Mindfulness‐based stress reduction for family carers of people with dementia
Abstract
Background
Caring for people with dementia is highly challenging, and family carers are recognised as being at increased risk of physical and mental ill‐health. Most current interventions have limited success in reducing stress among carers of people with dementia. Mindfulness‐based stress reduction (MBSR) draws on a range of practices and may be a promising approach to helping carers of people with dementia.
Objectives
To assess the effectiveness of MBSR in reducing the stress of family carers of people with dementia.
Search methods
We searched ALOIS ‐ the Cochrane Dementia and Cognitive Improvement Group's Specialized Register, the Cochrane Central Register of Controlled Trials (CENTRAL) (all years to Issue 9 of 12, 2017), MEDLINE (Ovid SP 1950 to September 2017), Embase (Ovid SP 1974 to Sepetmber 2017), Web of Science (ISI Web of Science 1945 to September 2017), PsycINFO (Ovid SP 1806 to September 2017), CINAHL (all dates to September 2017), LILACS (all dates to September 2017), World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), ClinicalTrials.gov, and Dissertation Abstracts International (DAI) up to 6 September 2017, with no language restrictions.
Selection criteria
Randomised controlled trials (RCTs) of MBSR for family carers of people with dementia.
Data collection and analysis
Two review authors independently screened references for inclusion criteria, extracted data, assessed the risk of bias of trials with the Cochrane 'Risk of bias' tool, and evaluated the quality of the evidence using the GRADE instrument. We contacted study authors for additional information, then conducted meta‐analyses, or reported results narratively in the case of insufficient data. We used standard methodological procedures expected by Cochrane.
Main results
We included five RCTs involving 201 carers assessing the effectiveness of MBSR. Controls used in included studies varied in structure and content. Mindfulness‐based stress reduction programmes were compared with either active controls (those matched for time and attention with MBSR, i.e. education, social support, or progressive muscle relaxation), or inactive controls (those not matched for time and attention with MBSR, i.e. self help education or respite care). One trial used both active and inactive comparisons with MBSR. All studies were at high risk of bias in terms of blinding of outcome assessment. Most studies provided no information about selective reporting, incomplete outcome data, or allocation concealment.
1. Compared with active controls, MBSR may reduce depressive symptoms of carers at the end of the intervention (3 trials, 135 participants; standardised mean difference (SMD) ‐0.63, 95% confidence interval (CI) ‐0.98 to ‐0.28; P<0.001; low‐quality evidence). We could not be certain of any effect on clinically significant depressive symptoms (very low‐quality evidence).
Mindfulness‐based stress reduction compared with active control may decrease carer anxiety at the end of the intervention (1 trial, 78 participants; mean difference (MD) ‐7.50, 95% CI ‐13.11 to ‐1.89; P<0.001; low‐quality evidence) and may slightly increase carer burden (3 trials, 135 participants; SMD 0.24, 95% CI ‐0.11 to 0.58; P=0.18; low‐quality evidence), although both results were imprecise, and we could not exclude little or no effect. Due to the very low quality of the evidence, we could not be sure of any effect on carers' coping style, nor could we determine whether carers were more or less likely to drop out of treatment.
2. Compared with inactive controls, MBSR showed no clear evidence of any effect on depressive symptoms (2 trials, 50 participants; MD ‐1.97, 95% CI ‐6.89 to 2.95; P=0.43; low‐quality evidence). We could not be certain of any effect on clinically significant depressive symptoms (very low‐quality evidence).
In this comparison, MBSR may also reduce carer anxiety at the end of the intervention (1 trial, 33 participants; MD ‐7.27, 95% CI ‐14.92 to 0.38; P=0.06; low‐quality evidence), although we were unable to exclude little or no effect. Due to the very low quality of the evidence, we could not be certain of any effects of MBSR on carer burden, the use of positive coping strategies, or dropout rates.
We found no studies that looked at quality of life of carers or care‐recipients, or institutionalisation.
Only one included study reported on adverse events, noting a single adverse event related to yoga practices at home
Authors' conclusions
After accounting for non‐specific effects of the intervention (i.e. comparing it with an active control), low‐quality evidence suggests that MBSR may reduce carers' depressive symptoms and anxiety, at least in the short term.
There are significant limitations to the evidence base on MBSR in this population. Our GRADE assessment of the evidence was low to very low quality. We downgraded the quality of the evidence primarily because of high risk of detection or performance bias, and imprecision.
In conclusion, MBSR has the potential to meet some important needs of the carer, but more high‐quality studies in this field are needed to confirm its efficacy.
PICO
Plain language summary
Mindfulness‐based stress reduction for family carers of people with dementia
Review question
How effective is mindfulness‐based stress reduction (MBSR) in reducing stress‐related problems of family carers of people with dementia?
Background
Dementia has become a public health burden worldwide. Caring for people with dementia is highly stressful, thus carers are more likely to suffer from psychological problems, such as depression and anxiety, than general population. Mindfulness‐based stress reduction is a potentially promising intervention to target these issues. More information is needed about whether MBSR can help family carers of people with dementia.
Study characteristics
We searched for evidence up to September 2017 and found five randomised controlled trials (clinical trials where people are randomly assigned to one of two or more treatment groups) comparing MBSR to a variety of other interventions. We reported the effects of MBSR programmes compared with active controls (interventions in which participants received a similar amount of attention to those in the MBSR group, such as social support or progressive muscle relaxation) or inactive controls (interventions in which participants received less attention than those in the MBSR group, such as self help education).
Key results
We were able to analyse study data from five randomised controlled trials involving a total of 201 carers. Findings from three studies (135 carers) showed that carers receiving MBSR may have a lower level of depressive symptoms at the end of treatment than those receiving an active control treatment. However, we found no clear evidence of any effect on depression when MBSR was compared with an inactive control treatment. Mindfulness‐based stress reduction may also lead to a reduction in carers' anxiety symptoms at the end of treatment. Mindfulness‐based stress reduction may slightly increase carers' feelings of burden. However, the results on anxiety and burden were very uncertain. We were unable to draw conclusions about carers' coping strategies and the risk of dropping out of treatment due to the very low quality of the evidence.
None of the studies measured quality of life of carers or people with dementia, or the rate of admission of people with dementia to care homes or hospitals.
Only one included study reported on adverse events, noting one minor adverse event (neck strain in one participant practising yoga at home)
Quality of the evidence
We considered the quality of the evidence to be low or very low, mainly because the studies were small and the way they were designed or conducted put them at risk of giving biased results. Consequently, we have limited confidence in the results.
Conclusion
To summarise, the review provides preliminary evidence on the effect of MBSR in treating some stress‐related problems of family carers of people with dementia. More good‐quality studies are needed before we can confirm whether or not MBSR is beneficial for family carers of people with dementia.
Authors' conclusions
Summary of findings
| MBSR (mindfulness‐based stress reduction) compared to active control for family carers of people with dementia | ||||||
| Patient or population: family carers of people with dementia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with active control | Risk with MBSR | |||||
| Depressive symptoms | ‐ | SMD 0.63 SD lower | ‐ | 135 | ⊕⊕⊝⊝ | Moderate effect size;c lower score represents lower depressive symptoms |
| Anxiety | The mean anxiety was 47.4 score. | MD 7.5 score lower | ‐ | 78 | ⊕⊕⊝⊝ | Moderate effect size;c lower score represents lower level of anxiety |
| Carer burden | ‐ | SMD 0.24 SD higher | ‐ | 135 | ⊕⊕⊝⊝ | Small effect size;c lower score represents lower level of carer burden |
| Dropout rates | Study population | RR 1.39 | 166 | ⊕⊝⊝⊝ | Important effect sizee | |
| 88 per 1000 | 122 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWe downgraded the quality of evidence by one level due to serious concern about high risk of bias in blinding of participants and personnel. | ||||||
| MBSR (mindfulness‐based stress reduction) compared to inactive control for family carers of people with dementia | ||||||
| Patient or population: family carers of people with dementia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with inactive control | Risk with MBSR | |||||
| Depressive symptoms | The mean depressive symptoms was 14.2 score. | MD 1.97 score lower | ‐ | 50 | ⊕⊕⊝⊝ | Small effect size;c lower score represents lower depressive symptoms |
| Anxiety | The mean anxiety was 47.8 score. | MD 7.27 score lower | ‐ | 33 | ⊕⊕⊝⊝ | Moderate effect size;c lower score represents lower level of anxiety |
| Carer burden | The mean carer burden was 26.4 score. | MD 1.6 score lower | ‐ | 17 | ⊕⊕⊝⊝ | Small effect size;c lower score represents lower level of carer burden |
| Dropout rates | Study population | RR 2.00 | 20 | ⊕⊝⊝⊝ | Important effect sizee | |
| 100 per 1000 | 200 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aAs suggested by Ryan 2016a, we downgraded the quality of evidence by one level due to serious concern about imprecision. | ||||||
Background
Description of the condition
Dementia has become a public health priority due to the ageing population. Approximately 46.8 million people are estimated to live with dementia globally, in low‐ and high‐income regions, and the figure increases by 9.9 million annually (Prince 2015). Dementia leads to progressive cognitive deficits, functional impairment, and behavioural changes. Over time, people with dementia become unable to function independently and require increasing amounts of care from others. Such care lasts for a median of 6.5 years and is most often provided by family members of the person with dementia (Haley 1997).
Caring for relatives with dementia is highly challenging. Indeed, family carers of people with dementia are vulnerable to a range of physical and psychological morbidities, including cardiovascular diseases (Mausbach 2007), depression, anxiety (Cooper 2007; Cuijpers 2005), and even early mortality (Schulz 1999). These and other issues may negatively influence carers' quality of life (Thomas 2006). Carers who feel overburdened are likely to institutionalise people with dementia at an earlier stage, putting pressure on the public healthcare budget (Spijker 2008). Consequently, there is a great need to help family carers of people with dementia to cope with the stress they encounter.
Various psychosocial interventions have been developed for family carers of people with dementia (Acton 2001; Peacock 2003; Pinquart 2006; Selwood 2007; Sörensen 2002). These interventions often include a number of components and aim variously to provide knowledge, practical skills (e.g. behaviour management or communication skills), social support and stress reduction. Their efficacy in reducing the distress felt by family carers is generally mild to moderate (Acton 2001; Peacock 2003; Pinquart 2006; Selwood 2007; Sörensen 2002). Furthermore, no matter how skilful or competent carers are, they are highly likely to experience stress and to feel overwhelmed at times in real‐life situations. This is where the skills of mindfulness may come in, allowing carers to relate to the challenges they face in new ways (Kabat‐Zinn 2013).
Description of the intervention
Mindfulness is described as "the awareness that emerges through paying attention on purpose, in the present moment, and non‐judgmentally to the unfolding of experience" (Kabat‐Zinn 2013). Mindfulness‐based stress reduction (MBSR) is a widely used programme that involves a range of practices with a focus on stress reduction, such as gentle mindful movement (awareness of the body), a body scan (to nurture awareness of the body region by region), and meditation (awareness of the breath) (Cullen 2011).
How the intervention might work
It is common for people caring for a relative with dementia to worry and ruminate over past events or over an uncontrollable future related to the progression of the illness. Worry and rumination are closely related to the distress felt by carers of people with dementia. With a relatively intensive mindfulness training in MBSR, the carers of people with dementia can learn to be present with the difficulties they face, and to accept unconditionally their own emotions and thoughts (which may be distressing or dysfunctional). This process of present‐moment awareness and non‐judgmental acceptance has been reported to reduce worry and rumination (Borders 2010; Jain 2007).
Mindfulness‐based stress reduction has shown promising benefits in clinical and non‐clinical populations, including people facing chronic pain, coping with cancer, or parenting children with autism, among others (Baer 2003; Grossman 2004; Keng 2011). The positive effects across various samples indicate that mindfulness training might enhance the ability to cope with stress even in extraordinary circumstances, such as dementia caring (Oken 2010; Whitebird 2013).
Why it is important to do this review
The amount of research on MBSR for carers of people with dementia has increased in recent years (Brown 2016; Oken 2010; Whitebird 2013). It is important to conduct a systematic review and meta‐analysis to synthesise the evidence. Previous reviews on a similar topic did not use a systematic search strategy (Hurley 2014); did not provide a quantitative synthesis by using meta‐analytic techniques (Hurley 2014; Jaffray 2015; Li 2016); or did not focus specifically on the population of family carers of people with dementia (Dharmawardene 2016; Jaffray 2015; Li 2016). In this review we have comprehensively searched the published and unpublished literature, and quantitatively evaluated the evidence for MBSR for family carers of people with dementia.
Objectives
To assess the effectiveness of MBSR in reducing the stress of family carers of people with dementia.
Methods
Criteria for considering studies for this review
Types of studies
We included individually randomised, parallel‐group controlled trials. We did not consider cluster‐randomised or cross‐over trials.
Types of participants
The participants were family carers of people with any type of dementia living in the community. The relationship of family carers to people with dementia could be spouses, children, other family members, friends, or neighbours. We did not consider care workers who were paid to provide care to people with dementia.
Types of interventions
We included studies of MBSR, which was developed by Jon Kabat‐Zinn in 1979 (Kabat‐Zinn 2013). The standard MBSR programme might be slightly adapted for the study population to accommodate their physical limitations or time commitment, or both. We applied no restrictions to the 'dosage' (i.e. the number or length of individual sessions or the duration of the trials), units of delivery (i.e. groups, individuals, or a mixture of them), or mode of delivery (i.e. face‐to‐face, telephone‐based, Internet‐based, or others).
Acceptable comparators were active‐control interventions, waiting list, or usual care. Specifically, 'active‐control interventions' refers to interventions matched in time and attention with MBSR, with the aim to control for the non‐specific effects of MBSR programmes (such as contact with researchers or social support from other participants). Participants in waiting‐list controls had the opportunity to receive MBSR after a waiting period. Participants in usual care controls could receive the support which was usually available in their community.
Types of outcome measures
We included outcomes measured at the end of the intervention period and at later follow‐ups.
Primary outcomes
-
Depressive symptoms. If the study specified a cut‐off score to identify clinically significant depressive symptoms,we also reported the presence of clinically significant depressive symptoms as an outcome.
Secondary outcomes
For carers:
-
anxiety;
-
carer burden;
-
coping style;
-
quality of life;
-
dropout rates.
For people with dementia:
-
quality of life;
-
institutionalisation.
Search methods for identification of studies
We identified trials for inclusion by searching electronic databases and other resources.
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois), the Cochrane Dementia and Cognitive Improvement Group Specialized Register, on 6 September 2017. The search terms used were: mindfulness OR meditation.
ALOIS is a study‐based register and is maintained by the Information Specialists for the Cochrane Dementia and Cognitive Improvement Group (CDCIG). It contains studies in the areas of dementia prevention, dementia treatment, and cognitive enhancement in healthy people.
We identified studies for inclusion from the following sources.
-
Monthly searches of a number of major healthcare databases: MEDLINE, Embase, CINAHL (Cumulative Index to Nursing and Allied Health Literature), PsycINFO, and LILACS (Latin American and Caribbean Health Science Information Database).
-
Monthly searches of a number of trial registers: ISRCTN; UMIN (Japan's Trial Register); the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) Search Portal (which covers ClinicalTrials.gov; ISRCTN; the Chinese Clinical Trials Register; the German Clinical Trials Register; the Iranian Registry of Clinical Trials; and the Netherlands National Trials Register, as well as others).
-
Quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library).
-
Six‐monthly searches of a number of grey literature sources: ISI Web of Science Conference Proceedings; Index to Theses; Australasian Digital Theses.
Details of the search strategies used for the retrieval of reports of trials from the healthcare databases, CENTRAL, and conference proceedings can be viewed on the Dementia and Cognitive Improvement website (dementia.cochrane.org/searches).
We performed additional searches in many of the sources listed above to cover the time frame from the last searches performed for ALOIS to ensure that the review search was as up‐to‐date and comprehensive as possible. The search strategies used are presented in Appendix 1.
Searching other resources
We checked the reference lists of all relevant reviews to identify more trials (Dharmawardene 2016; Hurley 2014; Jaffray 2015; Li 2016). We sought unpublished data by contacting researchers and other people with an interest in the field. Where possible, we also contacted the corresponding authors of identified randomised controlled trials for additional information about other relevant studies. There were no language restrictions.
Data collection and analysis
We used Review Manager 5 for Mac to conduct data entry and calculation of effect sizes (RevMan 2014), and Stata/SE version 14.1 for Mac (StataCorp LP) to conduct our investigation of heterogeneity and publication bias, and subgroup, meta‐regression, and sensitivity analyses.
Selection of studies
Two review authors (ZL, YYS) independently selected studies in two stages. First, we screened the titles and abstracts of citations obtained by the searches. Second, we obtained the full texts of potentially eligible studies to identify whether studies fulfilled the inclusion criteria. Any disagreements were resolved by discussion or by consulting a third review author (BLZ). We listed the studies that we excluded at the full‐text stage with detailed reasons for their exclusion in the Characteristics of excluded studies table.
Data extraction and management
Two review authors (ZL, YYS) independently extracted data based on the recommendations in Section 7.3.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). The collected information included the authors; publication date; country; funding source; study design; eligibility criteria; characteristics of the study population (including age, gender, and relationship to person with dementia); characteristics of interventions (sessions, dosage, duration, mode of delivery); types and contents of comparators; outcomes and results. For results, we collected outcome measures used, time of assessment, and statistics (numbers of participants, means, standard deviations or other summary statistics). Any disagreements were resolved by discussion or by consulting a third review author (BLZ). If important information was unreported, we contacted the original investigators for the missing information. We presented the information in the Characteristics of included studies table.
Assessment of risk of bias in included studies
Two review authors (ZL, YYS) independently assessed the risk of bias of included studies using the Cochrane 'Risk of bias' tool (Higgins 2011b). The sources of bias included the following.
-
Selection bias. We assessed random sequence generation and allocation concealment.
-
Performance bias. Although it is not possible to blind personnel or participants (or both) to psychosocial interventions of this nature, we nevertheless made judgements about the potential influence of performance bias on treatment effects. For instance, performance bias may be less of a concern for interventions delivered via the Internet than for those delivered face‐to‐face.
-
Detection bias. We judged whether outcome assessors were blinded to allocation.
-
Attrition bias. We appraised the comparability of carer characteristics between the completers and the dropouts and the methods used by the study to deal with missing data, including whether or not there was an intention‐to‐treat analysis.
-
Reporting bias. We searched for protocols of included trials, and then determined if all of the outcomes listed in the protocols were reported in the trials.
-
Other bias. We mainly described inexplicable baseline imbalances (presumably if these occurred despite a low risk of bias in the domains mentioned) on the variables known to impact carer stress (including gender, age, relationship to individuals with dementia, and baseline assessment of depression or anxiety) under this domain.
We rated the risk of bias in each domain as 'high risk', 'unclear risk', or 'low risk' according to the Cochrane 'Risk of bias' tool (Higgins 2011b). Any disagreements were resolved by discussion or by consulting a third review author (BLZ). We requested missing information related to the 'Risk of bias' assessment from the original investigators.
We included all studies in the initial analyses. We excluded studies assessed as being at high risk of selection, detection, or attrition bias in sensitivity analyses (see Sensitivity analysis).
Measures of treatment effect
For dichotomous data, we used the risk ratio (RR) as the measure of treatment effect with its 95% confidence interval (CI). For continuous data, we calculated the standardised mean difference (SMD) if trials used different psychometric scales, or the mean difference (MD) if trials used the same scale, with 95% CIs. If possible, we used immediate postintervention values to calculate the main treatment effect.
Unit of analysis issues
The participant allocated to a comparison group in the included trials was the unit of analysis.
Dealing with missing data
We evaluated the missing data and dropout rates for each included trial, and we displayed the results in this full review. If necessary, we requested the missing data from the original investigators. We preferred intention‐to‐treat analyses from the included studies for meta‐analysis. We reported any imputation methods used in the original studies.
Assessment of heterogeneity
We assessed clinical or methodological variation across studies by comparing important characteristics of interventions (e.g. duration, intensity), participants (e.g. age, gender), and the comparisons (whether the control groups were active or inactive). We assessed statistical heterogeneity by visual inspection of forest plots, test of significant level (P value), and the I2 statistic. We rated the level of heterogeneity across studies as low (I2 = 25%), moderate (I2 = 50%), or high (I2 = 75%) (Higgins 2003). The methods we employed to investigate heterogeneity included sensitivity, subgroup, and meta‐regression analyses (a detailed description is given in Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
We did not assess reporting biases, as a minimum of 10 studies is required for the meaningful interpretation of funnel plots (Egger 1997; Sterne 2011).
Data synthesis
We undertook a meta‐analysis only if we judged elements of trials (including participants, interventions, comparisons, and outcomes) to be sufficiently similar and essential data were available. Due to a considerable amount of clinical heterogeneity across included studies, we used the random‐effects model to pool the results by inverse variance methods. When meta‐analysis was not appropriate, we presented the findings of these studies narratively. Our main analyses were effects of MBSR at the end of treatment. If studies assessed the persistence of intervention effects with post‐treatment follow‐ups, we conducted separate analyses for outcomes measured within three months, three to six months, and six months to one year from the end of treatment.
Subgroup analysis and investigation of heterogeneity
If we identified moderate or high levels of heterogeneity in the meta‐analyses, we carefully considered the following characteristics as potential effect modifiers:
-
nature and dose of the active intervention;
-
nature of the control intervention;
-
levels of carers' depressive symptoms at baseline;
-
levels of carer burden at baseline.
If there were sufficient studies, we considered conducting subgroup analyses based on these potential effect modifiers.
Sensitivity analysis
We conducted sensitivity analyses for the following considerations.
-
To investigate heterogeneity, we excluded any individual studies for which the 95% CI of the treatment effect did not overlap with others.
-
We compared the pooled results by excluding individual studies at high risk of selection, detection, or attrition bias, as described in Assessment of risk of bias in included studies.
-
We compared the pooled results of the effects of MBSR on the reduction of levels of carer burden that was measured by different scales in the same study.
Presentation of results: GRADE and 'Summary of findings' tables
We used GRADE methods to rate the quality of the evidence (high, moderate, low, or very low) behind each effect estimate in the review (Guyatt 2011). This rating refers to our level of confidence that the estimate reflects the true effect, taking into account the risk of bias in the included studies, inconsistency between studies, imprecision in the effect estimate, indirectness in addressing our review question, and the risk of publication bias. We produced 'Summary of findings' tables for the comparisons MBSR versus active or inactive controls for family carers of people with dementia, including the following outcomes:
-
depressive symptoms of carers;
-
anxiety of carers;
-
carer burden;
-
dropout rates of carers.
Results
Description of studies
For a detailed description of trials, see: Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies; and Characteristics of studies awaiting classification sections.
Results of the search
Our comprehensive literature searches identified 403 records, of which 301 were left after duplicates were removed. We excluded 265 records based on screening of title/abstract. We obtained the full‐text articles for the remaining 36 records, which we screened for inclusion or exclusion (see Figure 1 for the PRISMA flow diagram). We included five trials in the review. We also identified one relevant study awaiting classification (see Characteristics of studies awaiting classification) and one relevant ongoing study (see Characteristics of ongoing studies).

Study flow diagram.
Included studies
A detailed description of the characteristics of the five included trials is provided in Characteristics of included studies. The following is a succinct overview.
Study populations
Three of the included trials recruited only family carers of people with dementia (Brown 2016; Oken 2010; Whitebird 2013); the remaining two studies recruited carers of people with dementia and other neurocognitive disorders, O'Donnell 2013, or chronic disease (Hou 2014). We included only the subsample of carers of people with dementia from Hou 2014 in this review. Sample sizes in the final analyses ranged from 24 in O'Donnell 2013 to 141 in Hou 2014. The mean age of the carers ranged from 57.5 in Hou 2014 to 71.3 in O'Donnell 2013, reflecting the carers' varied relationships to the people with dementia. The proportion of spousal carers ranged from 26% in Whitebird 2013 to 86% in O'Donnell 2013; other carers were adult offspring, other relatives, or friends. More than 80% of carers in all the included studies were women. Two of the included trials specified levels of carer burden or stress as an inclusion criterion (Hou 2014; Whitebird 2013).
Interventions
The included interventions were modelled on original MBSR in Kabat‐Zinn 2013, with six to eight weekly sessions covering body scan, mindful hatha yoga, sitting meditation, and other mindfulness practices. One trial also employed a cognitive theoretical framework in addition to MBSR (Oken 2010).
Comparisons
The Oken 2010 study used two comparison arms (group education (active) and respite only (inactive)). The other trials each had one comparison arm. These were education and social support (active) (Brown 2016; Whitebird 2013), progressive muscle relaxation (active) (O'Donnell 2013), and self help (inactive) (Hou 2014).
Outcomes
The most commonly defined primary outcome was depressive symptoms of carers. Studies reported measuring depressive symptoms of carers using the Center for Epidemiological Studies Depression Scale (CESD; Radloff 1977), the Geriatric Depression Scale (GDS; Yesavage 1983), or the depressive symptoms subscale of the Profile of Mood States (POMS; McNair 1971). Two included studies also identified clinically significant depressive symptoms, defined as a score of 4 or more on the GDS (O'Donnell 2013), or a score of 16 or more on the CESD (Hou 2014). Two included studies assessed carers' levels of anxiety using the State‐Trait Anxiety Inventory (STAI; Spielberger 1983). Two trials measured carer burden with the Zarit Burden Interview (ZBI; Zarit 1980); one trial used the subjective stress burden subscale of the Montgomery Borgatta Caregiver Burden Scale (MBCBS; Montgomery 2002); another trial used both the Revised Memory and Behavior Problems Checklist (RMBPC; Teri 1992), which was specifically designed for dementia studies, and the Caregiver Appraisal Tool (CAT; Lawton 1989), which was more general to carer studies. One included trial measured coping style using the Coping Responses Inventory (CRI; Moos 1988). No included studies reported on quality of life of carers or people with dementia, or on institutionalisation.
The MBSR programme included in two of the studies had been modified to accommodate the physical limitations of carers (Brown 2016; O'Donnell 2013). Only one study, Hou 2014, reported adverse events occurring when practicing yoga at home (it was unclear whether any adverse events occurred in the other studies). Reports of dropout rates in the MBSR group ranged from 2.6% in Whitebird 2013 to 20% in O'Donnell 2013 and Oken 2010.
Excluded studies
We excluded 28 studies (29 articles) after full‐text screening with detailed reasons (see Characteristics of excluded studies). The main reason for exclusion was that the intervention was not MBSR.
Risk of bias in included studies
For an overview of our judgements about each 'Risk of bias' item for individual trials and across all trials, see Figure 2 and Figure 3. For details on the risk of bias of the included trials, see Characteristics of included studies.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Three studies reported adequate sequence generation (low risk of bias), but two were assessed as having an unclear risk of bias in this domain. We rated two studies as at low risk and three as unclear risk of bias for allocation concealment.
Blinding
As it is not possible to blind personnel or participants (or both) to psychosocial interventions of this nature, we rated the risk of performance bias in all included studies as high. This was also true of detection bias due to the lack of blinding and the self report tools used to measure outcomes in the included studies.
Incomplete outcome data
We assessed the risk of attrition bias based on the percentage of missing data, the reasons given for loss of participants in each group, and the method that studies used to deal with incomplete outcome data. We rated two studies as high risk and the remaining studies as unclear risk, mainly due to incomplete information (e.g. concerning the reasons for attrition or the imputation method used for missing data).
Selective reporting
To assess selective outcome reporting, we checked whether publications reported all outcomes described in a protocol. We rated all included trials as being at unclear risk of reporting bias.
Other potential sources of bias
Regarding comparability of baseline characteristics between groups, we rated one study as high risk, one as unclear risk, and the remaining studies as low risk of bias.
Effects of interventions
See: Summary of findings for the main comparison MBSR compared to active control for family carers of people with dementia; Summary of findings 2 MBSR compared to inactive control for family carers of people with dementia
See summary of findings Table for the main comparison and summary of findings Table 2.
1. MBSR versus active controls immediately after the intervention period
Four trials compared MBSR with active‐control interventions (i.e. education, social support, or progressive muscle relaxation).
Primary outcomes
Depressive symptoms
Three trials reported depressive symptoms of carers as continuous outcomes that could be meta‐analysed. Pooling the effects in a random‐effects meta‐analysis demonstrated that MBSR might decrease depressive symptoms of carers compared with the active‐control interventions: standardised mean difference (SMD) ‐0.63 (95% confidence interval (CI) ‐0.98 to ‐0.28; P<0.001; 3 trials; 135 participants; low‐quality evidence, downgraded one level for serious concern about high risk of bias, and one level for serious concern about imprecision) (Analysis 1.1; Figure 4). One trial also reported carer depression as a categorical outcome. Due to the very low quality of the evidence, we were uncertain whether or not MBSR had any effect on the risk of clinically significant depressive symptoms when compared with progressive muscle relaxation: risk ratio (RR) 0.33 (95% CI 0.04 to 2.77; P = 0.31; 1 trial; 24 participants; very low‐quality evidence, downgraded two levels for very serious concern about imprecision and one level for serious concern about high risk of bias) (Analysis 1.2).

Forest plot of comparison: 1 MBSR versus active control at immediately postintervention, outcome: 1.1 Depressive symptoms.
Secondary outcomes
Anxiety
Mindfulness‐based stress reduction may reduce carers' anxiety compared with the active‐control group (i.e. education and social support) immediately after the intervention period: mean difference (MD) ‐7.50 (95% CI ‐13.11 to ‐1.89; P = 0.009; 1 trial; 78 participants; low‐quality evidence, downgraded one level for serious concern about imprecision and one level for serious concern about high risk of bias) (Analysis 1.3).
Carer burden
Three trials reported data on carer burden that could be meta‐analysed. Pooling the effects in a random‐effects meta‐analysis showed that MBSR may slightly increase levels of carer burden compared with active controls immediately after the intervention period, but the result was too imprecise for us to be sure of the size or direction of the effect: SMD 0.24 (95% CI ‐0.11 to 0.58; P = 0.18; 3 trials; 135 participants; low‐quality evidence, downgraded one level for serious concern about imprecision and one level for serious concern about high risk of bias) (Analysis 1.4; Figure 5).

Forest plot of comparison: 1 MBSR versus active control at immediately postintervention, outcome: 1.4 Carer burden.
Coping style
Due to the very low quality of the evidence, we were uncertain whether or not MBSR might improve the use of positive coping strategies compared with the active‐control group (i.e. education) immediately after the intervention period: MD 6.00 (95% CI ‐4.59 to 16.59; P = 0.27; 1 trial; 19 participants; very low‐quality evidence, downgraded two levels for very serious concern about imprecision and one level for high risk of bias) (Analysis 1.5).
Carers' quality of life
None of the included trials reported this outcome.
Dropout rates
Four trials reported data on rates of dropout from treatment that could be meta‐analysed. Pooling the effects in a random‐effects meta‐analysis, we were uncertain, due to the very low quality of the evidence, whether or not there was a higher risk of dropout in the MBSR group than in the active‐control group: RR 1.39 (95% CI 0.32 to 5.99; P = 0.66; 4 trials; 166 participants; very low‐quality evidence, downgraded two levels for very serious concern about imprecision and one level for high risk of bias) (Analysis 1.6; Figure 6).

Forest plot of comparison: 1 MBSR versus active control at immediately postintervention, outcome: 1.7 Dropout rates.
Quality of life of people with dementia
None of the included trials reported this outcome.
Institutionalisation
None of the included trials reported this outcome.
2. MBSR versus inactive controls immediately after the intervention period
Two trials compared MBSR with inactive controls (i.e. self help education or respite‐only control). We considered outcomes immediately after the end of the intervention period for each trial.
Primary outcomes
Depressive symptoms
Two trials reported data on depressive symptoms of carers that could be meta‐analysed. Pooling the effects in a random‐effects meta‐analysis, there was no clear effect on depressive symptoms of carers in the MBSR group compared with the inactive controls immediately after the intervention period; the result was imprecise and compatible with an effect in either direction: MD ‐1.97 (95% CI ‐6.89 to 2.95; P = 0.43; 2 trials; 50 participants; low‐quality evidence, downgraded one level for serious concern about imprecision and one level for high risk of bias) (Analysis 2.1; Figure 7). One trial reported carer depression as a categorical outcome. Due to the very low quality of the evidence, we were uncertain whether or not MBSR may reduce the risk of clinically significant depressive symptoms compared with the inactive control (i.e. self help education) immediately after the intervention: RR 0.69 (95% CI 0.26 to 1.78; P = 0.44; 1 trial; 33 participants; very low‐quality evidence, downgraded two levels for very serious concern about imprecision and one level for high risk of bias) (Analysis 2.2).

Forest plot of comparison: 2 MBSR versus inactive control at immediately postintervention, outcome: 2.1 Depressive symptoms.
Secondary outcomes
Anxiety
The results showed that MBSR may reduce carers' levels of anxiety compared with the inactive control (i.e. self help education) immediately after the intervention period, although due to imprecision, we could not exclude little or no effect: MD ‐7.27 (95% CI ‐14.92 to 0.38; P = 0.06; 1 trial; 33 participants; low‐quality evidence, downgraded one level for serious concern about imprecision and one level for high risk of bias) (Analysis 2.3).
Carer burden
Compared with the inactive control (i.e. respite‐only control), we were uncertain whether or not MBSR could reduce carer burden after the intervention period due to the very low quality of the evidence: MD ‐1.60 (95% CI ‐19.48 to 16.28; P = 0.86; 1 trial; 17 participants; very low‐quality evidence, downgraded two levels for very serious concern about imprecision and one level for high risk of bias) (Analysis 2.4).
Coping style
Again due to the very low quality of the evidence, we were uncertain whether or not MBSR had any effect on the use of positive coping strategies compared with the respite‐only control immediately after the intervention period: MD 7.90 (95% CI ‐5.41 to 21.21; P = 0.24; 1 trial; 17 participants; very low‐quality evidence, downgraded two levels for very serious concern about imprecision and one level for high risk of bias) (Analysis 2.5).
Carers' quality of life
None of the included trials reported this outcome.
Dropout rates
We were uncertain whether or not there was a significant difference in dropout rates in the MBSR group compared with the respite‐only control: RR 2.00 (95% CI 0.21 to 18.69; P = 0.54; 1 trial; 20 participants; very low‐quality evidence, downgraded two levels for very serious concern about imprecision and one level for high risk of bias) (Analysis 2.6).
Quality of life of people with dementia
None of the included trials reported this outcome.
Institutionalisation
None of the included trials reported this outcome.
3. MBSR versus controls at postintervention follow‐up
Two trials reported data at follow‐up within three months. Their results were not suitable for meta‐analysis because they compared MBSR with different kinds of control groups (i.e. active or inactive control).
-
Compared with the active‐control intervention, the results for depressive symptoms suggested that there may be little or no effect of MBSR: MD ‐0.16 (95% CI ‐0.71 to 0.39; P = 0.57; 1 trial; 38 participants; low‐quality evidence, downgraded one level for serious concern about imprecision and one level for high risk of bias) (Analysis 3.1). The results for carer burden favoured the active‐control intervention, but was too imprecise for us to be sure of the size or direction of the effect: MD 6.62 (95% CI ‐4.92 to 18.16; P = 0.26; 1 trial; 38 participants; low‐quality evidence, downgraded one level for serious concern about imprecision and one level for high risk of bias) (Analysis 3.2).
-
Compared with the inactive control, the results for carers' depressive symptoms slightly favoured MBSR but were too imprecise for us to be sure of the size or direction of the effect: MD ‐3.00 (95% CI ‐8.52 to 2.52; P = 0.29; 1 trial; 31 participants; low‐quality evidence, downgraded one level for serious concern about imprecision and one level for high risk of bias) (Analysis 4.1). Due to the very low quality of the evidence, we were uncertain whether or not MBSR had any effect on the risk of clinically significant depressive symptoms at follow‐up within three months: RR 0.55 (95% CI 0.20 to 1.49; P = 0.24; 1 trial; 31 participants; very low‐quality evidence, downgraded two levels for very serious concern about imprecision and one level for high risk of bias) (Analysis 4.2). There may also be a reduction in anxiety in the MBSR group at follow‐up within three months, although due to imprecision we could not rule out little or no effect: MD ‐6.92 (95% CI ‐14.60 to 0.76; P = 0.08; 1 trial; 31 participants; low‐quality evidence, downgraded one level for serious concern about imprecision and one level for high risk of bias) (Analysis 4.3).
Two trials reported data at follow‐up between three and six months. Both trials compared MBSR to an active‐control intervention.
-
Compared with the active‐control intervention, the results for carers' depressive symptoms slightly favoured MBSR, although due to imprecision we could not rule out little or no effect: MD ‐3.20 (95% CI ‐6.80 to 0.40; P = 0.08; 1 trial; 78 participants; low‐quality evidence, downgraded one level for serious concern about imprecision and one level for high risk of bias) (Analysis 5.1). Again due to the very low quality of the evidence, we were uncertain whether or not MBSR had any effect on the risk of clinically significant depressive symptoms: RR 1.00 (95% CI 0.25 to 4.00; P = 1.00; 1 trial; 24 participants; very low‐quality evidence, downgraded two levels for very serious concern about imprecision and one level for high risk of bias) (Analysis 5.2). Compared to an active‐control intervention, MBSR may reduce levels of anxiety: MD ‐6.50 (95% CI ‐12.00 to ‐1.00; P = 0.02; 1 trial; 78 participants; low‐quality evidence, downgraded one level for serious concern about imprecision and one level for high risk of bias) (Analysis 5.3). The results for carer burden also slightly favoured the active‐control intervention, but again due to imprecision we could not rule out little or no effect: MD 0.90 (95% CI ‐0.55 to 2.35; P = 0.22; 1 trial; 78 participants; low‐quality evidence, downgraded one level for serious concern about imprecision and one level for high risk of bias) (Analysis 5.4).
4. Subgroup and sensitivity analyses
We did not conduct subgroup analyses due to low levels of heterogeneity in most of the meta‐analyses (I2 = 0%) and an insufficient number of included studies in the comparisons (n < 5).
One included study measured carer burden using two different scales (Oken 2010). The effect of MBSR versus control on carer burden immediately after the intervention period did not change significantly after including outcomes from the alternative scale in the meta‐analyses (MBSR versus active control: 3 trials, 135 participants, SMD 0.20, 95% CI ‐0.14 to 0.55, P = 0.24; MBSR versus inactive control: 1 trial, 17 participants, MD ‐1.50, 95% CI ‐20.12 to 17.15, P = 0.87). Since all of the included trials had a high risk of detection bias, we did not conduct sensitivity analyses by excluding individual studies at high risk of bias.
Discussion
Summary of main results
This review of five randomised controlled trials with a total of 201 family carers of people with dementia evaluated the effects of MBSR on stress‐related outcomes. We conducted two comparisons according to the type of control intervention: MBSR versus active control and MBSR versus inactive control.
MBSR versus active control
For our primary review outcome, we found from a meta‐analysis of 3 studies (135 participants) that there may be a reduction in depressive symptoms for carers receiving MBSR compared with an active‐control intervention immediately after the intervention period (low‐quality evidence). One small study (24 participants) reported clinically significant depressive symptoms in participants, but we could not be certain of any effect on this outcome (very low‐quality evidence).
Regarding our secondary outcomes, MBSR may reduce carers' anxiety and slightly increase carer burden compared with active control immediately after the intervention period (low‐quality evidence), although both results were imprecise, and there could also have been little or no effect. Due to the very low quality of the evidence, we could not be sure of any effect on carers' coping style, nor could we determine whether carers were more or less likely to drop out of treatment. There were no data on quality of life of carers or people with dementia, or on institutionalisation.
Some included trials followed participants up to six months after the end of the intervention, but all results at postintervention follow‐up were very imprecise due to small sample sizes.
MBSR versus inactive control
For our primary review outcome of depressive symptoms, the results of a meta‐analysis of 2 studies (50 participants) were imprecise and showed no clear evidence of any effect (low‐quality evidence). We could not be certain of any effect on clinically significant depressive symptoms (1 trial, 33 participants, very low‐quality evidence).
Regarding our secondary outcomes, MBSR may be associated with a reduction of levels of anxiety immediately after the intervention period when compared with an inactive control, although we could not exclude little or no effect (low‐quality evidence). Due to the very low quality of the evidence, we could not be certain of any effects of MBSR on carer burden, the use of positive coping strategies, or dropout rates. There were no data on quality of life of carers or people with dementia, or on institutionalisation.
There were very few data from one small trial at follow‐up within three months, but the results were all very imprecise.
Adverse events
More information is needed. Only one included study reported on adverse events, noting one adverse event in a man aged 80 who reported neck strain from home practice, suggesting that MBSR practices performed at home, especially those including yoga practices, should be appropriately adapted to the physical limitations of older carers.
Overall completeness and applicability of evidence
We used an extensive search strategy including a comprehensive range of databases and other sources relevant to the focus of the review. It is therefore unlikely that we missed references that meet our eligibility criteria. Another strength of this review is that we included both active and inactive types of control groups. However, the applicability of the evidence was limited by several study characteristics. First, much emphasis of the included trials was placed on measures of depressive symptoms and carer burden, rather than other outcomes such as levels of anxiety, coping style, institutionalisation, or quality of life for carers or people with dementia. Stress theory suggests that stressors such as dementia caregiving may manifest their effects in multiple ways (Almeida 2005; Pearlin 1990). As a consequence, a focus on depression or burden as outcomes may not fully capture what carers of people with dementia might get out of MBSR. Second, the small number of included studies as well as insufficient numbers of participants might be underpowered to detect evidence of small effects favouring MBSR over control, or vice versa, indicating that further large‐scale and rigorously designed research is needed in this field. Moreover, as no studies have assessed effects longer than six‐month follow‐up, the evidence can be applied only to short‐term effects.
Quality of the evidence
We assessed the quality of the evidence as low to very low mainly due to high risk of detection and performance bias and imprecision of study results (summary of findings Table for the main comparison; summary of findings Table 2).
Potential biases in the review process
As described in Assessment of reporting biases, due to the limited data available, we were unable to generate funnel plots. We cannot exclude the possibility of publication bias.
Agreements and disagreements with other studies or reviews
We identified one recent review that concerns mindfulness‐based interventions for family carers of people with dementia (Kor 2017). This review included both randomised controlled trials and quasi‐experimental studies, unlike our review, which focused exclusively on randomised controlled trials, which is the best available evidence. The reviews also differed in terms of inclusion criteria of interventions or outcomes. We also considered the impact of different types of control groups (i.e. active or inactive control) on the effect of MBSR. In other words, this Cochrane Review differentiates the specific and non‐specific effect of MBSR versus controls for family carers of people with dementia.
Despite differences in types of interventions, assessed outcomes, data comparison and analyses between this review and ours, both reviews found that evidence of MBSR for carers is still preliminary, and that based on the available evidence, no clear conclusion could be reached.
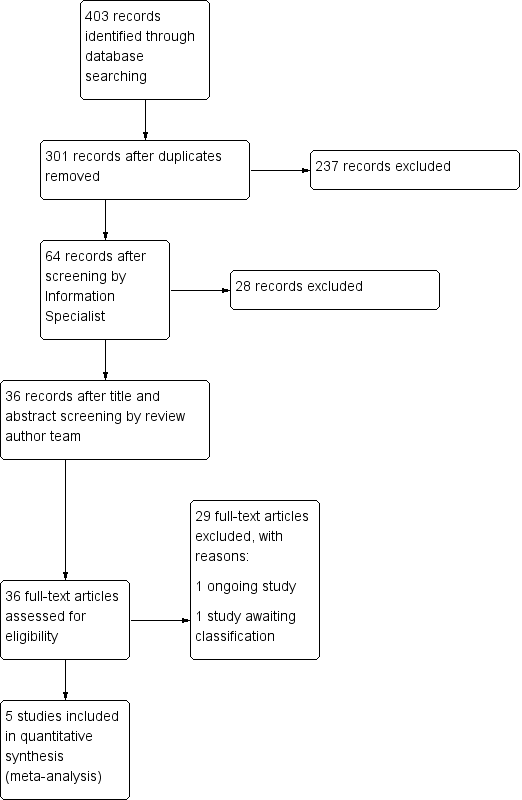
Study flow diagram.
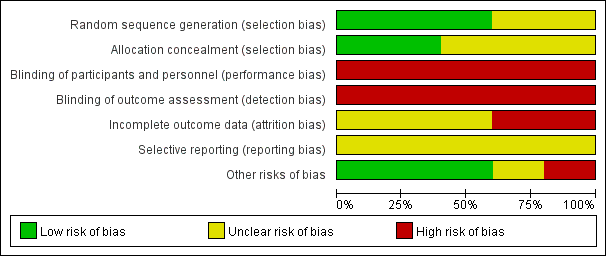
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
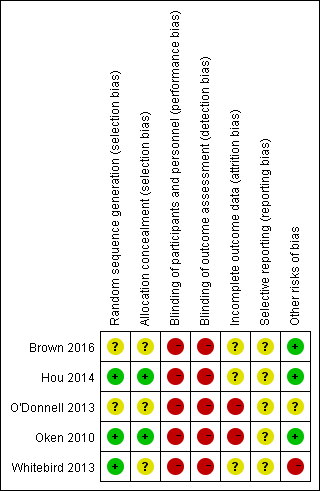
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 MBSR versus active control at immediately postintervention, outcome: 1.1 Depressive symptoms.

Forest plot of comparison: 1 MBSR versus active control at immediately postintervention, outcome: 1.4 Carer burden.

Forest plot of comparison: 1 MBSR versus active control at immediately postintervention, outcome: 1.7 Dropout rates.
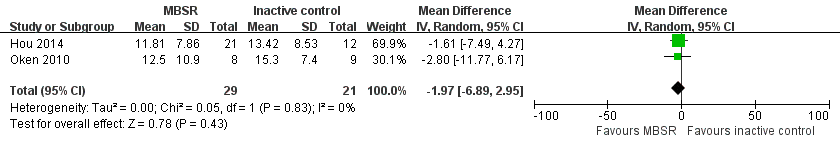
Forest plot of comparison: 2 MBSR versus inactive control at immediately postintervention, outcome: 2.1 Depressive symptoms.

Comparison 1 MBSR versus active control immediately postintervention, Outcome 1 Depressive symptoms.

Comparison 1 MBSR versus active control immediately postintervention, Outcome 2 Clinically significant depressive symptoms.

Comparison 1 MBSR versus active control immediately postintervention, Outcome 3 Anxiety.

Comparison 1 MBSR versus active control immediately postintervention, Outcome 4 Carer burden.

Comparison 1 MBSR versus active control immediately postintervention, Outcome 5 Coping style.

Comparison 1 MBSR versus active control immediately postintervention, Outcome 6 Dropout rates.

Comparison 2 MBSR versus inactive control immediately postintervention, Outcome 1 Depressive symptoms.

Comparison 2 MBSR versus inactive control immediately postintervention, Outcome 2 Clinically significant depressive symptoms.

Comparison 2 MBSR versus inactive control immediately postintervention, Outcome 3 Anxiety.

Comparison 2 MBSR versus inactive control immediately postintervention, Outcome 4 Carer burden.

Comparison 2 MBSR versus inactive control immediately postintervention, Outcome 5 Coping style.

Comparison 2 MBSR versus inactive control immediately postintervention, Outcome 6 Dropout rates.
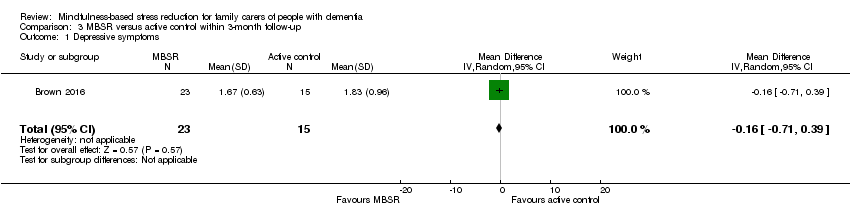
Comparison 3 MBSR versus active control within 3‐month follow‐up, Outcome 1 Depressive symptoms.
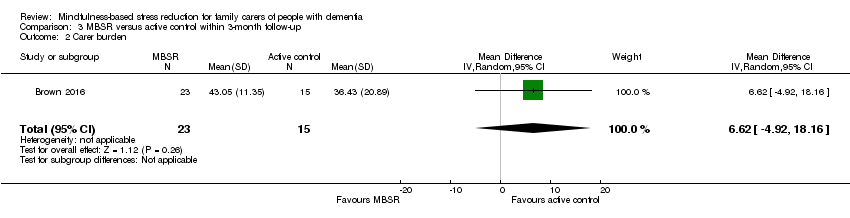
Comparison 3 MBSR versus active control within 3‐month follow‐up, Outcome 2 Carer burden.
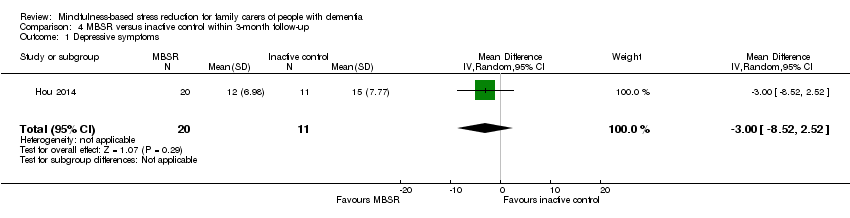
Comparison 4 MBSR versus inactive control within 3‐month follow‐up, Outcome 1 Depressive symptoms.

Comparison 4 MBSR versus inactive control within 3‐month follow‐up, Outcome 2 Clinically significant depressive symptoms.
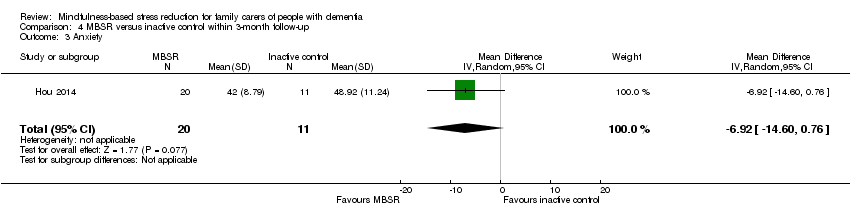
Comparison 4 MBSR versus inactive control within 3‐month follow‐up, Outcome 3 Anxiety.
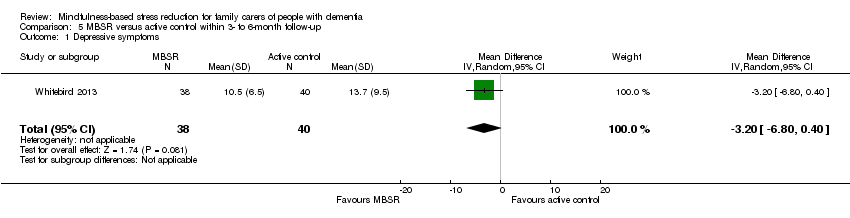
Comparison 5 MBSR versus active control within 3‐ to 6‐month follow‐up, Outcome 1 Depressive symptoms.

Comparison 5 MBSR versus active control within 3‐ to 6‐month follow‐up, Outcome 2 Clinically significant depressive symptoms.

Comparison 5 MBSR versus active control within 3‐ to 6‐month follow‐up, Outcome 3 Anxiety.

Comparison 5 MBSR versus active control within 3‐ to 6‐month follow‐up, Outcome 4 Carer burden.
| MBSR (mindfulness‐based stress reduction) compared to active control for family carers of people with dementia | ||||||
| Patient or population: family carers of people with dementia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with active control | Risk with MBSR | |||||
| Depressive symptoms | ‐ | SMD 0.63 SD lower | ‐ | 135 | ⊕⊕⊝⊝ | Moderate effect size;c lower score represents lower depressive symptoms |
| Anxiety | The mean anxiety was 47.4 score. | MD 7.5 score lower | ‐ | 78 | ⊕⊕⊝⊝ | Moderate effect size;c lower score represents lower level of anxiety |
| Carer burden | ‐ | SMD 0.24 SD higher | ‐ | 135 | ⊕⊕⊝⊝ | Small effect size;c lower score represents lower level of carer burden |
| Dropout rates | Study population | RR 1.39 | 166 | ⊕⊝⊝⊝ | Important effect sizee | |
| 88 per 1000 | 122 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWe downgraded the quality of evidence by one level due to serious concern about high risk of bias in blinding of participants and personnel. | ||||||
| MBSR (mindfulness‐based stress reduction) compared to inactive control for family carers of people with dementia | ||||||
| Patient or population: family carers of people with dementia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with inactive control | Risk with MBSR | |||||
| Depressive symptoms | The mean depressive symptoms was 14.2 score. | MD 1.97 score lower | ‐ | 50 | ⊕⊕⊝⊝ | Small effect size;c lower score represents lower depressive symptoms |
| Anxiety | The mean anxiety was 47.8 score. | MD 7.27 score lower | ‐ | 33 | ⊕⊕⊝⊝ | Moderate effect size;c lower score represents lower level of anxiety |
| Carer burden | The mean carer burden was 26.4 score. | MD 1.6 score lower | ‐ | 17 | ⊕⊕⊝⊝ | Small effect size;c lower score represents lower level of carer burden |
| Dropout rates | Study population | RR 2.00 | 20 | ⊕⊝⊝⊝ | Important effect sizee | |
| 100 per 1000 | 200 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aAs suggested by Ryan 2016a, we downgraded the quality of evidence by one level due to serious concern about imprecision. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Depressive symptoms Show forest plot | 3 | 135 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐0.98, ‐0.28] |
| 2 Clinically significant depressive symptoms Show forest plot | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 2.77] |
| 3 Anxiety Show forest plot | 1 | 78 | Mean Difference (IV, Random, 95% CI) | ‐7.5 [‐13.11, ‐1.89] |
| 4 Carer burden Show forest plot | 3 | 135 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.11, 0.58] |
| 5 Coping style Show forest plot | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 6.0 [‐4.59, 16.59] |
| 6 Dropout rates Show forest plot | 4 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.32, 5.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Depressive symptoms Show forest plot | 2 | 50 | Mean Difference (IV, Random, 95% CI) | ‐1.97 [‐6.89, 2.95] |
| 2 Clinically significant depressive symptoms Show forest plot | 1 | 33 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.26, 1.78] |
| 3 Anxiety Show forest plot | 1 | 33 | Mean Difference (IV, Random, 95% CI) | ‐7.27 [‐14.92, 0.38] |
| 4 Carer burden Show forest plot | 1 | 17 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐19.48, 16.28] |
| 5 Coping style Show forest plot | 1 | 17 | Mean Difference (IV, Random, 95% CI) | 7.90 [‐5.41, 21.21] |
| 6 Dropout rates Show forest plot | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.21, 18.69] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Depressive symptoms Show forest plot | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.71, 0.39] |
| 2 Carer burden Show forest plot | 1 | 38 | Mean Difference (IV, Random, 95% CI) | 6.62 [‐4.92, 18.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Depressive symptoms Show forest plot | 1 | 31 | Mean Difference (IV, Random, 95% CI) | ‐3.0 [‐8.52, 2.52] |
| 2 Clinically significant depressive symptoms Show forest plot | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.20, 1.49] |
| 3 Anxiety Show forest plot | 1 | 31 | Mean Difference (IV, Random, 95% CI) | ‐6.92 [‐14.60, 0.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Depressive symptoms Show forest plot | 1 | 78 | Mean Difference (IV, Random, 95% CI) | ‐3.20 [‐6.80, 0.40] |
| 2 Clinically significant depressive symptoms Show forest plot | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.25, 4.00] |
| 3 Anxiety Show forest plot | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐6.5 [‐12.00, 1.00] |
| 4 Carer burden Show forest plot | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.55, 2.35] |

