐Self‐rated was completed at the beginning and end of each phase of the study." (p. 771) AND: "Subjects were seen and rated each month during the maintenance phase." (p. 771). Very incomplete data in the text/tables (just full vs partial remission and relapse, but no presentation of clear data about HAM‐D, GAS, and SAS‐SR). |
| Other bias | Low risk | Insufficient treatment adherence: control of plasma drug concentrations. Allegiance bias/conflict of interest: no indication for conflict of interest. Attention bias: no differences between the groups. Participants were seen and rated each month during the maintenance phase. |
| Methods | Design: NRCT Phases: acute (12 weeks), continuation (16 weeks), maintenance (52 weeks) Comparison groups: nefazodone vs CBASP vs combination Funded by: Bristol‐Myers Squibb | |
| Participants | Number of participants randomized (NRCT: number of participants included): 329 Criteria for relapse/recurrence: "Two definitions of relapse were utilized. Any patient who scored higher than 15 on the HAM‐D was considered at risk for a relapse of MDD. In all such cases, an independent evaluator completed the DSM‐IV criteria checklist for MDD, and if the patient met DSM‐IV symptom criteria, the treating clinician was notified. A confirmatory visit was scheduled within 14 days and the HAM‐D and MDD criteria checklist assessment were repeated. Patients meeting MDD criteria were evaluated by an independent senior investigator to confirm relapse. In addition, an investigator could declare a relapse on de facto grounds in the case of an exacerbation of depressive symptomatology with marked incapacity and clinically significant suicidal ideation, including psychiatric hospitalizations resulting from such exacerbations. Patients not meeting relapse criteria but continuing to score higher than 15 on the HAM‐D were followed every other week until their outcome was clarified." (p. 77) Age distribution in sample (mean): nefazodone: 43.1 (SD 9.7) years; CBASP: 44.0 (SD 10.8) years; combination: 44.6 (SD 9.4) years Sex distribution in sample (% women): nefazodone: 58.7; CBASP: 66.3; combination: 67.8 Diagnoses in sample: nefazodone: 32.6% chronic major depressive disorder, 41.3% double depression, 26.1% recurrent depressive disorder with incomplete remission between episodes; CBASP: 33.7% chronic major depressive disorder, 46.1% double depression, 20.2% recurrent depressive disorder with incomplete remission between episodes; combination: 32.2% chronic major depressive disorder, 42.1% double depression, 26.6% recurrent depressive disorder with incomplete remission between episodes Depression severity at continuation/maintenance baseline: unclear Age of onset (mean): nefazodone: 26.3 (SD 13.1) years; CBASP: 28.1 (SD 13.5) years; combination: 27.0 (SD 12.9) years Length current/last major episode in months (mean): nefazodone: 92.4 (SD 114.0); CBASP: 105.6 (SD 144.0); combination: 99.6 (SD 120.0) | |
| Interventions | Continuation treatment (16 weeks) Nefazodone (participants = 91) Name (class and type): nefazodone (SNDRI) Planned dosage of drug: 300–600 mg/day Dosage of drug (mean): 499 (SD 115) mg/day CBASP (participants = 88) Name (class and type): CBASP Planned number of sessions: 6 Number of sessions (mean): 6 (SD 1) Combination (participants = 150) Name (class and type): combination (SNDRI + CBASP) Planned number of sessions + dosage of drug: 6 sessions + 300–600 mg/day Number of sessions + dosage of drug (mean): 5.9 (SD 1.1) sessions + 479 (SD 108) mg/day Notes: "Pharmacotherapists were directed not to provide any psychotherapeutic interventions." (p. 76) | |
| Outcomes | Relapse/recurrence Dropout any | |
| Notes | Probably conflict of interest because of funding and connection of the authors to pharmaceutical industry. | |
| Methods | Design: NRCT Phases: acute (12 weeks), continuation (16 weeks), maintenance (76 weeks) Comparison groups: sertraline vs imipramine Funded by: grant from Pfizer (NY) | |
| Participants | Number of participants randomized (NRCT: number of participants included): 386 Criteria for relapse/recurrence: "A full remission of depression was defined as a CGI improvement score (CGI‐I) (Guy, 1976) of 1 or 2 (very much or much improved) and a Hamilton Depression Rating Scale score (HRSD [HAM‐D]) (Hamilton, 1960) ≤ 7. A satisfactory therapeutic response (partial remission) was defined as a CGI‐I ≥2, a HRSD ≤ 15 with a ≥ 50% decrease from baseline, and a CGI severity score (CGI‐S) ≤ 3 (i.e. no more than mild depression). A patient whose scores dropped below a 'satisfactory therapeutic response' for a 4‐week period was considered relapsed." (p. 29) Age distribution in sample (mean): sertraline: 40.2 (SD 9.7) years; imipramine: 43.1 (SD 9.6) years Sex distribution in sample (% women): sertraline: 68.2; imipramine: 57.1 Diagnoses in sample: sertraline: 49.0% chronic major depressive disorder, 51.0% double depression; imipramine: 45.0% chronic major depressive disorder, 55.0% double depression Depression severity at continuation/maintenance baseline (mean): sertraline: 6.7 (SD 3.7); imipramine: 6.9 (SD 3.5) Mean age of onset: unclear Length current/last major episode (mean): sertraline: 73.2 (SD 98.4) months; imipramine: 76.8 (SD 114.0) months | |
| Interventions | Continuation treatment (16 weeks) Sertraline (participants = 239) Name (class and type): sertraline (SSRI) Planned dosage of drug: 50–200 mg/day Dosage of drug (mean): 149 (SD 55) mg/day Imipramine (participants = 147) Name (class and type): imipramine (TCA) Planned dosage of drug: 50–300 mg/day Dosage of drug: 227 (SD 73) mg/day Notes: "Psychotherapy was not allowed during the study unless it had started at least 3 months before acute phase randomisation and continued throughout all stages of the study without change." (p. 28) 60% of the participants received ongoing psychotherapy during the continuation phase. | |
| Outcomes | Relapse/recurrence HAM‐D‐24 mean Dropout any Q‐LES‐Q score Dropout due to adverse event | |
| Notes | Probably conflict of interest because of funding (authors = members of industry who financed study). Further randomized comparison on maintenance treatment of the sertraline group with placebo in the publication of Keller (1998). | |
| Methods | Design: RCT Phases: acute (10–12 weeks), continuation (16 weeks), maintenance (104.4 weeks) Comparison groups: desipramine vs placebo Funded by: supported by grant R01‐MH37103 from the National Institute of Mental Health and from a fund established in the New York Community Trust by DeWitt‐Wallace. | |
| Participants | Number of participants randomized (NRCT: number of participants included): 27 Criteria for relapse/recurrence: "Recurrence was defined as HAM‐D scores > 12 and GAS scores < 60 on three successive ratings over a period of 4 weeks or at least one rating meeting these criteria and an urgent need for alternative treatment for recurrence of depressive symptoms." (p. 233) Age distribution in sample (mean): desipramine: 34.4 (SD 9.6) years; placebo: 39.0 (SD 11.2) years Sex distribution in sample (% women): desipramine: 43.0; placebo: 46.0 Diagnoses in sample: 100% dysthymia Depression severity at continuation/maintenance baseline (mean): desipramine: 3.1 (SD 2.5); placebo: 3.9 (SD 5.2) Age of onset (mean): desipramine: 14.5 (SD 10.4) years; placebo: 12.3 (SD 8.0) years Length current/last major episode: unclear | |
| Interventions | Maintenance treatment (104.4 weeks) Desipramine (participants = 14) Name (class and type): desipramine (TCA) Dosage of drug: unclear Dosage of drug (mean): 223 (SD 90) mg/day Placebo (participants = 13) Name (class and type): placebo Planned dosage of placebo: unclear Dosage of placebo (mean): 240 (SD 60) mg/day (dummy dosage) Notes: participants in the placebo arm were tapered down by 25% per week during the first month of maintenance treatment followed by receiving identical placebo tablets. 43% of participants from the desipramine group and 38% of participants from the placebo group were in stable long‐term psychotherapy during the study, a non‐significant difference. | |
| Outcomes | Relapse/recurrence | |
| Notes | Analysis of the dysthymic subgroup of Kocsis et al. 1996 and some additional participants with dysthymia. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind maintenance phase |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Ratings were done by study clinicians who were blinded to treatment assignment, but may have guessed the maintenance treatment based on side effects, potentially biasing ratings of outcome." (p. 235) |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data for the available outcome. |
| Selective reporting (reporting bias) | High risk | No study protocol available, only recurrence rates were reported, HAM‐D, GAS, and SASR was also measured. |
| Other bias | Low risk | Insufficient treatment adherence: serum level control. Allegiance bias/conflict of interest: no indication for a conflict of interest. Attention bias: same approach in both conditions (quote: "monthly 20–30 minute appointments to monitor clinical status and manage side effects. Therapists provided support and encouragement, and medication compliance was discussed throughout." (p. 232) |
CBASP: Cognitive Behavioral Analysis System of Psychotherapy; CGI: Clinical Global Impression; CIGP‐CD: Cognitive‐Interpersonal Group Psychotherapy for Chronic Depression; CT: cognitive therapy; DSM‐III‐R: Diagnostic and Statistical Manual of Mental Disorders 3rd Edition – Revised; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders 4th Edition; GAS: Global Assessment Scale; HAM‐D: Hamilton Depression Rating Scale; HRSD: Hamilton Rating Scale for Depression (also known as HAM‐D); IPT: interpersonal psychotherapy; LOCF: last observation carried forward; MAOI: monoamine oxidase inhibitor; MDD: major depressive episode; NR: not reported; NRCT: non‐randomized controlled trial; Q‐LES‐Q: Quality of Life Enjoyment and Satisfaction Questionnaire; RCT: randomized controlled trial; SAS‐SR: Social Adjustment Scale – Self‐Report; SD: standard deviation; SF‐36: 36‐item Short‐Form Health Survey; SNDRI: selective noradrenaline‐dopamine reuptake inhibitor; SSRI: selective serotonin reuptake inhibitor; SWLS: Satisfaction With Life Scale; TCA: tricyclic antidepressant.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Included participants were remitted, some with and some without treatment. The interval between acute and continuation treatment was too long in some cases (> 1 year). | |
| Participants did not meet the criteria of persistent depression (duration < 2 years). | |
| Participants did not meet the criteria of persistent depression (duration < 2 years). | |
| Participants did not meet the criteria of persistent depression (duration < 2 years). | |
| No response during acute treatment required for entering MBCT. | |
| Acute treatment with long‐term follow‐up | |
| No comparator (pilot study) | |
| No response during acute treatment required to enter continuation trial. | |
| Participants did not meet the criteria of persistent depression (duration < 2 years). | |
| Participants did not meet the criteria of persistent depression (duration < 2 years). | |
| Participants did not meet the criteria of persistent depression (duration < 2 years); just 5% participants with double depression. | |
| Participants did not meet the criteria of persistent depression (duration < 2 years); duration of episode maximum 2 years for being eligible. | |
| No response during acute treatment required for entering MBCT. | |
| No response during acute treatment required to enter intervention. | |
| Partly meeting criteria for a PDD diagnosis; exact amount of persistent depressed participants unclear. | |
| No response during acute treatment required to enter continuation trial. | |
| < 80% of participants with a PDD diagnosis. | |
| Participants did not meet the criteria of persistent depression (duration < 2 years). | |
| No response during acute treatment required to enter continuation trial. |
MBCT: mindfulness‐based cognitive therapy; PDD: persistent depressive disorder.
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Design: RCT Phases: continuation (approximately 26 weeks) Comparison groups: T‐CT (telephone‐delivered cognitive‐behavioral continuation therapy) vs usual care Funded by: University of Zurich |
| Participants | Estimated enrolment: 218 Ages: 18 years to 75 years Sexes Eligible for Study: All Diagnoses: Recurrent major depressive disorder or chronic/persistent depressive disorder based on the criteria of the Diagnostic and Statistical Manual of Mental Disorders (DSM‐5) |
| Interventions | Continuation treatment (approximately 26 weeks) Behavioral: telephone‐administered continuation therapy Other: Usual care |
| Outcomes | Primary outcome: Relapse of a major depressive episode (time frame: 6 months, 12 months, and 18 months after baseline) Secondary outcomes: Well‐weeks (time frame: 6 months, 12 months, and 18 months after baseline) Depressive symptoms (time frame: baseline, 3 months, 6 months, and 12 months after baseline) Health‐related quality of life (time frame: baseline, 3 months, 6 months, and 12 months after baseline) Anxiety symptoms (time frame: baseline, 3 months, 6 months, and 12 months after baseline) Psychosocial functioning (time frame: 6 months and 12 months after baseline) Cost of health care utilization (time frame: baseline, 6 months, and 12 months after baseline) Cost‐effectiveness (time frame: baseline, 6 months, and 12 months after baseline) Other Pre‐specified Outcome Measures: T‐CT acceptability (time frame: 6 months after baseline) Treatment satisfaction (time frame: baseline, and 6 months after baseline) Self‐confidence (time frame: baseline, 6 months, and 12 months after baseline) Physical activity (time frame: baseline, 6 months, and 12 months after baseline) Self‐efficacy for depression self‐management (time frame: baseline, 3 months, 6 months, and 12 months after baseline) Self‐management behaviours (time frame: baseline, 3 months, 6 months, and 12 months after baseline) Interpersonal emotion regulation skills (time frame: baseline, 6 months, and 12 months after baseline) Therapeutic alliance (time frame: baseline, 3 months, and 6 months after baseline) |
| Notes |
DSM‐5: Diagnostic and Statistical Manual of Mental Disorders 5th Edition; RCT: randomized controlled trial; T‐CT: telephone‐delivered cognitive‐behavioral continuation therapy
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse/recurrence Show forest plot | 4 | 383 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.21, 0.79] |
| Analysis 1.1  Comparison 1 Pharmacological continuation and maintenance therapies versus placebo, Outcome 1 Relapse/recurrence. | ||||
| 2 Dropout due to any reason Show forest plot | 4 | 386 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.39, 2.11] |
| Analysis 1.2  Comparison 1 Pharmacological continuation and maintenance therapies versus placebo, Outcome 2 Dropout due to any reason. | ||||
| 3 Depression severity Show forest plot | 3 | 333 | Mean Difference (IV, Random, 95% CI) | ‐4.79 [‐8.49, ‐1.09] |
| Analysis 1.3  Comparison 1 Pharmacological continuation and maintenance therapies versus placebo, Outcome 3 Depression severity. | ||||
| 4 SF‐36 Social Functioning score Show forest plot | 1 | 161 | Mean Difference (IV, Random, 95% CI) | 10.80 [3.04, 18.56] |
| Analysis 1.4  Comparison 1 Pharmacological continuation and maintenance therapies versus placebo, Outcome 4 SF‐36 Social Functioning score. | ||||
| 5 SF‐36 Emotional Role score Show forest plot | 1 | 161 | Mean Difference (IV, Random, 95% CI) | 20.70 [7.43, 33.97] |
| Analysis 1.5  Comparison 1 Pharmacological continuation and maintenance therapies versus placebo, Outcome 5 SF‐36 Emotional Role score. | ||||
| 6 SF‐36 Role Physical score Show forest plot | 1 | 161 | Mean Difference (IV, Random, 95% CI) | 2.10 [‐9.76, 13.96] |
| Analysis 1.6  Comparison 1 Pharmacological continuation and maintenance therapies versus placebo, Outcome 6 SF‐36 Role Physical score. | ||||
| 7 Dropout due to any type of adverse event Show forest plot | 3 | 333 | Odds Ratio (M‐H, Random, 95% CI) | 3.53 [0.67, 18.70] |
| Analysis 1.7  Comparison 1 Pharmacological continuation and maintenance therapies versus placebo, Outcome 7 Dropout due to any type of adverse event. | ||||
| 8 Any type of adverse event Show forest plot | 1 | 161 | Odds Ratio (M‐H, Random, 95% CI) | 1.47 [0.70, 3.09] |
| Analysis 1.8  Comparison 1 Pharmacological continuation and maintenance therapies versus placebo, Outcome 8 Any type of adverse event. | ||||
| 9 Relapse/recurrence sensitivity analysis Show forest plot | 4 | 360 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.16, 0.89] |
| Analysis 1.9  Comparison 1 Pharmacological continuation and maintenance therapies versus placebo, Outcome 9 Relapse/recurrence sensitivity analysis. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse/recurrence Show forest plot | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.14, 0.93] |
| Analysis 2.1  Comparison 2 Psychological continuation and maintenance therapies versus attention placebo/non‐specific control, Outcome 1 Relapse/recurrence. | ||||
| 2 Dropout due to any reason Show forest plot | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.41, 1.81] |
| Analysis 2.2  Comparison 2 Psychological continuation and maintenance therapies versus attention placebo/non‐specific control, Outcome 2 Dropout due to any reason. | ||||
| 3 Depression severity Show forest plot | 1 | 82 | Mean Difference (IV, Random, 95% CI) | ‐4.00 [‐7.05, ‐0.95] |
| Analysis 2.3  Comparison 2 Psychological continuation and maintenance therapies versus attention placebo/non‐specific control, Outcome 3 Depression severity. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse/recurrence Show forest plot | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.43, 3.49] |
| Analysis 3.1  Comparison 3 Psychological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies, Outcome 1 Relapse/recurrence. | ||||
| 2 Dropout due to any reason Show forest plot | 1 | 179 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.30, 1.03] |
| Analysis 3.2  Comparison 3 Psychological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies, Outcome 2 Dropout due to any reason. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse/recurrence Show forest plot | 1 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.44, 3.44] |
| Analysis 4.1  Comparison 4 Combined psychological and pharmacological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies alone, Outcome 1 Relapse/recurrence. | ||||
| 2 Dropout due to any reason Show forest plot | 2 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.90, 2.29] |
| Analysis 4.2  Comparison 4 Combined psychological and pharmacological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies alone, Outcome 2 Dropout due to any reason. | ||||
| 3 Depression severity Show forest plot | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 2.8 [0.38, 5.22] |
| Analysis 4.3  Comparison 4 Combined psychological and pharmacological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies alone, Outcome 3 Depression severity. | ||||
| 4 Depression severity – follow‐up Show forest plot | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐3.26, 5.06] |
| Analysis 4.4  Comparison 4 Combined psychological and pharmacological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies alone, Outcome 4 Depression severity – follow‐up. | ||||
| 5 Health‐related quality of life Show forest plot | 1 | 35 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐1.63, 0.63] |
| Analysis 4.5  Comparison 4 Combined psychological and pharmacological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies alone, Outcome 5 Health‐related quality of life. | ||||
| 6 Health‐related quality of life – follow‐up Show forest plot | 1 | 33 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐0.56, 1.76] |
| Analysis 4.6  Comparison 4 Combined psychological and pharmacological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies alone, Outcome 6 Health‐related quality of life – follow‐up. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse/recurrence Show forest plot | 1 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.57, 4.01] |
| Analysis 5.1  Comparison 5 Combined psychological and pharmacological continuation and maintenance therapies versus psychotherapeutic continuation and maintenance therapies alone, Outcome 1 Relapse/recurrence. | ||||
| 2 Dropout due to any reason Show forest plot | 1 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.45, 1.51] |
| Analysis 5.2  Comparison 5 Combined psychological and pharmacological continuation and maintenance therapies versus psychotherapeutic continuation and maintenance therapies alone, Outcome 2 Dropout due to any reason. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dropout due to any reason Show forest plot | 1 | 73 | Risk Ratio (M‐H, Random, 95% CI) | 4.35 [1.19, 15.87] |
| Analysis 6.1  Comparison 6 Imipramine (TCA) versus desipramine (TCA), Outcome 1 Dropout due to any reason. | ||||
| 2 Dropout due to any type of adverse event Show forest plot | 1 | 73 | Odds Ratio (M‐H, Random, 95% CI) | 1.49 [0.23, 9.60] |
| Analysis 6.2  Comparison 6 Imipramine (TCA) versus desipramine (TCA), Outcome 2 Dropout due to any type of adverse event. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse/recurrence Show forest plot | 1 | 376 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.84, 1.91] |
| Analysis 7.1  Comparison 7 Imipramine (TCA) versus sertraline (SSRI), Outcome 1 Relapse/recurrence. | ||||
| 2 Dropout due to any reason Show forest plot | 1 | 386 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.48, 1.38] |
| Analysis 7.2  Comparison 7 Imipramine (TCA) versus sertraline (SSRI), Outcome 2 Dropout due to any reason. | ||||
| 3 Depression severity Show forest plot | 1 | 377 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.97, 1.77] |
| Analysis 7.3  Comparison 7 Imipramine (TCA) versus sertraline (SSRI), Outcome 3 Depression severity. | ||||
| 4 Health‐related quality of life Show forest plot | 1 | 347 | Mean Difference (IV, Random, 95% CI) | ‐4.30 [‐7.31, ‐1.29] |
| Analysis 7.4  Comparison 7 Imipramine (TCA) versus sertraline (SSRI), Outcome 4 Health‐related quality of life. | ||||
| 5 Dropout due to any type of adverse event Show forest plot | 1 | 386 | Odds Ratio (M‐H, Random, 95% CI) | 1.99 [0.60, 6.65] |
| Analysis 7.5  Comparison 7 Imipramine (TCA) versus sertraline (SSRI), Outcome 5 Dropout due to any type of adverse event. | ||||
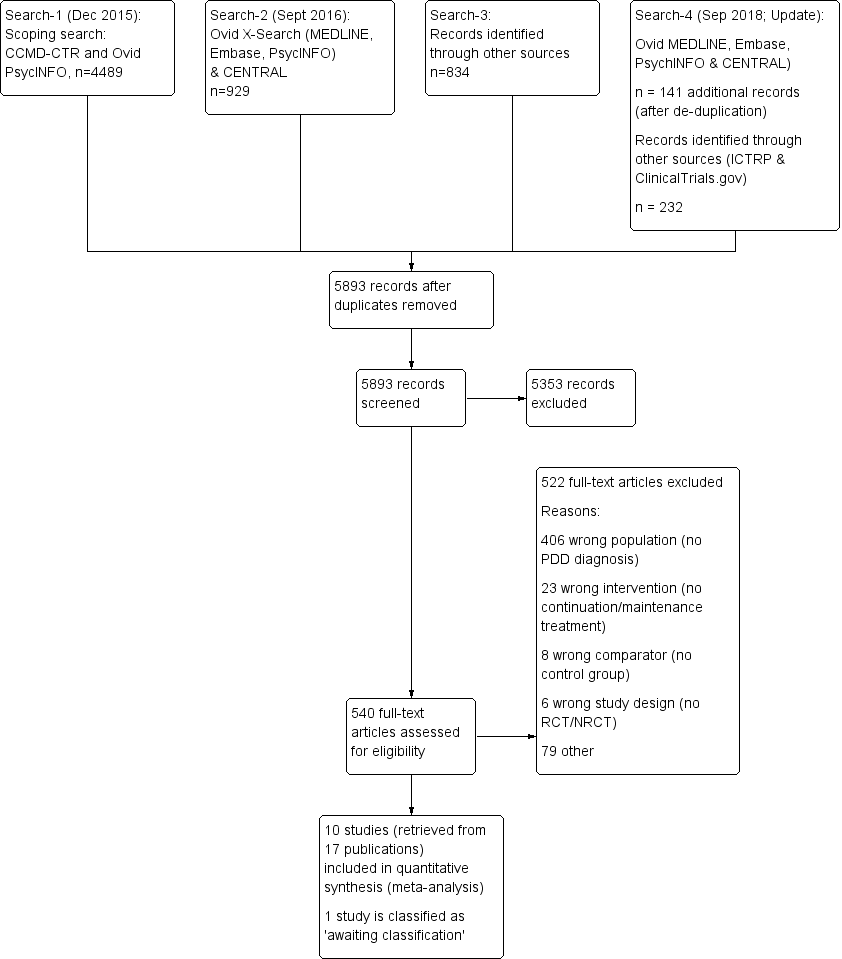
Study flow diagram. NRCT: non‐randomized controlled trial; PDD: persistent depressive disorder; RCT: randomized controlled trial.
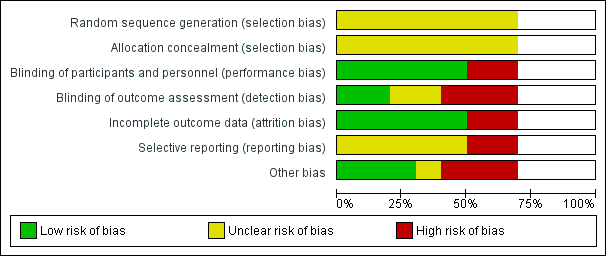
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included seven randomized controlled trials. Blank space in rows containing no information indicate missing information on the 'Risk of bias' scale for the three non‐randomized controlled trials.
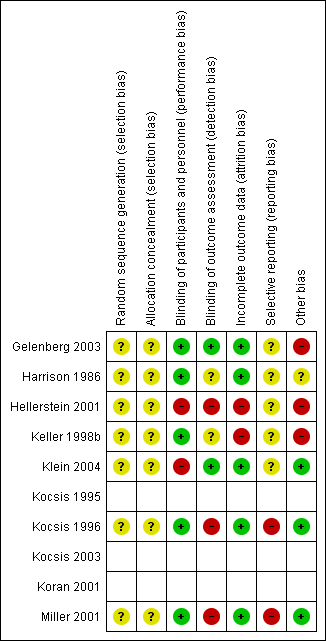
Risk of bias summary: review authors' judgements about each risk of bias item for each included randomized controlled trial (seven studies). Blank space in rows containing no information indicate missing information on the 'Risk of bias' scale for the three non‐randomized controlled trials.

Risk of bias graph non‐randomized controlled trials (NRCT): review authors' judgement about each risk of bias item presented as percentages across all included NRCTs (three studies).
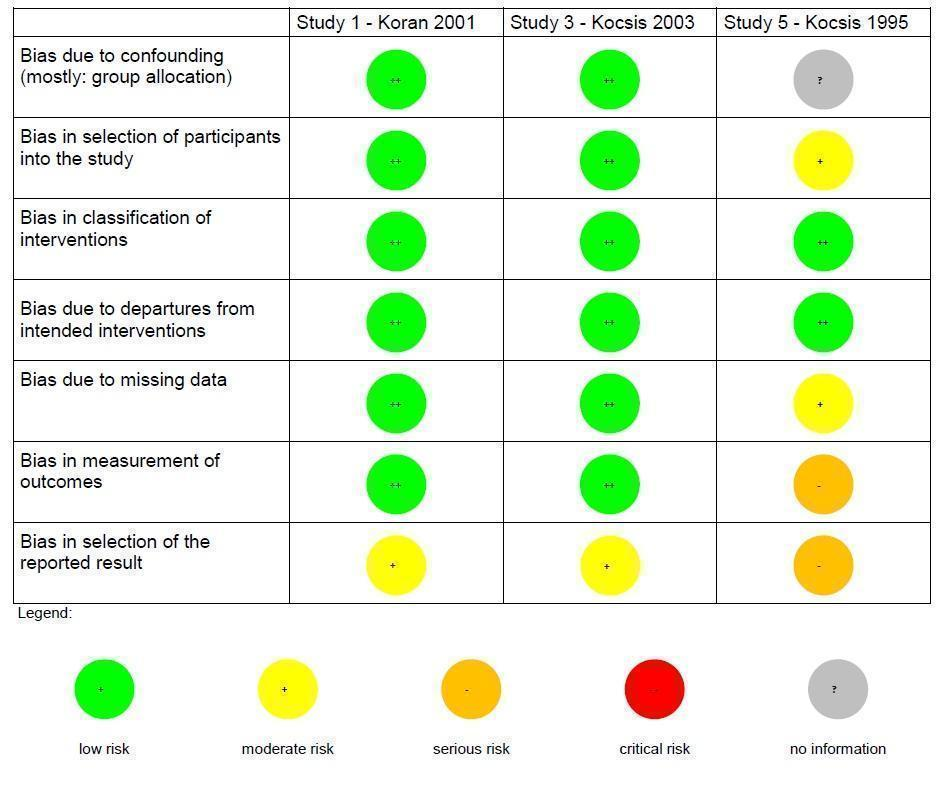
Risk of bias summary non‐randomized controlled trials (NRCT): review authors' judgements about each risk of bias item for each included NRCT (three studies).

Forest plot of comparison: 1 Medication versus placebo, outcome: 1.1 Relapse/recurrence.
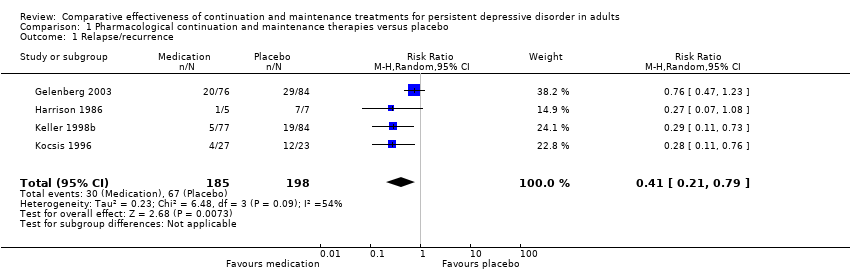
Comparison 1 Pharmacological continuation and maintenance therapies versus placebo, Outcome 1 Relapse/recurrence.

Comparison 1 Pharmacological continuation and maintenance therapies versus placebo, Outcome 2 Dropout due to any reason.

Comparison 1 Pharmacological continuation and maintenance therapies versus placebo, Outcome 3 Depression severity.
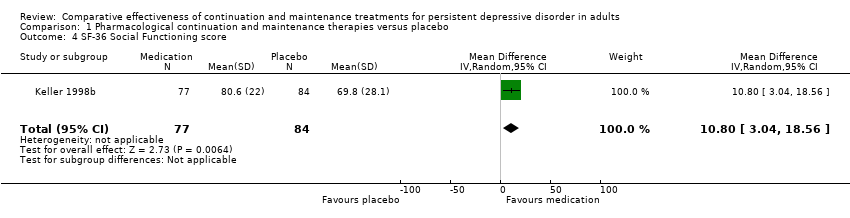
Comparison 1 Pharmacological continuation and maintenance therapies versus placebo, Outcome 4 SF‐36 Social Functioning score.

Comparison 1 Pharmacological continuation and maintenance therapies versus placebo, Outcome 5 SF‐36 Emotional Role score.
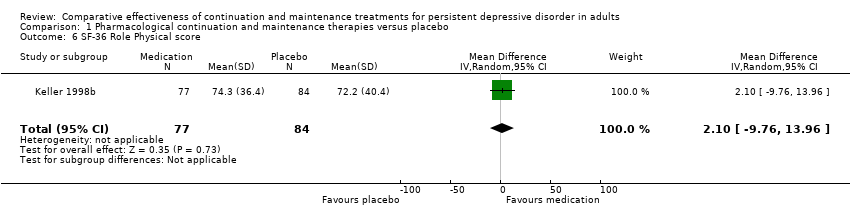
Comparison 1 Pharmacological continuation and maintenance therapies versus placebo, Outcome 6 SF‐36 Role Physical score.

Comparison 1 Pharmacological continuation and maintenance therapies versus placebo, Outcome 7 Dropout due to any type of adverse event.

Comparison 1 Pharmacological continuation and maintenance therapies versus placebo, Outcome 8 Any type of adverse event.
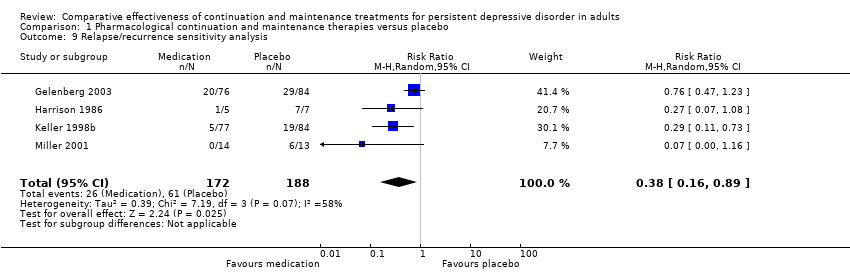
Comparison 1 Pharmacological continuation and maintenance therapies versus placebo, Outcome 9 Relapse/recurrence sensitivity analysis.
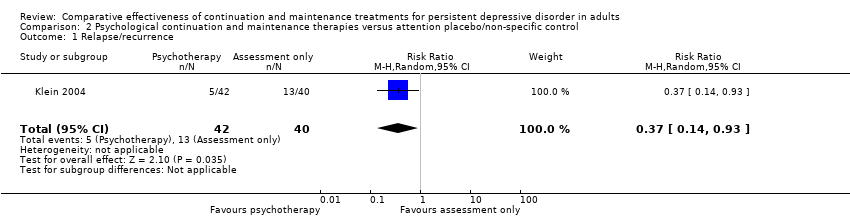
Comparison 2 Psychological continuation and maintenance therapies versus attention placebo/non‐specific control, Outcome 1 Relapse/recurrence.
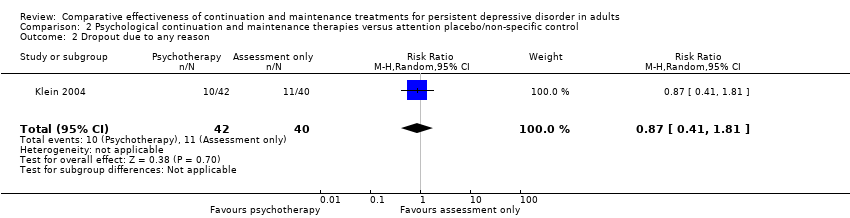
Comparison 2 Psychological continuation and maintenance therapies versus attention placebo/non‐specific control, Outcome 2 Dropout due to any reason.

Comparison 2 Psychological continuation and maintenance therapies versus attention placebo/non‐specific control, Outcome 3 Depression severity.

Comparison 3 Psychological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies, Outcome 1 Relapse/recurrence.

Comparison 3 Psychological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies, Outcome 2 Dropout due to any reason.
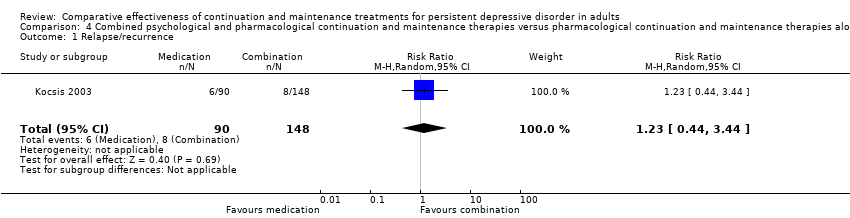
Comparison 4 Combined psychological and pharmacological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies alone, Outcome 1 Relapse/recurrence.
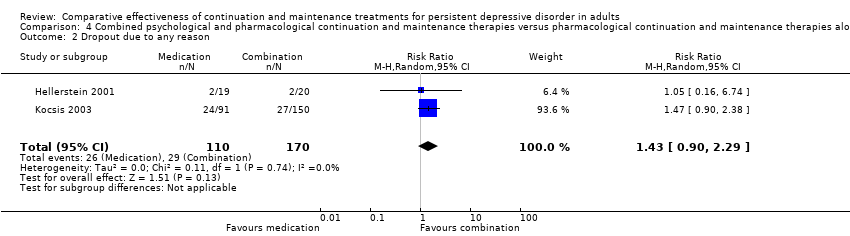
Comparison 4 Combined psychological and pharmacological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies alone, Outcome 2 Dropout due to any reason.
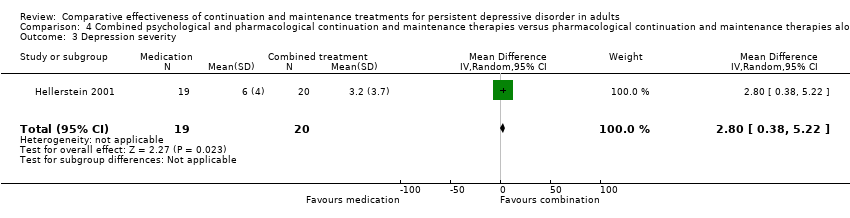
Comparison 4 Combined psychological and pharmacological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies alone, Outcome 3 Depression severity.
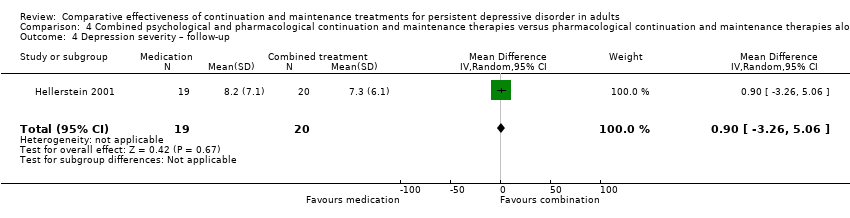
Comparison 4 Combined psychological and pharmacological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies alone, Outcome 4 Depression severity – follow‐up.

Comparison 4 Combined psychological and pharmacological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies alone, Outcome 5 Health‐related quality of life.

Comparison 4 Combined psychological and pharmacological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies alone, Outcome 6 Health‐related quality of life – follow‐up.

Comparison 5 Combined psychological and pharmacological continuation and maintenance therapies versus psychotherapeutic continuation and maintenance therapies alone, Outcome 1 Relapse/recurrence.

Comparison 5 Combined psychological and pharmacological continuation and maintenance therapies versus psychotherapeutic continuation and maintenance therapies alone, Outcome 2 Dropout due to any reason.

Comparison 6 Imipramine (TCA) versus desipramine (TCA), Outcome 1 Dropout due to any reason.
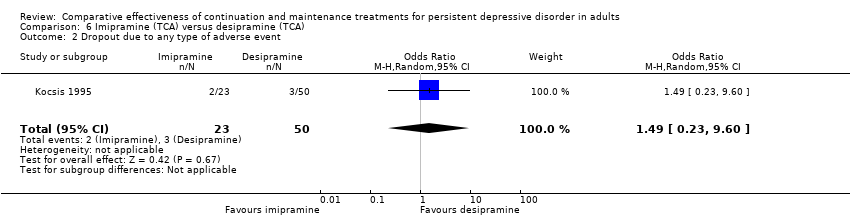
Comparison 6 Imipramine (TCA) versus desipramine (TCA), Outcome 2 Dropout due to any type of adverse event.
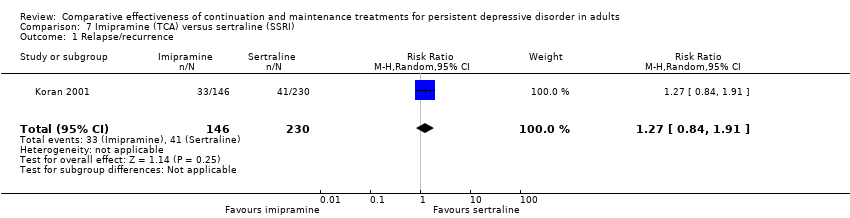
Comparison 7 Imipramine (TCA) versus sertraline (SSRI), Outcome 1 Relapse/recurrence.

Comparison 7 Imipramine (TCA) versus sertraline (SSRI), Outcome 2 Dropout due to any reason.
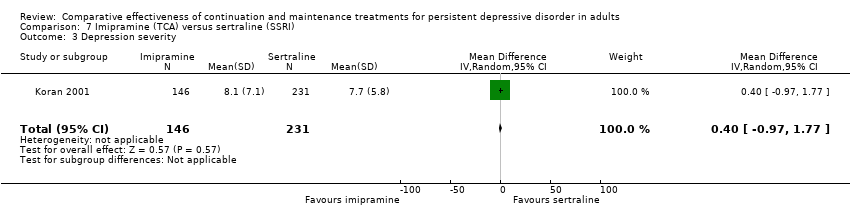
Comparison 7 Imipramine (TCA) versus sertraline (SSRI), Outcome 3 Depression severity.
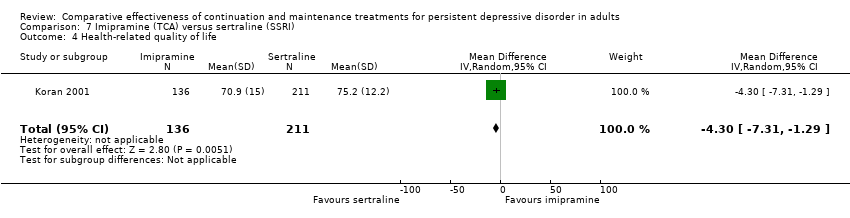
Comparison 7 Imipramine (TCA) versus sertraline (SSRI), Outcome 4 Health‐related quality of life.

Comparison 7 Imipramine (TCA) versus sertraline (SSRI), Outcome 5 Dropout due to any type of adverse event.
| Pharmacological continuation and maintenance treatment compared with placebo for persistent depressive disorder | ||||||
| Patient or population: people with persistent depressive disorder Settings: outpatient treatment Intervention: pharmacological continuation or maintenance treatment (sertraline, phenelzine, nefazodone, desipramine) Comparison: tablet placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Pharmacotherapy | |||||
| Relapse/recurrence (end of intervention) | 338 per 1000 | 139 per 1000a | RR 0.41 (0.21 to 0.79) | 383 | ⊕⊕⊕⊝b | See Characteristics of included studies table for the criteria of relapse/recurrence. |
| Dropout due to any reason (end of intervention) | 255 per 1000 | 230 per 1000a | RR 0.90 (0.39 to 2.11) | 386 | ⊕⊕⊝⊝c, d | "Dropout due to any reason" was all reported dropouts due to other reasons than relapse/recurrence. 1 study only reported dropouts in the first month of the maintenance treatment phase (Kocsis 1996). As the maintenance treatment lasted 24 months, the dropout rate in this study was very likely to be underestimated. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aAssumed risk calculated as the proportion of participants on placebo with the outcome (relapse/recurrence or dropout any) in the four included studies, multiplied by 1000. bDowngraded due to limitations in the design and implementation of available studies suggesting high likelihood of bias (there were studies with high or unclear risk of bias in almost all RoB‐Domains (except detection bias)). cDowngraded due to unexplained heterogeneity between studies (I² = 64%). Due to the small number of included studies, subgroup or meta‐regression analyses were not performed. In two studies, dropout rates were higher in the intervention group, in two studies they were lower. dDowngraded due to imprecision of results (the overall confidence interval was wide and the confidence intervals of two included studies are also very wide). | ||||||
| Related acute‐phase study | Study ID | Treatment arms | Continuation/maintenance (treatment duration) | Study design | Diagnosis |
| Keller 1998b | Sertraline Imipramine | Continuation (16 weeks) | NRCT | Chronic major depressive disorder, double depression | |
| Sertraline Placebo | Maintenance (76 weeks) | RCT | Chronic major depressive disorder, double depression | ||
| Harrison 1986 | Phenelzine Placebo | Continuation (26 weeks) | RCT | Dysthymia, double depression | |
| Keller 2000 | Nefazodone CBASP Combination | Continuation (16 weeks) | NRCT | Chronic major depressive disorder, double depression, recurrent depressive disorder with incomplete interepisode remission | |
| Nefazodone Placebo | Maintenance (52 weeks) | RCT | Chronic major depressive disorder, double depression, recurrent depressive disorder with incomplete interepisode remission | ||
| CBASP Assessment only | Maintenance (52 weeks) | RCT | Chronic major depressive disorder, double depression, recurrent depressive disorder with incomplete interepisode remission | ||
| Hellerstein 2001 | Fluoxetine Fluoxetine + group psychotherapy | Continuation (16 weeks) | RCT | Dysthymia | |
| Marin 1994 | Imipramine Desipramine | Continuation (16–20 weeks) | NRCT | Dysthymia, double depression | |
| Desipramine Placebo | Maintenance (104 weeks) | RCT | Chronic major depressive disorder, dysthymia, double depression | ||
| Desipramine Placebo | Maintenance (104 weeks) | RCT | Dysthymia | ||
| *These groups are partially overlapping (see above). CBASP: Cognitive Behavioral Analysis System of Psychotherapy; NRCT: non‐randomized controlled trial; RCT: randomized controlled trial. | |||||
| Risk of bias (ROBINS‐I tool) | Rating | Explanation of judgement | Possible ratings |
| Bias due to confounding | 5 | No information how participants were allocated to groups in the acute treatment. | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias in selection of participants into the study | 2 | Different length of drugs and procedures during acute treatment (participants of 3 protocols were included for analyses of continuation treatment). | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias in classification of interventions | 1 | Intervention was well defined: IMI and DMI were continued on an open basis at the same final dose achieved during the acute phase. | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias due to departures from intended interventions | 1 | No indication for departures from intended interventions, check of plasma levels was performed. | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias due to missing data | 2 | Proportions of missing participants differed substantially across interventions: 26% in the IMI group and 6% in the DMI group, in the IMI group 4 participants did not comply with the follow‐up assessment, reasons for dropout were reported; but proportion of dropout due to dissatisfaction with treatment was similar for IMI and DMI (7% and 6%). | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias in measurement of outcomes | 3 | Participants in the IMI protocols were seen and rated once at week 26 of treatment. Participants on the DMI protocol were seen and rated every 2 weeks through week 26. Lack of blinding: participants and raters were aware of the treatment. (see p. 214) | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias in selection of the reported result | 3 | Not all predefined outcomes were reported separately for both groups. Some data were assessed every 2 weeks, these data were not reported. In general, data were not reported for HAM‐D and GAS for the DMI vs IMI. | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| DMI: desipramine; GAS: Global Assessment Scale; HAM‐D: Hamilton Depression Rating Scale; IMI: imipramine. | |||
| Risk of bias (ROBINS‐I tool) | Rating | Explanation of judgement | Possible ratings |
| Bias due to confounding | 1 | Randomization before acute phase, exclusion of cross‐over participants. | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias in selection of participants into the study | 1 | All eligible participants were included, cross‐over participants were excluded; acute phase treatment had the same length and measurement times for all groups. | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias in classification of interventions | 1 | Intervention status was well described (planned and actual dose of medication as well as number of CBASP sessions). | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias due to departures from intended interventions | 1 | Medication doses as well as number of CBASP sessions were within the planned range. | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias due to missing data | 1 | Number of missing data was low and comparable in all groups (2–3%). | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias in measurement of outcomes | 1 | Methods of outcome assessment were comparable across intervention groups. Quote: "Trained independent evaluators unaware of treatment assignment completed the HAM‐D‐24 at each assessment visit." (p. 77), no means and standard deviations reported, unclear if other subscales were evaluated. | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias in selection of the reported result | 2 | No study protocol existed, but all measures mentioned in the methods section were reported in the outcome section. | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| CBASP: Cognitive Behavioral Analysis System of Psychotherapy; HAM‐D: Hamilton Depression Rating Scale. | |||
| Risk of bias (ROBINS‐I tool) | Rating | Explanation of judgement | Possible ratings |
| Bias due to confounding | 1 | Randomization before acute phase. | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias in selection of participants into the study | 1 | All possible participants were included (direct and cross‐over). All measures existing from the beginning of the intervention. The study flow was clearly described since acute treatment. | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias in classification of interventions | 1 | Intervention status is well defined (dose ranges are described in section 2.3 and 3.4). | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias due to departures from intended interventions | 1 | Assignment to intervention. Quote: "For both treatment groups, 10% of patients had dose increases aimed at improving outcome" (p. 31); same for both groups with regard to the main outcome; adapting dose is usual practice; no deviation from intended treatment, they counted the tablets. | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias due to missing data | 1 | Quote: "For all patients, including drop outs, pill counts indicated compliance rates of 88.7% for imipramine and 84.7% for sertraline. No differences were found between diagnostic groups or between acute and crossover patients." (p. 31); Proportions of and reasons for missing participants were similar across intervention groups; less than 5% dropout for the main outcome; proportions of missing data were comparable and are addressed in the analyses with LOCF. | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias in measurement of outcomes | 1 | Information from Rush et al., 1998 (study protocol): reliable ratings (p. 593); quote: "continued on the same double‐blind medication dose for an additional 16 weeks" (p. 593); assessment methods comparable across groups. | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias in selection of the reported result | 2 | Outcomes correspond to the ones named in the protocol, but protocol just for acute phase; not all measures used during the acute phase were used in continuation phase. | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| LOCF: last observation carried forward. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse/recurrence Show forest plot | 4 | 383 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.21, 0.79] |
| 2 Dropout due to any reason Show forest plot | 4 | 386 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.39, 2.11] |
| 3 Depression severity Show forest plot | 3 | 333 | Mean Difference (IV, Random, 95% CI) | ‐4.79 [‐8.49, ‐1.09] |
| 4 SF‐36 Social Functioning score Show forest plot | 1 | 161 | Mean Difference (IV, Random, 95% CI) | 10.80 [3.04, 18.56] |
| 5 SF‐36 Emotional Role score Show forest plot | 1 | 161 | Mean Difference (IV, Random, 95% CI) | 20.70 [7.43, 33.97] |
| 6 SF‐36 Role Physical score Show forest plot | 1 | 161 | Mean Difference (IV, Random, 95% CI) | 2.10 [‐9.76, 13.96] |
| 7 Dropout due to any type of adverse event Show forest plot | 3 | 333 | Odds Ratio (M‐H, Random, 95% CI) | 3.53 [0.67, 18.70] |
| 8 Any type of adverse event Show forest plot | 1 | 161 | Odds Ratio (M‐H, Random, 95% CI) | 1.47 [0.70, 3.09] |
| 9 Relapse/recurrence sensitivity analysis Show forest plot | 4 | 360 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.16, 0.89] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse/recurrence Show forest plot | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.14, 0.93] |
| 2 Dropout due to any reason Show forest plot | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.41, 1.81] |
| 3 Depression severity Show forest plot | 1 | 82 | Mean Difference (IV, Random, 95% CI) | ‐4.00 [‐7.05, ‐0.95] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse/recurrence Show forest plot | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.43, 3.49] |
| 2 Dropout due to any reason Show forest plot | 1 | 179 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.30, 1.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse/recurrence Show forest plot | 1 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.44, 3.44] |
| 2 Dropout due to any reason Show forest plot | 2 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.90, 2.29] |
| 3 Depression severity Show forest plot | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 2.8 [0.38, 5.22] |
| 4 Depression severity – follow‐up Show forest plot | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐3.26, 5.06] |
| 5 Health‐related quality of life Show forest plot | 1 | 35 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐1.63, 0.63] |
| 6 Health‐related quality of life – follow‐up Show forest plot | 1 | 33 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐0.56, 1.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse/recurrence Show forest plot | 1 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.57, 4.01] |
| 2 Dropout due to any reason Show forest plot | 1 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.45, 1.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dropout due to any reason Show forest plot | 1 | 73 | Risk Ratio (M‐H, Random, 95% CI) | 4.35 [1.19, 15.87] |
| 2 Dropout due to any type of adverse event Show forest plot | 1 | 73 | Odds Ratio (M‐H, Random, 95% CI) | 1.49 [0.23, 9.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse/recurrence Show forest plot | 1 | 376 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.84, 1.91] |
| 2 Dropout due to any reason Show forest plot | 1 | 386 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.48, 1.38] |
| 3 Depression severity Show forest plot | 1 | 377 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.97, 1.77] |
| 4 Health‐related quality of life Show forest plot | 1 | 347 | Mean Difference (IV, Random, 95% CI) | ‐4.30 [‐7.31, ‐1.29] |
| 5 Dropout due to any type of adverse event Show forest plot | 1 | 386 | Odds Ratio (M‐H, Random, 95% CI) | 1.99 [0.60, 6.65] |

